Abstract
Global remodelling of the chromatin landscape occurs during senescence, although its functional consequence is still unclear. In this study, Tasdemir et al (1) show that the epigenetic regulator BRD4 is required for expression of the pro-inflammatory “senescence-associated secretory phenotype” (SASP) and immune clearance of senescent cells in vitro and in vivo. Their results could be useful in design of novel therapies to treat aging-related diseases, including cancer.
Cellular senescence is a stable arrest of cell proliferation triggered by replicative exhaustion or stresses such as DNA damage, oxidative stress and aberrant oncogenic activation. In particular, oncogene-induced senescence (OIS) is important in tumour suppression, as numerous reports have demonstrated that it occurs in precancerous tissues in humans and mice, and acts as a barrier to tumorigenesis. On the other hand, other modes of senescence are involved in embryonic and foetal development and wound healing, and accumulation of senescent cells in aged tissues promotes tissue aging (2). Van Deursen and co-workers recently showed that elimination from tissues of cells expressing a hallmark molecular marker of senescence, p16, promotes healthy aging (3).
Senescent cells display important and unique properties including changes in morphology, chromatin organization, gene expression and metabolism. Senescent cells also express an inflammatory signature, the so-called “senescence-associated secretory phenotype” (SASP): they produce a wide range of inflammatory cytokines, growth factors and matrix metalloproteinases which operate in a cell-autonomous manner to reinforce senescence, but also communicate with and modify the microenvironment. SASP factors can contribute to tumour suppression by triggering “senescence surveillance”, an immuno-mediated clearance of senescent cells. However, chronic inflammation is also a known driver of tumorigenesis and accumulating evidence indicates that chronic SASP can also boost cancer and aging-related diseases. To date, a number of signalling systems have been implicated in expression of the SASP, including DNA damage, p38 MAPK and mTOR signalling as upstream drivers, and NFκB and C/EBPβ as transcription activators (2). However, the role of epigenetic programming has been less clear, although depletion of H3K27me3 from SASP genes is associated with their activation (4). A better understanding of how the SASP is controlled and how it might be targeted to promote tumour suppression and minimize other pro-tumorigenic and pro-aging effects could provide a valuable weapon for cancer therapy.
Lowe and colleagues shed light on this matter by establishing a new player underlying the regulation of SASP genes that is responsible for the interplay between senescent cells and tissue microenvironment. They show that in senescent cells the dramatic reprogramming of the pattern of gene expression depends on dynamic remodelling of enhancer landscapes. Through genome-wide chromatin profiling, the authors found that H3K27ac, an epigenetic mark of active enhancers, is selectively remodelled in cells induced to senescence by activation of an H-RASV12 oncogene, compared to proliferating and quiescent cells. More interestingly, they found that BRD4, an acetylated histone-binding protein, is recruited to super-enhancer elements during OIS and these regions are adjacent to the SASP genes. As a very interesting aside, the authors note that many of the SASP-associated super-enhancers activated in senescence are already modified by H3K4me1 (on its own a marker of inactive enhancers) in proliferating cells, suggesting that these enhancers are already poised for activation prior to activation of senescence by H-RASV12. This, in turn, suggests that the senescent phenotype is physiologically pre-programmed into cells, perhaps in a cell type and trigger specific manner, and perhaps in such a way that cells can epigenetically measure and integrate cumulative diverse stresses over a lifespan, leading to onset of senescence after reaching a pre-specified threshold of “lifetime” cumulative stress exposure.
