Abstract
Background
The optimal treatment for hypertrophic scar and keloid remains controversial. Therefore, the aim of this systematic review and meta-analysis was to compare the effectiveness of intralesional injection of botulinum toxin type A compared with placebo and intralesional injection of corticosteroid compared with placebo in patients with hypertrophic scar and keloid.
Material/Methods
Six databases were searched using Medical Subject Headings (MeSH) keywords and included Web of Science, PubMed, EMBASE, the Cochrane Library, WanFang, and CNKI from their inception to March 1 2019, without language restriction. Randomized controlled trials (RCTs) and prospective controlled trials (PCTs) were identified that compared intralesional injection of botulinum toxin type A with placebo and corticosteroid with placebo in hypertrophic scar and keloid. The quality of controlled trials was assessed by the Newcastle-Ottawa Scale (NOS).
Results
Comparison of intralesional botulinum toxin type A and corticosteroid showed significant differences in the Visual Analog Scale (VAS) (P<0.001) (WMD, −4.30; 95% CI, −4.44 to −4.16) and effective rate (P=0.012) (RR=0.82; 95% CI, 0.70–0.96). Intralesional injection of botulinum toxin type A compared with placebo showed significant differences in the VAS (P<0.001) (WMD, 1.41; 95% CI, 1.21–1.62), the width of scar (P=0.00) (WMD, −0.15; 95% CI, −0.19 to −0.10) and Vancouver Scar Scale (VSS) (P=0.003) (WMD, −0.69; 95% CI, −1.14 to −0.23).
Conclusions
Systematic review and meta-analysis showed that injection of intralesional botulinum toxin type A was more effective in the treatment of hypertrophic scar and keloid than injection of intralesional corticosteroid or placebo.
MeSH Keywords: 17-Hydroxycorticosteroids; Botulinum Toxins, Type A; Cicatrix, Hypertrophic; Keloid; Meta-Analysis
Background
The formation of scar tissue is part of the normal healing process that follows injury to the skin or following surgery, but some individuals are prone to develop hypertrophic scar and keloid. Hypertrophic scar is excessive fibrosis of the skin that is confined to the area of injury and regresses over time. Keloid is excessive fibrosis of the skin that extends beyond the area of injury and does not regress. Severe hypertrophic scar and keloid can also involve the joints and mouth and can occur following severe injury, including burns, and can impair the quality of life for affected individuals. Traditional treatments for hypertrophic scar and keloid include massage therapy, silicone gel treatment, laser therapy, light therapy, and radiotherapy [1]. Several emerging treatment options include intralesional cryotherapy, intralesional injection with 5-fluorouracil (5FU), interferon, and bleomycin [2].
Treating hypertrophic scar and keloids can be a complicated and difficult procedure. Before treatment, an evaluation of the lesion should be performed to include the size, location, and any pain or tenderness and it is important to understand the expectation of treatment for each patient and to have a multidisciplinary therapeutic approach [3–5]. Although several treatments have been used for hypertrophic scar and keloid, there is still no gold standard and for the majority of patients, management is driven by individual clinical experience [6]. Intralesional injection with corticosteroid is commonly used but complications such as pain and itch occur after injections [7]. Therefore, recent studies have investigated the efficacy of injection with botulinum toxin type A and the inhibition of hypertrophic scars and keloids and the lack of discomfort and other adverse events [8,9]. However, intralesional botulinum toxin type A has not been routinely used in clinical practice and there have been few multicenter clinical trials with a sufficiently large number of participants to provide the evidence to support its use in the treatment of hypertrophic scar and keloid.
Therefore, the aim of this systematic review and meta-analysis was to compare the effectiveness of intralesional injection of botulinum toxin type A compared placebo with intralesional injection of corticosteroid compared with placebo in patients with hypertrophic scar and keloid.
Material and Methods
Eligibility criteria
Randomized controlled trials (RCTs) and prospective controlled trials (PCTs) were identified that compared intralesional injection of botulinum toxin type A with corticosteroid and placebo in hypertrophic scar and keloid. Animal studies, reviews articles, clinical practice guidelines, commentaries, case reports, and published letters were excluded. Low-quality studies with small patient numbers (≤4) were also excluded. Inclusion criteria required that the study compared the clinical effects of intralesional injection with botulinum toxin type A, corticosteroid and placebo, a minimum of one month of follow-up after treatment, and one or more outcomes of interest documented after injection. Exclusion criteria were non-English language and non-Chinese language publications, studies that described only intralesional botulinum toxin type A or corticosteroid or placebo, animal experiments, reviews, clinical practice guidelines, commentaries, case reports and letters, and low-quality studies.
Information sources
Six databases were searched, including Web of Science, PubMed, EMBASE, Cochrane Library, WanFang, and China Academic Journals (CNKI) from their inception to March 1, 2019 and without language restriction, using Medical Subject Headings (MeSH) keywords. Only RCTs or PCTs that compared botulinum toxin type A treatment groups with corticosteroid treatment groups or with placebo were identified.
