Abstract
The translocase of the outer mitochondrial membrane (TOM) complex is a preprotein translocase that mediates transport of nuclear-encoded mitochondrial proteins across the outer mitochondrial membrane. Here we report the purification of this protein complex from Arabidopsis. On blue-native gels the Arabidopsis TOM complex runs at 230 kD and can be dissected into subunits of 34, 23, 21, 8, 7, and 6 kD. The identity of four subunits could be determined by immunoblotting and/or direct protein sequencing. The 21- and the 23-kD subunits exhibit significant sequence homology to the TOM20 preprotein receptor from other organisms. Analysis by two-dimensional isoelectric focusing/Tricine sodium dodecyl sulfide-polyacrylamide gel electrophoresis revealed the presence of further forms for Arabidopsis TOM20. All TOM20 proteins comprise a large cytoplasmically exposed hydrophilic domain, which is degraded upon trypsination of intact mitochondria. Clones encoding four different forms of Arabidopsis TOM20 were identified and sequenced. The deduced amino acid sequences are rather conserved in the N-terminal half and in the very C-terminal part, but include a highly variable glycine-rich region close to the C terminus. Implications on the function of plant TOM complexes are discussed. Based on peptide and nucleic acid sequence data, the primary structure for Arabidopsis TOM40 is presented.
Prerequisites for protein transport into mitochondria are targeting information of the proteins to be transported and a mitochondrial “protein import apparatus” that decodes the targeting information and mediates translocation of proteins across the organellar membranes (for review, see Neupert, 1997; Mori and Terada 1998; Braun and Schmitz, 1999; Voos et al., 1999). The targeting information often is localized on N-terminal extensions, termed presequences, which are removed within the organelles by processing peptidases (Braun and Schmitz, 1998). Central components of the protein import apparatus are translocase complexes: the preprotein translocase of the outer mitochondrial membrane (the so-called TOM complex) and the two preprotein translocases of the inner mitochondrial membrane (called TIM complexes). These translocases were first described for the fungi yeast and Neurospora crassa (for review, see Meisinger et al., 1999; Rassow et al., 1999; Ryan et al., 2000). In yeast the TOM complex consists of nine subunits termed TOM72, TOM70, TOM40, TOM37, TOM22, TOM20, TOM7, TOM6, and TOM5, according to their molecular masses (nomenclature according to Pfanner et al., 1996). The fungal translocases of the outer mitochondrial membrane contain two to three pores for preprotein translocation that are formed by a “TOM core complex” made out of TOM40, TOM22, and the small TOM components (Künkele et al., 1998; Ahting et al., 1999; Verschoor and Lithgow, 1999). TOM40 is directly involved in pore formation (Hill et al., 1998), TOM6 and TOM7 modulate the dynamics of the pore (Alconada et al., 1995; Hönlinger et al., 1996), and TOM5 and TOM22 mediate the interaction of the pore with preprotein receptors (Kiebler et al., 1993; Dietmeier et al., 1997). TOM22 is a multifunctional protein as it also is involved in preprotein binding on both sides of the outer mitochondrial membrane and as it was reported to be important for the assembly of the TOM core complex (van Wilpe et al., 1999). Besides TOM22, fungi contain at least two further preprotein receptors, TOM20 and TOM70, which dynamically interact with the core complex and exhibit different, but overlapping, specificities for the recognition of mitochondrial preproteins. Two additional putative preprotein receptors were so far only identified in yeast: TOM37, which interacts with TOM70 (Gratzer et al., 1995), and TOM72, which represents a TOM70 homolog (Bömer et al., 1996; Schlossmann et al., 1996). Thus, the TOM complex from yeast contains up to five distinct preprotein receptors.
The purification of the preprotein TOM from mammals has not been reported up to now, but some proteins were identified that potentially form part of the TOM complex from mammals (for review, see Mori and Terada, 1998): TOM20, metaxin I and II, and TOM34. Mammalian TOM20 is a preprotein receptor and can functionally replace TOM20 from yeast (Goping et al., 1995). The two metaxins exhibit some sequence similarity to TOM37 from yeast and were also shown to play a role in preprotein recognition at the mitochondrial surface (Armstrong et al., 1997, 1999). Furthermore, TOM34 was reported to represent a functionally important component of the translocation machinery of the outer mitochondrial membrane from mammals (Nuttall et al., 1997; Chewawiwat et al., 1999). Very recently, mammalian counterparts of TOM22 and TOM40 were identified and functionally characterized (Saeki et al., 2000; Suzuki et al., 2000; Yano et al., 2000). On blue-native (BN) gels both subunits form part of a 400-kD protein complex that also includes TOM20 and several other unidentified proteins with molecular masses of 5 to 10 kD (Suzuki et al., 2000). Hence, the mammalian TOM complex seems to have a similar subunit composition as the TOM complex from fungi.
A plant TOM complex was purified from potato tuber (Jänsch et al., 1998a, 1998b; Braun and Schmitz, 1999). The protein complex is comparatively small and only comprises six subunits of 36, 23, 9, 8, 7, and 6 kD. An additional subunit of about 70 kD possibly forms part of the potato TOM complex. Based on sequence homology the 36-kD protein could be classified as a TOM40 homolog and most likely constitutes the pore of the TOM complex from plant mitochondria. The four small TOM components are tightly linked to TOM40 and seem to be counterparts of yeast TOM5, TOM6, and TOM7 (the 7-kD protein from potato has significant sequence homology to TOM7 from yeast). In contrast, the 23-kD protein interacts dynamically with the potato TOM complex. The protein was designated TOM20 because it exhibits some sequence homology to TOM20 from other organisms and because it contains a tetratricopeptide motif described for some preprotein receptors from other organisms (Heins and Schmitz, 1996). However, sequence homology is very low and the plant protein also seems to be anchored to the outer mitochondrial membrane differently than TOM20 proteins from mammals and fungi. The role of potato TOM20 in mitochondrial preprotein recognition was demonstrated by the inhibition of in vitro protein import into potato mitochondria by antibodies directed against this protein (Heins and Schmitz, 1996). Very recently the 9-kD subunit of the potato TOM complex was suggested to be a counterpart of fungal TOM22 based on sequence similarity (Macasev et al., 2000). The putative plant TOM22 homolog is comparatively small and lacks the acidic domain that is involved in preprotein recognition on the cytoplasmically exposed side of the outer mitochondrial membrane from fungi. In summary, the potato TOM complex seems to have a different structure than the corresponding protein complex from other organisms. Most notably it possibly contains only one receptor for preproteins on the cytoplasmic side of the outer mitochondrial membrane. No counterparts for TOM37 and TOM70 could be identified.
In an attempt to verify these results and to further explore the structure of the plant preprotein TOM we purified the TOM complex from Arabidopsis. The protein complex has a similar subunit composition like the TOM complex from potato as monitored by two-dimensional PAGE. However, besides the 23-kD band an additional band at 21 kD could be resolved on our gels. Two-dimensional isoelectric focusing (IEF)/Tricine SDS-PAGE further separated the two protein bands into four to six spots, which all crossreacted with an antibody directed against TOM20 from potato. Direct protein sequencing and characterization of cDNA clones verified the existence of at least four TOM20 forms in Arabidopsis. They all are susceptible toward trypsin treatment of intact mitochondria and represent candidates for receptors with distinct substrate specificity.
