Abstract
Rising global prevalence and incidence of obesity lead to increased cardiovascular-renal complications and cancers. Epidemiological studies reported a worldwide trend towards suboptimal sleep duration and poor sleep quality in parallel with this obesity epidemic. From rodents and human models, it is highly plausible that abnormalities in sleep, both quantity and quality, impact negatively on energy metabolism. While excess dietary intake and physical inactivity are the known drivers of the obesity epidemic, promotion of healthy sleep habits has emerged as a new target to combat obesity. In this light, present review focuses on the existing literature examining the relationship between sleep physiology and energy homeostasis. Notably, sleep dysregulation perturbs the metabolic milieu via alterations in hormones such as leptin and ghrelin, eating behavior, neuroendocrine and autonomic nervous systems. In addition, shift work and trans-meridian air travel may exert a negative influence on the hypothalamic-pituitary-adrenal axis and trigger circadian misalignment, leading to impaired glucose tolerance and increased fat accumulation. Amassing evidence has also suggested that uncoupling of the circadian clock can increase the risk of adverse metabolic health. Given the importance of sleep in maintaining energy homeostasis and that it is potentially modifiable, promoting good sleep hygiene may create new avenues for obesity prevention and treatment.
Keywords: Sleep, Energy metabolism, Leptin, Ghrelin, Pituitary-adrenal system, Circadian rhythm
INTRODUCTION
The global prevalence of obesity has doubled since 1980, affecting 107.7 million children and 603.7 million adults in 2015, with more than two-thirds of the United States population being either overweight or obese.1 This rapid surge in obesity is driven mainly by lifestyle factors such as physical inactivity, unhealthy dietary choices and patterns.2 Emerging evidence suggests that sleep disturbances (e.g., suboptimal sleep duration, poor sleep quality, circadian misalignment and insomnia), may contribute to obesity and type 2 diabetes mellitus (T2DM).2 Physiologically, sleep duration declines during transition from infancy, puberty to late adulthood.3 Optimal sleep duration has been a matter of controversy but recent consensus of the American Academy of Sleep Medicine defines short sleep duration as <8–10 hours and <7 hours per day in adolescents and adults aged 18–60 years respectively, considering the potential risks in association with the development of cardiometabolic disease and death.4–6 Due to increasing demand from school, work and leisure activities2, along with the prevalent usage of Internet and electronic devices, there is a global phenomenon for a shift in both adolescents and adults to have shorter sleep duration compared to a few decades ago. Based on the 2014 U.S. Behavioral Risk Factor Surveillance System, more than one-third of adults were short sleepers, particularly prevalent in those who were young, obese (body mass index [BMI] ≥30 kg/m2) or with low socioeconomic and education status.7 In China, a meta-analysis of 17 cross-sectional population-based studies reported that every one in six adults had insomnia, which was again more commonly seen in young individuals.8 Paradoxically, long sleep duration (>9 hours per day) was also observed in 23%–37% of the general population in developed countries9, and a meta-analysis of 137 prospective cohort studies reported a dose-response relationship with incident cardiovascular disease and death, attesting a U-shaped relationship between sleep duration and health outcomes across all age groups.10,11 Alarmingly, poor sleep quality (defined as global Pittsburgh Sleep Quality Index score >5) was also independently associated with an increase in BMI and worse glycemic control.6 Given that obesity is closely linked to multiple chronic diseases, notably diabetes and cancer which are the leading causes of morbidities and mortality in many parts of the world12, the perplexing relationship between sleep and energy metabolism call for more research and clinical attention, as sleep is an essential part of life and a potentially modifiable behavioral risk factor for metabolic health. Here, we review some of the important findings in this field, and discuss the mechanisms linking sleep, energy metabolism and obesity.
SLEEP AND NEUROHORMONAL DYSREGULATION
Sleep and satiety
The appetite center, located at the ventromedial and arcuate nuclei of the hypothalamus, is regulated by hormones including leptin and ghrelin. Leptin is an adipocyte-derived hormone which suppresses appetite, whilst ghrelin is mainly a stomach-derived hunger-promoting peptide.13,14 High total energy and fat intake, night-time snacking and binge eating tendencies have been reported to be associated with sleep curtailment.15,16 The potential mechanisms mediating the effects of sleep debt on obesity are complex, which include alterations in eating behavior (e.g., skipping meals, snacking, and irregular meal times), increased ghrelin to leptin ratio and activation of hedonic pathways.15,17,18 Several small scale experimental studies involving the healthy population (number of subjects, 11 to 26 in each study) examined the effect of sleep restriction on dietary intake and patterns under the controlled environment, which included objective sleep measurements and planned meals in both phases of short and habitual sleep.19,20 Sleep restriction was reported to be associated with an increase in total energy, total fat and saturated fatty acids intake19, as well as high consumption of carbohydrate-rich night-time snacks.20 These were accompanied by a lack of compensatory increase or even with a reduction in 24-hour energy expenditure, leading to positive energy balance.19,20 In keeping with these findings, an inverse relationship between sleep duration and total energy and macronutrient intake was evident in epidemiological cohorts.15,17,21,22 In a meta-analysis of 14,906 Europeans from the Cohorts for the Heart and Aging Research in Genomic Epidemiology Consortium, younger people (aged 20–64 years) with short sleep duration were independently associated with higher relative saturated fatty acids intake, whereas older women (aged 65–80 years) with similar exposure demonstrated higher relative carbohydrate, lower relative total fat and polyunsaturated fatty acids consumption.22 In the 2005–2010 U.S. National Health and Nutrition Examination Survey involving 15,199 community-dwelling adults, short sleepers reported frequent snacking and increased total glucose intake.17 Similarly, amongst 2,828 Chinese adults, those with less than 7-hour sleep had excess fat intake than the group with 7- to 9-hour sleep.21
Accumulating evidence suggests that sleep disruption may interfere with the feeding and satiety signals at the hypothalamic feeding circuits.23 A shift towards increased hunger in short sleepers was driven by decreased leptin level with or without a concomitant change in the diurnal rhythm amplitude of leptin, increased ghrelin level or both.24,25 In healthy young adults with stable caloric intake and activity levels, 6 days of sleep restriction (4 hours in bed per night) was independently associated with a 26% reduction in leptin than sleep extension (12 hours in bed per night), on top of a flattened diurnal profile of leptin secretion.25 In the prospective Wisconsin Sleep Cohort Study involving 1,024 healthy adults, compared with those with 8-hour of sleep, short sleepers (5-hour of sleep per day) had a 16% decrease in leptin and 15% increase in ghrelin level.24 Interestingly, diminished activity of the appetitive desire and food stimulus evaluation regions within the frontal, insular and cingulate cortices, along with enhanced activity of amygdala are other plausible pathways linking sleep curtailment to obesity.26–28 The disrupted neural circuits led to hedonic hunger and preferences for highly palatable and rewarding energy-dense food26–28, albeit with inter-individual variation in the magnitude of change in eating behavior proportional to the severity of sleep curtailment.26 On the other hand, short sleepers have prolonged wakefulness that promotes obesogenic eating behavior e.g., meal-skipping, frequent snacking and increase intake of low-quality diet.17,29,30 Late-time eating, which was defined as caloric intake after 8:00 PM, also significantly predicted an increase in BMI, suggesting that eating late at night may lead to obesity.29 Moreover, decreased nocturnal ghrelin levels were found in men with insomnia compared with their age- and body weight-matched healthy control (n=25), while no difference of leptin levels was found between groups.31
Sleep and growth hormone-insulin like growth factor-1 axis
The growth hormone (GH)-insulin like growth factor-1 (IGF-1) axis also plays a role in the regulation of adiposity and glucose homeostasis. Under a normal sleep/wake cycle, there is a spontaneous nocturnal GH pulse during the restorative slow wave sleep (SWS) that occurs within the first 3 hours of sleep, with both demonstrating a dose-dependent relationship.32 Increasing evidence suggests that there are biological interactions of GH/IGF-1 axis with sleep dysregulation. Sleep curtailment and late chronotype are associated with suppressed GH and IGF-1 pulsatility, followed by a compensatory increase in IGF-binding protein 3 (IGFBP-3) level via the negative feedback loop.33 In a 4-year prospective cohort study of normoglycemic adults, low circulating IGF-1 level had a 50% excess risk of developing either impaired glucose tolerance or T2DM, consequent to decreased peripheral insulin sensitivity and hyperinsulinemia.34 In the Danish-Monitoring Trends in Cardiovascular Diseases study, after a mean follow-up of 15 years, either a low IGF-1 or high IGFBP-3 level was independently associated with a relative risk (RR) of 1.94–2.22 of incident coronary heart disease, after adjustment for other cardiovascular risk factors.35 Several mechanisms were postulated to explain the pleiotropic effects of GH/IGF-1 axis on the retardation of atherosclerosis e.g., reduced systemic and vascular oxidative stress (low interleukin-6 and tumor necrosis factor-alpha levels), decreased aortic stiffness and control of de novo hepatic lipid metabolism.35,36
In contrast to the aforementioned findings, another experimental sleep restriction (4 hours in bed per night for six consecutive nights) study involving 11 healthy young men revealed a biphasic pattern of nocturnal GH release, with the first pulse occurred at 3-hour prior to the sleep onset and the second peak corresponded to the usual circadian rhythm as seen during the sleep extension period (12 hours in bed per night for a week).32 This prolonged exposure to elevated GH level may interact with the hunger-promoting ghrelin centrally or stimulate peripheral glucose and lipid catabolism, resulting in insulin resistance and positive energy balance.37 These findings were supported by some but not all observational studies, which reported a U-shaped relationship between IGF-1 to IGFBP-3 ratio, anthropometric traits (e.g., BMI and waist circumference) or risk of metabolic syndrome.38,39 To this end, a better understanding of the cellular-microenvironment interactions and their downstream signaling cascades integrating sleep, GH/IGF-1 axis and metabolic functions is warranted.