Search strategy
In accordance with the requirements of the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement [10,11], two different search strategies were used for PubMed. First, the terms searched included “cicatrix” (MeSH term) OR “cicatrix” (All Fields) OR “scar” (All Fields) AND “botulinum toxin” (MeSH term) OR “botulinum” (All Fields) AND “toxins” (All Fields) OR “botulinum toxin” (All Fields) OR “botulinum” (All Fields) AND “toxin” (All Fields) OR “botulinum toxin” (All Fields) AND “adrenal cortex hormones” (MeSH terms) OR “adrenal” (All Fields) AND “cortex” (All Fields) AND “hormones” (All Fields) OR “adrenal cortex hormones” (All Fields) OR “corticosteroid” (All Fields). Second, “cicatrix” (MeSH terms) OR “cicatrix” (All Fields) OR “scar” (All Fields) AND “botulinum toxins” (MeSH terms] OR “botulinum” (All Fields) AND “toxins” (All Fields) OR “botulinum toxins” (All Fields) OR “botulinum” (All Fields) AND “toxin” (All Fields) OR “botulinum toxin” (All Fields) OR “botulinum toxins” (MeSH terms) OR “botulinum” (All Fields) AND “toxins” (All Fields) OR “botulinum toxins” (All Fields) AND “placebo” (MeSH terms) OR “placebos” (All Fields) OR “placebo” (All Fields).
Study selection
Two authors reviewed and screened the titles and abstracts of all publications screened. The full text of all possible relevant studies was reviewed. If there were disagreements, a third reviewer was included until a consensus was achieved. Only studies that met the inclusion criteria were included in the final analysis.
Data collection process and risk of bias in individual studies
Data were extracted and assessed independently by the two reviewers and then confirmed by a third reviewer and a fourth more experienced reviewer. The extracted information contained the name of the first author, the publication year, country, number of patients, duration of follow-up, and type of study, the treatment including use and dose, the location of the skin lesions, and the outcome indicators. Also, the Newcastle-Ottawa Scale (NOS) was used to evaluate the risk of bias in individual studies in the meta-analysis [12].
Case-control studies were included and were chosen from three aspects, selection (a maximum of 4 items and 4 scores), comparability (a maximum of one item and 2 scores) and exposure (a maximum of 3 items and 3 scores). The total scores were 9 and articles that achieved a score >5 were regarded as high-quality studies.
Summary measures
This meta-analysis was conducted using Stata version 15.0 software (Stata Corporation, College Station, TX, USA). The risk ratio (RR) was used to analyze dichotomous data (effective rate) and continuous data, including the Visual Analog Scale (VAS), the Vancouver Scar Scale (VSS), and the width of the scar were analyzed using the weighted mean difference (WMD), both with a 95% confidence interval (CI) [13].
Synthesis of results and publication bias across studies
To ensure the objectivity and rationality of the meta-analysis, the chi-squared (χ2) test was used to assess the study heterogeneity, and I2 >50% indicated statistical heterogeneity, supporting the use of a random effects model (or variance components model). If I2 <50%, the fixed effects model was applied because there was no significant statistical heterogeneity [14]. Otherwise, the publication bias of this meta-analysis was evaluated by Egger’s test [15].
Results
Study selection and risk of bias within studies
Figure 1 shows the flow diagram of the systematic review of the literature and selection of studies to compare intralesional injection of botulinum toxin type A compared with intralesional injection of corticosteroid for the treatment of hypertrophic scar and keloid. The initial literature search identified 3,475 publications. After screening, 15 clinical trials with 639 participants, published between 2006 and 2018 in seven different countries were identified, with a mean follow-up of 6 months, which met the inclusion criteria. All the included studies were full-text publications that were written in English or Chinese and included 11 randomized controlled trials (RCTs) and 4 prospective controlled trials (PCTs) [16–30]. Table 1 summarizes the characteristics of the studies identified by the systematic review of the literature and included in the meta-analysis. The risk of bias in individual studies was assessed by the Newcastle-Ottawa Scale (NOS) with a mean score of 7.14 of 9.
Figure 1.
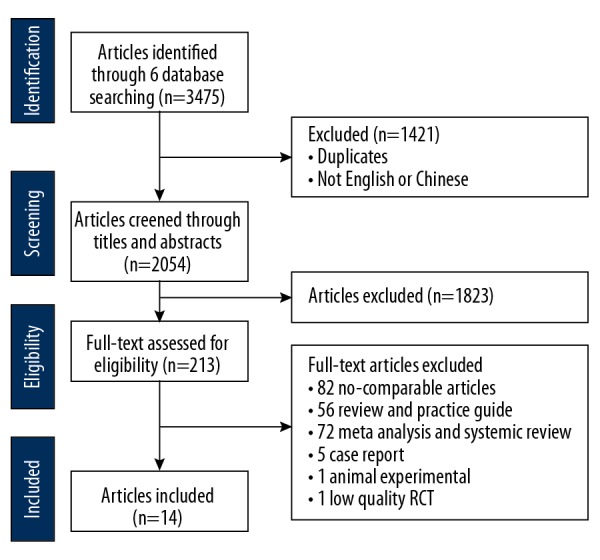
Flow diagram of the systematic review of the literature and selection of studies to compare intralesional injection of botulinum toxin type A compared with intralesional injection of corticosteroid for the treatment of hypertrophic scar and keloid.