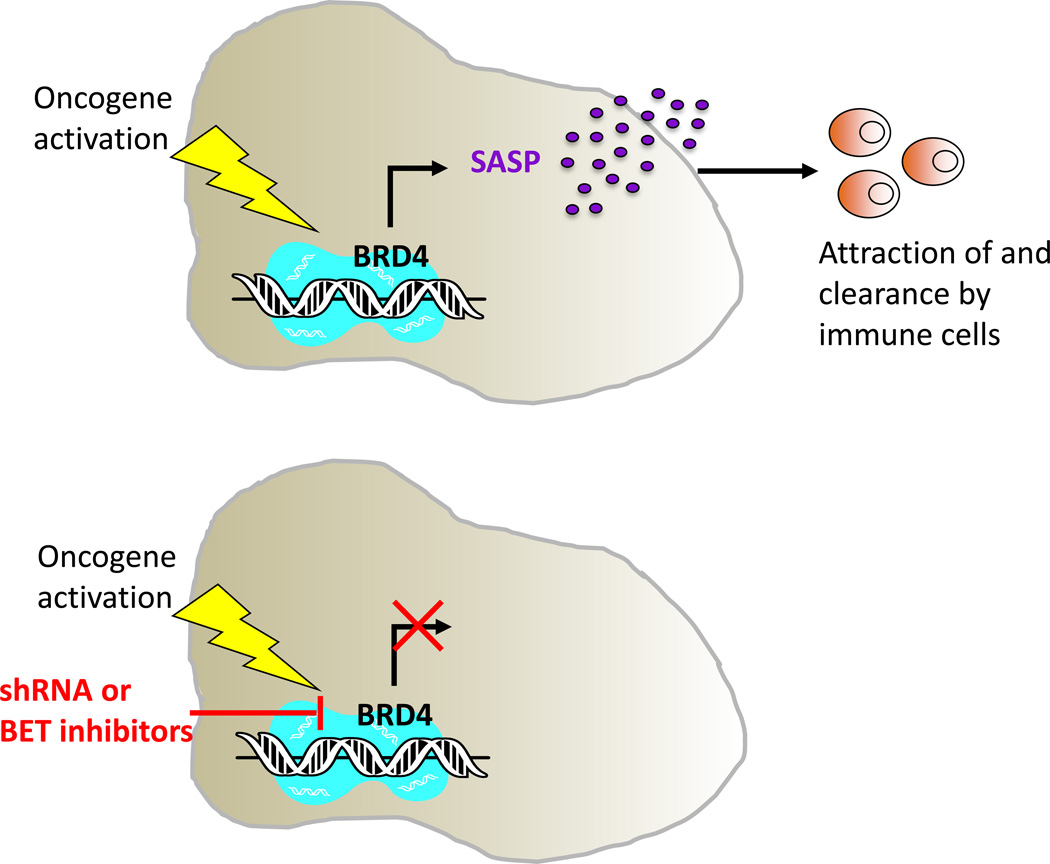
Taking advantage of genetic and pharmacologic inactivation of BRD4, Lowe and colleagues found that BRD4 is necessary for the execution of senescence program. They showed that BRD4 inhibition suppresses most SASP components analysed without overcoming the proliferation arrest, and impairs the paracrine function of SASP to communicate and to disrupt the function and fate of neighbouring cells. Moreover, BRD4 inactivation disrupts immune clearance of senescent cells (Fig. 1). The authors provide evidence of in vivo relevance using a mouse liver OIS model, in which the induction and immune-mediated elimination of OIS hepatocytes can be monitored. They observed that the genetic and pharmacologic inhibition of BRD4 leads to an accumulation of oncogene-expressing hepatocytes and compromises their immune-mediated clearance due to a marked reduction of immune cell infiltration. Although the profound reorganization in the epigenome upon senescence has been long described, its functional consequence has remained elusive. In this light Lowe and colleagues provide new insights showing a novel role for the epigenetic regulator BRD4 as a gatekeeper for the proper execution of senescence as a tumour suppressive mechanism.
Figure 1. BRD4 is required for the execution of the senescence program.
(Top) Lowe and colleagues show that BRD4 regulates SASP expression and immune surveillance during OIS. (Bottom) Genetic or pharmacologic inactivation of BRD4 (by shRNA or BET inhibitors) breaks this link and causes the collapse of this tumour-suppressive mechanism.
A role for BRD4 in tumor suppression is unexpected, given the long-standing observation that it is pro-oncogenic and considered to be a valuable drug target in many tumours including leukaemias, lung cancer, multiple myeloma and melanoma. Inhibitors of BRD4 and other Bromodomain and Extraterminal (BET) family members are currently in phase I clinical trials, including against haematological malignancies. In part, BET inhibitors are thought to exert their anti-cancer effects by blocking expression of oncogenic genes, such as c-MYC, BCL-2 and CDK4 (5). How can the dual oncogenic and tumor suppressive role of BRD4 be reconciled? The authors suggest that BRD4 function depends on cellular context and genetic makeup of target cells. Whatever the reconciliation, this study has important implications for application of BET inhibitors as novel chemotherapies. Although BET inhibition might be beneficial against established tumors, this strategy might also unmask latent tumors through suppression of immune clearance. In some respects similar, the BRAF inhibitor, vemurafenib, although a valuable tool to combat BRAFV600E mutant melanoma, paradoxically unmasks the oncogenic potential of latent H-RAS oncogenes in skin keratinocytes to promote cutaneous squamous cell carcinomas (6).
However, the present study also has other more positive translational implications: it establishes small-molecule inhibition of BRD4 as a candidate therapeutic strategy to suppress the pro-aging and pro-tumorigenic activities of SASP. Since senescent cells accumulate with age and are present at sites of aging-related pathologies the ability of BET inhibitors to prevent or ameliorate the pro-aging and pro-tumorigenic effects of senescent cells should be explored. Notably, mice lacking Cxcr2 (the receptor for multiple SASP factors (Cxcl1 (Groα), Cxcl2 (Groβ), Cxcl5 (Ena-78) and Cxcl8 (Il-8)) are resistant to spontaneous and inflammation-driven cancer (7). So, BET inhibitors might exhibit chemopreventative/tumor suppressive activity by suppressing inflammation-driven cancer. More speculatively, BET inhibitors might suppress the pro-aging effects of SASP outwith a cancer context. Also in line with the potential of BET inhibitors to promote healthy aging, c-MYC heterozygous mice were recently shown to exhibit extended healthspan and lifespan (8). A major caveat here though is that, although BET inhibitors likely act in part by repression of C-MYC expression, C-MYC is by no means their only target.