RESULTS
The preparation of pure mitochondria from green Arabidopsis tissue proved to be a very difficult task. Therefore, dark-grown cell lines were cultivated as a starting material for organelle preparations. Following the protocol given in “Materials and Methods,” 1 g of Arabidopsis mitochondria can be obtained from 700 g of Arabidopsis cells.
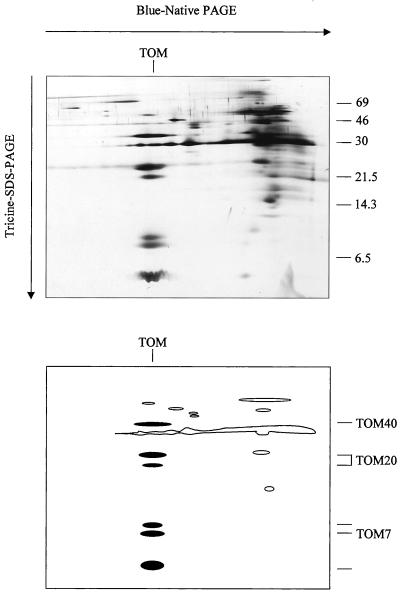
The TOM complex is considered to be a rather dynamic structure with receptors reversibly binding to the components directly involved in pore formation. Hence, biochemical preparations of the TOM complex should be carried out under very gentle conditions. Our isolation protocol is based on the preparation of outer mitochondrial membranes by a combination of two Suc gradient centrifugations and solubilization of the membrane proteins with digitonin. The Arabidopsis TOM complex is subsequently separated from other proteins of the outer mitochondrial membrane by blue-native (BN)-PAGE. The complex forms a band at 230 kD and can be electroeluted in intact form (data not shown). If the BN gel electrophoresis is combined with a second gel electrophoresis, which is carried out under denaturing conditions, the subunits of the TOM complex are separated and form a vertical row (Fig. 1). Six dominant protein bands are resolved with apparent molecular masses of 34, 23, 21, 8, 7, and 6 kD. In additional, a faint band at about 50 kD is visible in the same row on the gels. The composition of the Arabidopsis TOM complex resembles the one reported for potato (Jänsch et al., 1998a) with two exceptions: one of the small potato TOM proteins seems to be absent in Arabidopsis and one extra subunit is present in the 20 kD range.
Figure 1.
Purification of the TOM complex from Arabidopsis. Total outer mitochondrial membrane protein from Arabidopsis was solubilized by 5% (w/v) digitonin and was directly analyzed by two-dimensional BN-PAGE/Tricine SDS-PAGE as described under “Materials and Methods.” The gel was silver stained. Sizes of standard proteins are given on the right in kilodaltons. A scheme of the gel is presented in the bottom part of the figure. TOM subunits are marked in black.
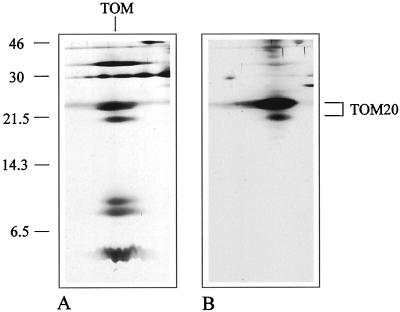
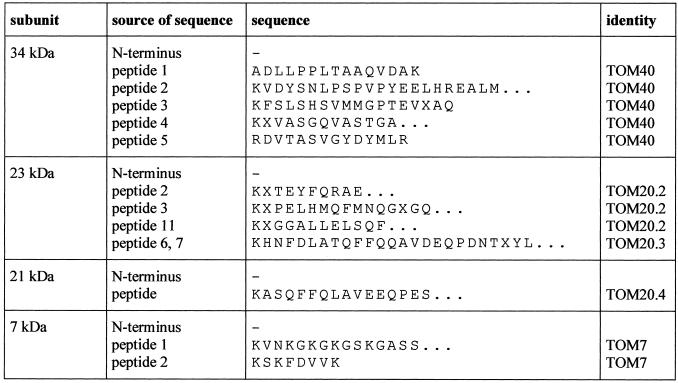
To identify the subunits of the Arabidopsis TOM complex the two-dimensional gels were blotted and immunostained. An antibody directed against TOM20 from potato specifically reacted with the 23-kD subunit from Arabidopsis, but also showed some cross reaction with the band at 21 kD (Fig. 2). Hence, the 21-kD protein possibly represents a second form of TOM20. To further investigate the identity of the 21- and 23-kD bands, both proteins were subjected to direct protein sequencing, but proved to be N-terminally blocked for cyclic Edman degradation. Therefore, peptides for both proteins were generated, separated by HPLC, and sequenced (Fig. 3). Comparison of the sequences of four peptides of the 23-kD band and of one peptide of the 21-kD band with TOM20 from potato revealed significant sequence homologies. Partial sequencing of the 36- and 7-kD bands allowed the identification of these proteins as counterparts of TOM40 and TOM7 from other organisms. The identity of the other proteins is so far unclear.
Figure 2.
Immunological identification of TOM20. A, Silver-stained gel of the purified TOM complex from Arabidopsis. B, Western blot of an identical gel after immunostaining with an antibody directed against potato TOM20 (Heins and Schmitz 1996). The numbers on the left indicate the masses of standard proteins in kilodaltons.
Figure 3.
N-terminal sequences of peptides of the TOM proteins from Arabidopsis. Amino acids are given in the one-letter code. The identity of the TOM20 sequences refers to the sequences deduced from TOM20 clones as given in Figure 4. The peptide sequences for the 34- and 7-kD subunits match to amino acid sequences encoded by the Arabidopsis P1 clones MZE19 (accession no. AP002050) and MBK23 (accession no. AB005233) and resemble fungal TOM40 and TOM7 (Fig. 9). Peptide sequences of Arabidopsis TOM20.4 and TOM40 were submitted to the Swiss-Prot database (accession nos. P82805 and Q9LHE5).
In an attempt to identify clones encoding TOM proteins in Arabidopsis the complete TOM20 sequence of potato was used to probe Arabidopsis DNA databases. More than 15 sequence entries with significant homologies were found and carefully analyzed. On the basis of the nearly completed genomic sequence of Arabidopsis, four different regions were found to encode proteins highly similar to potato TOM20 (Table I). Three of these genes completely match entries in Arabidopsis expressed sequence tag (EST) databases and hence are expressed. EST clones encoding the three different TOM20 proteins were obtained from the Arabidopsis Biological Resource Center and completely sequenced on both strands (Table I). All clones encode complete open reading frames, 3′-non-coding regions including poly(A) tails, and 5′-non-coding regions containing stop codons in frame with the coding sequence. The deduced amino acid sequences were termed AtTOM20–1, AtTOM20–2, and AtTOM20–3 (Table I). A fourth AtTOM20 protein (AtTOM20–4) can be predicted from a genomic clone, but corresponding ESTs are absent in the Arabidopsis EST databases.
Table I.