Table 1.
Characteristics of the studies identified by systematic review of the literature and included in the meta-analysis.
| Study first author | Year | Country | Treatment | No. of patients | Follow-up (months) | Study type | NOS score | |
|---|---|---|---|---|---|---|---|---|
| Chang et al. [16] | 2014 | Taiwan | BoNT-A | Placebo | 6 | RCT | 7 | |
| Chang et al. [17] | 2014 | Taiwan | BoNT-A | Placebo | 6 | RCT | 8 | |
| Zelken et al. [18] | 2015 | Taiwan | BoNT-A | Placebo | 27 | PCT | 6 | |
| Pruksapong et al. [19] | 2007 | Thailand | BoNT-A | Placebo | 25 | 6 | RCT | 6 |
| Li et al. [20] | 2018 | China | BoNT-A | Placebo | 6 | RCT | 7 | |
| Hu et al. [21] | 2018 | China | BoNT-A | Placebo | 6 | RCT | 7 | |
| Chen et al. [22] | 2018 | China | BoNT-A | Placebo | 38 | 12–24 | PCT | 8 |
| Gassner et al. [23] | 2006 | America | BoNT-A | Placebo | 31 | 6 | PCT | 7 |
| Lee et al. [24] | 2017 | Korea | BoNT-A | Placebo | 30 | 6 | RCT | 8 |
| Ziade et al. [25] | 2014 | France | BoNT-A | Placebo | 24 | 12 | PCT | 7 |
| Wang et al. [26] | 2009 | China | BoNT-A | Corticosteroid | 27 | 6 | RCT | 5 |
| Liu et al. [27] | 2017 | China | BoNT-A | Corticosteroid | 80 | 6 | RCT | 6 |
| Zhao et al. [28] | 2016 | China | BoNT-A | Corticosteroid | 86 | 5 | RCT | 5 |
| Zhang et al. [29] | 2017 | China | BoNT-A | Corticosteroid | 100 | 1 | RCT | 6 |
| Shaarawy et al. [30] | 2015 | Egypt | BoNT-A | Corticosteroid | 24 | 7 | RCT | 7 |
BoNT-A – botulinum toxin type A; RCT – randomized controlled trial; PCT – prospective controlled trial; NOS – Newcastle-Ottawa Scale.
Study characteristics
Other information extracted from the identified publications included the different sites of the scars, the specific use and dose of botulinum toxin type A, corticosteroid, and placebo in the treatment and control groups, as shown in Tables 2–4. From the included studies, the mean age of the patients was 33.7 years with the most common site for hypertrophic scars and keloids being on the face (55.87%) (Table 2). Patients and the mode of delivery were similar between the treatment group and control group, with the exception of treatment dose due to the different drug formulations used.
Table 2.
Location of the hypertrophic scar or keloid in the studies included in the meta-analysis.
| Participants with hypertrophic scars or keloids n=639 prevalence | |||
|---|---|---|---|
| Face (n=357) | Forehead | 87 | 13.62% |
| Ear | 32 | 5.01% | |
| Upper lip | 117 | 18.31% | |
| Other sites | 121 | 18.94% | |
| Trunk (n=90) | Chest wall | 55 | 8.61% |
| Back | 9 | 1.41% | |
| Abdomen | 26 | 4.07% | |
| Extremity | 68 | 10.64% | |
| Not stated | 124 | 19.41% | |
Table 3.
Use and dose of botulinum toxin type A and placebo.
| Study first author | Year | Country | Use | Dose | |
|---|---|---|---|---|---|
| BoNT-A | Placebo (NS) | ||||
| Chang et al. [16] | 2014 | Taiwan | Intralesional injection | 15 units | 0.6 ml |
| Chang et al. [17] | 2014 | Taiwan | Intralesional injection | 6.07±0.64 units | 0.17–0.27 ml |
| Zelken et al. [18] | 2015 | Taiwan | Intralesional injection | 20 units | 0.5 ml |
| Pruksapong et al. [19] | 2007 | Thailand | Intralesional injection | 18.05±5.54 units | 1.3–2.46 ml |
| Li et al. [20] | 2018 | China | Intralesional injection | 5 units/point | 0.1 ml/point |
| Hu et al. [21] | 2018 | China | Intralesional injection | 10 units/cm | 0.2 ml/cm |
| Chen et al. [22] | 2018 | China | Intralesional injection | 1–2 units/point | Not stated |
| Gassner et al. [23] | 2006 | America | Intralesional injection | <2 cm (15 units) 2–4 cm (30 units) >4 cm (45 units) |
<2 cm (0.2 ml) 2–4 cm (0.4 ml) >4 cm (0.6 ml) |
| Lee et al. [24] | 2017 | Korea | Intralesional injection | 30 units | 1.2 ml |
| Ziade et al. [25] | 2014 | France | Intralesional injection | 15–40 units | 1.5–4 ml |
BoNT-A – botulinum toxin type A; NS – normal saline.
Table 4.
