Abstract
Background
Rheumatoid arthritis model (CIA) rats were treated by tail vein injection of IL-10-modified bone marrow mesenchymal stem cells (BMSCs) to investigate its feasibility and intrinsic molecular mechanism.
Material/Methods
The CIA rat model was established by induction type II collagen, and IL-10-modified BMSCs was established by transfecting BMSCs with adenovirus. IL-10-modified BMSCs were used to treat the CIA rats. The therapeutic effect was evaluated by measuring the changes in body weight, ankle swelling, and forced swimming time, as well as observation of synovial hyperplasia and cartilage tissue repair by HE staining. Western blot analysis and ELISA were used to detect gene expression.
Results
After 4 weeks and 8 weeks of treatment with IL10-BMSCs, the body weight, swelling value, resting time, and forced swimming struggle time of CIA rats were significantly higher than those of BMSCs-treated and -untreated CIA rats (P<0.05). Compared to BMSCs-treated CIA model rats, after treatment with IL10-BMSCs, the repair rate of osteoarticular cartilage was higher and the inhibition of synovial proliferation was better, and serum IL-17, IL-1β, and TNF-α levels were lower. We found that the protein level of SIRT1 in peripheral blood mononuclear cells was lower, the protein level in spleen was higher, and phosphorylation of p65 protein in peripheral blood mononuclear cells was reduced.
Conclusions
The efficacy of tail vein injection of IL-10-modified BMSCs in treatment of CIA rats was superior to that of BMSCs alone, which may be related to the more pronounced suppression of IL-10-modified BMSCs in peripheral blood inflammation and spleen immune response.
MeSH Keywords: Arthritis, Juvenile; Inflammation; Mesenchymal Stromal Cells; Receptors, Interleukin-10
Background
Rheumatoid arthritis (RA) is an autoimmune disease characterized mainly by chronic inflammation of the symmetric peripheral joints, joint swelling and pain, articular cartilage damage and deformity, and eventual dysfunction [1]. For patients with RA, synovial hyperplasia and tissue inflammation can cause joint and surrounding tissue damage, resulting in joint deformity and dysfunction. Lesions can affect all joints with synovial membranes, with the hands and feet being the most commonly affected. Statistical studies had shown that the global population incidence rate of RA is about 1–2%, and the ratio of male to female patients is 1: 2.5. In China, the prevalence of RA is lower than the global average, at about 0.2–0.4% [2]. At present, the therapeutic drugs for RA mainly achieve the delay of disease progression by controlling inflammation [3]. With the deepening of RA research, various new agents had been developed to treat RA, such as tumor necrosis factor-α inhibitors and interleukin-1 antibodies [4,5]. In addition, some therapeutic drugs targeting immune cells have also been developed and applied clinically; for example, the CTLA4-IgG1 fusion protein developed by Repligen has been approved for clinical use in the treatment of graft-versus-host response [6], and CD2 receptor antibodies and anti-T-cell therapies are at different stages of clinical research [7,8].
Bone marrow mesenchymal stem cells (BMSCs) are bone marrow stromal cells that not only have mechanical support for hematopoietic stem cells (HSCs) in the bone marrow, but also secrete various growth factors (e.g., IL-6, IL-11, LIF, M-CSF, and SCF) to support hematopoiesis [9,10]. BMSCs can differentiate into various types of cells, such as osteoblasts, chondrocytes, and adipocytes [11,12]. Previous research has shown that BMMSCs regulate immune responses, including suppressing the proliferation of T lymphocytes and inhibiting the production of inflammatory mediators [13,14]. These capabilities of BMSCs can be used to treat various autoimmune diseases, such as rheumatoid arthritis [15], multiple sclerosis [16], systemic lupus erythematosus [17], and Crohn’s disease [18]. Many studies [19] have shown that BMSCs transplantation affects the treatment of RA. To improve the efficacy of BMSCs transplantation in the treatment of RA, researchers have tried to change the injection site of BMSCs, combined with other drugs and other methods, but few studies have explored using genetic engineering methods to transform BMSCs for the treatment of RA.
IL-10 is a cytokine with immunosuppression and regulation effects [20], but this has rarely been investigated in RA patients [21,22]. Research using animal RA models showed that IL-10 suppresses the immune response and inflammatory reaction in vivo, and helps to relieve RA symptoms [23,24]. In the present study, we established a CIA rat model and used IL-10-modified BMSCs to treat the CIA rats to investigate its feasibility and intrinsic molecular mechanism.
Material and Methods
Experimental animals and grouping
Clean-grade Wistar rats (female, 3 weeks old, 140–160 g) adapted for feeding (room temperature 20–24°C, 12-h/12-h light/dark cycle, and 60% humidity) for 1 week were used as the experimental animals in this study, and they were randomly divided into a normal group, a BMSCs group, an IL10-BMSCs group, and a negative control group.
Rats in the normal group were fed normally. Rats in the BMSCs group were used to establish the CIA rat model and were treated with BMSCs. Rats in the IL10-BMSCs group were used to establish the CIA rat model and were transplanted with the IL-10-modified BMSCs for treatment. Rats in the negative control group were only used to establish the CIA rat model, but were not treated.
Ethics statement
This study was performed in strict accordance with the NIH guidelines for the care and use of laboratory animals. Animal welfare and use of experiment complied with the Guide for the Care and Use of Laboratory Animals. Our experiments were approved by the Ethics Committee of The Third Hospital of Hebei Medical University.
CIA rat model
For primary immunization, anesthetize rats received intraperitoneal injection of 2% sodium pentobarbital (Sinopharm Chemical Reagent Co., Shanghai, China). The dorsal skin was shaved, then sterilized with 75% alcohol. Intradermal injection was performed by selecting 4–6 points from the base of the tail to the back with 1 mL of chicken collagen type II 1 mg/mL emulsion. The normal group rats were injected with an equal volume of sterile saline.