Clones encoding TOM20 forms of Arabidopsis
| TOM20-1a | TOM20-2a | TOM20-3a | TOM20-4 | |
|---|---|---|---|---|
| Expressed Sequence Tag (EST) | T45288 | T44475 | R86802 | – |
| Accession no. | AJ296023 | AJ296024 | AJ296025 | – |
| Other ESTs with identical sequence | – | Z26778; Z26777 | R90391; AI995262; AA042456; AA006022; AA598240 | – |
| Genomic clones | AB026649 (chromosome 3) | AC004557 (chromosome 1) | AB026649 (chromosome 3) | AB023040 (chromosome 5) |
| Length of the sequenced clone | 828 bp | 1,018 bp | >782 bp | – |
| Open reading frame of the sequenced clone | 564 bp | 630 bp | 606 bp | 561 bpb |
| 5′ Non-coding region of the sequenced clone | 70 bp including in frame stop | 62 bp including in frame stop | 28 bp including in frame stop | – |
| 3′ Non-coding region of the sequenced clone (including PolyA) | 194 bp | 326 bp | 148 bp | – |
| Poly(A) | 20 bp | 16 bp | >15 bp |
The complete nucleotide sequences of clones encoding TOM20-1, TOM20-2, and TOM20-3 were submitted to sequence databanks and are available under the accession nos. AJ296023, AJ296024, and AJ296025. The partial amino acid sequence of TOM20-4 is available under P82805.
As predicted by “GeneBuilder.”
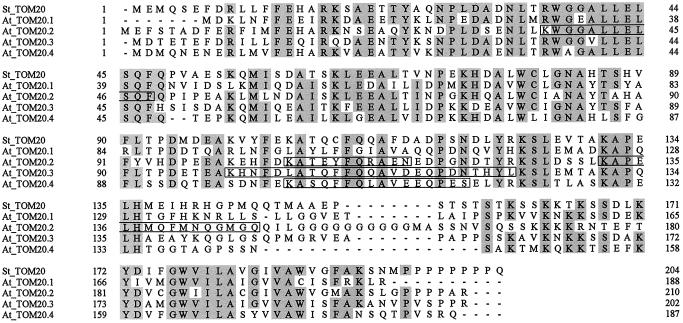
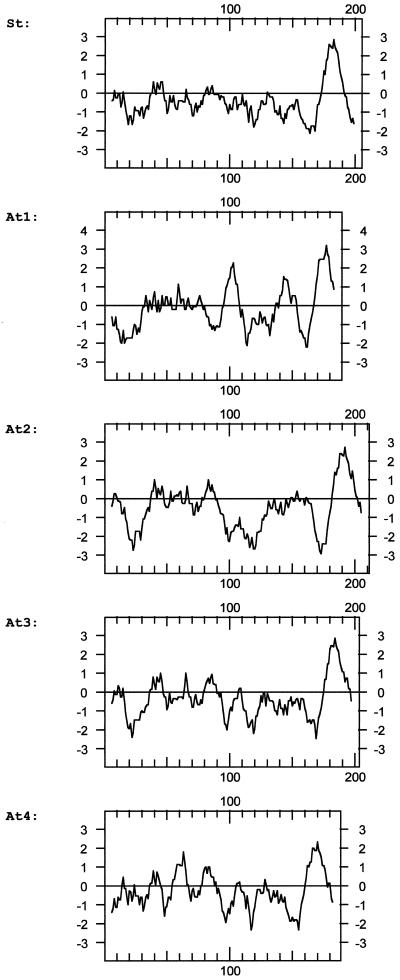
Sequence comparisons between the four TOM20 forms revealed sequence identities between 35% and 60% (Fig. 4); sequence identities between the Arabidopsis proteins and potato TOM20 are in the same range. The N-terminal half of the proteins and the very C-terminal part are highly conserved, whereas a region close to the C terminus is variable and rich in Gly residues. All proteins are predicted to have a large hydrophilic domain and a membrane anchor at the C terminus (Fig. 5).The amino acid sequences determined for the 21- and 23-kD proteins of the Arabidopsis TOM complex completely match sequence stretches of the proteins deduced from the DNA sequences (Fig. 4): three peptides of the 23-kD band correspond to AtTOM20–2 (calculated molecular mass of 23.2 kD), one peptide of the 23-kD band corresponds to AtTOM20–3 (calculated molecular mass of 22.6 kD), and the peptide of the 21-kD band corresponds to AtTOM20–4 (calculated molecular mass of 21.0 kD). Hence, the gene encoding AtTOM20–4 is also expressed. No peptide sequence matches TOM20–1, which has a calculated molecular mass of 21.3 kD.
Figure 4.
Sequence comparison of the four TOM20 proteins from Arabidopsis (At) and TOM20 from potato (St). Amino acids are underlayed in gray if conserved in at least four of the five sequences. The peptides given in Figure 3 are indicated by boxes.
Figure 5.
Comparison of hydrophobicity profiles of the four TOM20 proteins from Arabidopsis (At) and TOM20 from potato (St). The profiles were calculated according to Kyte and Doolittle (1982) using a window of 11 amino acids. The numbers at the x axis refer to the amino acid positions and the numbers on the y axis refer to hydrophobicity.
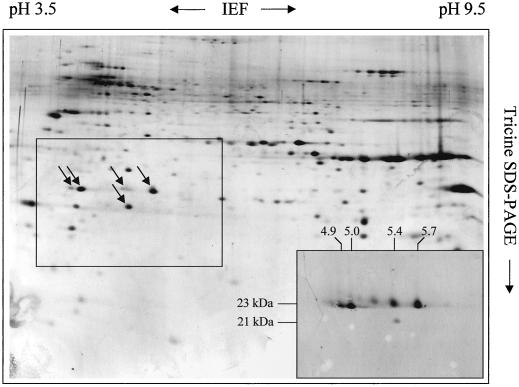
To investigate the number of different TOM20 proteins present in our TOM complex preparations the proteins of a fraction containing outer mitochondrial membranes from Arabidopsis were separated by two-dimensional IEF/Tricine SDS-PAGE and blotted onto filter membranes (Fig. 6). Immunostaining of the blots with the TOM20 antibody directed against TOM20 from potato revealed three dominant and three faint protein spots with pI between 4.9 and 5.7 (the calculated pI for the Arabidopsis TOM20 proteins predicted from DNA lie between 4.9 and 5.8 and are given in Table II). Hence, the number of TOM20 forms in Arabidopsis might be higher than four. In an alternate manner, two of the four identified TOM20 forms are partially post-translationally modified, giving rise to the occurrence of additional spots on the two-dimensional gels.
Figure 6.
Separation of multiple TOM20 forms from Arabidopsis. Total outer mitochondrial protein from Arabidopsis was separated by two-dimensional IEF/Tricine SDS-PAGE as described in “Materials and Methods.” The gel was silver stained. Some spots in the central frame specifically reacted with an antibody directed against TOM20 from potato (western blot in the right corner at the botton of the gel). The numbers on top and on the left of the western blot refer to the pI and molecular masses of the immunopositive spots.
Table II.
Features of TOM20 proteins of Arabidopsis
| TOM20-1 | TOM20-2 | TOM20-3 | TOM20-4a | |
|---|---|---|---|---|
| No. of amino acids | 188 | 210 | 202 | 187 |
| Calculated molecular mass (kDa) | 21.3 | 23.2 | 22.6 | 21.0 |
| Calculated isoelectric point | 5.8 | 5.5 | 4.9 | 5.4 |
As predicted by “GeneBuilder.”