Sleep and hypothalamic-pituitary-adrenal axis
Sleep disturbances, including short sleep, sleep debt, and circadian misalignment, may also disrupt glucose homeostasis and cause metabolic perturbations via other hormonal and cellular signaling cascades.23,24,40 In a pilot study conducted in 11 healthy young men, decreased glucose tolerance, increased evening cortisol level and sympathetic over-activity were observed after sleep restriction (4 hours in bed per night for six consecutive nights) compared with the measurements taken after a sleep-restorative period (12 hours in bed per night for seven consecutive nights), indicating that sleep debt had a detrimental effect on glucose metabolism and endocrine function.40
The bidirectional relationships between sleep and the activity of hypothalamic-pituitary-adrenal (HPA) axis may exert negative metabolic consequences. Dysregulation of the HPA axis and its circadian rhythm can mediate the impact of sleep disturbances on cardiometabolic risk, mainly via the actions of two counter-regulatory hormones, namely the glucocorticoids and catecholamines. Physiologically, cortisol level peaks after 30–45 minutes of awakening (defined as cortisol awakening response [CAR]), followed by a sharp decline over the next 3 hours, more gradual decline over the rest of the day, and reaching nadir during the first half of sleep cycle.41 Existing studies examining the associations between sleep and circadian variability of cortisol secretion is inconclusive, due to differences in the sleep measurements (actigraphy/polysomnography vs. self-reported sleep parameters), definitions used for sleep duration and quality, and the number of real-time sampling points for modelling of 24-hour cortisol profile. Several reports indicated that short sleepers have flattened diurnal cortisol slope and raised evening cortisol followed by an aggravated CAR, yielding an increase in total daily cortisol secretion which ends up in a state of hypercortisolism and catabolism.42–44 In school-age children, high CAR and subsequent diurnal cortisol level were inversely associated with short sleep duration, low sleep efficiency and low frequency of SWS.45,46 Conversely, two other studies found no association between objective sleep duration and salivary awakening cortisol44 or 24-hour urinary cortisol level.47 Poor sleep quality has been reported to be an independent stressor for excess cortisol and catecholamines secretion, particularly with greater magnitude in adolescents at late puberty.42,44 Increased HPA axis activation has also been reported in subjects with insomnia.48,49 Furthermore, it has been suggested that a gender difference on this stress reaction related to sleep disturbances which may be modulated by the type of sleep disorders involved, calling for more studies to investigate the impact of sex hormones on sleep and energy balance.44,50
Sympathetic over-activity may also contribute to the neurohormonal dysregulation linking sleep curtailment to energy imbalance. In addition to a 1.5-hour delay in the nocturnal nadir of cortisol level, experimental sleep restriction with 4 hours in bed per night for six consecutive nights in healthy volunteers had shown a concomitant 24-hour lowering of heart rate variability (measured by the autocorrelation coefficient of consecutive interbeat intervals) especially during awakening, which reflected an increase in cardiac sympathetic activity and/or diminished vagal tone, compared with sleep extension phase (12 hours in bed per night for seven consecutive nights).25 Physiologically, sleep onset is associated with decreased catecholamines release, reaching a nocturnal nadir an hour into a restful sleep.51 Amongst short sleepers, the secretion of norepinephrine was pathologically elevated during early awakening in early morning, between 3:00 AM and 6:00 AM, with epinephrine showing a similar trend.44,51 Furthermore, patients with mild to moderate hypertension with acute sleep restriction reported an early morning elevation in blood pressure (systolic blood pressure of 7 mmHg and diastolic blood pressure of 4 mmHg) and heart rate (5.5 beats per minute) with potential risk of silent cardiovascular event, as sequelae of their blunted nocturnal dipping related to a surge in norepinephrine release.52
SLEEP, OBESITY AND DIABETES
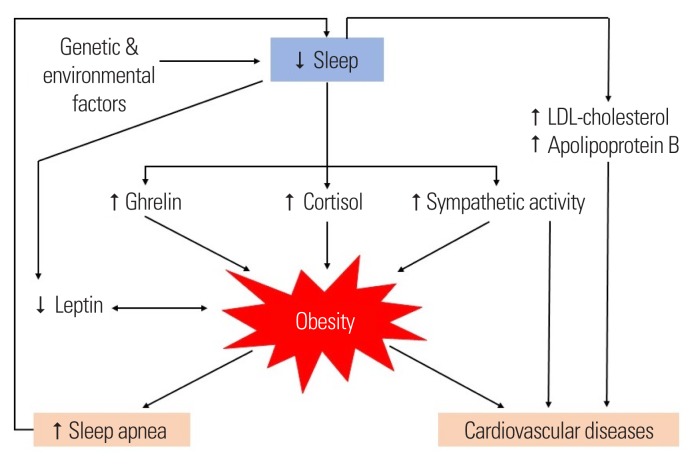
Multiple studies have consistently demonstrated significant associations between sleep curtailment with decreased resting and postprandial energy expenditure (~5% and 20%, respectively), increased appetite and insulin resistance in general population.2,11 In addition, a randomized cross-over study demonstrated a 30% decrease in the intracellular insulin sensitivity within adipocytes from the subcutaneous fat samples of seven healthy adults collected after sleep restriction compared with after normal sleep.53 It has also been demonstrated that short sleep duration is associated with atherogenic dyslipidemia.54,55 Once obesity sets in, there may be a vicious cycle as obesity associated comorbidities such as obstructive sleep apnea may further compromise sleep quality in affected individuals (Fig. 1). Taken together, evidence to date suggests that sleep curtailment affects the resiliency of neurohormonal stress response and predisposes to metabolic disturbances with possible long-term cardiovascular risk. Besides sleep curtailment, there have been a wealth of clinical evidence supporting the association of sleep disturbances, both quantity and quality, with obesity and diabetes (Tables 1 and 2).11,56–103 However, the associations of insomnia with obesity and glucose metabolism is under debate.
Figure 1.
Association between sleep, obesity, cardiovascular risks and possible underlying neurohormonal dysregulation. LDL, low-density lipoprotein.
Table 1.
Clinical studies examining the association between sleep duration with obesity and diabetes
| Author (year) | Type of study/country or region | Study population (sample size) | Definition of sleep disturbances | Method of sleep assessment | Age (yr) and male (%) | Outcome |
|---|---|---|---|---|---|---|
| Sleep duration and overweight/obesity | ||||||
| Ayas et al. (2003)11 | Prospective (mean follow-up: 10 yr)/the United States | Female married registered nurses from the Nurses’ Health Study (n=70,026) | Short sleep: ≤5 hr/day Long sleep: ≥9 hr/day Reference: 8 hr/day |
Self-reported sleep duration | 40–65, 0% | New-onset obesity Short sleep: RR, 1.18; 95% CI, 0.96–1.44 Long sleep: RR, 1.29; 95% CI, 1.05–1.59 |
| Patel et al. (2006)56 | Prospective study (follow-up: 16 yr)/the United States | Female nurses from the Nurses’ Health Study (n=68,183) | Sleep duration was categorized as: ≤5, 6, 7, 8, and ≥9 hr/day Reference: 7 hr/day |
Self-reported | 39–65, 0% | New-onset obesity Sleep duration ≤5 hr/day: HR, 1.15; 95% CI, 1.04–1.27 Sleep duration 6 hr/day: HR, 1.06; 95% CI, 1.01–1.12 Sleep duration ≥9 hr/day: HR, 1.03; 95% CI, 0.93–1.14 ≥15 kg weight gain Sleep duration ≤5 hr/day: HR, 1.28; 95% CI, 1.15–1.42 Sleep duration 6 hr/day: HR, 1.10; 95% CI, 1.04–1.17 Sleep duration ≥9 hr/day: HR, 1.04; 95% CI, 0.92–1.16 |
| Chaput et al. (2008)57 | Prospective study (follow-up: 6±0.9 yr)/Canada | General population form the Quebec Family Study (n=276; nonobese: n=224) | Short sleep: 5–6 hr/day Long sleep: 9–10 hr/day Reference: 7–8 hr/day |
Self-reported | 21–64, 42.4% | Weight gain Short-duration sleepers gained 1.84 kg; 95% CI, 1.08–2.61 Long-duration sleepers gained 1.49 kg; 95% CI, 0.92–2.48 New-onset obesity Short-duration sleepers: OR, 1.27; 95% CI, NS Long-duration sleepers: OR, 1.21; 95% CI, NS |
| López-García et al. (2008)58 | Prospective study (follow-up: 2 yr)/Spain | Elderly population (n=3,235) | Sleep duration was categorized as: ≤5, 6, 7, 8, 9, and ≥10 hr/day Reference: 7 hr/day |
Interview | Male, 71.6±8.0; female, 72.1±7.6, 43.6% | ORs of obesity Sleep ≤5 hr: OR, 1.33; 95% CI, 1.00–1.77 Sleep 8 hr: OR, 1.39; 95% CI, 1.11–1.75 ORs of severe obesity Sleep ≤5 hr: OR, 2.08; 95% CI, 1.31–3.32 Sleep 8 hr: OR, 1.82; 95% CI, 1.21–2.73 Sleep 9 hr: OR, 1.57; 95% CI, 1.00–2.47 Weight gain ≥5 kg In women sleeping ≤5 hr: OR, 3.41; 95% CI, 1.34–8.69 In women sleeping 8 hr: OR, 3.03; 95% CI, 1.29–7.12 In women sleeping 9 hr: OR, 3.77; 95% CI, 1.55–9.17 In total or men: no significant findings |
| Stranges et al. (2008)59 | Prospective study (follow-up: 1997–1999 to 2003–2004)/the United Kingdom | White-collar British civil servants from the Whitehall II Study (n=10,308) | Short sleep: ≤5 hr/day Normal sleep: 7 hr/day |
Self-reported | 35–55, 72.1% | Changes in BMI Short sleep: β, –0.06; 95% CI, −0.26–0.14 Changes in WC Short sleep: β, 0.44; 95% CI, −0.23–1.12 New-onset obesity Short sleep: OR, 1.05; 95% CI, 0.60–1.82 |
| Nishiura et al. (2010)60 | Prospective study (follow-up: 4 yr)/Japan | Nonobese Japanese male workers (n=2,632) | Short sleep: <6 hr/day Normal sleep: 7–7.9 hr/day |
Self-reported | 40–59, 100% | New-onset obesity Short sleep: OR, 2.46; 95% CI, 1.41–4.31 |
| Watanabe et al. (2010)61 | Prospective study (follow-up: 1 yr)/Japan | Employees for an electric power company (n=23,212) | Sleep duration was categorized as: <5, 5–<6, 6–<7, 7–<8, 8–<9, and ≥9 hr/day Reference: 7–<8 hr/day |
Self-reported | 39.8±9.6, 86.3% | Weight gain Male with sleep <5 hr/day: β, 0.016; 95% CI, 0.024–0.146; P<0.01 Male with sleep <5 hr/day: β, 0.013; 95% CI, 0.001–0.061; P=0.04 Male with sleep ≥9 hr/day: β, 0.018; 95% CI, 0.079–0.340; P<0.01 New-onset obesity Male with sleep <5 hr/day: OR, 1.91; 95% CI, 1.36–2.67 Male with sleep 5–6 hr/day: OR, 1.50; 95% CI, 1.24–1.80 No significant association between sleep duration and weight gain or obesity was found for women. |
| Itani et al. (2011)62 | Prospective study (follow-up: 7 yr)/Japan | Workers in a local government organization (n=22,743) | Short sleep: <5 hr/day Normal sleep: 5–7 hr/day |
Self-reported | NS, 95.4% | New-onset obesity Male subjects with short sleep: RR, 1.20; 95% CI, 1.09–1.32 Female subjects with short sleep: RR, 1.71; 95% CI, 1.11–2.87 |
| Lyytikäinen et al. (2011)63 | Prospective study (follow-up: 5–7 yr)/Finland | Middle-aged municipal employees from the Helsinki Health Study (n=7,027) | Short sleep: ≤5 hr/day Long sleep: ≥9 hr/day Reference: 7 hr/day |
Self-reported | 40–60, 18.5% | Weight gain ≥5 kg Female with short sleep: OR, 1.42; 95% CI, 1.01–2.01 Female with long sleep: OR, 1.30; 95% CI, 0.97–1.76 Male with short sleep: OR, 0.88; 95% CI, 0.44–1.74 Male with long sleep: OR, 1.06; 95% CI, 0.48–2.32 |
| Kobayashi et al. (2012)64 | Prospective study (follow-up: 3 yr)/Japan | Healthy population (n=11,136) | Sleep duration was categorized as: ≤5, 6, 7, and ≥8 hr/day Reference: 7 hr/day |