To strengthen immunity, 1 week after the first immunization, each CIA rat was intraperitoneally injected with bovine type II collagen 1 mg/mL emulsion 0.5 mL. Normal group rats were injected with an equal volume of sterile saline.
Isolation, culture, and identification of rat BMSCs
A 4-week-old female Wistar rat was selected for isolation of rat femur and tibia BMSCs by density gradient centrifugation. Rats were sacrificed by dislocating the cervical spine and placed in 75% alcohol for 15 min. Femurs and iliac bones were taken under aseptic conditions. We washed the medullary cavity of the femurs and iliac bones with 20 mL of serum-free LG-DMEM medium plus 1 U/mL streptomycin (BBI Life Science, Shanghai, China). L-DMEM (Sigma-Aldrich, MA, USA) added to 10% FBS (Gibco, MA, USA) was used to culture BMSCs in a T25 flask (NEST, Wuxi, China) with 3×105 cells/cm2.
The second-generation rat bone marrow mesenchymal stem cells were harvested to obtain a single-cell suspension at a concentration of 1×106/ml. We added 10 μl rat PE-CD90, FIFC-CD44, PE-CD29, and PE-CD45 anti-human antibodies, incubated at 4°C for 30 min and washed with PBS (135 mM NaCl, 2.7 mM KCl, 1.5 mM KH2PO4, 8 mM K2HPO4, pH=7.2). After fixing with 4% paraformaldehyde, we performed flow cytometry analysis (LSR II, BD Pharmingen, USA). All of the above antibodies were purchased from BD Pharmingen.
Building and transplanting the IL10-BMSCs
The rat IL-10 gene sequence (NC_005112.4) was queried on the NCBI, and the ends of the IL-10 sequence were added the ends of the Sal I enzyme, then artificially synthesized (Sangon Biotech, Shanghai, China). Construction of pDc316-IL10 recombinant plasmid on pDc316 plasmid recovered from Sal I enzyme (NEB, CT, USA) under the action of T4 DNase. The pDc316-IL10 was transfected into HEK293 (ATCC, VA, USA) according to the Lipofectamine 2000 liposome instructions and the adenovirus was harvested after repeated freezing and thawing. IL-10-modified BMSCs were established by transfecting BMSCs with adenovirus.
At 2 weeks after the booster immunization, CIA rats were scored by arthritis index (AI), and rats with a score greater than 2 were selected as successful rats for the CIA model. After 48 h of adenovirus infection of BMSCs, and when the percentage of green fluorescent positive cells reached 90% as assessed by fluorescence microscopy, the BMSCs was digested by trypsin and prepared into 2×104cells/mL of single-cell suspension. We added 10 μmol/L Brdu (Sigma-Aldrich, MA, USA) into the single-cell suspension, followed by culturing for 48 h. After we harvested these cells, 2×104 normal BMSCs cells were injected into the rats in the BMSCs group via tail vein injection, 2×104 IL 10-MSCs cells were injected into the rats in the IL 10-BMSCs group via tail vein injection, and rats in the normal group and negative control group were injected with equal amounts of PBS.
Growth, swelling, and organ index
We stipulated that 0W was not measured before immunization, 3W was before transplantation, 7W was 4 weeks after BMSCs transplantation, and 12W was 8 weeks after BMSCs transplantation. At the 4 time points above, we measured the weight of rats, the diameter of ankle joints of the same site, and the organ index. Organ index=Spleen or thymus mass (mg)/rat body weight (g).
Behaviors
Forced swimming was used to test the behavioral changes in rats. The forced swimming time was 8: 30–11: 30 during the day and 20: 30–23: 30 at night. One day before the experiment, the rats were placed in a transparent plexiglass cylinder 54 cm in height, 93 cm in height, and 60 cm in depth. The water temperature was 20–24°C, and 1 rat per cylinder was adapted to swim for 1 min. The day of the experiment was the same as the day before the experiment, the irrelevant experimenters randomly selected 6 min for video recording and recorded the cumulative immobility time and struggling time of rats. Immobility time refers to the fact that the rat stops struggling to float in the water, or that only tiny limb movements are used to keep the head above the water.
HE staining
The synovial tissues of the knee joints and their surrounding tissues were taken from rats of each group, fixed in 10% neutral buffered formalin, decalcified, dehydrated, rendered translucent, immersed in wax, and embedded in paraffin. Paraffin blocks were serially sectioned (thickness 4 μm), and then they were placed at room temperature for 10 min, and placed in a 60°C oven for about 45 min. Then, samples were soaked in hematoxylin dye (Solarbio, Beijing, China) for 5 min, washed by ddH2O for 1 min, backed to blue by 60°C ddH2O for 1 h, stained by eosin Y staining solution (Solarbio, Beijing, China) for 2 min, washed by ddH2O for 1 min, and subjected to gradient dehydration by alcohol. Samples were mounted after being rendered transparent by xylene, and then observed with a microscope.
Western blotting
Rats were anesthetized with an intraperitoneal injection of 2% sodium pentobarbital, and were fixed in supine position on a clean bench. We collected 5–10 mL of rat peripheral blood using the abdominal aorta blood collection method. The serum was removed by centrifugation, and peripheral blood mononuclear cells were isolated by density gradient centrifugation.