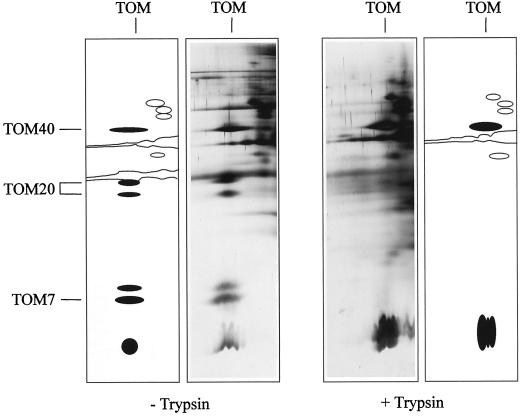
Protease susceptibility of the components of the TOM complex from Arabidopsis was investigated by trypsination of intact Arabidopsis mitochondria prior to the preparation of the outer mitochondrial membranes (Fig. 7). On BN gels the TOM complex shifts to a size of less than 200 kD. Arabidopsis TOM40 (34 kD) and possibly also the 6-kD protein resist the protease treatment. In contrast, all TOM20 forms and also TOM7 and the 8-kD subunit are effectively degraded by the trypsination of the mitochondria and therefore are assumed to contain cytoplasmic domains.
Figure 7.
Trypsination of intact Arabidopsis mitochondria causes degradation of TOM20. Outer mitochondrial membranes were prepared from trypsinated mitochondria (+ Trypsin) and from untreated mitochondria (− Trypsin). The TOM complex of both preparations was resolved by two-dimensional BN/Tricine SDS-PAGE and silver stained. Schemes of the gels are given beside the gels. TOM subunits are marked in black.
DISCUSSION
Dark-grown Arabidopsis cell cultures proved to be a suitable starting material for the isolation of mitochondria and the subsequent purification of mitochondrial enzymes. This is the first report of a biochemical characterization of a component of the mitochondrial protein import apparatus from Arabidopsis. The purified Arabidopsis TOM complex has a similar molecular mass as the corresponding complex from potato and runs at 230 kD on BN gels. In contrast, the TOM complex from yeast prepared by BN gel electrophoresis runs at 400 kD (Dekker et al., 1996, 1998). Thus, the plant TOM complex seems to have a simpler structure and possibly comprises only one type of receptor, TOM20. Of the six resolved protein bands of the Arabidopsis TOM complex, four proteins could be identified by immunostaining and/or direct protein sequencing: the 34-kD protein corresponds to TOM40 from other organisms, the 7-kD protein is homolog to TOM7, and the 23- and 21-kD proteins resemble TOM20. No data could be generated to identify the 8- and 6-kD proteins up to now. Possibly the 8-kD protein represents a TOM22 homolog that has a comparatively low molecular mass due to the absence of the cytoplasmically exposed domain for preprotein recognition as proposed by Macasev et al. (2000). In addition, a protein of about 50 kD possibly represents another subunit of the Arabidopsis TOM complex because it is resolved in the same vertical row on BN gels as the other TOM subunits. However, if this protein forms part of the TOM complex it most likely is present in substoichiometric amounts because it is weakly stained on our gels (Fig. 1).
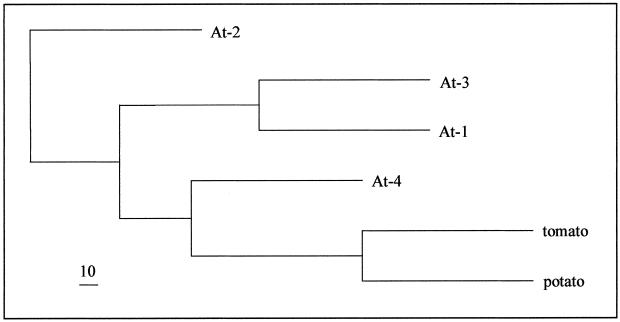
Sequence identity between potato TOM20 and the four TOM20 forms from Arabidopsis lies in the range of 50%. As reflected by the tree in Figure 8, TOM20–4 is most similar to potato and tomato TOM20, TOM20–1 and TOM20–3 from Arabidopsis are closely related proteins and are positioned on one branch, and TOM20–2, which contains 10 successive Gly residues in the variable region close to the C terminus, is the least related. The sequence identity between TOM20 from plants and TOM20 from mammals and fungi is very low (about 20%) and at the borderline of significance (Heins and Schmitz, 1996, Jänsch et al., 1998b). The plant TOM20s contain a tetratricopeptide motif reported for TOM20 proteins from other organisms, but they lack an N-terminal membrane anchor sequence. Instead, they are predicted to be anchored by their C-termini. Therefore, it remains an open question whether the plant TOM20s on one side and the fungal and mammalian TOM20s on the other side are truly related and descendants of the same protein, or if they represent different proteins that became similar due to convergence.
Figure 8.
Phylogram of the four TOM20 proteins from Arabidopsis and TOM20 from potato and tomato. An alignment was calculated with ClustalW. The phylogenetic analysis was performed using the programs Seqboot, Protdist, Neighbor, Consensus, and Drawgramm of the Phylip program package. The scale bar in the left bottom corner indicates a bootstrap value of 10. The TOM20 sequence from tomato was deduced from the EST clone AI486270.
It is interesting that TOM20 from Arabidopsis is present in four to six different forms. In contrast, the TOM components of yeast are present only in one form with the exception of TOM70 and TOM72, which exhibit 50% sequence identity (Bömer et al., 1996; Schlossmann et al., 1996). Also for N. crassa there were no reports on TOM components occurring in more than one form. However, the genome sequencing projects for mammals and plants already uncovered that many if not most proteins of higher eukaryotes are encoded by several related genes. The complete sequencing of the chromosomes 2 and 4 of Arabidopsis revealed large duplicated regions and also a high number of genes arranged in tandem repeats (Lin et al., 1999; Mayer et al., 1999). The physiological importance of the presence of different forms of proteins is an interesting field of study. In several cases tissue-specific, developmental, or physiological stage specific expression of related genes was reported (Gazzarrini et al., 1999; Genger et al., 1999; Lemoine et al., 1999; Torki et al., 1999). The regulation of the genes encoding TOM20 proteins in Arabidopsis remains to be investigated. At least three of the TOM20 genes are expressed in dark-grown tissue cultures from Arabidopsis. The sequences of the different Arabidopsis TOM20 forms are less similar than usually reported for isoforms, possibly reflecting different functions of the proteins. Especially the highly variable Gly-rich region of the Arabidopsis TOM20s could determine differential substrate specificity. Hence, plant mitochondria would basically contain one type of preprotein receptor, which is present in multiple forms that recognize different sets of preproteins. An interesting question is whether different forms of TOM20 can occur simultaneously in individual protein complexes.
The genome sequencing project and the EST sequencing projects proved to be a very fruitful background for the investigation of the Arabidopsis TOM subunits. The four TOM20 forms are encoded by chromosome 1 (AtTOM20–1 and AtTOM20–3), chromosome 3 (AtTOM20–2), and chromosome 5 (AtTOM20–4). The genes encoding TOM20–1 and TOM20–3, which are comparatively similar, are arranged as a tandem repeat. Furthermore, a region encoding an incomplete form of TOM20 is present on chromosome 5 and possibly represents a pseudogene. The presence of pseudogenes for TOM20 was also reported for humans (Hernández et al., 1999a, 1999b). The TOM20 forms of Arabidopsis are encoded by six exons, respectively, which have very conserved exon/intron boundaries.