Given this complexity it will be essential to carefully evaluate and test all the pros and cons of clinical application of BET inhibitors. Initially, BET inhibitors will continue to be tested for their anti-cancer activity in clinical trials, both alone and in combination with other agents. If a molecule in this class is ultimately approved for treatment of cancer, it will then be possible to assess other activities, such as suppression of inflammation, in large numbers of humans receiving BET inhibitors as anti-cancer agents. Given an acceptable toxicity profile and supporting empirical data, it might then be feasible to test BET inhibitors in short term protocols for their ability to combat features of aging linked to senescence and senescence-associated inflammation. Encouragingly, BET inhibition has already been shown to be anti-inflammatory in mouse models and to limit lipopolysaccharide-induced endotoxic shock and bacteria-induced sepsis (9). As a possible precedent for this route to pre-emptively tackle other conditions of aging, the TAME (Targeting Aging with Metformin) study will hopefully test the ability of metformin to prevent common diseases of aging, and low doses of everolimus have been shown to promote response to influenza vaccination in the elderly (10). While the FDA currently does not approve aging as a treatable indication, it is hoped that studies such as these will open the way for testing other small molecules, such as BET inhibitors and their derivatives and so-called “senolytics” that kill senescent cells, for their ability to promote healthy aging and suppression of disease, such as cancer. While the current generation of BET inhibitors is no doubt lacking in selectivity and too prone to toxicity to be used to target aging in relatively healthy adults, understanding the mechanisms by which BRD4 regulates the SASP could pave the way for more specific therapeutic strategies to combat cancer and promote healthy aging.
Acknowledgments
The authors are supported by NIA. Grant number: P01 AG031862
Footnotes
The authors disclose no potential conflicts of interest.
References
- 1.Tasdemir N, Banito A, Alonso-Curbelo D, Roe JS, Camiolo M, Tschaharganeh D, et al. BRD4 connects enhancer remodeling to senescence immune surveillance. Cancer Discovery. 2016 doi: 10.1158/2159-8290.CD-16-0217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Salama R, Sadaie M, Hoare M, Narita M. Cellular senescence and its effector programs. Genes Dev. 2014;28:99–114. doi: 10.1101/gad.235184.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baker DJ, Childs BG, Durik M, Wijers ME, Sieben CJ, Zhong J, et al. Naturally occurring p16(Ink4a)-positive cells shorten healthy lifespan. Nature. 2016;530:184–189. doi: 10.1038/nature16932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shah PP, Donahue G, Otte GL, Capell BC, Nelson DM, Cao K, et al. Lamin B1 depletion in senescent cells triggers large-scale changes in gene expression and the chromatin landscape. Genes Dev. 2013;27:787–799. doi: 10.1101/gad.223834.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Filippakopoulous P, Qi J, Picaud S, Shen Y, Smith WB, Federov O, et al. Selective inhibition of BET bromodomains. Nature. 2010;468:1067–1073. doi: 10.1038/nature09504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Su F, Viros A, Milagre C, Trunzer K, Bollag G, Spleiss O, et al. RAS mutations in cutaneous squamous-cell carcinomas in patients treated with BRAF inhibitors. N. Engl J Med. 2012 Jan 19;366(3):207–215. doi: 10.1056/NEJMoa1105358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jamieson T, Clarke M, Steele CW, Samuel MS, Nuemann J, Jung A, et al. Inhibition of CXCR2 profoundly suppresses inflammation-driven and spontaneous tumorigenesis. J Clin Invest. 2012;122:3127–3144. doi: 10.1172/JCI61067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hofmann JW, Zhao X, De Cecco M, Peterson AL, Pagliardi L, Manivannan J, et al. Reduced expression of MYC increases longevity and enhances healthspan. Cell. 2015;160:477–488. doi: 10.1016/j.cell.2014.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nicodeme E, Jeffrey KL, Schaefer U, Beinke S, Dewell S, Chung CW, et al. Suppression of inflammation by a synthetic histone mimic. Nature. 2010 Dec 23;468(7327):1119–1123. doi: 10.1038/nature09589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mannick JB, Del Giudice G, Lattanzi M, Valiante NM, Praestgaard J, Huang B, et al. mTOR inhibition improves immune function in the elderly. Sci Transl Med. 2014 Dec 24;6(268) doi: 10.1126/scitranslmed.3009892. 268ra179. [DOI] [PubMed] [Google Scholar]