Self-reported | ≥20, 44.0% | Weight gain Sleep ≤5: β coefficient, 0.03; 95% CI, 0.03–1.1; P=0.02 Sleep ≥8: β coefficient, 0.01; 95% CI, −0.03–0.1; P=0.34 New-onset obesity Sleep ≤5: OR, 1.5; 95% CI, 1.1–2.0 Sleep ≥8: OR, 1.3; 95% CI, 0.9–1.8 |
| Yiengprugsawan et al. (2012)65 | Prospective study (follow-up: 4 yr)/Thailand | Distance learners at Sukhothai Thammathirat Open University (n=60,569) | Sleep duration was categorized as <6, 6, 7, 8 and ≥9 hr/day; Short sleep: <6 hr/day Long sleep: ≥9 hr/day Reference: 7 hr/day |
Self-reported | 35.6 (20–49), 45.2% | Overweight Female with short sleep: OR, 1.33; 95% CI, 1.18–1.51 Female with long sleep: OR, 1.22; 95% CI, 1.07–1.39 Male with short sleep: OR, 1.13; 95% CI, 1.00–1.28 Male with long sleep: OR, 1.03; 95% CI, 0.91–1.16 New-onset obesity Female with short sleep: OR, 1.49; 95% CI, 1.32–1.68 Female with long sleep: OR, 1.36; 95% CI, 1.20–1.53 Male with short sleep: OR, 1.36; 95% CI, 1.21–1.52 Male with long sleep: OR, 1.16; 95% CI, 1.03–1.30 |
| Nagai et al. (2013)66 | Prospective study (follow-up: 11 yr)/Japan | General population (n=9,658) | Short sleep: ≤5 hr/day Long sleep: ≥9 hr/day Reference: 7 hr/day |
Self-reported | 40–79, NS | Weight gain ≥5 kg Total subjects with short sleep: OR, 0.93; 95% CI, 0.73–1.20 Total subjects with long sleep: OR, 1.05; 95% CI, 0.91–1.20 BMI ≥25 kg/m2 with short sleep: OR, 0.86; 95% CI, 0.58–1.29 BMI ≥25 kg/m2 with long sleep: OR, 1.36; 95% CI, 1.09–1.70 New-onset obesity Short sleep: OR, 1.08; 95% CI, 0.77–1.51 Long sleep: OR, 1.06; 95% CI, 0.86–1.29 |
| Ohkuma et al. (2013)67 | Cross-sectional study/Japan | Japanese patients with T2DM (n=4,870) | Sleep duration was categorized as: <4.5, 4.5–5.4, 5.5–6.4, 6.5–7.4, 7.5–8.4, and ≥8.5 hr/day; Reference: 6.5–7.4 hr/day |
Self-reported | ≥20, 57% | ORs (95% CIs) for obesity Sleep <4.5 hr/day: OR, 1.78; 95% CI, 1.26–2.52 Sleep ≥8.5 hr/day: OR, 1.24; 95% CI, 0.97–1.58 P for quadratic trend: <0.001 |
| Sayón-Orea et al. (2013)68 | Prospective study (median follow-up: 6.5 yr)/Spain | General population from the SUN Mediterranean Cohort (n=10,532) | Sleep duration was categorized as <5, 5–<7, 7–<8, ≥8 hr/night; Reference: 7–<8 hr/day | Self-reported | 39±12, NS | New-onset obesity Total with sleep <5 hr/night: HR, 1.94; 95% CI, 1.19–3.18 Male with sleep <5 hr/night: HR, 2.09; 95% CI, 1.18–3.69 Female with sleep <5 hr/night: HR, 1.26; 95% CI, 0.44–3.57 Total with sleep ≥8 hr/night: HR, 1.13; 95% CI, 0.89–1.43 Male with sleep ≥8 hr/night: HR, 0.88; 95% CI, 0.64–1.21 Female with sleep ≥8 hr/night: HR, 1.43; 95% CI, 0.97–2.10 |
| Xiao et al. (2013)69 | Prospective study (follow-up: 7.5 yr)/the United States | General population from the National Institutes of Health-AARP Diet and Health Study (n=83,377) | Sleep duration was categorized as <5, 5–6, 7–8, ≥9 hr/day; Reference: 7–8 hr/day |
Self-reported | 51–72, 51.8% | Weight gain ≥5 kg Male with sleep <5 hr/day: OR, 1.27; 95% CI, 1.07–1.52 Female with sleep <5 hr/day: OR, 1.30; 95% CI, 1.12–1.51 Male with sleep ≥9 hr/day: OR, 1.16; 95% CI, 0.99–1.36 Female with sleep ≥9 hr/day: OR, 1.02; 95% CI, 0.88–1.17 New-onset obesity Male with sleep <5 hr/day: OR, 1.45; 95% CI, 1.06–1.99 Female with sleep <5 hr/day: OR, 1.37; 95% CI, 1.04–1.79 Sleep duration ≥9 hr/day Male with sleep ≥9 hr/day: OR, 1.12; 95% CI, 0.84–1.49 Female with sleep ≥9 hr/day: OR, 0.91; 95% CI, NS |
| Vgontzas et al. (2014)70 | Prospective study (total follow-up: 7.5 yr; women: 4.5 yr; men: 10.5 yr)/the United States | General population from the Penn State Cohort (n=815) | Sleep duration was categorized as ≤5, 5–6, 6–7, ≥7 hr/night; Reference: ≥7 hr/night |
Self-reported (subjective) and PSG (objective) | 48.9±13.4, 50.5% | New-onset obesity Subjective sleep duration ≤5 hr/night: OR, 1.08; 95% CI, 0.48–2.41 Objective sleep duration ≤5 hr/night: OR, 0.51; 95% CI, 0.22–1.18 |
| Gutiérrez-Repiso et al. (2014)71 | Prospective study (follow-up: 11 yr)/Spain | General population from the Pizarra cohort study (n=1,145) | Short sleep: ≤7 hr/night Normal sleep: ≥8 hr/night | Self-reported | 18–65, 38.8% | ORs of becoming obese in subjects with short sleep At the 6-yr follow-up: OR, 1.99; 95% CI, 1.12–3.55 At the 11-yr follow-up: OR, 2.73; 95% CI, 1.47–5.04 |
| Kim et al. (2015)72 | Prospective study (follow-up: 2.6 yr)/Korea | General population from the ARIRANG Study (n=3,862) | Short sleep: <6 hr/day Long sleep: ≥10 hr/day Reference: 6–7.9 hr/day |
Self-reported | 40–70, 41.1% | New-onset metabolic syndrome Short sleep: OR, 1.41; 95% CI, 1.06–1.88 Long sleep: OR, 0.68; 95% CI, 0.39–1.17 High WC Short sleep: OR, 1.30; 95% CI, 0.98–1.69 Long sleep: OR, 0.97; 95% CI, 0.62–1.50 |
| Zhang et al. (2015)73 | Cross-sectional study/China | School-aged children (n=3,086) | Sleep duration was categorized as: ≤8.00, 8.01–9.00, 9.01–10.00, and >10 hr/night; Reference: >10 hr/night Sleep compensated group: (weekend [or holiday] sleep duration–weekday sleep duration)/weekday sleep duration×100% ≥10% Reference: noncompensated group |
Self-reported | 7–14, 52.1% | Risk of being overweight/obese Weekdays sleep ≤8.00 hr/night: OR, 1.995; 95% CI, 0.917–4.219 Weekends sleep ≤8.00 hr/night: OR, 2.691; 95% CI, 1.513–4.785 Long holidays sleep ≤8.00 hr/night: OR, 2.921; 95% CI, 1.630–5.323 Sleep compensation during weekends: OR, 1.197; 95% CI, 1.004–1.493 Sleep compensation during holidays: OR, 1.309; 95% CI, 1.052–1.630 |
|
| ||||||
| Sleep duration and risk of diabetes | ||||||
| Ayas et al. (2003)11 | Prospective (mean follow-up: 10 yr)/the United States | Female married registered nurses from the Nurses’ Health Study (n=70,026) | Short sleep: ≤5 hr/day Long sleep: ≥9 hr/day Reference: 8 hr/day |
Self-reported | 40–65, 0% | New-onset symptomatic diabetes Short sleep: RR, 1.34; 95% CI, 1.04–1.72 Long sleep: RR, 1.35; 95% CI, 1.04–1.75 |
| Mallon et al. (2005)74 | Prospective (mean follow-up: 12 yr)/Sweden | General population (n=1,170) | Short sleep: ≤5 hr/night Long sleep: ≥9 hr/night Reference: 5–8 hr/night |
Self-reported | 45–65, 47.0% | New-onset diabetes Male with short sleep: RR, 2.8; 95% CI, 1.1–7.3 Female with short sleep: RR, 1.8; 95% CI, 0.5–6.8 Male with long sleep: NS Female with long sleep: RR, 2.9; 95% CI, 0.6–15.0 |
| Yaggi et al. (2006)75 | Prospective study (follow-up: 15–17 yr)/the United States | Men from the Massachusetts Male Aging Study without diabetes (n=1,139) | Average sleep duration was divided into: ≤5, 6, 7, 8, and >8 hr/night; Reference: 7 hr/night |
Self-reported | 40–70, 100% | New-onset diabetes Sleep ≤5 hr/night: RR, 1.95; 95% CI, 0.95–4.01 Sleep >8 hr/night: RR, 3.12; 95% CI, 1.53–6.37 Further adjusted for testosterone Sleep ≤5 hr/night: RR, 1.51; 95% CI, 0.71–3.19 Sleep >8 hr/night: RR, 2.81; 95% CI, 1.34–5.90 |
| Gangwisch et al. (2007)76 | Prospective study (follow-up: 8–10 yr)/the United States | General population from the First National Health and Nutrition Examination Survey I (n=8,992) | Short sleep: ≤5 hr/night Long sleep: ≥9 hr/night Reference: 7 hr/night |
Self-reported | 32–86, 37% | New-onset diabetes Short sleep: OR, 1.47; 95% CI, 1.03–2.09 Long sleep: OR, 1.52; 95% CI, 1.06–2.18 |
| Hayashino et al. (2007)77 | Prospective study (median follow-up: 4.2 yr)/Japan | Asian workers from High-risk and Population Strategy for Occupational Health Promotion Study (n=6,509) | Short sleep: sleep duration <6 hr/day Long sleep: ≥9 hr/day Reference: 6–7 hr/day |
Self-reported | 38.2 (19–69), 73.9% | New-onset diabetes Short sleep: HR, 1.15; 95% CI, 0.76–1.74 Long sleep: HR, 1.03; 95% CI, 0.62–1.70 |
| Beihl et al. (2009)78 | Prospective study (follow-up: 5 yr)/the United States | General population from the Insulin Resistance Atherosclerosis Study (n=900) |
Short sleep: ≤7 hr/night Long sleep: ≥9 hr/night Reference: 8 hr/night |
Self-reported | 40–69, 43.3% | New-onset diabetes Non-Hispanic whites and Hispanics with short sleep: OR, 2.36; 95% CI, 1.11–5.00 Non-Hispanic whites and Hispanics with long sleep: OR, 2.15; 95% CI, 0.50–9.30 African American with short sleep: OR, 0.63; 95% CI, 0.14–2.90 African American with long sleep: OR, 0.39; 95% CI, 0.02–7.19 |
| Chaput et al. (2009)79 | Longitudinal study (mean follow-up: 6.0±0.9 yr)/Canada | General population from the Quebec Family Study (n=276) | Short sleep: ≤6 hr/night Long sleep: ≥9 hr/night Reference: 7–8 hr/night |
Self-reported | 21–64, 42.4% | New-onset T2DM or IGT Short sleep: OR, 2.42; 95% CI, 1.49–3.33 Long sleep: OR, 2.31; 95% CI, 1.41–3.15 |
| Xu et al. (2010)80 | Prospective study (follow-up: 6 yr)/the United States | General population from the National Institutes of Health-American Association of Retired Persons Diet and Health cohort (n=164,399) | Average sleep duration was divided into: <5, 5–6, 7–8, and ≥9 hr/night; Reference: 7–8 hr/night Day napping was categorized as: none, <1 hr, and ≥1 hr/day; Reference: none |
Self-reported | 50–71, 56.8% | New-onset diabetes Sleep <5 hr/night: OR, 1.34; 95% CI, 1.20–1.50 Sleep 5–6 hr/night: OR, 1.06; 95% CI, 1.01–1.11 Sleep ≥9 hr/night: OR, 1.09; 95% CI, 0.97–1.22 Day napping <1 hr/day: OR, 1.23; 95% CI, 1.18–1.29 Day napping ≥1 hr/day: OR, 1.55; 95% CI, 1.45–1.66 Hours of day napping×night sleep on diabetes: P<0.0001; among participants with no napping, only short night sleeping was associated with higher occurrence of diabetes (OR, 1.32), whereas among those with ≥1 hr of napping, both long (OR, 1.55) and short (OR, 1.78) sleeping was associated with higher risk. |
| Kita et al. (2012)81 | Prospective, occupational-based study (follow-up: 4 yr)/Japan | Local government employees (n=3,570) | Short sleep: ≤5 hr/day Reference: >7 hr/day |
Self-reported | 35–55, 79.0% | New-onset diabetes Short sleep: OR, 5.37; 95% CI, 1.38–20.91 |
| von Ruesten et al. (2012)82 | Prospective study (mean follow-up: 7.8 yr)/Germany | General population from the European Prospective Investigation into Cancer and Nutrition-Potsdam Study (n=23,620) | Short sleep: <6 hr/day Long sleep: ≥9 hr/day Reference: 7–<8 hr/day |
Self-reported | 35–65, 38.6% | New-onset diabetes Short sleep: HR, 1.06; 95% CI, 0.80–1.40 Long sleep: HR, 1.05; 95% CI, 0.82–1.33 |
| Holliday et al. (2013)83 | Prospective study (mean follow-up: 2.3 yr)/Australia | General population from the 45 and Up Study (n=212,388) | Average sleep duration was categorized as: <6, 6–<7, 7–<8, 8–<9, 9–<10, and ≥10 hr/day; Reference: 7–<8 hr/day |
Self-reported | ≥45, 47.3% | New-onset diabetes Sleep <6 hr: HR, 1.29; 95% CI, 1.08–1.53 Sleep ≥10 hr: HR, 1.03; 95% CI, 0.88–1.19 |
| Gutiérrez-Repiso et al. (2014)71 | Prospective study (follow-up: 11 yr)/Spain | General population from the Pizarra cohort study (n=1,145) | Short sleep: ≤7 hr/night Reference: ≥8 hr/night |