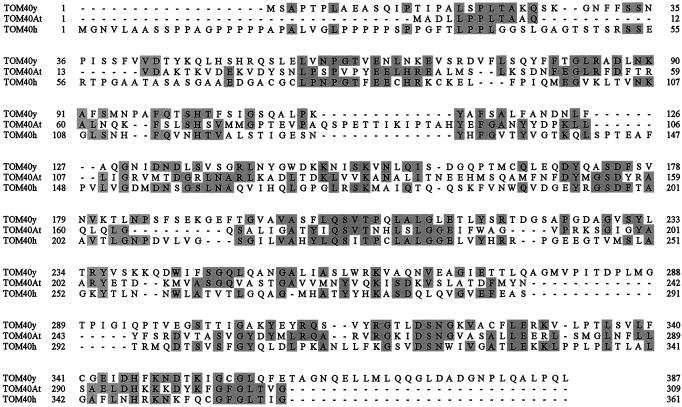
Very recently a large number of Arabidopsis ESTs were published that represent new sequences (Asamizu et al., 2000). The peptide sequences of Arabidopsis TOM40 exhibit 100% identity to the amino acid sequences deduced by some of the novel ESTs and to a putative protein sequence encoded by a genomic clone of Arabidopsis chromosome 1 (P1 clone MZE19). Arabidopsis TOM40 comprises 309 amino acids, has a calculated molecular mass of 34 kD, and exhibits between 25% and 28% sequence identity to TOM40 from fungi and mammals (Fig. 9).
Figure 9.
Sequence comparisons between TOM40 from different organisms. TOM40y, yeast TOM40 (S12773); TOM40At, Arabidopsis TOM40 (Q9LHE5); and TOM40h, human TOM40 (AAC82343).
Taking all data together, protein transport into mitochondria seems to be a rather conserved mechanism between animals, plants, and fungi. Mitochondrial presequences always have a similar amino acid composition and many components of the protein import machineries are likewise present in all organisms investigated. However, there are some exceptions. The mitochondrial processing peptidase that cleaves off mitochondrial presequences of preproteins after their import has been completed forms part of the cytochrome c reductase complex in plants, but not in mammals or fungi (Braun et al., 1992; Braun and Schmitz, 1995). Animal mitochondria have a preprotein receptor, TOM34, which is not related to any of the well-characterized fungal receptors (Nuttall et al., 1997; Chewawiwat et al., 1999). The TOM complex of plant mitochondria seems to have a simpler structure and possibly only contains one type of receptor that occurs in multiple forms. An investigation of the expression and function of the TOM20 forms of Arabidopsis is under way in our laboratory.
MATERIALS AND METHODS
Cultivation of Arabidopsis Cell Lines
Arabidopsis cell lines were cultivated in the dark at 24°C to 26°C, 30% humidity, and gentle shaking (90 rpm). The cultivation medium contained 3% (w/v) Suc, Murashige and Skoog basal salt mixture (Sigma, Germany), nicotinic acid (0.5 mg L−1), pyridoxol-HCl (0.5 mg L−1), thiamine-HCl (100 μg L−1), myo-inositol (100 mg L−1), 2,4-dichlorphenoxyacetic acid (1 mg L−1), and ampicilline (100 mg L−1), pH 5.7. Inoculation of 50 mL of medium in a 300-mL Erlenmeyer vessel with 1 g Arabidopsis cells yielded about 8 g of cells after 7 d of cultivation.
Preparation of Mitochondria
About 700 g Arabidopsis cells were filtered through two layers of muslin and suspended in 1,400 mL of ice-cold “grinding buffer” (450 mm Suc, 1.5 mm EGTA, 0.2% [w/v] bovine serum albumin [BSA], 0.6% [w/v] polyvinylpyrrolidone 40, 10 mm dithiothreitol [DTT], 0.2 mm phenylmethylsulfonyl fluoride [PMSF], and 15 mm MOPS [3-(N-morpholino)-propanesulfonic acid]/KOH, pH 7.4). The cells were disrupted by homogenizing for three periods of 15 s using a Waring blender. Mitochondria were enriched by a three-step centrifugation: two centrifugations at 3,000g for 5 min (organelles in supernatant) and one centrifugation at 17,000g for 10 min (organelles in pellet). The mitochondrial fraction was resuspended in “washing buffer” (300 mm Suc, 1 mm EGTA, 0.2 mm PMSF, and 10 mm MOPS/KOH, pH 7.2) and layered on top of three-step Percoll gradients (six gradients of 30 mL each containing 10 mL of 18% [v/v], 10 mL of 23% [v/v], and 10 mL of 40% [v/v] Percoll in 0.3 m Suc and 10 mm MOPS/KOH, pH 7.2). After centrifugation for 45 min at 70,000g the mitochondria can be isolated from the 23%/40% interphase. To remove the Percoll the purified mitochondria were centrifuged twice in “resuspension buffer” (0.4 m mannitol, 1 mm EGTA, 0.2 mm PMSF, and 10 mm Tricine/KOH, pH 7.2) for 10 min at 12,000g. The yield of a typical preparation lies at 1.0 g of mitochondria (about 100 mg of mitochondrial protein) per 700 g of Arabidopsis cells.
Purification of the TOM Complex from Arabidopsis
As a first step to enrich the TOM complex the outer mitochondrial membranes are prepared from freshly prepared organelles. One gram of mitochondria were resuspended in 6 mL of “swelling buffer” (2 mm PMSF and 5 mm KiPO4, pH 7.2) for 6 min on ice. Another 12 mL of swelling buffer was added and after 4 min the mitochondria were ruptured in a Potter homogenizer. Outer membrane vesicles were separated from mitoplasts and unbroken mitochondria by centrifugation through Suc step gradients (six gradients of 6.5 mL each containing 1 mL of 60% [w/v], 4 mL of 32% [w/v], and 1.5 mL of 15% [w/v] Suc in 1 mm EDTA, 1 mm PMSF, and 10 mm MOPS/KOH, pH 7.2). After centrifugation at 2°C for 1 h at 92,000g outer membranes can be collected from the 15%/30% interphases and were adjusted to 50% (w/v) Suc. Another Suc step gradient was formed by layering a two-step Suc gradient on top of the outer membrane suspension (three gradients of 11.5 mL each containing 5 mL of outer membrane suspension [50% (w/v) Suc], 5 mL of 32%, and 1.5 mL of 0% [w/v] Suc in 1 mm EDTA, 1 mm PMSF, and 10 mm MOPS/KOH, pH7.2). Outer membranes were made to float through this gradient at 2°C for 5 h at 170,000g and were removed from the 0%/32% interphase. The fraction was diluted 1:4 with a “dilution buffer” (1 mm EDTA, 1 mm PMSF, and 10 mm MOPS/KOH, pH7.2) and pelleted by centrifugation at 100,000g for 90 min. The yield of a typical preparation lies at about 0.5 mg of outer mitochondrial membrane protein/100 mg of total mitochondrial protein from Arabidopsis.