Tissue or cell lysates were separated by SDS-page and then transferred to PVDF membranes. Primary antibody was selected as follows: SIRT1 (ab110304, 1: 2000), p65 (ab16502, 1: 1000), p-p65(ab86299,1: 2000), PD-1 (ab214421, 1: 1000), TGF-β1 (ab92486, 1: 1000), Foxp3 (ab20034, 1: 1000) or GAPDH (ab8246, 1: 3000). Second antibody was selected as follows: goat anti-rabbit (ab150077, 1: 1000), or goat anti-rat (ab150117, 1: 1000). Primary antibody was incubated overnight at 4°C and second antibody was incubated for 1 h at room temperature. Unless otherwise specified, all antibodies were purchased from ABCAM (Cambridge, UK)
ELISA assay for IL-1β, TNF-α, IL-10, and IL-17
Blood was drawn from about 1-cm capillaries under the eyelids, and serum was obtained after centrifuging at 3000 rpm for 5 min. IL-1β (MitcSciences, USA), TNF-α (Sangon Biotech, Shanghai, China), IL-10 (Sangon Biotech, Shanghai, China), and IL-17 (Sangon Biotech, Shanghai, China) ELISA kit for rats were used to detect the IL-1β, TNF-α, TGF-β1, and IL-17 levels, respectively, in serum.
Statistical analysis
SPSS 20.0 was used for statistical analysis. Data are expressed as mean ± standard deviation. The t test was used to compare differences between 2 groups. One-way ANOVA was used to compare differences between different groups, and Duncan method was used as the post-test. P<0.05 indicated that a difference was statistically significant.
Results
Cultivation and identification of BMSCs
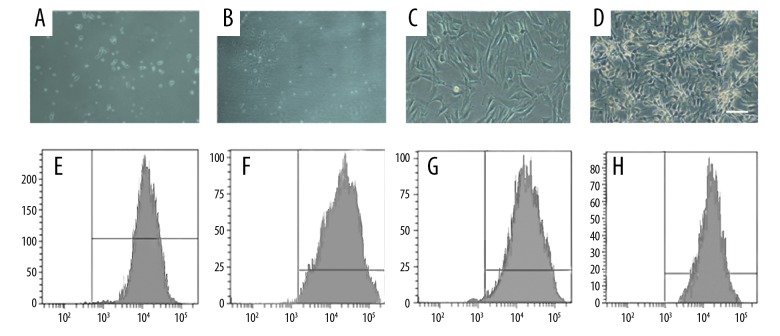
After 24 h of primary culturing of BMSCs, we observed a small number of adherent cells that were spindle-shaped or polygonal (Figure 1A). After 3–5 days of continuous cultivation, we observed cell clones. Most of the clones were composed of spindle-shaped, multifibrillating, fibroblast-like cells (Figure 1B, 1C), and a few of the cloned cells were round and “cobblestone” like a mixture of hematopoietic cells. There was up to 90% fusion after 10–15 days of cultivation. The passaged cells were basically adherent at 24 h and showed a uniform long spindle shape with rapid proliferation. At 4–5 days after cell passage, the density of BMSCs cells reached 90%, and cells were arranged in spirals or parallel bundles (Figure 1D). We found that the cells can be transmitted for more than 10 generations and kept the morphology and proliferation ability under light microscopy that was passed by 5×103 cells/cm2.
Figure 1.
Culture of BMSCs and identification of their surface molecular markers. (A) Spindle-shaped morphology of the marrow cells at day 1 (×10). (B, C) The number and size of the colonies gradually increased on days 3–5 (C×20, D×20). (D) A homogeneous population of BMSCs in the first passage of cultured marrow cells (×10). (E–H) Assay of cell surface antigens on rat mesenchymal stem cells.
Flow cytometry was used to identify surface molecular markers of BMSCs, showing that CD29 (Figure 1E), CD44 (Figure 1F), and CD90 (Figure 1G) were positive and CD45 (Figure 1H) was negative, indicating that BMSCs met the qualification requirements.
IL10-BMSCs reduced RA symptoms in CIA rats
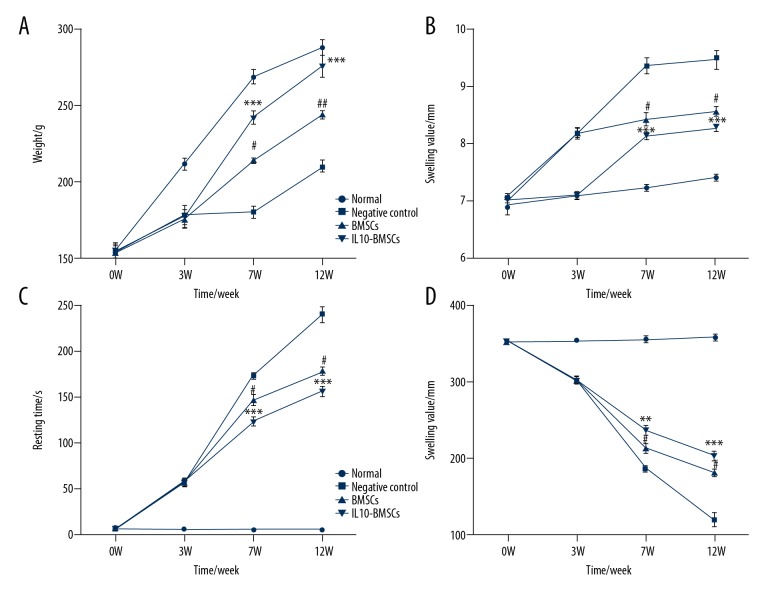
Normal group rats had normal eating activity, body weight gradually increased, and the ankle joint gradually increased with the extension of study time; therefore, there was an upward trend in the determination of the normal rat ankle swelling value. In the forced swimming test, normal group rats were struggling most of the time. However, CIA model rats were depressed and had mild hair loss symptoms during weeks 0–3 of modeling, eating was significantly reduced, the hindlimb walking gradually became difficult, and the weight gain was slow. At 7W and 12W, the weight and struggle time of rats in the BMSCs group were significantly increased compared to CIA group rats without any treatment (P<0.05). However, the weight (Figure 2A), swelling value (Figure 2B), resting time (Figure 2C), and struggle time (Figure 2D) of rats in the BMSCs group were significantly less than in the IL10-BMSCs group (P<0.05). These results suggest that IL10-BMSCs treatment improves the symptoms of RA in CIA group rats, and the effect was stronger than the treatment without modified BMSCs.