Self-reported | 18–65, 38.8% | New-onset diabetes At the 6-yr follow-up, short sleep: OR, 1.96; 95% CI, 1.10–3.50 At the 11-yr follow-up, short sleep: OR, 1.28; 95% CI, 0.60–2.69 |
| Heianza et al. (2014)84 | Prospective study (follow-up: 8 yr)/Japan | Workers (n=38,987) | Average sleep duration was categorized as: <5.5, 5.5–<6.5, 6.5–<7.0, 7.0–7.5, >7.5–8.0, or >8.0 hr/day; Short sleep: <5.5 or 5.5–<6.5 hr/day; Reference: 7.0–7.5 hr/day |
Self-reported | 18–83, 64.2% | New-onset diabetes Sleep <5.5 hr: OR, 1.53; 95% CI, 1.19–1.97 Sleep 5.5–<6.5 hr: OR, 1.25; 95% CI, 1.10–1.42 Sleep >8 hr/day: OR, 1.03; 95% CI, 0.81–1.30 In age ≤45 yr, sleep <5.5 hr: OR, 1.61; 95% CI, 1.08–2.42 In age 46–59 yr, sleep <5.5 hr: OR, 1.56; 95% CI, 1.10–2.22 In age ≥60 yr, sleep <5.5 hr: OR, 1.72; 95% CI, 0.75–3.92 |
| Lou et al. (2015)85 | Prospective study (median follow-up: 5 yr)/China | General population (n=11,842) | Short sleep: ≤6 hr/night Long sleep: ≥8 hr/night Reference: 6–8 hr/night |
Self-reported | 44.8±14.7, 45.4% | New-onset T2DM Short sleep: RR, 1.67; 95% CI, 1.34–2.16 Long sleep: RR, 1.45; 95% CI, 1.02–1.77 |
| Kim et al. (2015)72 | Prospective study (follow-up: 2.6 yr)/Korea | General population (n= 3,862) | Short sleep: <6 hr/day Long sleep: ≥10 hr/day Reference: 6–7.9 hr/day |
Self-reported | 40–70, 41.1% | High blood glucose Short sleep: OR, 1.31; 95% CI, 0.96–1.79 Long sleep: OR, 0.56; 95 CI, 0.29–1.04 |
| Han et al. (2016)86 | Prospective Study (follow-up: 3–4.75 yr)/China | Retired employees from the Dongfeng-Tongji cohort (n=16,399) | Sleep duration was categorized as: <7, 7–<8 (reference), 8–<9, 9–<10, and ≥10 hr/night; afternoon napping was divided into: no napping (0 min, reference), 1–30, 31–60, 61–90, and >90 min |
Self-reported | 62.5, 43.2% | New-onset diabetes Sleep <7 hr/night: HR, 0.93; 95% CI, 0.72–1.19 Sleep ≥10 hr/night: HR, 1.42; 95% CI, 1.08–1.87 Napping >90 min: HR, 1.28; 95% CI, 1.03–1.59 Sleep duration ≥10 hr/night and napping >60 min: HR, 1.72; 95% CI, 1.03–2.85 |
|
| ||||||
| Sleep duration and glycemic control | ||||||
| Knutson et al. (2006)87 | Cross-sectional study/the United States | African-American women and men with diabetes (n=161) | Perceived sleep debt: the difference between weekday sleep duration and preferred sleep duration | Self-reported | 57±12, 26.1% | Glycemic control (lnHbA1c) Sleep debt in patients without diabetic complications: β, 0.51; P=0.04 Sleep debt in patients with 1 or more diabetic complications: β, −0.005; P=0.85 |
| Kim et al. (2013)88 | Cross-sectional study/Korea | Korean patients with diabetes (n=2,134) | Sleep duration was categorized as: <6, 6, 7, 8, and ≥9 hr/day; Reference: 7 hr/day |
Self-reported | 61.7±12.3, 49.9% | OR of high HbA1c (≥7.0%) Total with sleep <6 hr/day: OR, 1.15; 95% CI, 0.85–1.60 Total with sleep ≥9 hr/day: OR, 1.38; 95% CI, 0.93–2.03 Female with sleep <6 hr/day: OR, 1.46; 95% CI, 0.96–2.21 Female with sleep ≥9 hr/day: OR, 1.31; 95% CI, 0.75–2.27 Age <65 yr with sleep <6 hr/day: OR, 1.34; 95% CI, 0.86–2.09 Age <65 yr with sleep ≥9 hr/day: OR, 1.33; 95% CI, 0.84–2.41 |
| Ohkuma et al. (2013)67 | Cross-sectional study/Japan | Japanese patients with T2DM (n=4,870) | Sleep duration was categorized as: <4.5, 4.5–5.4, 5.5–6.4, 6.5–7.4, 7.5–8.4, and ≥8.5 hr/day | Self-reported | ≥20, 57% | Adjusted geometric means (95% CIs) of HbA1c Sleep <4.5 hr/day: 7.52 (7.38–7.67) Sleep 6.5–7.4 hr/day: 7.32 (7.28–7.37) Sleep ≥8.5 hr/day: 7.43 (7.34–7.52) P for quadratic trend: 0.004 |
| Wang et al. (2015)89 | Cross-sectional study/China | Patients with diabetes from the baseline survey of the REACTION Study (n=56,032) | Sleep duration was categorized as: <6, 6–7.9, 8–8.9, and ≥9 hr/night; Reference: 6–7.9 hr/night |
Self-reported | Sleep <6 hr/night: 61.2±8.8, 37%; Sleep ≥9 hr/night: 61.4±9.7, 38%; Reference: 60.5±8.8, 39% |
Poor glycemic control (HbA1c ≥7.0%) Sleep <6 hr/night: OR, 1.09; 95% CI, 0.99–1.21 Sleep ≥9 hr/night: OR, 1.11; 95% CI, 1.05–1.18 |
| Kong et al. (2017)90 | Cross-sectional study/Hong Kong | Hong Kong Chinese patients with T2DM (n=3,508) | Sleep duration: the period between bedtime and wake-up time on weekdays and weekends | Self-reported | 53.9±8.7, 59% | Sleep duration difference between weekdays and weekends is curvilinearly associated with both HbA1c and FPG. One hour more sleep during weekends than weekdays was associated with a decrease in HbA1c (−0.13%; 95 % CI, −0.24 to −0.02). |
Values are presented as range, mean±standard deviation, or mean (range).
RR, relative risk; CI, confidence interval; HR, hazard ratio; OR, odds ratio; NS, not specified; BMI, body mass index; WC, waist circumference; T2DM, type 2 diabetes mellitus; PSG, polysomnography; IGT, impaired glucose tolerance; FPG, fasting plasma glucose.
Table 2.
Clinical studies examining the association between sleep quality with obesity and diabetes
| Author (year) | Type of study/country or region | Study population (sample size) | Definition of sleep disturbances | Method of sleep assessment | Age (yr) and male (%) | Outcome |
|---|---|---|---|---|---|---|
| Sleep quality and weight gain/obesity | ||||||
| Lyytikäine et al. (2011)91 | Prospective study (follow-up: 5–7 yr)/Finland | Middle-aged municipal employees from the Helsinki Health Study (n=7,022) | Trouble falling asleep, waking up several times per night, trouble staying asleep, or waking up early feeling tired: the corresponding sleep problems ≥15 nights in the past 4 weeks | Self-reported (the Jenkins Sleep Questionnaire) | 40–60, 18.5% | Weight gain of ≥5 kg Female with trouble falling asleep: OR, 1.49; 95% CI, 1.09–2.03 Female with waking up several times per night: OR, 1.34; 95% CI, 1.09–1.65 Female with trouble staying asleep: OR, 1.29; 95% CI, 1.02–1.62 Female with waking up tired: OR, 1.04; 95% CI, 0.83–1.30 Male: NS |
| Huang et al. (2013)92 | Cross-sectional study/China | Patients with insomnia (n=141) | Slow wave sleep time and rapid eye movement sleep time | PSG | Insomnia: 42.2±9.8, 44% Healthy: 38.9±12.4, 51% |
BMI Slow wave sleep time (min): β, −0.013; 95% CI, −0.026 to −0.001; P=0.043 Rapid eye movement sleep time (min): β, 0.007; 95% CI, −0.008 to 0.023; P=0.352 |
| Piccolo et al. (2013)93 | Prospective study (follow-up: 4.8±0.6 yr)/the United States | General population from the Boston Area Community Health Survey (n=4,145) | Restless sleep: experiencing restless sleep much of the time during the past week | Self-reported | 30–79, NS | New-onset obesity Experiencing restless sleep: OR, 1.66; 95% CI, 1.10–2.49 |
| Sivertsen et al. (2014)94 | Prospective study (follow-up: 11 yr)/Norway | General population from the Nord-Trøndelag Health Studies (n=24,715) | Insomnia: “often” or “almost every night” had difficulties in initiating or maintaining sleep in the preceding month, in addition to reporting impaired work performance caused by insomnia during the preceding year | Self-reported | 32–66, 43.1% | New-onset obesity Insomnia: OR, 1.13; 95% CI, 0.96–1.33 |
| Vgontzas et al. (2014)70 | Prospective study (total follow-up: 7.5 yr; women: 4.5 yr; men: 10.5 yr)/the United States | General population (n=815) | Insomnia: a complaint of insomnia with a duration of ≥1 yr Poor sleep: a moderate-to-severe complaint of difficulty falling asleep, difficulty staying asleep, early morning awakening, or non-restorative sleep. Normal sleep: absence of either of these two categories. |
Self-reported | 48.9±13.4, 50.5% | New-onset obesity Insomnia with adjustment for subjective sleep duration: OR, 0.48; 95% CI, 0.15–1.53 Poor sleep with adjustment for subjective sleep duration: OR, 1.78; 95% CI, 1.02–3.13 Insomnia with adjustment for objective sleep duration: OR, 0.59; 95% CI, 0.20–1.77 Poor sleep with adjustment for objective sleep duration: OR, 1.76; 95% CI, 1.03–3.00 |
| Tan et al. (2015)95 | Cross-sectional study/Finland | Overweight middle-aged men (n=211) | OSA: an AHI of 5 or greater with EDS or an AHI of 15 or greater, regardless of associated symptoms Insomnia: DIS and/or DMS and/or NRS, and lasted for at least 1 month during the last 3 months Reference: overweight participants free from any sleep disorders |
Specialist physician diagnosis (through Vitalmed sleep questionnaire and PSG) | 30–65, 100% | BMI Reference, 15.8±4.3; OSA, 19.7±6.0 (P<0.05); insomnia, 18.5±5.9; OSA+insomnia, 19.7±7.1 (P<0.05) Waist circumference (cm) Reference, 98.0±7.6; OSA, 110.4±9.0 (P<0.05); insomnia, 106.5±10.2 (P<0.05); OSA+insomnia, 111.4±14.7 (P<0.05) Fat mass trunk (kg) Reference, 15.8±4.3; OSA, 19.7±6.0 (P<0.05); insomnia, 18.5±5.9 (P<0.05); OSA+insomnia, 19.7±7.1 (P<0.05) Fat mass android region (kg) Reference, 2.9±0.9; OSA, 3.7±1.1 (P<0.05); insomnia, 3.5±1.2 (P<0.05); OSA+insomnia, 3.8±1.4 (P<0.05) |
|
| ||||||
| Sleep quality and risk of diabetes | ||||||
| Nilsson et al. (2004)96 | Prospective study (mean follow-up: 14.8±2.4 yr)/Sweden | Healthy men from the Malmo Preventive Project (n=6,599) | Sleep disturbances: had either or both positive reply to difficulty in falling asleep or generally use sleeping pills more than 3 times a week | Self-reported questionnaire | 35–51, 100% | New-onset diabetes Sleep disturbances: OR, 1.52; 95% CI, 1.05–2.20 |
| Björkelund et al. (2005)97 | Prospective study (follow-up: 32 yr)/Sweden | Swedish women from the Population Study of Women in Gothenburg (n=661) | Sleep complaints: sleep problems (without specified time frame) and/or having consulted a doctor for sleep problems and/or hospital admission for this reason | Self-reported | 70–92, 0% | New-onset diabetes Sleep complaints: RR, 1.04; 95% CI, 0.91–1.18 |
| Mallon et al. (2005)74 | Prospective study (mean follow-up: 12 yr)/Sweden | General population (n=1,170) | DIS: had severe difficulties (scores 4 and 5) in initiating sleep DMS: had severe difficulties (scores 4 and 5) in maintaining sleep |
Self-reported (the Uppsala Sleep Inventory) | 45–65, 47.0% | New-onset diabetes Male with DIS: RR, 2.4; 95% CI, 0.7–8.6 Male with DMS: RR, 4.8; 95% CI, 1.9–12.5 Female with DIS: NS Female with DMS: RR, 1.8; 95% CI, 0.5–6.0 |
| Meisinger et al. (2005)98 | Prospective study (mean follow-up: 7.5 yr)/Germany | General population (n=8,269) | DIS: often had trouble falling asleep DMS: often woke up during the night |
Self-reported | 25–74, 49.9% | New-onset diabetes Male with DIS: HR, 1.10; 95% CI, 0.59–2.03 Female with DIS: HR, 1.42; 95% CI, 0.81–2.50 Male with DMS: HR, 1.60; 95% CI, 1.05–2.45 Female with DMS: HR, 1.60; 95% CI, 1.05–2.45 |
| Hayashino et al. (2007)77 | Prospective study (median follow-up: 4.2 yr)/Japan | Asian healthy workers from High-risk and Population Strategy for Occupational Health Promotion Study (n=6,509) |