The second step to purify the TOM complex is based on gentle solubilization of outer membrane proteins with detergents and on BN-PAGE. One hundred micrograms of outer mitochondrial membrane proteins are resuspended in 75 μL of 750 mm aminocaproic acid, 0.5 mm EDTA, and 50 mm BisTris/HCl, pH 7.0. Membrane proteins are solubilized by adding the same volume of ice-cold “digitonin solution” (10% [w/v] digitonin, 750 mm aminocaproic acid, 0.5 mm EDTA, and 50 mm BisTris [2-[bis(hydroxyethyl)amino]-2-(hydroxymethyl)-1-propane-1,3-diol]/HCl, pH 7.0). Insoluble material is removed by centrifugation at 50,000g at 2°C for 20 min and the supernatant is supplemented with 15 μL of Coomassie Blue solution (5% [w/v] Coomassie Blue and 750 mm aminocaproic acid). The suspension can be loaded directly into the pockets of a BN gel. BN gel electrophoresis is carried out as described in Jänsch et al. (1996). The Arabidopsis TOM complex forms a faint band at about 230 kD, which is visible without staining. The band was cut out and the protein complex was electroeluted in an “electroelution buffer” (25 mm Tricine, 7.5 mm Bis-Tris, pH 7.0, and 0.1 mm PMSF) using the electroeluter from CBS Scientific (Del Mar, CA). The yield of a typical preparation lies at 50 μg of TOM complex/0.5 mg of outer mitochondrial membrane protein.
Two-Dimensional PAGE
To analyze the subunit composition of the TOM complex from Arabidopsis two different two-dimensional gel electrophoresis systems were used, which are based on BN-PAGE or IEF in the first dimension and on Tricine SDS-PAGE in the second gel dimension. A protocol for BN-PAGE is given in Jänsch et al. (1996). Stripes of BN gels containing separated outer membrane proteins were cut out, treated with “denaturation solution” (1% [w/v] SDS and 1% [w/v] β-mercaptoethanol) for 30 min, and subsequently transferred horizontally on Tricine-SDS polyacrylamide gels. A protocol for Tricine SDS-PAGE as a second gel dimension for BN gels is given in Schägger et al., 1994.
IEF was carried out using Immobiline DryStrip gels (18 cm) with non-linear pH gradients (pH 3–10) and the IPGphor isoelectric focusing system (Amersham Pharmacia Biotech, Sweden). One hundred micrograms of outer mitochondrial membrane protein were resuspended in 10 μL of “lysis solution” (8 m urea, 4% [w/v] Triton X-100, 40 mm Tris base, 50 mm DTT, and 0.1 mm PMSF), incubated for 1 h, and subsequently supplemented with 340 μL of DryStrip “rehydration solution” (8 m urea, 2% [w/v] Triton X-100, 0.5% [w/v] immobilized pH gradient buffer, a trace of bromphenol blue, and 20 mm DTT) according to the manufacturer's instructions (Berkelman and Stenstedt, 1998). The solution was directly applied onto a dry gel stripe, rehydration took place at 30 V for 12 h and focusing in four steps took place at 500 V (1 h), 500 to 1,000 V (1 h), 1,000 to 8,000 V (4 h), and 8,000 V (6 h). Gelstrips were incubated with “equilibration buffer” (50 mm Tris-HCl, pH 8.8, 6 m urea, 30% [v/v] glycerol, 2% [w/v] SDS, 66 mm DTT, and a trace of bromphenol blue) and transferred horizontally onto a Tricine-SDS polyacrylamide gel as described by Berkelman and Stenstedt (1998). Tricine SDS-PAGE was carried out as outlined by Schägger et al. (1994).
Two-dimensional gels were silver stained (Heukeshoven and Dernick, 1986) or blotted onto filter membranes (see below).
Identification of Proteins by Immunoblotting and Direct Amino Acid Sequencing
Blotting of gels was carried out using the TransBlot cell from Bio-Rad (Germany). For immunostaining, gels were blotted onto nitrocellulose membranes in “transfer buffer I” (20 mm Tris base, 20% [v/v] methanol, and 150 mm Gly) for 6 h at 200 mA. Blots were incubated with antibodies raised against TOM20 from potato (dilution: 1:1,000) overnight and staining of immunopositive bands was carried out using the Vectastain ABC-Kit (Vector Laboratories, Burlingame, CA) according to the manufacturer's instructions. For direct protein sequencing, gels were blotted onto polyvinylidene difluoride membranes in “transfer buffer II” (20 mm Tris-HCl, pH 8.8, 0.04% [w/v] SDS, 1 mm DTT, and 20% [v/v] methanol) for 18 h at 300 mA. Proteins were stained with Ponceau S, cut out, and directly subjected to Edman degradation or digested overnight with endoproteinase Lys C (Boehringer, Germany) to generate peptides. The separation of peptides and the direct amino acid sequence determination was described previously (Braun et al., 1994).
Trypsination of Intact Mitochondria
To obtain topological information on the TOM subunits, freshly prepared Arabidopsis mitochondria were incubated with Trypsin for 20 min at 20°C. Trypsin concentration was 0.25 mg/mg of mitochondrial protein. The reaction was stopped by adding Trypsin inhibitor (10 mg/mg of Trypsin). The outer mitochondrial membrane of the trypsinated organelles and of untreated organelles (control) was subsequently prepared as outlined above and the TOM complex was visualized by two-dimensional BN-PAGE/Tricine SDS-PAGE.
DNA Analysis
TOM20 from potato (X92491) was used to probe the Arabidopsis database at http://www.Arabidopsis.org/search.html. Several ESTs encoding parts of putative TOM20 homologs could be identified. The ESTs T44475, R86802, and T45288 were obtained from the Arabidopsis Biological Resource Center (http://aims.cps.msu.edu/aims) and were completely sequenced on both strands. Nucleic acid sequences and deduced amino acid sequences were analyzed by programs available on the Internet (calculation of molecular weights and pI: Compute pI/Mw tool at http://www.expasy.ch/tools/pitool.html; alignments and phylogenetic trees: ClustalW at http://www2.ebi.ac.uk/clustalw; and exon and intron prediction: GeneBuilder at http://www.itba.mi.cnr.it/webgene). Hydrophobicity profiles were calculated using the DNA strider software package.
ACKNOWLEDGMENTS
The EST clones T45288, T44475, and R86802 were kindly provided by the Arabidopsis Biological Resource Center. We thank Gabi Kühne and Dagmar Lewejohann for the cultivation of Arabidopsis cell lines and expert technical assistance.
Footnotes
This work was supported by the Deutsche Forschungsgemeinschaft and the Fonds der Chemischen Industrie.