Use and dose of botulinum toxin type A and corticosteroid.
| Study first author | Year | Country | Use | Dose | |
|---|---|---|---|---|---|
| BoNT-A | Placebo (NS) | ||||
| Wang et al. [26] | 2009 | China | Intralesional injection | 5 units/point | 5 units/point |
| Liu et al. [27] | 2017 | China | Intralesional injection | 5 units/point | 5 units/point |
| Zhao et al. [28] | 2016 | China | Intralesional injection | ≤55 units/time | 0.2 ml/cm2 |
| Zhang et al. [29] | 2017 | China | Intralesional injection | ≤55 units/time | 0.2 ml/cm2 |
| Shaarawy et al. [30] | 2015 | Egypt | Intralesional injection | 5 units/cm3 | 10 mg/cm3 (≤80 mg in total) |
BoNT-A – botulinum toxin type A; NS, normal saline.
Individual studies and synthesis of results for the primary outcomes
The Visual Analog Scale (VAS) was commonly used to evaluate the degree of pain and was reflected by the VAS scores [31]. Although different hospitals use different types of this scale, the fundamental principle of its use was uniform and included a 10-point scale (0 for no pain, and 10 for severe pain). This advantage of the VAS was that it was easy for patients to use.
Of the 15 included studies, 10 studies assessed VAS after intralesional injection, including 463 participants in total. Data to compare intralesional botulinum toxin type A with placebo were analyzed using a fixed effects model that identified low study heterogeneity (I2=10.1%) and there was a significant difference in VAS at about 6 months after injections, with a weighted mean difference (WMD)=1.41 (P<0.001; 95% CI, 1.21–1.62) (Figure 2, Table 5). After applying the fixed effects model, because of the low heterogeneity (I2=0), the forest plot of intralesional botulinum toxin type A compared with intralesional corticosteroid injection showed that there was also a significant difference in the VAS scores, with WMD=−4.30 (P<0.001, 95% CI, −4.44 to −4.16) (Figure 3, Table 5).
Figure 2.
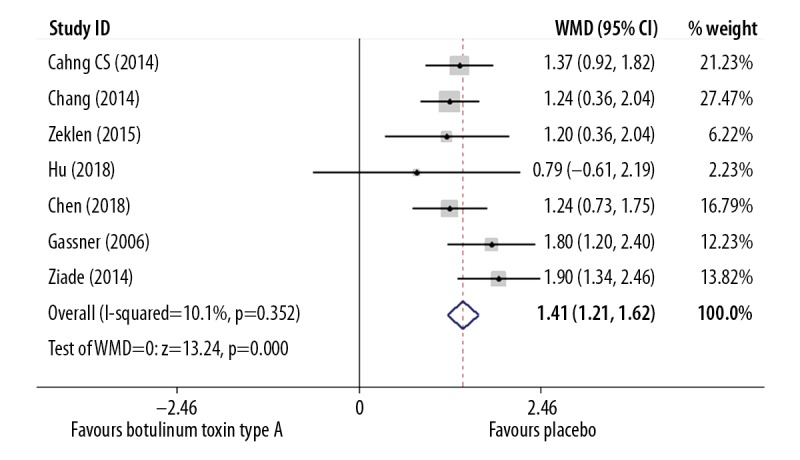
Forest plot to compare the visual analog scale (VAS) findings between the group treated with intralesional injection of botulinum toxin type A and the placebo group.
Table 5.
Pooled outcome indicators for patients with hypertrophic scar and keloid.
| Outcomes | No. of studies | No. of participants | Heterogeneity/I2 | Analysis model | Q Statistic, P-value | WMD/RR (95% CI) |
|---|---|---|---|---|---|---|
| VAS (botulinum vs. place) | 7 | 250 | 10.1% | Fixed-effects | <0.0001 | 1.41 (1.21–1.62) |
| VAS (botulinum vs. corticosteroid) | 3 | 213 | 0.0% | Fixed-effects | <0.0001 | −4.30 (−4.44 to −4.16) |
| VSS | 6 | 203 | 31.4% | Fixed-effects | 0.003 | −0.69 (−1.14 to −0.23) |
| Width of scar | 4 | 164 | 0.0% | Fixed-effects | 0.000 | −0.15 (−0.19 to −0.10) |
| Effective rate | 2 | 104 | 12.2% | Fixed-effects | 0.012 | 0.82 (0.70–0.96) |
VAS – visual analog scale; VSS – Vancouver Scar Scale; CI – confidence interval; WMD – weighted mean difference; RR – risk ratio.
Figure 3.