DIS: sometimes or often had difficulties initiating sleeping DMS: sometimes or often had difficulties maintaining sleeping |
Self-reported | 38.2 (19–69), 73.9% | New-onset diabetes Sometimes DIS: HR, 1.42; 95% CI, 1.05–1.91 Often DIS: HR,1.61; 95% CI, 1.00–2.58 Sometimes DMS: HR, 1.31; 95% CI, 0.97–1.76 Often DMS: 1.37; 95% CI, 0.87–2.16 |
| Kita et al. (2012)81 | Prospective, occupational-based study (follow-up: 4 yr)/Japan | Local government employees (n=3,570) | Any sleep difficulties participants may have experienced ≥3 times a week during the previous month in sleep induction, awakening during the night, early morning awakening, self-perceived insufficient sleep duration, and overall quality of sleep | Self-reported questionnaire | 35–55, 79.0% | New-onset diabetes Awakening during the night: OR, 5.03; 95% CI, 1.43–17.64 Self-perceived insufficient sleep duration: OR, 6.76; 95% CI, 2.09–21.87 Unsatisfactory overall quality of sleep: OR, 3.71; 95% CI, 1.37–10.07 |
| Piccolo et al. (2013)93 | Prospective study (mean follow-up: 4.8±0.6 yr)/the United States | General population from the Boston Area Community Health Survey (n=4,145) | Restless sleep: experiencing restless sleep much of the time during the past week | Self-reported | 30–79, NS | New-onset T2DM Restless sleep: OR, 1.05; 95% CI, 0.67–1.64 |
| Sivertsen et al. (2014)94 | Prospective study (follow-up: 11 yr)/Norway | General population from the Nord-Trøndelag Health Studies (n=24,715) | Insomnia: “often” or “almost every night” had difficulties in initiating or maintaining sleep in the preceding month, in addition to reporting impaired work performance caused by insomnia during the preceding year | Self-reported | 32–66, 43.1% | New-onset T2DM Insomnia: OR, 1.07; 95% CI, 0.82–1.41 |
| Lou et al. (2015)85 | Prospective study (follow-up: 5 yr)/China | General population (n=11,842) | Poor sleep: had difficulties with initiating and maintaining sleep ≥8 days per month on average during the previous year | Self-reported | 44.8±14.7, 45.4% | New-onset T2DM Poor sleep quality: RR, 1.91; 95% CI, 1.31–2.74 Poor sleep quality with short sleep duration (≤ 6 hr/night): RR, 6.21; 95% CI, 2.78–11.81 |
| Lee et al. (2016)99 | Prospective study (median follow-up period: 2.5 yr)/Korea | General population from the family cohort study in primary care (the FACTS) (n=563) | Poor sleep quality: the score of the PSQI ≥5 | Self-reported | 10–75, 45.6% | New-onset T2DM RR, 2.64; 95% CI, 1.03–6.78 |
|
| ||||||
| Sleep quality and glycemic control | ||||||
| Knutson et al. (2006)87 | Cross-sectional study/the United States | African-American women and men with diabetes (n=161) | Modified PSQI score: PSQI score after removing the sleep duration component to assess sleep quality independently from sleep quantity | Self-reported questionnaire | 57±12, 26.1% | Glycemic control (lnHbA1c) Modified PSQI score in patients without diabetic complications: β, −0.014; P=0.16 Modified PSQI score in patients with at least 1 diabetic complications: β, 0.043; P=0.002 |
| Wan Mahmood et al. (2013)100 | Cross-sectional study/Ireland | Caucasian patients with T2DM (n=114) | Poor sleep quality: the score of PSQI >5 | Self-reported questionnaire | NS, 54.4% | Log HbA1c Poor sleep quality: β, 0.038; P=0.826 |
| Cho et al. (2014)101 | Cross-sectional study/Korea | Patients with T2DM (n=614) | Sleep apnea: SDQ-SA ≥36 for males and ≥32 for females; Poor sleep: PSQI score ≥5 Insomnia: any difficulty in falling asleep, maintaining sleep, early morning waking, and non-restorative sleep occurring at least three times per week over the preceding month |
Self-reported questionnaires | 59.7±11.1, 62.1% | Postprandial glucose Sleep apnea score (SDQ-SA): r=0.100, P=0.032 HbA1c No significant association between HbA1c values and poor sleep, insomnia |
| Nefs et al. (2015)102 | Cross-sectional study/the Netherlands | Dutch adults with T1DM (n=267) or T2DM (n=361), (total n=628) | Poor sleep quality: PSQI score >5 | Self-reported questionnaire | T1DM: 47±16, 41% T2DM: 62±9, 54% |
Most recent HbA1c, % (mmol/mol) In T1DM, good sleep quality vs. poor sleep quality: 7.5±0.9 (58±10) vs. 7.5±1.1 (59±12), P=0.68; In T2DM, good sleep quality vs. poor sleep quality: 7.1±1.3 (54±14) vs. 7.3±1.3 (57±14), P=0.09 |
| Osonoi et al. (2015)103 | Cross-sectional study/Japan | Patients with T2DM (n=724) | Poor sleep quality: PSQI score ≥9 Average sleep quality: PSQI score 6–8 Good sleep quality: PSQI score ≤5 |
Self-reported questionnaire | 57.8±8.6, 62.9% | Fasting blood glucose (mg/dL) Good sleep: 132±31; average sleep: 136±31; poor sleep: 141±32 (P>0.05) HbA1c (%) Good sleep: 6.9±1.0; average sleep: 7.1±1.1; poor sleep: 7.1±0.8; (P>0.05) |
Values are presented as range or mean± standard deviation.
OR, odds ratio; CI, confidence interval; NS, not specified; PSG, polysomnography; BMI, body mass index; OSA, obstructive sleep apnea; AHI, apnea hypopnea index; EDS, excessive daytime sleepiness; DIS, difficulty in initiating sleep; DMS, difficulty in maintaining sleep; NRS, nonrestorative sleep; RR, relative risk; HR, hazard ratio; T2DM, type 2 diabetes mellitus; PSQI: the Pittsburgh Sleep Quality Index; SDQ-SA, the Sleep Disorders Questionnaire Sleep Apnea subscale; T1DM, type 1 diabetes mellitus.
Insomnia is a common sleep problem worldwide and affects 15% of the Chinese population.8 It is defined as difficulty in initiating sleep (DIS), difficulty in maintaining sleep (DMS) and/or early morning awakening (EMA) at least three times per week with associated impairment of daytime functioning.104 Nonrestorative sleep (NRS) is also suggested by the International Classification of Sleep Disorders as a subtype of insomnia.104
Although the pathophysiology of insomnia is still not fully understood, it has long been considered to be a disorder of hyperarousal during both daytime and nighttime, which is associated with increased activation of whole-body and brain metabolism, hyperactivity of HPA axis and hormonal dysregulation.49 A study involving 1,042 monozygotic and 828 dizygotic twin pairs demonstrated a 10% phenotypic correlation between insomnia and obesity, suggesting a shared genetic mechanism that underlies these conditions.105 However, the findings about this association were inconsistent in other clinical studies, which mostly reported either no association or an inverse relationship between insomnia and BMI. In a cross-sectional study conducted in 211 Finnish men aged 30–65 years, among the overweight or obese participants (n=163), those with insomnia (n=40) had higher fat mass in the trunk and android regions than the group without sleep disorder (n=76, P<0.05), but no between-group differences in BMI or total fat mass.95 There was also no difference in BMI when comparing 141 Chinese patients with primary insomnia with 55 healthy volunteers (22.54±2.76 kg/m2 vs. 22.84±3.28 kg/m2, P=0.526).92 However, this study found that among patients with insomnia, there was a significant negative correlation between the amount of SWS and BMI after controlling for potential confounders (β, −0.013; 95% confidence interval [CI], −0.026 to −0.001; P=0.043), suggesting that impaired sleep quality in subjects with insomnia may be the culprit linking insomnia with obesity. By contrast, some researchers had reported positive relationships between insomnia and obesity indices. The prospective Helsinki Health Study which included 7,022 middle-aged respondents demonstrated that DIS (odds ratio [OR], 1.65; 95% CI, 1.22–2.22) and DMS (OR, 1.41; 95% CI, 1.13–1.75) were associated with a weight gain of 5 kg or more after 5 to 7 years of follow-up, being more evident in women than in men.91 In the prospective Penn State Cohort involving 815 nonobese adults with a follow-up duration of 7.5 years, a moderate to severe complaint of any DIS, DMS, EMA, or NRS was associated with an increased incidence of obesity (OR, 1.76–1.78), while insomnia tended towards significance after adjusted for sleep duration, emotional stress, and other potential confounders.70 Another prospective study conducted in Norwegian population (n=24,715) reported that insomnia was associated with an 18% increased risk (95% CI, 1.06–1.33) of incident obesity after 11 years of follow up, which was independent of baseline demographics, anxiety and depression. However, this association was negated when further adjusted for other comorbidities, such as angina, T2DM and hypertension (OR, 1.09; 95% CI, 0.97–1.24).94 Taken together, these inconclusive results may be related to the adoption of different definitions of insomnia and obesity.
Although conflicting results have been reported for obesity, it appears that most studies support an association between insomnia and diabetes risk. In a meta-analysis of 107,756 participants from 13 independent cohorts, those with insomnia had stronger association with incident diabetes (DIS: RR, 1.57; 95% CI, 1.25–1.97; DMS: RR, 1.84; 95% CI, 1.39–2.43), compared with participants with short sleep duration of ≤5–6 hours per night (RR, 1.28; 95% CI, 1.03–1.60).106 In another larger meta-analysis of nearly 1.1 million participants from 36 studies, insomnia (RR, 1.38; 95% CI, 1.18–1.62) and its subtypes (DIS: RR, 1.55; 95% CI, 1.23–1.95; DMS: RR, 1.72; 95% CI, 1.45–2.05) also demonstrated an independent association with excess risk of developing diabetes.107
Short sleep duration with coexistent insomnia may further aggravate the risk of future diabetes. In the Penn State Cohort of 1,741 participants, participants with insomnia and sleep curtailment (<5-hour per day) had an OR of 2.95 (95% CI, 1.24–7.03) for diabetes after adjustment for age, race, sex, BMI, smoking, alcohol use, depression and sleep-disordered breathing, compared with those who had >6-hour sleep per day and without insomnia.108 Conversely, in the retrospective Freiburg Insomnia Cohort involving 328 patients with primary insomnia (203 women and 125 men; mean age, 44.3±12.2 years), those with concomitant short sleep duration did not have increased risk of T2DM (OR, 1.39; 95% CI, 0.34–5.67 for first night of short sleep and OR, 2.30; 95% CI, 0.48–10.96 for second night of short sleep), perhaps due to a small number of patients with T2DM (n=9).109 Indeed, there are possible pathogenetic mechanisms underpinning the link between insomnia and risk of diabetes, including reduction of glucose tolerance40, alteration of HPA axis and sympathetic nervous system110, and elevated markers of chronic inflammation.111 Hence, well-designed studies with sufficient sample size are required to unravel the relationship between insomnia and diabetes.