Figure 2.
Results of rat weight, swollen ankle, and forced swimming test. (A) Changes in body weight at different time in different groups of rats. (B) Changes in swelling of ankle joints at different times in different groups of rats. (C, D) Rat resting time (C) and struggling time (D) in forced swimming test. Compared with negative control group, # P<0.05, ## P<0.01, and ### P<0.001. Compared with BMSCs group, * P<0.05, ** P<0.01, *** P<0.001.
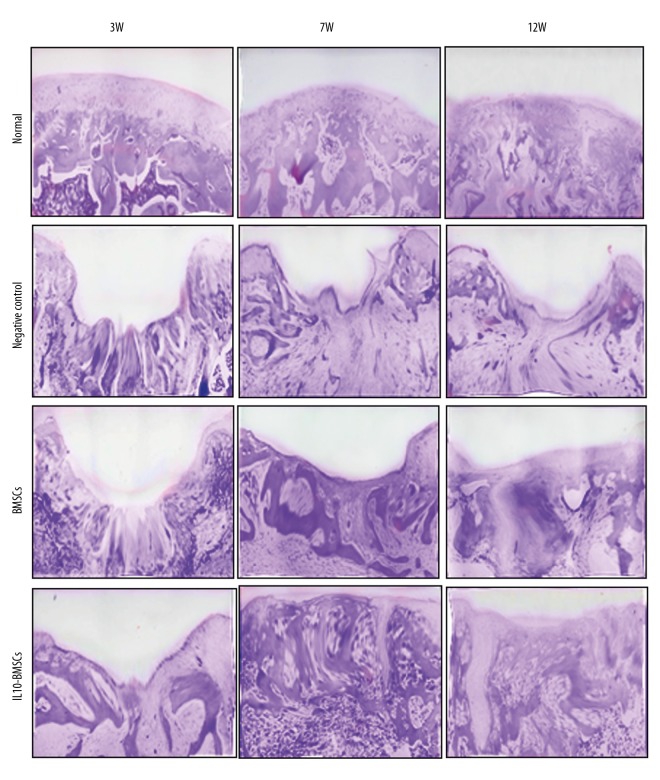
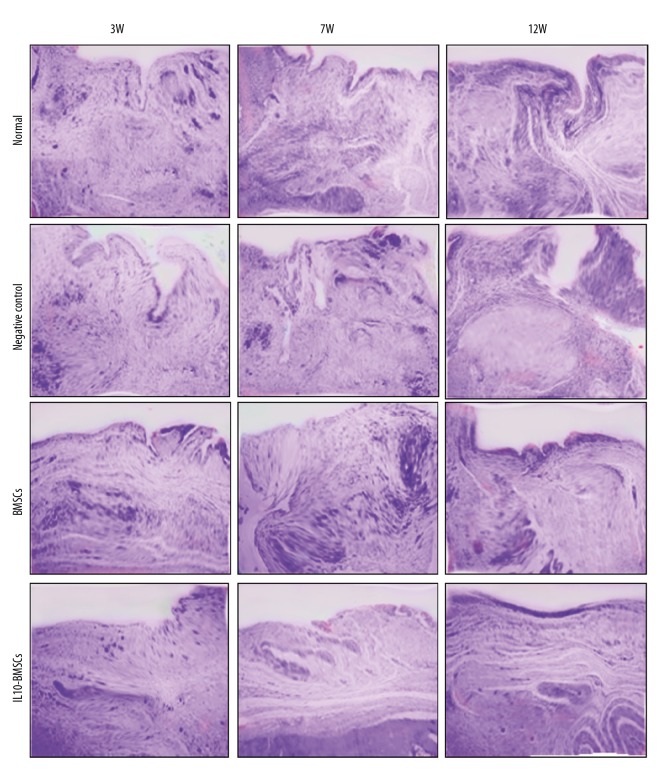
IL10-BMSCs reduced synovial hyperplasia and promoted cartilage tissue repair
Rat synovial membrane and its surrounding tissues were stained with HE at 21 days after the CIA model was established, and results show (Figures 3, 4) that the cartilage surface of the normal group rats was smooth, and synovial tissue cells were arranged neatly. However, synovial hyperplasia occurred in CIA model rats, and some of the articular cartilage showed significant erosion or ulceration, and we found synovial, articular cartilage, and moderate-to-severe mixed inflammatory cell infiltration in the joint cavity. Synovial hyperplasia and cartilage tissue damage of rats in the negative control group worsened over time. After 4 weeks of treatment with BMSCs, although no new cartilage was formed in the cartilage injury site in the rats, fibroblast-like cells were observed in the cartilage layer. After 4 weeks of treatment with IL10-BMSCs, a small amount of new cartilage appeared in the IL10-BMSCs group, and the boundary between the new cartilage and the cartilage in situ was clear, but the arrangement of chondrocytes was still disorderly. For synovial hyperplasia, rats in the BMSCs group and the IL10-BMSCs group were significantly better than the negative control group at 7W.
Figure 3.
Histological analyses by H&E staining at the indicated times during the osteochondral repair in 4 groups (×40).