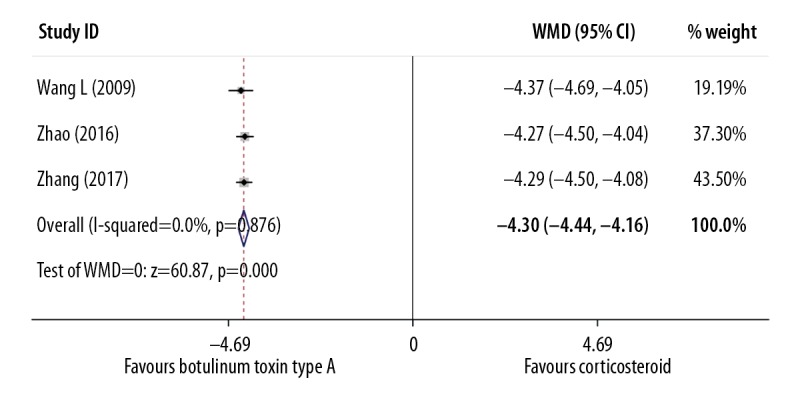
Forest plot to compare the visual analog scale (VAS) findings between the group treated with intralesional injection of botulinum toxin type A and the group treated with intralesional injection of corticosteroid.
The Vancouver Scar Scale (VSS) contained the four components of melanin pigmentation (0–3 points), scar height (0–4 points), vascularity (0–3 points), and pliability (0–5 points), with a maximal score of 15 points that indicated the most severe scar formation [32]. The VSS outcome measure was used in six included studies involving 203 study participants and was measured at the mean follow-up duration of six months after injection. Meta-analysis on the VSS outcome showed a significant difference between the group treated with botulinum toxin type A and the placebo group (WMD=−0.69) (P=0.003; 95% CI, −1.14 to −0.23) and a fixed effects model was used (I2=31.4%) (Figure 4, Table 5).
Figure 4.
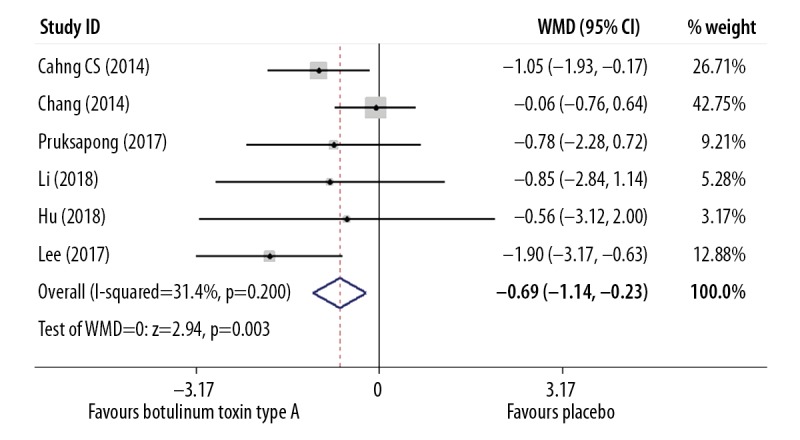
Forest plot to compare the Vancouver Scar Scale (VSS) findings between the group treated with intralesional injection of botulinum toxin type A and the placebo group.
Effective rates for intralesional injection of botulinum toxin type A compared with intralesional injection of corticosteroid
The clinical efficacy of each therapeutic strategy was calculated using the formula: n (effective events)/n (total events). Two studies that included 104 participants reported this outcome. The fixed effects model was used (I2=12.2%) and showed a significant difference between injection of intralesional botulinum toxin type A and intralesional injection of corticosteroid, with an RR=0.82 (P=0.012; 95% CI=0.70–0.96) (Figure 5, Table 5).
Figure 5.
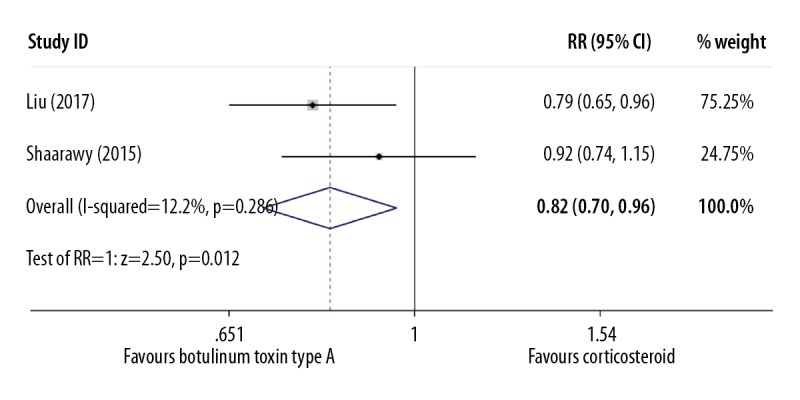
Forest plot to compare the effectiveness rate between the group treated with intralesional injection of botulinum toxin type A and the group treated with intralesional injection of corticosteroid.
Width of the scar as an additional outcome
The width of the scar was measured in mm to two decimal points. This additional outcome was included in four studies with 164 participants. Meta-analysis to compare intralesional botulinum toxin type A and intralesional placebo used the fixed effects model because of low study heterogeneity (I2=0.00%) and a significant difference was identified with (WMD=−0.15) (P=0.000; 95% CI, −0.19 to −0.10) (Figure 6, Table 5). To select the optimal results, this outcome was selected for the longest follow-up duration of six months.
Figure 6.