SLEEP AND GENES REGULATING THE CIRCADIAN CLOCK
Clock genes may play a key role in linking sleep and the effect of circadian disruptions on metabolic functions.23 A central clock which is located within the suprachiasmatic nuclei of the hypothalamus orchestrates the environmental light/dark cycles with human physiology and behavior. Genetic or environmental perturbations of this synchronized molecular mechanism can lead to metabolic disturbances. In the core clock mechanism, the brain and muscle Arnt like protein-1 (BMAL1)/circadian locomotor output cycles kaput (CLOCK) heterodimer binds to the E-box elements in the promoter regions of Per and Cry genes, thus activating their transcription proteins with feedback inhibition on the BMAL1/CLOCK.112 Six hours of sleep curtailment was sufficient to reduce BMAL1 binding to the E-box elements of Per gene.23 Other important regulators that integrate the effects of circadian clock on metabolic pathways include the clock-controlled genes and certain transcription factors e.g., REV-ERBα, retinoid-related orphan receptor α, and peroxisome proliferator-activated receptor α.2,113 These genes can also be categorized by type of regulations e.g., circadian cycle only (BMAL1), sleep-wake cycle only (Homer1a) or both (Per). Hence, the effects of sleep on gene expression, subsequent epigenetic modifications and metabolic perturbations require further translational research to improve our understanding about the genes involved and the details of the regulation.23
Coordination of the sleep/wake cycle as well as the metabolic and fasting/feeding cycle are all essential to maintain a normal circadian oscillatory system for healthy bodily functions. Glucose homeostasis is regulated by both the central clock that synchronizes sleep and feeding, and peripheral tissue clocks that coordinate one’s behavior with glucose synthesis and utilization.114 When BMAL1/CLOCK heterodimer colocalizes with the pancreatic transcription factor pancreatic and duodenal homeobox 1 on the regulatory sites of beta-cell cycling genes, pancreatic beta-cell maturation is enhanced with possible pulsatile insulin secretion according to the circadian rhythmicity.114 Similarly, BMAL1-ablation in mice reduces insulin exocytosis from pancreatic beta-cell, resulting in hyperglycemia.23 Based on the genome-wide association studies, individuals with T2DM and melatonin receptor 1B (MTNR1B) risk allele may have high melatonin level that interacts with dietary intake, with subsequent impairment in glucose metabolism and increased risk of T2DM.23
Conditions that are often associated with circadian misalignment are shift work and trans-meridian air travel (jet lag). Shift work can desynchronize the central and peripheral circadian clocks, which further perturbs the glucose metabolism by decreasing insulin sensitivity, independent of sleep curtailment.23,115 In healthy adults, 3-week sleep curtailment with concomitant circadian misalignment is demonstrated to reduce the resting metabolic rate and impair pancreatic beta-cell secretion.116 In mice model, chronic jet lag disrupts the circadian cycle by uncoupling the central and peripheral clocks in the adipose tissue, and triggers leptin resistance leading to development of obesity, an effect independent of other risk factors.117 In the large prospective Nurses’ Health Study 2, early chronotype combined with increasing years of rotating night shift work aggravated diabetes risk, even after adjustment for patients’ attributes, family history of diabetes, diet, physical activity and self-reported sleep duration.118 Interestingly, late chronotype alone predicted a 51% (hazard ratio, 1.51; 95% CI, 1.13–2.03) excess risk of developing T2DM, which was attenuated by longer night shift exposure, presumably due to less interference to the “usual” circadian rhythm.118 These findings support the notion that chronotype-adapted work schedules can possibly reduce the circadian misalignment and prevent future cardiometabolic risk.
The circadian clock has been demonstrated to regulate the diurnal fluctuations of certain human metabolites such as the fatty acids and amino acids, independent of the fasting/feeding cycle.119 In the first human metabolomics study conducted during a 48-hour sleep/wake cycle, 27 of the 171 measured metabolites (e.g., fatty acids, tryptophan, taurine, and serotonin) were significantly elevated with sleep curtailment.120 This creates novel opportunities for potential therapeutic intervention targeting the key clock-regulated metabolic pathways and application of these noninvasive biomarkers for disease prediction and monitoring.
CONCLUSION
Good sleep hygiene is crucial to maintain optimal functions of the neuroendocrine and appetite regulation systems. To date, there is clear evidence in support of a causal relationship between sleep, circadian misalignment and cardiometabolic risk. Thus, health promotion campaigns should look beyond conventional strategies by emphasizing the pivotal role of improving sleep environment to curb the obesity epidemic. Nonetheless, there is a pressing need for future research to delineate the molecular intersections between sleep, circadian clock and metabolic pathways, as well as to explore potential preventive and therapeutic approaches to optimize sleep and circadian rhythm in predisposed individuals.
Footnotes
CONFLICTS OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- 1.GBD 2015 Obesity Collaborators. Afshin A, Forouzanfar MH, Reitsma MB, Sur P, Estep K, et al. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med. 2017;377:13–27. doi: 10.1056/NEJMoa1614362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McHill AW, Wright KP., Jr Role of sleep and circadian disruption on energy expenditure and in metabolic predisposition to human obesity and metabolic disease. Obes Rev. 2017;18(Suppl 1):15–24. doi: 10.1111/obr.12503. [DOI] [PubMed] [Google Scholar]
- 3.Iglowstein I, Jenni OG, Molinari L, Largo RH. Sleep duration from infancy to adolescence: reference values and generational trends. Pediatrics. 2003;111:302–7. doi: 10.1542/peds.111.2.302. [DOI] [PubMed] [Google Scholar]
- 4.Consensus Conference Panel. Watson NF, Badr MS, Belenky G, Bliwise DL, Buxton OM, et al. Joint consensus statement of the American Academy of Sleep Medicine and Sleep Research Society on the recommended amount of sleep for a healthy adult: methodology and discussion. Sleep. 2015;38:1161–83. doi: 10.5665/sleep.4886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paruthi S, Brooks LJ, D’Ambrosio C, Hall WA, Kotagal S, Lloyd RM, et al. Recommended amount of sleep for pediatric populations: a consensus statement of the American Academy of Sleep Medicine. J Clin Sleep Med. 2016;12:785–6. doi: 10.5664/jcsm.5866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee SW, Ng KY, Chin WK. The impact of sleep amount and sleep quality on glycemic control in type 2 diabetes: a systematic review and meta-analysis. Sleep Med Rev. 2017;31:91–101. doi: 10.1016/j.smrv.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 7.Liu Y, Wheaton AG, Chapman DP, Cunningham TJ, Lu H, Croft JB. Prevalence of healthy sleep duration among adults: United States, 2014. MMWR Morb Mortal Wkly Rep. 2016;65:137–41. doi: 10.15585/mmwr.mm6506a1. [DOI] [PubMed] [Google Scholar]
- 8.Cao XL, Wang SB, Zhong BL, Zhang L, Ungvari GS, Ng CH, et al. The prevalence of insomnia in the general population in China: a meta-analysis. PLoS One. 2017;12:e0170772. doi: 10.1371/journal.pone.0170772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bin YS, Marshall NS, Glozier N. Sleeping at the limits: the changing prevalence of short and long sleep durations in 10 countries. Am J Epidemiol. 2013;177:826–33. doi: 10.1093/aje/kws308. [DOI] [PubMed] [Google Scholar]
- 10.Jike M, Itani O, Watanabe N, Buysse DJ, Kaneita Y. Long sleep duration and health outcomes: a systematic review, meta-analysis and meta-regression. Sleep Med Rev. 2017 Jul 5; doi: 10.1016/j.smrv.2017.06.011. [Epub]. [DOI] [PubMed] [Google Scholar]
- 11.Ayas NT, White DP, Al-Delaimy WK, Manson JE, Stampfer MJ, Speizer FE, et al. A prospective study of self-reported sleep duration and incident diabetes in women. Diabetes Care. 2003;26:380–4. doi: 10.2337/diacare.26.2.380. [DOI] [PubMed] [Google Scholar]
- 12.Heymsfield SB, Wadden TA. Mechanisms, pathophysiology, and management of obesity. N Engl J Med. 2017;376:254–66. doi: 10.1056/NEJMra1514009. [DOI] [PubMed] [Google Scholar]
- 13.Nakazato M, Murakami N, Date Y, Kojima M, Matsuo H, Kangawa K, et al. A role for ghrelin in the central regulation of feeding. Nature. 2001;409:194–8. doi: 10.1038/35051587. [DOI] [PubMed] [Google Scholar]
- 14.Zigman JM, Elmquist JK. Minireview: from anorexia to obesity. The yin and yang of body weight control. Endocrinology. 2003;144:3749–56. doi: 10.1210/en.2003-0241. [DOI] [PubMed] [Google Scholar]
- 15.Dashti HS, Scheer FA, Jacques PF, Lamon-Fava S, Ordovás JM. Short sleep duration and dietary intake: epidemiologic evidence, mechanisms, and health implications. Adv Nutr. 2015;6:648–59. doi: 10.3945/an.115.008623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Colles SL, Dixon JB, O’Brien PE. Night eating syndrome and nocturnal snacking: association with obesity, binge eating and psychological distress. Int J Obes (Lond) 2007;31:1722–30. doi: 10.1038/sj.ijo.0803664. [DOI] [PubMed] [Google Scholar]
- 17.Kant AK, Graubard BI. Association of self-reported sleep duration with eating behaviors of American adults: NHANES 2005–2010. Am J Clin Nutr. 2014;100:938–47. doi: 10.3945/ajcn.114.085191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ding M, Keiley MK, Garza KB, Duffy PA, Zizza CA. Food insecurity is associated with poor sleep outcomes among US adults. J Nutr. 2015;145:615–21. doi: 10.3945/jn.114.199919. [DOI] [PubMed] [Google Scholar]
- 19.St-Onge MP, Roberts AL, Chen J, Kelleman M, O’Keeffe M, RoyChoudhury A, et al. Short sleep duration increases energy intakes but does not change energy expenditure in normal-weight individuals. Am J Clin Nutr. 2011;94:410–6. doi: 10.3945/ajcn.111.013904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nedeltcheva AV, Kilkus JM, Imperial J, Kasza K, Schoeller DA, Penev PD. Sleep curtailment is accompanied by increased intake of calories from snacks. Am J Clin Nutr. 2009;89:126–33. doi: 10.3945/ajcn.2008.26574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shi Z, McEvoy M, Luu J, Attia J. Dietary fat and sleep duration in Chinese men and women. Int J Obes (Lond) 2008;32:1835–40. doi: 10.1038/ijo.2008.191. [DOI] [PubMed] [Google Scholar]
- 22.Dashti HS, Follis JL, Smith CE, Tanaka T, Cade BE, Gottlieb DJ, et al. Habitual sleep duration is associated with BMI and macronutrient intake and may be modified by CLOCK genetic variants. Am J Clin Nutr. 2015;101:135–43. doi: 10.3945/ajcn.114.095026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arble DM, Bass J, Behn CD, Butler MP, Challet E, Czeisler C, et al. Impact of sleep and circadian disruption on energy balance and diabetes: a summary of workshop discussions. Sleep. 2015;38:1849–60. doi: 10.5665/sleep.5226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taheri S, Lin L, Austin D, Young T, Mignot E. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med. 2004;1:e62. doi: 10.1371/journal.pmed.0010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spiegel K, Leproult R, L’hermite-Balériaux M, Copinschi G, Penev PD, Van Cauter E. Leptin levels are dependent on sleep duration: relationships with sympathovagal balance, carbohydrate regulation, cortisol, and thyrotropin. J Clin Endocrinol Metab. 2004;89:5762–71. doi: 10.1210/jc.2004-1003. [DOI] [PubMed] [Google Scholar]
- 26.Greer SM, Goldstein AN, Walker MP. The impact of sleep deprivation on food desire in the human brain. Nat Commun. 2013;4:2259. doi: 10.1038/ncomms3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.St-Onge MP, Wolfe S, Sy M, Shechter A, Hirsch J. Sleep restriction increases the neuronal response to unhealthy food in normal-weight individuals. Int J Obes (Lond) 2014;38:411–6. doi: 10.1038/ijo.2013.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Benedict C, Brooks SJ, O’Daly OG, Almèn MS, Morell A, Åberg K, et al. Acute sleep deprivation enhances the brain’s response to hedonic food stimuli: an fMRI study. J Clin Endocrinol Metab. 2012;97:E443–7. doi: 10.1210/jc.2011-2759. [DOI] [PubMed] [Google Scholar]