Figure 4.
Typical synovium in each group stained with H&E at 3, 7, and 12 weeks (×40).
At 12W, the new cartilage was obvious and the synovial hyperplasia was obviously improved in rats of the BMSCs group. In the IL10-BMSCs group, the cartilage tissue had a smooth and transparent surface and was well associated with adjacent cartilage, the cartilage repair was significantly better than that of the BMSCs group, and the synovial hyperplasia was of the lowest grade, which was close to that of the normal group. These results suggest that IL10-BMSCs treatment reduces synoviocyte hyperplasia and cartilage tissue repair in CIA rats, and the effect was better than that of unmodified BMSCs.
IL10-BMSCs reduced the circulating inflammation of CIA rats
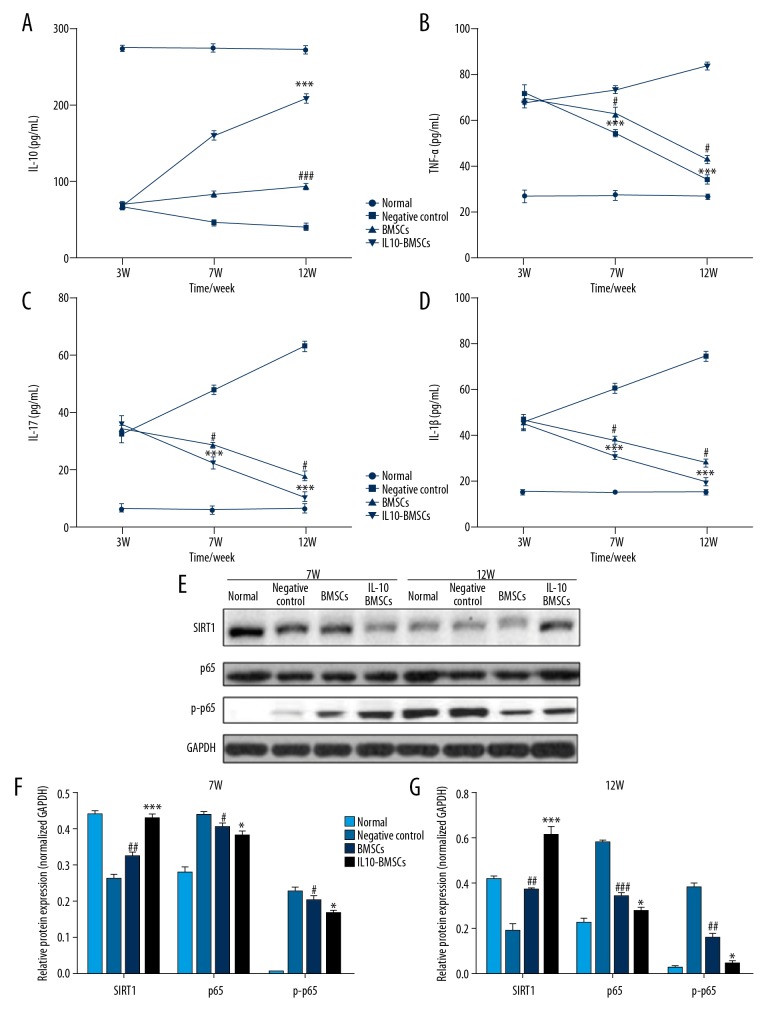
Serum inflammatory cytokines were detected by use of ELISA kits, and we found that the serum IL-10 levels in CIA model rats decreased and the serum levels of TNF-α, IL-17, and IL-1β were increased after the completion of modeling. After 4 weeks and 8 weeks with different treatments, the serum level of IL-10 (Figure 5A) in the IL10-BMSCs group was significantly higher than that in the negative group and BMSCs group (P<0.05), and the serum level of TNF-α (Figure 5B), IL-17 (Figure 5C), and IL-1β (Figure 5D) in the IL10-BMSCs group was significantly lower than that in the negative group and BMSCs group (P<0.05).
Figure 5.
IL10-BMSCs reduced the circulating inflammation of CIA rats (n=5). (A–D) The serum levels of IL-10 (A), TNF-α (B), IL-17 (C), and IL-1β (D) at different times in different groups. (E–G) Western blotting was used to detect the expression of SIRT1, p65, and p-p65 protein in peripheral blood mononuclear cells. Compared with negative control group, # P<0.05, ## P<0.01, and ### P<0.001. Compared with BMSCs group, * P<0.05, ** P<0.01, *** P<0.001.
In addition, Western blotting was used to measure the expression of SIRT1 protein and phosphorylation of p65 protein in peripheral blood mononuclear cells. As shown in Figure 5E–5G, IL10-BMSCs significantly inhibited the phosphorylation of p65 protein and promoted the expression of SIRT1 protein in peripheral blood mononuclear cells of CIA rats. These results suggest that IL10-BMSCs inhibits the inflammation of peripheral blood mononuclear cells in CIA rats by inhibiting the SIRT1/p65-mediated NF-κB signaling pathway.