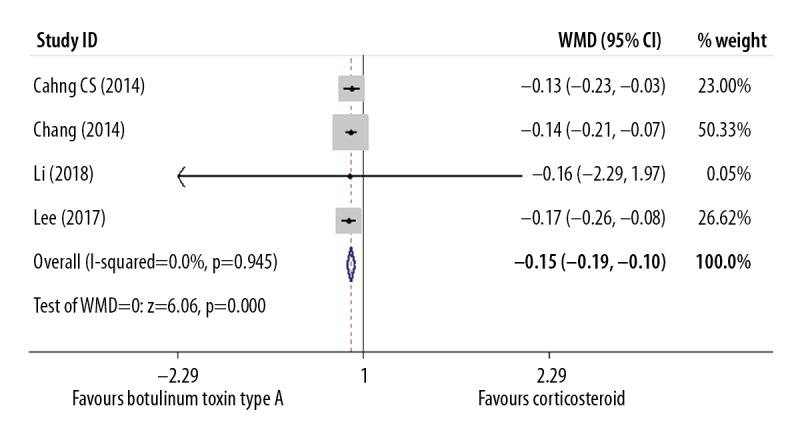
Forest plot to compare the width of the scar between the group treated with intralesional injection of botulinum toxin type A and the placebo group.
Publication bias across studies
Because of the limited number of included studies, Egger’s test was used to detect the publication bias in the meta-analysis (P=0.883).
Discussion
Scar formation is part of the normal healing process, but hypertrophic scar and keloid are a challenge for plastic surgeons worldwide, not only because they cause functional impairment but because they can have cosmetic effects that can impair social interactions and quality of life [33]. Treatments for hypertrophic scar and keloid include physical approaches, laser therapy, light therapy, autologous transfer of adipose tissue, and surgery [34]. However, because of incomplete understandings of the mechanism of scarring, each treatment has limitations and comparison and analysis of methods to treat and prevent hypertrophic scar and keloid are required [35]. To our knowledge, this was the first systematic review and meta-analysis to compare intralesional botulinum toxin type A with intralesional corticosteroid for the treatment of hypertrophic scar and keloid.
The findings of this meta-analysis showed that patients who received intralesional injections with botulinum toxin type A had a significantly lower incidence and severity of pain compared with patients who received intralesional injections with corticosteroid. Also, the efficacy rate was significantly increased in the botulinum toxin type A group compared with corticosteroid group. When compared with the placebo group, although intralesional injection with botulinum toxin type A was painful for some patients, it achieved significantly improved clinical efficacy which was measured by the Vancouver Scar Scale (VSS) and the width of the scar after injection.
The findings from the meta-analysis appeared to be a real reflection of clinical efficacy of intralesional injection with botulinum toxin type A rather than an artifact of statistical heterogeneity. Also, all the included studies were high-quality studies, which were likely to ensure the quality of the meta-analysis and the result from Egger’s test showed that there was no significant publication bias in the meta-analysis.
The findings of this meta-analysis are supported by the findings from previous studies and reviews of the literature. Liu et al. [36] and Fanous et al. [37], showed botulinum toxin type A inhibited hypertrophic scars and keloids in animal models. A literature review published by Austin et al. [38] also showed that botulinum toxin type A had the potential to prevent pathological scar formation in patients with a known individual history or family history of this condition. Li et al. [39] and Hao et al. [40] reported that botulinum toxin type A could promote the healing of scars, but that the mechanisms require further investigation. However, the number of participants in several previous studies have been small, which has prevented definitive conclusions to be made. However, in the present study, the large patient sample size (>600 participants) of the meta-analysis adds to the significance of the findings.
However, this study had several limitations. First, analysis of the cost-effectiveness of treatment hypertrophic scar and keloid was not performed, and future studies are needed due to its importance in planning healthcare policy. Botulinum toxin type A and corticosteroid have different costs according to different formulations used. Generally, botulinum toxin type A was more expensive than corticosteroid and was more difficult to source. Second, only four outcomes were assessed in this study, which is likely to have introduced bias into the analysis. The two scales used in this meta-analysis, the VAS and the VSS were both subjective, and there may have been bias introduced from the patient and clinician responses. Third, the majority of included studies were undertaken and reported in Asia (13/15, 86.7%) and the majority of patients were of Asian ethnicity. Therefore, the conclusions from this meta-analysis might not be representative of patients of other ethnicities. Importantly, individuals of African ethnicity are more likely to suffer from hypertrophic scars and keloid than individuals of European ethnicity [41,42]. However, in this meta-analysis, no individuals of African ethnicity were included because no eligible studies were identified.
Conclusions
The findings from this systematic review of the literature and meta-analysis showed that intralesional injection of botulinum toxin type A was more effective in inhibiting hypertrophic scar and keloid than intralesional injection of corticosteroid or placebo and was also associated with reduced pain following injection. This study has shown that further large-scale, controlled, high-quality studies should be performed to determine the most effective treatment protocols for the use of intralesional injection with botulinum toxin type A in patients with hypertrophic scar and keloid in all ethnic groups.
Footnotes
Conflict of interest
None.