LITERATURE CITED
- Ahting U, Thun C, Hegerl R, Typke D, Nargang FE, Neupert W, Nussberger S. The TOM core complex: the general protein import pore of the outer membrane of mitochondria. J Cell Biol. 1999;147:959–968. doi: 10.1083/jcb.147.5.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alconada A, Kübrich M, Moczko M, Hönlinger A, Pfanner N. The mitochondrial receptor complex: the small subunit Mom8b/ISP6 supports association of receptors with the general insertion pore and transfer of preproteins. Mol Cell Biol. 1995;15:6196–6205. doi: 10.1128/mcb.15.11.6196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong LC, Komiya T, Bergman BE, Mihara K, Bornstein P. Metaxin is a component of a preprotein import complex in the outer membrane of the mammalian mitochondrion. J Biol Chem. 1997;272:6510–6518. doi: 10.1074/jbc.272.10.6510. [DOI] [PubMed] [Google Scholar]
- Armstrong LC, Saenz AJ, Bornstein P. Metaxin 1 interacts with metaxin 2, a novel related protein associated with the mammalian mitochondrial outer membrane. J Cell Biochem. 1999;74:11–22. [PubMed] [Google Scholar]
- Asamizu E, Nakamura Y, Sato S, Tabata S. A large scale analysis of cDNA in Arabidopsis thaliana: generation of 12,028 non-redundant expressed sequence tags from normalized and size-selected cDNA libraries. DNA Res. 2000;30:175–180. doi: 10.1093/dnares/7.3.175. [DOI] [PubMed] [Google Scholar]
- Berkelman T, Stenstedt T. 2-D Electrophoresis using Immobilized pH Gradients: Principles and Methods. Uppsala: Amersham Pharmacia Biotech; 1998. [Google Scholar]
- Bömer U, Pfanner N, Dietmeier K. Identification of a third yeast mitochondrial Tom protein with tetratricopeptide repeats. FEBS Lett. 1996;382:153–158. doi: 10.1016/0014-5793(96)00156-1. [DOI] [PubMed] [Google Scholar]
- Braun HP, Emmermann M, Kruft V, Schmitz UK. The general mitochondrial processing peptidase from potato is an integral part of cytochrome c reductase of the respiratory chain. EMBO J. 1992;11:3219–3227. doi: 10.1002/j.1460-2075.1992.tb05399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun HP, Kruft V, Schmitz UK. Molecular identification of the 10 subunits of cytochrome c reductase from potato. Planta. 1994;193:99–106. doi: 10.1007/BF00191612. [DOI] [PubMed] [Google Scholar]
- Braun HP, Schmitz UK. Are the “core” proteins of the mitochondrial bc1 complex evolutionary relics of a processing peptidase? Trends Biochem Sci. 1995;20:171–175. doi: 10.1016/s0968-0004(00)88999-9. [DOI] [PubMed] [Google Scholar]
- Braun HP, Schmitz UK. Mitochondrial processing peptidase. In: Barrett AJ, Rawlings N, Woessner F, editors. Handbook of Proteolytic Enzymes. London: Academic Press; 1998. pp. 1372–1376. [Google Scholar]
- Braun HP, Schmitz UK. The protein-import apparatus of plant mitochondria. Planta. 1999;209:267–274. doi: 10.1007/s004250050632. [DOI] [PubMed] [Google Scholar]
- Chewawiwat N, Yano M, Terada K, Hoogenraad NJ, Mori M. Characterization of the novel mitochondrial import component, Tom34, in mammalian cells. J Biochem. 1999;125:721–727. doi: 10.1093/oxfordjournals.jbchem.a022342. [DOI] [PubMed] [Google Scholar]
- Dekker PJT, Müller H, Rassow J, Pfanner N. Characterization of the preprotein translocase of the outer mitochondrial membrane by blue native electrophoresis. Biol Chem. 1996;377:535–538. [PubMed] [Google Scholar]
- Dekker PJT, Ryan MT, Brix J, Müller H, Hönlinger A, Pfanner N. Preprotein translocase of the outer mitochondrial membrane: molecular dissection and assembly of the general import pore complex. Mol Cell Biol. 1998;18:6515–6524. doi: 10.1128/mcb.18.11.6515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietmeier K, Hönlinger A, Bömer U, Dekker PJT, Eckerskorn C, Lottspeich F, Pfanner N. Tom5 functionally links mitochondrial preprotein receptors to the general import pore. Nature. 1997;338:195–200. doi: 10.1038/40663. [DOI] [PubMed] [Google Scholar]
- Gazzarrini S, Lejay L, Gojon A, Ninnemann O, Frommer W, von Wiren N. Three functional transporters for constitutive, diurnally regulated, and starvation-induced uptake of ammonium into Arabidopsisroots. Plant Cell. 1999;11:937–948. doi: 10.1105/tpc.11.5.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genger RK, Kovac KA, Peacock WJ, Finnegan EJ. Multiple DNA methyltransferase genes in Arabidopsis thaliana. Plant Mol Biol. 1999;41:269–278. doi: 10.1023/a:1006347010369. [DOI] [PubMed] [Google Scholar]
- Goping IS, Millar DG, Shore GC. Identification of the human mitochondrial import receptor, huMas20p: complementation of delta-mas20 in yeast. FEBS Lett. 1995;373:45–50. doi: 10.1016/0014-5793(95)01010-c. [DOI] [PubMed] [Google Scholar]
- Gratzer S, Lithgow T, Bauer RE, Lamping E, Paltauf F, Kohlwein SD, Hauke V, Junn T, Schatz G, Horst M. Mas37p, a novel receptor subunit for protein import into plant mitochondria. J Cell Biol. 1995;129:25–34. doi: 10.1083/jcb.129.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heins L, Schmitz UK. A receptor for protein import into potato mitochondria. Plant J. 1996;9:829–839. doi: 10.1046/j.1365-313x.1996.9060829.x. [DOI] [PubMed] [Google Scholar]
- Hernández JM, Giner CP, Hernández-Yago J. Gene structure of the human mitochondrial receptor Tom20 and evolutionary study of its family of processed pseudogenes. Gene. 1999a;239:283–291. doi: 10.1016/s0378-1119(99)00409-6. [DOI] [PubMed] [Google Scholar]
- Hernández JM, Hernández CS, Giner CP, Donat V, Hernández-Yago J. Identification of psi3Tom20, a novel processed pseudogene of the human Tom20 gene, and complete characterization of psi1Tom20 and psi2Tom20. Mol Gen Genet. 1999b;262:207–211. doi: 10.1007/s004380051076. [DOI] [PubMed] [Google Scholar]
- Heukeshoven J, Dernick R. Silver staining of proteins. In: Radola BJ, editor. Elektrophorese Forum '86. Germany: Technische Universität München; 1986. pp. 22–27. [Google Scholar]
- Hill K, Model K, Ryan MT, Dietmeier K, Martin F, Wagner R, Pfanner N. Tom40 forms the hydrophilic channel of the mitochondrial import pore for preproteins. Nature. 1998;395:516–521. doi: 10.1038/26780. [DOI] [PubMed] [Google Scholar]
- Hönlinger A, Bömer U, Alconada A, Eckerskorn C, Lottspeich F, Dietmeier K, Pfanner N. Tom7 modulates the dynamics of the mitochondrial outer membrane translocase and plays a pathway-related role in protein import. EMBO J. 1996;15:2125–2137. [PMC free article] [PubMed] [Google Scholar]
- Jänsch L, Kruft V, Schmitz UK, Braun HP. New insights into the composition, molecular mass and stoichiometry of the protein complexes of plant mitochondria. Plant J. 1996;9:357–368. doi: 10.1046/j.1365-313x.1996.09030357.x. [DOI] [PubMed] [Google Scholar]