IL10-BMSCs reduced immune response in CIA rats
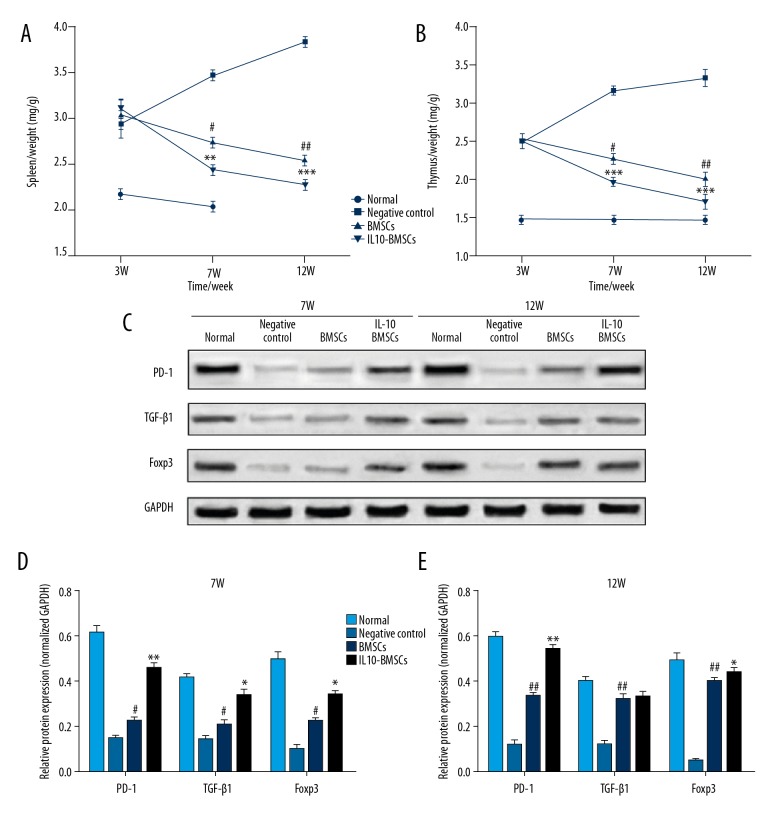
Excessive immune response is one of the main reasons for the development of RA diseases, and we found that the organ index of spleen and thymus in CIA rats was significantly higher than in normal rats (Figure 6A, 6B). As shown in Figure 6C–6E, after 4 weeks and 8 weeks with different treatments, the organ index of rat spleen and thymus in the IL 10-BMSCs group was significantly lower than that in the negative group and BMSCs group (P<0.05). Moreover, the expression of PD-1, TGF-β1, and Foxp3 protein in the spleen of IL10-BMSCs and BMSCs group rats increased gradually. More importantly, the expression of PD-1, TGF-β1, and Foxp3 protein in the spleen of IL10-BMSCs rats was significantly higher than in the BMSCs group (P<0.05).
Figure 6.
IL10-BMSCs reduced immune response in CIA rats (n=5). (A, B) The organ index of spleen (A) and thymus (B) in different groups at different times. (C–E) Western blotting was used to detect the expression of PD-1, TGF-β1, and Foxp3 protein in spleens at different times. Compared with negative control group, # P<0.05, ## P<0.01, and ### P<0.001. Compared with BMSCs group, * P<0.05, ** P<0.01, and *** P<0.001.
Discussion
IL-10 was first found to be synthesized and secreted by murine CD4+ Th2 cells and had the function of inhibiting the synthesis of IFN-γ by Th1-T cells. As the study progressed, the immunological characteristics of IL-10 were gradually revealed. The main cells for synthesizing IL-10 were monocytes and macrophages, and many other cells could also synthesize IL-10, including CD4+T cells, CD8+ T cells, and B cells. In terms of immunology, IL-10 has the ability to prevent the proliferation of antigen-specific T cells, inhibit the ability of the antigen-presenting cells to present, and inhibit the synthesis and expression of inflammatory cytokines and inflammatory mediators. IL-10 is a cytokine that inhibits the inflammatory response and immunosuppression of the body [25], so IL-10 was widely used in the treatment of autoimmune diseases as a natural immunosuppressive agent [26,27], which included RA [28].
When IL-10 was added to the culture medium of synoviocytes from patients with RA in vitro, we could detect the decreased expression of inflammatory factors such as IFN-γ, TNF-α, and IL-1β [29]. In addition, subcutaneous injection of IL-10 in clinical practice could not only reduce the existing drug dosage in patients with active RA, but also could reduce the serum levels of TNF-α and IL-1β in patients, and patients only showed mild, reversible, dose-dependent reduction of platelets and hemoglobin. These studies indicated that IL-10 may be an important substance for endogenous immunosuppression in patients with RA, and direct injection therapy was safe and had few adverse effects.
In the present study, based on observation of clinical symptoms such as weight, ankle swelling, and forced swimming test, as well as pathological perspectives such as cartilage repair and inhibition of synovial hyperplasia, we show that the efficacy of IL10-BMSCs in treating CIA rats is superior to that of unmodified BMSCs. We also found that the serum level of IL-10 in the IL10-BMSCs group was significantly higher than that in the BMSCs group (P<0.05), and the serum level of TNF-α, IL-17, and IL-1β in the IL10-BMSCs group was significantly lower than that in the BMSCs group. We found that IL10-BMSCs significantly inhibited the phosphorylation of p65 protein and promoted the expression of SIRT1 protein in peripheral blood mononuclear cells of CIA rats.
Previous studies had found that the serum level of IL-10 in RA patients was lower than that in healthy people, but inflammatory factors, such as IL-17 [30,31], IL-1β [32], and TNF-α [33], were higher. In addition, the direct mechanism in RA model animals or RA patients treated by injection of IL-10 was to increase the peripheral blood IL-10 level. In vitro, when the immune cell subsets were co-cultured with BMSCs, IFN-γ secretion was decreased from NK and Th1 cells, TNF-α was decreased from dendritic DC1 cells, but IL-4 was increased from Treg cells and Th2 cells, and IL-10 was increased from DC2 cells. This indicates that when BMSCs cells are in an inflammatory environment, they inhibit NK cells, Th1, and other inflammatory cells to express inflammatory mediators, promote anti-inflammatory cell signaling, and inhibit inflammatory cell signaling. In conclusion, exogenous injection of BMSCs can transform the body from a pro-inflammatory environment to an anti-inflammatory environment [34]. In the present study, the decrease of pro-inflammatory cytokines in BMSCs-treated CIA rats and increase in the content of anti-inflammatory cytokine IL-10 also confirms this point. More importantly, the IL10-BMSCs group had lower levels of pro-inflammatory cytokines than the BMSCs group, and the levels of anti-inflammatory cytokines were higher. This suggests that IL-10-modified BMSCs have a stronger ability to reverse the body’s progression from a pro-inflammatory environment to an anti-inflammatory environment than unmodified BMSCs.