Source of support: Departmental sources
References
- 1.Arno AI, Gauglitz GG, Barret JP, Jeschke MG. Up-to-date approach to manage keloids and hypertrophic scars: A useful guide. Burns. 2014;40:1255–66. doi: 10.1016/j.burns.2014.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gauglitz GG. Management of keloids and hypertrophic scars: Current and emerging options. Clin Cosmet Investig Dermatol. 2013;6:103–14. doi: 10.2147/CCID.S35252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nast A, Eming S, Fluhr J, et al. German S2k guidelines for the therapy of pathological scars (hypertrophic scars and keloids) J Dtsch Dermatol Ges. 2012;10(10):747–62. doi: 10.1111/j.1610-0387.2012.08012.x. [DOI] [PubMed] [Google Scholar]
- 4.Ogawa R. The most current algorithms for the treatment and prevention of hypertrophic scars and keloids. Plast Reconstr Surg. 2010;125:557–68. doi: 10.1097/PRS.0b013e3181c82dd5. [DOI] [PubMed] [Google Scholar]
- 5.Wolfram D, Tzankov A, Pülzl P, Piza-Katzer H. Hypertrophic scars and keloids – a review of their pathophysiology, risk factors, and therapeutic management. Dermatol Surg. 2009;35:171–81. doi: 10.1111/j.1524-4725.2008.34406.x. [DOI] [PubMed] [Google Scholar]
- 6.Gold MH, McGuire M, Mustoe TA, et al. Updated international clinical recommendations on scar management: Part 2 – algorithms for scar prevention and treatment. Dermatol Surg. 2014;40:825–31. doi: 10.1111/dsu.0000000000000050. [DOI] [PubMed] [Google Scholar]
- 7.Kim DY, Park HS, Yoon HS, Cho S. Efficacy of IPL device combined with intralesional corticosteroid injection for the treatment of keloids and hypertrophic scars with regards to the recovery of skin barrier function: A pilot study. J Dermatolog Treat. 2015;26:481–84. doi: 10.3109/09546634.2015.1024598. [DOI] [PubMed] [Google Scholar]
- 8.Omranifard M, Heidari M, Farajzadegan Z, et al. Botulinum toxin and burn induces contracture. Arch Plast Surg. 2016;43:609–11. doi: 10.5999/aps.2016.43.6.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gauglitz GG, Bureik D, Dombrowski Y, et al. Botulinum toxin A for the treatment of keloids. Skin Pharmacol Physiol. 2012;25:313–18. doi: 10.1159/000342125. [DOI] [PubMed] [Google Scholar]
- 10.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA Statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. Ann Intern Med. 2009;151:W65–94. doi: 10.7326/0003-4819-151-4-200908180-00136. [DOI] [PubMed] [Google Scholar]
- 11.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ. 2009;339:332–36. [PMC free article] [PubMed] [Google Scholar]
- 12.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–5. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 13.Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011] The Cochrane Collaboration. 2011. Available from [URL]: www.handbook.cochrane.org.
- 14.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang CS, Wallace CG, Hsiao YC, et al. Botulinum toxin to improve results in cleft lip repair: A double-blinded, randomized, vehicle-controlled clinical trial. PLoS One. 2014;9:e115690. doi: 10.1371/journal.pone.0115690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang CS, Wallace CG, Hsiao YC, et al. Botulinum toxin to improve results in cleft lip repair. Plastic Reconstr Surg. 2014;134:511–16. doi: 10.1097/PRS.0000000000000416. [DOI] [PubMed] [Google Scholar]
- 18.Zelken J, Yang SY, Chang CS, et al. Donor site aesthetic enhancement with preoperative botulinum toxin in forehead flap nasal reconstruction. Ann Plast Surg. 2016;77:535–38. doi: 10.1097/SAP.0000000000000625. [DOI] [PubMed] [Google Scholar]
- 19.Pruksapong C, Yingtaweesittikul S, Burusapat C. Efficacy of botulinum toxin a in preventing recurrence keloids: Double blinded randomized controlled trial study: Intraindividual subject. J Med Assoc Thai. 2017;100:280–86. [PubMed] [Google Scholar]
- 20.Li YH, Yang JM, Liu JQ, et al. A randomized, placebo-controlled, double-blind, prospective clinical trial of botulinum toxin type A in prevention of hypertrophic scar development in median sternotomy wound. Aesth Plast Surg. 2018;42:1364–69. doi: 10.1007/s00266-018-1187-x. [DOI] [PubMed] [Google Scholar]
- 21.Hu L, Zou Y, Chang SJ, et al. Effects of botulinum toxin on improving facial surgical scars: A prospective, split-scar, double-blind, randomized controlled trial. Plast Reconstr Surg. 2018;141:646–50. doi: 10.1097/PRS.0000000000004110. [DOI] [PubMed] [Google Scholar]