- 29.Baron KG, Reid KJ, Kern AS, Zee PC. Role of sleep timing in caloric intake and BMI. Obesity (Silver Spring) 2011;19:1374–81. doi: 10.1038/oby.2011.100. [DOI] [PubMed] [Google Scholar]
- 30.Stern JH, Grant AS, Thomson CA, Tinker L, Hale L, Brennan KM, et al. Short sleep duration is associated with decreased serum leptin, increased energy intake and decreased diet quality in postmenopausal women. Obesity (Silver Spring) 2014;22:E55–61. doi: 10.1002/oby.20683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Motivala SJ, Tomiyama AJ, Ziegler M, Khandrika S, Irwin MR. Nocturnal levels of ghrelin and leptin and sleep in chronic insomnia. Psychoneuroendocrinology. 2009;34:540–5. doi: 10.1016/j.psyneuen.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spiegel K, Leproult R, Colecchia EF, L’Hermite-Balériaux M, Nie Z, Copinschi G, et al. Adaptation of the 24-h growth hormone profile to a state of sleep debt. Am J Physiol Regul Integr Comp Physiol. 2000;279:R874–83. doi: 10.1152/ajpregu.2000.279.3.R874. [DOI] [PubMed] [Google Scholar]
- 33.Van Cauter E, Plat L, Copinschi G. Interrelations between sleep and the somatotropic axis. Sleep. 1998;21:553–66. [PubMed] [Google Scholar]
- 34.Sandhu MS, Heald AH, Gibson JM, Cruickshank JK, Dunger DB, Wareham NJ. Circulating concentrations of insulin-like growth factor-I and development of glucose intolerance: a prospective observational study. Lancet. 2002;359:1740–5. doi: 10.1016/S0140-6736(02)08655-5. [DOI] [PubMed] [Google Scholar]
- 35.Juul A, Scheike T, Davidsen M, Gyllenborg J, Jørgensen T. Low serum insulin-like growth factor I is associated with increased risk of ischemic heart disease: a population-based case-control study. Circulation. 2002;106:939–44. doi: 10.1161/01.CIR.0000027563.44593.CC. [DOI] [PubMed] [Google Scholar]
- 36.Sukhanov S, Higashi Y, Shai SY, Vaughn C, Mohler J, Li Y, et al. IGF-1 reduces inflammatory responses, suppresses oxidative stress, and decreases atherosclerosis progression in ApoE-deficient mice. Arterioscler Thromb Vasc Biol. 2007;27:2684–90. doi: 10.1161/ATVBAHA.107.156257. [DOI] [PubMed] [Google Scholar]
- 37.García-García F, Juárez-Aguilar E, Santiago-García J, Cardinali DP. Ghrelin and its interactions with growth hormone, leptin and orexins: implications for the sleep-wake cycle and metabolism. Sleep Med Rev. 2014;18:89–97. doi: 10.1016/j.smrv.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 38.Gram IT, Norat T, Rinaldi S, Dossus L, Lukanova A, Téhard B, et al. Body mass index, waist circumference and waist-hip ratio and serum levels of IGF-I and IGFBP-3 in European women. Int J Obes (Lond) 2006;30:1623–31. doi: 10.1038/sj.ijo.0803324. [DOI] [PubMed] [Google Scholar]
- 39.Yeap BB, Chubb SA, Ho KK, Setoh JW, McCaul KA, Norman PE, et al. IGF1 and its binding proteins 3 and 1 are differentially associated with metabolic syndrome in older men. Eur J Endocrinol. 2010;162:249–57. doi: 10.1530/EJE-09-0852. [DOI] [PubMed] [Google Scholar]
- 40.Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354:1435–9. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- 41.Fries E, Dettenborn L, Kirschbaum C. The cortisol awakening response (CAR): facts and future directions. Int J Psychophysiol. 2009;72:67–73. doi: 10.1016/j.ijpsycho.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 42.Kumari M, Badrick E, Ferrie J, Perski A, Marmot M, Chandola T. Self-reported sleep duration and sleep disturbance are independently associated with cortisol secretion in the Whitehall II study. J Clin Endocrinol Metab. 2009;94:4801–9. doi: 10.1210/jc.2009-0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang T, Poole EM, Vetter C, Rexrode KM, Kubzansky LD, Schernhammer E, et al. Habitual sleep quality and diurnal rhythms of salivary cortisol and dehydroepiandrosterone in postmenopausal women. Psychoneuroendocrinology. 2017;84:172–80. doi: 10.1016/j.psyneuen.2017.07.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang J, Ma RC, Kong AP, So WY, Li AM, Lam SP, et al. Relationship of sleep quantity and quality with 24-hour urinary catecholamines and salivary awakening cortisol in healthy middle-aged adults. Sleep. 2011;34:225–33. doi: 10.1093/sleep/34.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lemola S, Perkinson-Gloor N, Hagmann-von Arx P, Brand S, Holsboer-Trachsler E, Grob A, et al. Morning cortisol secretion in school-age children is related to the sleep pattern of the preceding night. Psychoneuroendocrinology. 2015;52:297–301. doi: 10.1016/j.psyneuen.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 46.Räikkönen K, Matthews KA, Pesonen AK, Pyhälä R, Paavonen EJ, Feldt K, et al. Poor sleep and altered hypothalamic-pituitary-adrenocortical and sympatho-adrenal-medullary system activity in children. J Clin Endocrinol Metab. 2010;95:2254–61. doi: 10.1210/jc.2009-0943. [DOI] [PubMed] [Google Scholar]
- 47.Rao MN, Blackwell T, Redline S, Punjabi NM, Barrett-Connor E, Neylan TC, et al. Association between sleep duration and 24-hour urine free cortisol in the MrOS Sleep Study. PLoS One. 2013;8:e75205. doi: 10.1371/journal.pone.0075205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Richardson GS. Human physiological models of insomnia. Sleep Med. 2007;8(Suppl 4):S9–14. doi: 10.1016/S1389-9457(08)70003-0. [DOI] [PubMed] [Google Scholar]
- 49.Bonnet MH, Arand DL. Hyperarousal and insomnia: state of the science. Sleep Med Rev. 2010;14:9–15. doi: 10.1016/j.smrv.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 50.Zhang J, Lam SP, Li SX, Ma RC, Kong AP, Chan MH, et al. A community-based study on the association between insomnia and hypothalamic-pituitary-adrenal axis: sex and pubertal influences. J Clin Endocrinol Metab. 2014;99:2277–87. doi: 10.1210/jc.2013-3728. [DOI] [PubMed] [Google Scholar]
- 51.Irwin M, Thompson J, Miller C, Gillin JC, Ziegler M. Effects of sleep and sleep deprivation on catecholamine and interleukin-2 levels in humans: clinical implications. J Clin Endocrinol Metab. 1999;84:1979–85. doi: 10.1210/jcem.84.6.5788. [DOI] [PubMed] [Google Scholar]
- 52.Lusardi P, Zoppi A, Preti P, Pesce RM, Piazza E, Fogari R. Effects of insufficient sleep on blood pressure in hypertensive patients: a 24-h study. Am J Hypertens. 1999;12(1 Pt 1):63–8. doi: 10.1016/S0895-7061(98)00200-3. [DOI] [PubMed] [Google Scholar]
- 53.Broussard JL, Ehrmann DA, Van Cauter E, Tasali E, Brady MJ. Impaired insulin signaling in human adipocytes after experimental sleep restriction: a randomized, crossover study. Ann Intern Med. 2012;157:549–57. doi: 10.7326/0003-4819-157-8-201210160-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kong AP, Wing YK, Choi KC, Li AM, Ko GT, Ma RC, et al. Associations of sleep duration with obesity and serum lipid profile in children and adolescents. Sleep Med. 2011;12:659–65. doi: 10.1016/j.sleep.2010.12.015. [DOI] [PubMed] [Google Scholar]
- 55.Zhan Y, Chen R, Yu J. Sleep duration and abnormal serum lipids: the China Health and Nutrition Survey. Sleep Med. 2014;15:833–9. doi: 10.1016/j.sleep.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 56.Patel SR, Malhotra A, White DP, Gottlieb DJ, Hu FB. Association between reduced sleep and weight gain in women. Am J Epidemiol. 2006;164:947–54. doi: 10.1093/aje/kwj280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chaput JP, Després JP, Bouchard C, Tremblay A. The association between sleep duration and weight gain in adults: a 6-year prospective study from the Quebec Family Study. Sleep. 2008;31:517–23. doi: 10.1093/sleep/31.4.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.López-García E, Faubel R, León-Muñoz L, Zuluaga MC, Banegas JR, Rodríguez-Artalejo F. Sleep duration, general and abdominal obesity, and weight change among the older adult population of Spain. Am J Clin Nutr. 2008;87:310–6. doi: 10.1093/ajcn/87.2.310. [DOI] [PubMed] [Google Scholar]
- 59.Stranges S, Cappuccio FP, Kandala NB, Miller MA, Taggart FM, Kumari M, et al. Cross-sectional versus prospective associations of sleep duration with changes in relative weight and body fat distribution: the Whitehall II Study. Am J Epidemiol. 2008;167:321–9. doi: 10.1093/aje/kwm302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nishiura C, Noguchi J, Hashimoto H. Dietary patterns only partially explain the effect of short sleep duration on the incidence of obesity. Sleep. 2010;33:753–7. doi: 10.1093/sleep/33.6.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Watanabe M, Kikuchi H, Tanaka K, Takahashi M. Association of short sleep duration with weight gain and obesity at 1-year follow-up: a large-scale prospective study. Sleep. 2010;33:161–7. doi: 10.1093/sleep/33.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Itani O, Kaneita Y, Murata A, Yokoyama E, Ohida T. Association of onset of obesity with sleep duration and shift work among Japanese adults. Sleep Med. 2011;12:341–5. doi: 10.1016/j.sleep.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 63.Lyytikäinen P, Rahkonen O, Lahelma E, Lallukka T. Association of sleep duration with weight and weight gain: a prospective follow-up study. J Sleep Res. 2011;20:298–302. doi: 10.1111/j.1365-2869.2010.00903.x. [DOI] [PubMed] [Google Scholar]
- 64.Kobayashi D, Takahashi O, Deshpande GA, Shimbo T, Fukui T. Association between weight gain, obesity, and sleep duration: a large-scale 3-year cohort study. Sleep Breath. 2012;16:829–33. doi: 10.1007/s11325-011-0583-0. [DOI] [PubMed] [Google Scholar]
- 65.Yiengprugsawan V, Banwell C, Seubsman SA, Sleigh AC Thai Cohort Study Team. Short sleep and obesity in a large national cohort of Thai adults. BMJ Open. 2012;2:e000561. doi: 10.1136/bmjopen-2011-000561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nagai M, Tomata Y, Watanabe T, Kakizaki M, Tsuji I. Association between sleep duration, weight gain, and obesity for long period. Sleep Med. 2013;14:206–10. doi: 10.1016/j.sleep.2012.09.024. [DOI] [PubMed] [Google Scholar]
- 67.Ohkuma T, Fujii H, Iwase M, Kikuchi Y, Ogata S, Idewaki Y, et al. Impact of sleep duration on obesity and the glycemic level in patients with type 2 diabetes: the Fukuoka Diabetes Registry. Diabetes Care. 2013;36:611–7. doi: 10.2337/dc12-0904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sayón-Orea C, Bes-Rastrollo M, Carlos S, Beunza JJ, Basterra-Gortari FJ, Martínez-González MA. Association between sleeping hours and siesta and the risk of obesity: the SUN Mediterranean Cohort. Obes Facts. 2013;6:337–47. doi: 10.1159/000354746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xiao Q, Arem H, Moore SC, Hollenbeck AR, Matthews CE. A large prospective investigation of sleep duration, weight change, and obesity in the NIH-AARP Diet and Health Study cohort. Am J Epidemiol. 2013;178:1600–10. doi: 10.1093/aje/kwt180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vgontzas AN, Fernandez-Mendoza J, Miksiewicz T, Kritikou I, Shaffer ML, Liao D, et al. Unveiling the longitudinal association between short sleep duration and the incidence of obesity: the Penn State Cohort. Int J Obes (Lond) 2014;38:825–32. doi: 10.1038/ijo.2013.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gutiérrez-Repiso C, Soriguer F, Rubio-Martín E, Esteva de Antonio I, Ruiz de Adana MS, Almaraz MC, et al. Night-time sleep duration and the incidence of obesity and type 2 diabetes: findings from the prospective Pizarra study. Sleep Med. 2014;15:1398–404. doi: 10.1016/j.sleep.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 72.Kim JY, Yadav D, Ahn SV, Koh SB, Park JT, Yoon J, et al. A prospective study of total sleep duration and incident metabolic syndrome: the ARIRANG study. Sleep Med. 2015;16:1511–5. doi: 10.1016/j.sleep.2015.06.024. [DOI] [PubMed] [Google Scholar]
- 73.Zhang B, Hao Y, Zhou J, Jia F, Li X, Tang Y, et al. The association between sleep patterns and overweight/obesity in Chinese children: a cross-sectional study. Neuropsychiatr Dis Treat. 2015;11:2209–16. doi: 10.2147/NDT.S90838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mallon L, Broman JE, Hetta J. High incidence of diabetes in men with sleep complaints or short sleep duration: a 12-year follow-up study of a middle-aged population. Diabetes Care. 2005;28:2762–7. doi: 10.2337/diacare.28.11.2762. [DOI] [PubMed] [Google Scholar]