The NF-κB signaling pathway is one of the most important signaling pathways in mammalian cells, and it is widely present in nodes of multiple cellular signaling pathways [35].When NF-κB is activated, it can regulate various downstream inflammatory factors and participate in the regulation of inflammatory responses [36]. SIRT1 is a broad histone deacetylase present in human cells, and it regulates the expression of multiple transcription factors, such as p53 [37], UCP2 [38], and NF-κB [36]. P65 is an important constituent protein in NF-κB, and it needs to be acetylated to play its role. In the inflammatory response, SIRT1 can use its deacetylation ability to reduce the acetylation of NF-κB p65 protein, thereby inhibiting the transcription levels of inflammatory genes, including IL-17, IL-1β, and TNF-α downstream of NF-κB. BMSCs can inhibit the body’s peripheral blood mononuclear cells SIRT1/p65-mediated NF-κB signaling pathway to achieve the effect of inhibiting the body’s inflammatory response, and IL10-BMSCs achieve this effect better than BMSCs.
Autoimmune lymphocyte abnormalities are one of the causes of RA, and we found that the organ index of spleen and thymus in CIA group rats was higher than that of normal rats, but decreased after treatment with IL10-BMSCs and BMSCs. This indicates that the immune response of CIA rats was stronger than that in normal rats, and the treatment of IL 10-BMSCs reduced the immune response of CIA rats.
Previous studies confirmed that BMSCs have immunosuppressive effects, and their mechanisms were related to low autoimmunity and inhibition of T lymphocyte immune response [39,40]. However, IL10-BMSCs group rats not only had significantly lower organ index than BMSCs group rats, but also had lower expression of PD-1, TGF-β1, and Foxp3 proteins in the spleen. PD-1 is a glycoprotein expressed on the cell surface and has immunosuppressive effects by interacting with receptor PD-L1 or PD-L2 in T cells to inhibit TCR signaling pathways [41,42]. Chemel et al. found that the expression of PD-1 in RA mice was significantly lower than that in the normal group, while low expression of PD-1 aggravated the progression of autoimmune reactions, which in turn stimulated the development of RA [43]. TGF-β1 is a polypeptide factor expressed on the cell surface or secreted into cells and has immunosuppressive effects; it plays an important regulatory role in the progression of RA disease [44,45]. Foxp3 is specifically expressed on CD4+CD25+Tregs, but once Foxp3 is deleted from CD4+CD25+Treg, it does not normally exert its immunosuppressive function, while enhancing the activity of CD4+CD25+ Treg cells was beneficial to the treatment of RA by cytokines [46, 47].
Conclusions
The efficacy of IL-10-modified BMSCs in treatment of CIA rats is better than that of unmodified BMSCs. This may be because IL10-BMSCs enter the body of CIA rats and can continue to increase IL-10 levels in the body, leading to stronger inflammatory suppression and immunosuppressive effects.
In the future, the effects of IL10-BMSC transplantation on the phenotypic changes of immune cells in RA model rats, especially those associated with inflammation, should be explored. In addition, altered microRNAs, lncRNAs, and other encoded mRNAs can also be screened by sequencing.
Footnotes
Conflict of interest
None.
Source of support: Departmental sources
References
- 1.Kyburz D, Finckh A. The importance of early treatment for the prognosis of rheumatoid arthritis. Swiss Med Wkly. 2013;143:w13865. doi: 10.4414/smw.2013.13865. [DOI] [PubMed] [Google Scholar]
- 2.Smolen JS, Burmester G-R, Combe B, et al. Head-to-head comparison of certolizumab pegol versus adalimumab in rheumatoid arthritis: 2-year efficacy and safety results from the randomised EXXELERATE study. Lancet. 2016;388:2763–74. doi: 10.1016/S0140-6736(16)31651-8. [DOI] [PubMed] [Google Scholar]
- 3.Singh JA, Saag KG, Bridges SL, Jr, et al. 2015 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Care Res. 2016;68:1–25. doi: 10.1002/acr.22783. [DOI] [PubMed] [Google Scholar]
- 4.Lequerré T, Avenel G, Vittecoq O. [Therapeutic update in rheumatoid arthritis]. La Revue De Medecine Interne. 2013;34:754–62. doi: 10.1016/j.revmed.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 5.Mcinnes IB, O’Dell JR. State-of-the-art: rheumatoid arthritis. Ann Rheum Dis. 2010;69:1898–906. doi: 10.1136/ard.2010.134684. [DOI] [PubMed] [Google Scholar]
- 6.Kremer JM, Westhovens R, Leon M, et al. Treatment of rheumatoid arthritis by selective inhibition of T-cell activation with fusion protein CTLA4Ig. N Engl J Med. 2003;349:1907–15. doi: 10.1056/NEJMoa035075. [DOI] [PubMed] [Google Scholar]