- 22.Chen H, Pan W, Zhang J, et al. The application of W-plasty combined Botox-A injection in treating sunk scar on the face. Medicine (Baltimore) 2018;97:e11427. doi: 10.1097/MD.0000000000011427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gassner HG, Brissett AE, Otley CC, et al. Botulinum toxin to improve facial wound healing: A prospective, blinded, placebo-controlled study. Mayo Clin Proc. 2006;81:1023–28. doi: 10.4065/81.8.1023. [DOI] [PubMed] [Google Scholar]
- 24.Lee SH, Min HJ, Kim YM, Cheon YW. The efficacy and safety of early postoperative botulinum toxin A injection for facial scars. Aesth Plast Surg. 2018;42:530–37. doi: 10.1007/s00266-017-1008-7. [DOI] [PubMed] [Google Scholar]
- 25.Ziade M, Domergue S, Batifol D, et al. Use of botulinum toxin type A to improve treatment of facial wounds: A prospective randomised study. J Plast Reconstr Aesthet Surg. 2013;66(2):209–14. doi: 10.1016/j.bjps.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 26.Wang L, Yi NZ, Fu MG, Fan ZH. [Observation of intractable itching in patients with keloid treated with botulinum toxin A injection]. Chin J Plast Surg. 2009;25:222–23. [in Chinese] [Google Scholar]
- 27.Liu B, Li J, Hu HQ. [Efficacy and safety of botulinum toxin type A on scar treatment]. Chin J Dermato Venerol Integ Trad W Med. 2017;16:25–27. [in Chinese] [Google Scholar]
- 28.Zhao YY, Lin S. [The application of botulinum toxin type A injection to treat the intractable itching of keloids]. Chinese Journal of Woman and Child Health Research. 2016;27:54–55. [in Chinese] [Google Scholar]
- 29.Zhang QL, Chen JN, Xia JB. [A clinical study of the injection of botulinum toxin A in the treatment of scar tissue]. China Medical Cosmetology. 2017;7:19–21. [in Chinese] [Google Scholar]
- 30.Shaarawy E, Hegazy RA, Abdel Hay RM. Intralesional botulinum toxin type A equally effective and better tolerated than intralesional steroid in the treatment of keloids: A randomized controlled trial. J Cosmet Dermatol. 2015;14:161–66. doi: 10.1111/jocd.12134. [DOI] [PubMed] [Google Scholar]
- 31.Heller GZ, Manuguerra M, Chow R. How to analyze the Visual Analogue Scale: Myths, truths and clinical relevance. Scand J Pain. 2016;13:67–75. doi: 10.1016/j.sjpain.2016.06.012. [DOI] [PubMed] [Google Scholar]
- 32.Thompson CM, Sood RF, Honari S, et al. What score on the Vancouver Scar Scale constitutes a hypertrophic scar? Results from a survey of North American burn-care providers. Burns. 2015;41:1442–48. doi: 10.1016/j.burns.2015.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tebble NJ, Thomas DW, Price P. Anxiety and self-consciousness in patients with minor facial lacerations. J Adv Nurs. 2004;47:417–26. doi: 10.1111/j.1365-2648.2004.03123.x. [DOI] [PubMed] [Google Scholar]
- 34.Finnerty CC, Jeschke MG, Branski LK, et al. Hypertrophic scarring: The greatest unmet challenge after burn injury. Lancet. 2016;388:1427–36. doi: 10.1016/S0140-6736(16)31406-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang J, Liao Y, Xia J, et al. Mechanical micronization of lipoaspirates for the treatment of hypertrophic scars. Stem Cell Res Ther. 2019;10:42. doi: 10.1186/s13287-019-1140-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu DQ, Li XJ, Weng XJ. Effect of BTXA on inhibiting hypertrophic scar formation in a rabbit ear model. Aesth Plast Surg. 2017;41:721–28. doi: 10.1007/s00266-017-0803-5. [DOI] [PubMed] [Google Scholar]
- 37.Fanous A, Bezdjian A, Caglar D. Treatment of keloid scars with botulinum toxin A versus triamcinolone in an athymic nude mice animal model. Plast Reconstr Surg. 2019;143(3):760–67. doi: 10.1097/PRS.0000000000005323. [DOI] [PubMed] [Google Scholar]
- 38.Austin E, Koo E, Jagdeo J. The cellular response of keloids and hypertrophic scars to botulinum toxin A: A comprehensive literature review. Dermatol Surg. 2018;44:149–57. doi: 10.1097/DSS.0000000000001360. [DOI] [PubMed] [Google Scholar]
- 39.Li YH, Liu JQ, Xiao D, et al. [Advances in the research of mechanism in prevention and treatment of scar with botulinum toxin type A and its clinical application]. Zhonghua Shao Shang Za Zhi. 2017;33:254–56. doi: 10.3760/cma.j.issn.1009-2587.2017.04.017. [in Chinese] [DOI] [PubMed] [Google Scholar]
- 40.Hao R, Li Z, Chen X, Ye W. Efficacy and possible mechanisms of botulinum toxin type A on hypertrophic scarring. J Cosmet Dermatol. 2018;17:340–46. doi: 10.1111/jocd.12534. [DOI] [PubMed] [Google Scholar]
- 41.Deitch EA, Wheelahan TM, Rose MP, et al. Hypertrophic burn scars: Analysis of variables. J Trauma. 1983;23:895–98. [PubMed] [Google Scholar]
- 42.Reckwell WB. Keloids and hypertrophic scars: A comprehensive review. Plastic Reconstr Surg. 1989;84:827. doi: 10.1097/00006534-198911000-00021. [DOI] [PubMed] [Google Scholar]