- 75.Yaggi HK, Araujo AB, McKinlay JB. Sleep duration as a risk factor for the development of type 2 diabetes. Diabetes Care. 2006;29:657–61. doi: 10.2337/diacare.29.03.06.dc05-0879. [DOI] [PubMed] [Google Scholar]
- 76.Gangwisch JE, Heymsfield SB, Boden-Albala B, Buijs RM, Kreier F, Pickering TG, et al. Sleep duration as a risk factor for diabetes incidence in a large U.S. sample. Sleep. 2007;30:1667–73. doi: 10.1093/sleep/30.12.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hayashino Y, Fukuhara S, Suzukamo Y, Okamura T, Tanaka T, Ueshima H, et al. Relation between sleep quality and quantity, quality of life, and risk of developing diabetes in healthy workers in Japan: the High-risk and Population Strategy for Occupational Health Promotion (HIPOP-OHP) Study. BMC Public Health. 2007;7:129. doi: 10.1186/1471-2458-7-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Beihl DA, Liese AD, Haffner SM. Sleep duration as a risk factor for incident type 2 diabetes in a multiethnic cohort. Ann Epidemiol. 2009;19:351–7. doi: 10.1016/j.annepidem.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 79.Chaput JP, Després JP, Bouchard C, Astrup A, Tremblay A. Sleep duration as a risk factor for the development of type 2 diabetes or impaired glucose tolerance: analyses of the Quebec Family Study. Sleep Med. 2009;10:919–24. doi: 10.1016/j.sleep.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 80.Xu Q, Song Y, Hollenbeck A, Blair A, Schatzkin A, Chen H. Day napping and short night sleeping are associated with higher risk of diabetes in older adults. Diabetes Care. 2010;33:78–83. doi: 10.2337/dc09-1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kita T, Yoshioka E, Satoh H, Saijo Y, Kawaharada M, Okada E, et al. Short sleep duration and poor sleep quality increase the risk of diabetes in Japanese workers with no family history of diabetes. Diabetes Care. 2012;35:313–8. doi: 10.2337/dc11-1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.von Ruesten A, Weikert C, Fietze I, Boeing H. Association of sleep duration with chronic diseases in the European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam study. PLoS One. 2012;7:e30972. doi: 10.1371/journal.pone.0030972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Holliday EG, Magee CA, Kritharides L, Banks E, Attia J. Short sleep duration is associated with risk of future diabetes but not cardiovascular disease: a prospective study and meta-analysis. PLoS One. 2013;8:e82305. doi: 10.1371/journal.pone.0082305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Heianza Y, Kato K, Fujihara K, Tanaka S, Kodama S, Hanyu O, et al. Role of sleep duration as a risk factor for type 2 diabetes among adults of different ages in Japan: the Niigata Wellness Study. Diabet Med. 2014;31:1363–7. doi: 10.1111/dme.12555. [DOI] [PubMed] [Google Scholar]
- 85.Lou P, Zhang P, Zhang L, Chen P, Chang G, Zhang N, et al. Effects of sleep duration and sleep quality on prevalence of type 2 diabetes mellitus: a 5-year follow-up study in China. Diabetes Res Clin Pract. 2015;109:178–84. doi: 10.1016/j.diabres.2015.04.012. [DOI] [PubMed] [Google Scholar]
- 86.Han X, Liu B, Wang J, Pan A, Li Y, Hu H, et al. Long sleep duration and afternoon napping are associated with higher risk of incident diabetes in middle-aged and older Chinese: the Dongfeng-Tongji cohort study. Ann Med. 2016;48:216–23. doi: 10.3109/07853890.2016.1155229. [DOI] [PubMed] [Google Scholar]
- 87.Knutson KL, Ryden AM, Mander BA, Van Cauter E. Role of sleep duration and quality in the risk and severity of type 2 diabetes mellitus. Arch Intern Med. 2006;166:1768–74. doi: 10.1001/archinte.166.16.1768. [DOI] [PubMed] [Google Scholar]
- 88.Kim BK, Kim BS, An SY, Lee MS, Choi YJ, Han SJ, et al. Sleep duration and glycemic control in patients with diabetes mellitus: Korea National Health and Nutrition Examination Survey 2007–2010. J Korean Med Sci. 2013;28:1334–9. doi: 10.3346/jkms.2013.28.9.1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang T, Lu J, Wang W, Mu Y, Zhao J, Liu C, et al. Sleep duration and snoring associate with hypertension and glycaemic control in patients with diabetes. Diabet Med. 2015;32:1001–7. doi: 10.1111/dme.12809. [DOI] [PubMed] [Google Scholar]
- 90.Kong AP, Choi KC, Zhang J, Luk A, Lam SP, Chan MH, et al. Curvilinear associations of sleep patterns during weekdays and weekends with glycemic control in type 2 diabetes: the Hong Kong Diabetes Registry. Acta Diabetol. 2017;54:151–62. doi: 10.1007/s00592-016-0923-4. [DOI] [PubMed] [Google Scholar]
- 91.Lyytikäinen P, Lallukka T, Lahelma E, Rahkonen O. Sleep problems and major weight gain: a follow-up study. Int J Obes (Lond) 2011;35:109–14. doi: 10.1038/ijo.2010.113. [DOI] [PubMed] [Google Scholar]
- 92.Huang L, Zhou J, Sun Y, Li Z, Lei F, Zhou G, et al. Polysomnographically determined sleep and body mass index in patients with insomnia. Psychiatry Res. 2013;209:540–4. doi: 10.1016/j.psychres.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 93.Piccolo RS, Yang M, Bliwise DL, Yaggi HK, Araujo AB. Racial and socioeconomic disparities in sleep and chronic disease: results of a longitudinal investigation. Ethn Dis. 2013;23:499–507. [PMC free article] [PubMed] [Google Scholar]
- 94.Sivertsen B, Lallukka T, Salo P, Pallesen S, Hysing M, Krokstad S, et al. Insomnia as a risk factor for ill health: results from the large population-based prospective HUNT Study in Norway. J Sleep Res. 2014;23:124–32. doi: 10.1111/jsr.12102. [DOI] [PubMed] [Google Scholar]
- 95.Tan X, Alén M, Cheng SM, Mikkola TM, Tenhunen J, Lyytikäinen A, et al. Associations of disordered sleep with body fat distribution, physical activity and diet among overweight middle-aged men. J Sleep Res. 2015;24:414–24. doi: 10.1111/jsr.12283. [DOI] [PubMed] [Google Scholar]
- 96.Nilsson PM, Rööst M, Engström G, Hedblad B, Berglund G. Incidence of diabetes in middle-aged men is related to sleep disturbances. Diabetes Care. 2004;27:2464–9. doi: 10.2337/diacare.27.10.2464. [DOI] [PubMed] [Google Scholar]
- 97.Björkelund C, Bondyr-Carlsson D, Lapidus L, Lissner L, Månsson J, Skoog I, et al. Sleep disturbances in midlife unrelated to 32-year diabetes incidence: the prospective population study of women in Gothenburg. Diabetes Care. 2005;28:2739–44. doi: 10.2337/diacare.28.11.2739. [DOI] [PubMed] [Google Scholar]
- 98.Meisinger C, Heier M, Loewel H MONICA/KORA Augsburg Cohort Study. Sleep disturbance as a predictor of type 2 diabetes mellitus in men and women from the general population. Diabetologia. 2005;48:235–41. doi: 10.1007/s00125-004-1604-3. [DOI] [PubMed] [Google Scholar]
- 99.Lee JA, Sunwoo S, Kim YS, Yu BY, Park HK, Jeon TH, et al. The effect of sleep quality on the development of type 2 diabetes in primary care patients. J Korean Med Sci. 2016;31:240–6. doi: 10.3346/jkms.2016.31.2.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wan Mahmood WA, Draman Yusoff MS, Behan LA, Di Perna A, Kyaw Tun T, McDermott J, et al. Association between sleep disruption and levels of lipids in Caucasians with type 2 diabetes. Int J Endocrinol. 2013;2013:341506. doi: 10.1155/2013/341506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cho EH, Lee H, Ryu OH, Choi MG, Kim SW. Sleep disturbances and glucoregulation in patients with type 2 diabetes. J Korean Med Sci. 2014;29:243–7. doi: 10.3346/jkms.2014.29.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nefs G, Donga E, van Someren E, Bot M, Speight J, Pouwer F. Subjective sleep impairment in adults with type 1 or type 2 diabetes: results from diabetes MILES-The Netherlands. Diabetes Res Clin Pract. 2015;109:466–75. doi: 10.1016/j.diabres.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 103.Osonoi Y, Mita T, Osonoi T, Saito M, Tamasawa A, Nakayama S, et al. Poor sleep quality is associated with increased arterial stiffness in Japanese patients with type 2 diabetes mellitus. BMC Endocr Disord. 2015;15:29. doi: 10.1186/s12902-015-0026-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.American Academy of Sleep Medicine. International classification of sleep disorders. Darien (IL): American Academy of Sleep Medicine; 2014. [Google Scholar]
- 105.Watson NF, Goldberg J, Arguelles L, Buchwald D. Genetic and environmental influences on insomnia, daytime sleepiness, and obesity in twins. Sleep. 2006;29:645–9. doi: 10.1093/sleep/29.5.645. [DOI] [PubMed] [Google Scholar]
- 106.Cappuccio FP, D’Elia L, Strazzullo P, Miller MA. Quantity and quality of sleep and incidence of type 2 diabetes: a systematic review and meta-analysis. Diabetes Care. 2010;33:414–20. doi: 10.2337/dc09-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Anothaisintawee T, Reutrakul S, Van Cauter E, Thakkinstian A. Sleep disturbances compared to traditional risk factors for diabetes development: systematic review and meta-analysis. Sleep Med Rev. 2016;30:11–24. doi: 10.1016/j.smrv.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 108.Vgontzas AN, Liao D, Pejovic S, Calhoun S, Karataraki M, Bixler EO. Insomnia with objective short sleep duration is associated with type 2 diabetes: a population-based study. Diabetes Care. 2009;32:1980–5. doi: 10.2337/dc09-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Johann AF, Hertenstein E, Kyle SD, Baglioni C, Feige B, Nissen C, et al. Insomnia with objective short sleep duration is associated with longer duration of insomnia in the Freiburg Insomnia Cohort compared to insomnia with normal sleep duration, but not with hypertension. PLoS One. 2017;12:e0180339. doi: 10.1371/journal.pone.0180339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Vgontzas AN, Chrousos GP. Sleep, the hypothalamic-pituitary-adrenal axis, and cytokines: multiple interactions and disturbances in sleep disorders. Endocrinol Metab Clin North Am. 2002;31:15–36. doi: 10.1016/S0889-8529(01)00005-6. [DOI] [PubMed] [Google Scholar]
- 111.Mullington JM, Haack M, Toth M, Serrador JM, Meier-Ewert HK. Cardiovascular, inflammatory, and metabolic consequences of sleep deprivation. Prog Cardiovasc Dis. 2009;51:294–302. doi: 10.1016/j.pcad.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Partch CL, Green CB, Takahashi JS. Molecular architecture of the mammalian circadian clock. Trends Cell Biol. 2014;24:90–9. doi: 10.1016/j.tcb.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Albrecht U. The circadian clock, metabolism and obesity. Obes Rev. 2017;18(Suppl 1):25–33. doi: 10.1111/obr.12502. [DOI] [PubMed] [Google Scholar]
- 114.Perelis M, Marcheva B, Ramsey KM, Schipma MJ, Hutchison AL, Taguchi A, et al. Pancreatic β cell enhancers regulate rhythmic transcription of genes controlling insulin secretion. Science. 2015;350:aac4250. doi: 10.1126/science.aac4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Leproult R, Holmbäck U, Van Cauter E. Circadian misalignment augments markers of insulin resistance and inflammation, independently of sleep loss. Diabetes. 2014;63:1860–9. doi: 10.2337/db13-1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Buxton OM, Cain SW, O’Connor SP, Porter JH, Duffy JF, Wang W, et al. Adverse metabolic consequences in humans of prolonged sleep restriction combined with circadian disruption. Sci Transl Med. 2012;4:129ra43. doi: 10.1126/scitranslmed.3003200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kettner NM, Mayo SA, Hua J, Lee C, Moore DD, Fu L. Circadian dysfunction induces leptin resistance in mice. Cell Metab. 2015;22:448–59. doi: 10.1016/j.cmet.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Vetter C, Devore EE, Ramin CA, Speizer FE, Willett WC, Schernhammer ES. Mismatch of sleep and work timing and risk of type 2 diabetes. Diabetes Care. 2015;38:1707–13. doi: 10.2337/dc15-0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Dallmann R, Viola AU, Tarokh L, Cajochen C, Brown SA. The human circadian metabolome. Proc Natl Acad Sci U S A. 2012;109:2625–9. doi: 10.1073/pnas.1114410109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Davies SK, Ang JE, Revell VL, Holmes B, Mann A, Robertson FP, et al. Effect of sleep deprivation on the human metabolome. Proc Natl Acad Sci U S A. 2014;111:10761–6. doi: 10.1073/pnas.1402663111. [DOI] [PMC free article] [PubMed] [Google Scholar]