- Jänsch L, Kruft V, Schmitz UK, Braun HP. Unique composition of the preprotein translocase of the outer mitochondrial membrane from plants. J Biol Chem. 1998a;273:17251–17257. doi: 10.1074/jbc.273.27.17251. [DOI] [PubMed] [Google Scholar]
- Jänsch L, Heins L, Schmitz UK, Braun HP. The TOM complex from plant mitochondria. In: Moller IM, Gardeström P, Glimelius K, Glaser E, editors. Plant Mitochondria: From Gene to Function. Leiden, The Netherlands: Blackhuys Publishers; 1998b. pp. 225–230. [Google Scholar]
- Kiebler M, Keil P, Schneider H, van der Klei IJ, Pfanner N, Neupert W. The mitochondrial receptor complex: a central role of MOM22 in mediating preprotein transfer from receptors to the general insertion pore. Cell. 1993;74:483–492. doi: 10.1016/0092-8674(93)80050-o. [DOI] [PubMed] [Google Scholar]
- Künkele KP, Heins S, Dombowski M, Nargang FE, Benz R, Thieffry M, Walz J, Lill R, Nussberger S, Neupert W. The preprotein translocation channel of the outer membrane of mitochondria. Cell. 1998;93:1009–1019. doi: 10.1016/s0092-8674(00)81206-4. [DOI] [PubMed] [Google Scholar]
- Kyte J, Doolittle RF. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982;157:105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Lemoine R, Burkle L, Barker L, Sakr S, Kuhn C, Regnacq M, Gaillard C, Delrot S, Frommer W. Identification of a pollen-specific sucrose transporter-like protein NtSUT3 from tobacco. FEBS Lett. 1999;454:325–330. doi: 10.1016/s0014-5793(99)00843-1. [DOI] [PubMed] [Google Scholar]
- Lin X, Kaul S, Rounsley S, Shea TP, Benito M-I, Town CD, Fujii CY, Mason T, Bowman CL, Barnstead M, Feldblyum TV, Buell CR, Ketchum KA, Lee J, Ronning CM, Koo HL, Moffat KS, Cronin LA, Shen M, Pai G, Van Aken S, Umayam L, Tallon LJ, Gill JE, Adams MD, Carrera AJ, Creasy TH, Goodman HM, Somerville CR, Copenhaver GP, Preuss D, Nierman WC, White O, Eisen JA, Salzberg SL, Fraser CM, Venter JC. Sequence analysis of chromosome 2 of the plant Arabidopsis thaliana. Nature. 1999;402:761–768. doi: 10.1038/45471. [DOI] [PubMed] [Google Scholar]
- Macasev D, Newbigin E, Whelan J, Lithgow T. How do plant mitochondria avoid importing chloroplast proteins? Components of the import apparatus Tom20 and Tom22 from Arabidopsis differ from their fungal counterparts. Plant Physiol. 2000;123:811–816. doi: 10.1104/pp.123.3.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer K, Schüler C, Wambutt R, Murphy G, Volckaert G, Pohl T, Düsterhöft A, Stiekema W, Entian K-D, Terryn N. Sequence and analysis of chromosome 4 of the plant Arabidopsis thaliana. Nature. 1999;402:769–777. doi: 10.1038/47134. [DOI] [PubMed] [Google Scholar]
- Meisinger C, Brix J, Model K, Pfanner N, Ryan MT. The preprotein translocase of the outer mitochondrial membrane: receptors and the general import pore. Cell Mol Life Sci. 1999;56:817–824. doi: 10.1007/s000180050028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori M, Terada K. Mitochondrial protein import in animals. Biochim Biophys Acta. 1998;1403:12–27. doi: 10.1016/s0167-4889(98)00021-4. [DOI] [PubMed] [Google Scholar]
- Neupert W. Protein import into mitochondria. Annu Rev Biochem. 1997;66:863–917. doi: 10.1146/annurev.biochem.66.1.863. [DOI] [PubMed] [Google Scholar]
- Nuttall SD, Hanson BJ, Mori M, Hoogenraad NJ. hTom34: A novel translocase for import of proteins into human mitochondria. DNA Cell Biol. 1997;16:1067–1074. doi: 10.1089/dna.1997.16.1067. [DOI] [PubMed] [Google Scholar]
- Pfanner N, Douglas MG, Endo T, Hoogenraad NJ, Jensen RE, Meijer M, Neupert W, Schatz G, Schmitz UK, Shore GC. Uniform nomenclature for the protein transport machinery of the mitochondrial membranes. Trends Biochem Sci. 1996;21:51–52. [PubMed] [Google Scholar]
- Rassow J, Dekker PJT, van Wilpe S, Meijer M, Soll J. The preprotein translocase of the mitochondrial inner membrane: function and evolution. J Mol Biol. 1999;286:105–120. doi: 10.1006/jmbi.1998.2455. [DOI] [PubMed] [Google Scholar]
- Ryan MT, Wagner R, Pfanner N. The transport machinery for the import of preproteins across the outer mitochondrial membrane. Int J Biochem Cell Biol. 2000;32:13–21. doi: 10.1016/s1357-2725(99)00114-4. [DOI] [PubMed] [Google Scholar]
- Saeki K, Suzuki H, Tsuneoka M, Maeda M, Iwamoto R, Hasuwa H, Shida S, Takahashi T, Sakaguchi M, Endo T, Miura Y, Mekada E, Mihara K. Identification of mammalian Tom22 as a subunit of the preprotein translocase of the mitochondrial outer membrane. J Biol Chem. 2000;275:31996–32002. doi: 10.1074/jbc.M004794200. [DOI] [PubMed] [Google Scholar]
- Schägger H, Cramer WA, von Jagow G. Analysis of molecular masses and oligomeric states of protein complexes by Blue native electrophoresis and isolation of membrane protein complexes by two-dimensional native electrophoresis. Anal Biochem. 1994;217:220–230. doi: 10.1006/abio.1994.1112. [DOI] [PubMed] [Google Scholar]
- Schlossmann J, Lill R, Neupert W, Court DA. Tom71, a novel homologue of the mitochondrial preprotein receptor Tom70. J Biol Chem. 1996;271:17890–17895. doi: 10.1074/jbc.271.30.17890. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Okazawa Y, Komiya T, Saeki K, Mekada E, Kitada S, Ito A, Mihara K. Characterization of rat TOM40, a central component of the preprotein translocase of the mitochondrial outer membrane. J Biol Chem. 2000;275:37930–37936. doi: 10.1074/jbc.M006558200. [DOI] [PubMed] [Google Scholar]
- Torki M, Mandaron P, Thomas F, Quigley F, Mache R, Falconet D. Differential expression of a polygalacturonase gene family in Arabidopsis thaliana. Mol Gen Genet. 1999;261:948–952. doi: 10.1007/s004380051042. [DOI] [PubMed] [Google Scholar]
- van Wilpe S, Ryan MT, Hill K, Maarse AC, Meisinger C, Brix J, Dekker PJT, Moczko M, Wagner R, Meijer M, Guiard B, Hönlinger A, Pfanner N. Tom22 is a multifunctional organizer of the mitochondrial preprotein translocase. Nature. 1999;401:485–489. doi: 10.1038/46802. [DOI] [PubMed] [Google Scholar]
- Verschoor A, Lithgow T. A first glimpse at the structure of the TOM translocase from the mitochondrial outer membrane. J Cell Biol. 1999;147:905–907. doi: 10.1083/jcb.147.5.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voos W, Martin H, Krimmer T, Pfanner N. Mechanisms of protein translocation into mitochondria. Biochim Biophys Acta. 1999;1422:235–254. doi: 10.1016/s0304-4157(99)00007-6. [DOI] [PubMed] [Google Scholar]
- Yano M, Hoogenraad N, Terada K, Mori M. Identification and functional analysis of human tom22 for protein import into mitochondria. Mol Cell Biol. 2000;20:7205–7213. doi: 10.1128/mcb.20.19.7205-7213.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]