- 7.Rao DA, Gurish MF, Marshall JL, et al. Pathologically expanded peripheral T helper cell subset drives B cells in rheumatoid arthritis. Nature. 2017;542:110. doi: 10.1038/nature20810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen L, Wang D, Lu S, et al. Increased circulating follicular regulatory T cells are associated with lower auto-antibodies in stable remission rheumatoid arthritis patients. Arthritis Rheumatol. 2018:70. doi: 10.1002/art.40430. [DOI] [PubMed] [Google Scholar]
- 9.Ohishi M, Schipani E. Bone marrow mesenchymal stem cells. J Cell Biochem. 2010;109:277–82. doi: 10.1002/jcb.22399. [DOI] [PubMed] [Google Scholar]
- 10.Krampera M, Glennie S, Dyson J, et al. Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood. 2003;101:3722–29. doi: 10.1182/blood-2002-07-2104. [DOI] [PubMed] [Google Scholar]
- 11.Wu GW, Liu XX, Wu MX, et al. Experimental study of millimeter wave-induced differentiation of bone marrow mesenchymal stem cells into chondrocytes. Int J Mol Med. 2009;23:461–67. doi: 10.3892/ijmm_00000152. [DOI] [PubMed] [Google Scholar]
- 12.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–47. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 13.Liu Z, Wang W, Gao J, et al. Isolation, culture, and induced multiple differentiation of Mongolian sheep bone marrow-derived mesenchymal stem cells. In Vitro Cell Dev Biol Anim. 2015;51:464–74. doi: 10.1007/s11626-013-9725-y. [DOI] [PubMed] [Google Scholar]
- 14.Sánchezberná I, Santiagodíaz C, Jiménezalonso J. [Immunomodulatory properties of stem mesenchymal cells in autoimmune diseases]. Med Clin (Barc) 2015;144:88–91. doi: 10.1016/j.medcli.2014.01.014. [in Spanish] [DOI] [PubMed] [Google Scholar]
- 15.Qiao Z, Ma L. Study on mechanism of bone marrow mesenchymal stem cell in treating patients with rheumatoid arthritis. Blood. 2009;114:4486. [Google Scholar]
- 16.Yamout B, Hourani R, Salti H, et al. Bone marrow mesenchymal stem cell transplantation in patients with multiple sclerosis: A pilot study. J Neuroimmunol. 2010;227:185–89. doi: 10.1016/j.jneuroim.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 17.Vagnani S, Tani C, Carli L, et al. AB0190 treatment with bone marrow mesenchymal stromal cells in a murine model of systemic lupus erythematosus. Ann Rheum Dis. 2015;74:954. [Google Scholar]
- 18.Dave M, Martino LD, Somoza R, et al. 971c Human bone marrow derived mesenchymal stem cells mediate immunosuppression in vitro and in experimental Crohn’s disease. Gastroenterology. 2016;150:S196. [Google Scholar]
- 19.Kastrinaki MC, Sidiropoulos P, Roche S, et al. Functional, molecular and proteomic characterisation of bone marrow mesenchymal stem cells in rheumatoid arthritis. Ann Rheum Dis. 2008;67:741–49. doi: 10.1136/ard.2007.076174. [DOI] [PubMed] [Google Scholar]
- 20.Moore KW, De WMR, Coffman RL, et al. Interleukin-10 and the interleukin-10 receptor. Ann Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 21.Eskdale J, Mcnicholl J, Wordsworth P, et al. Interleukin-10 microsatellite polymorphisms and IL-10 locus alleles in rheumatoid arthritis susceptibility. Lancet. 1998;352:1282–83. doi: 10.1016/S0140-6736(05)70489-X. [DOI] [PubMed] [Google Scholar]
- 22.Sabry D, Elamir A, Mahmoud RH, et al. Role of LncRNA-AF085935, IL-10 and IL-17 in rheumatoid arthritis patients with chronic hepatitis C. J Clin Med Res. 2017;9:416–25. doi: 10.14740/jocmr2896w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guggino G, Giardina A, Ferrante A, et al. The in vitro addition of methotrexate and/or methylprednisolone determines peripheral reduction in Th17 and expansion of conventional Treg and of IL-10 producing Th17 lymphocytes in patients with early rheumatoid arthritis. Rheumatol Int. 2015;35:171–75. doi: 10.1007/s00296-014-3030-2. [DOI] [PubMed] [Google Scholar]
- 24.Hernández-Bello J, Oregón-Romero E, Vázquez-Villamar M, et al. Aberrant expression of interleukin-10 in rheumatoid arthritis: Relationship with IL10 haplotypes and autoantibodies. Cytokine. 2017;95:88–96. doi: 10.1016/j.cyto.2017.02.022. [DOI] [PubMed] [Google Scholar]
- 25.Fujii SI, Lotze MT. Interleukin-10 (IL-10) Humana Press; 2007. pp. 165–79. [Google Scholar]
- 26.Dambuza IM, He C, Choi JK, et al. IL-12p35 induces expansion of IL-10 and IL-35-expressing regulatory B cells and ameliorates autoimmune disease. Nat Commun. 2017;8:719. doi: 10.1038/s41467-017-00838-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Groux H, Cottrez F. The complex role of interleukin-10 in autoimmunity. J Autoimmun. 2003;20:281–85. doi: 10.1016/s0896-8411(03)00044-1. [DOI] [PubMed] [Google Scholar]
- 28.Clair EWS. Interleukin 10 treatment for rheumatoid arthritis. Ann Rheum Dis. 1999;58(Suppl 1):I99. doi: 10.1136/ard.58.2008.i99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Möttönen T, Paimela L, Leirisalorepo M, et al. Only high disease activity and positive rheumatoid factor indicate poor prognosis in patients with early rheumatoid arthritis treated with “sawtooth” strategy. Ann Rheum Dis. 1998;57:533. doi: 10.1136/ard.57.9.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baek SH, Lee SG, Park YE, et al. Increased synovial expression of IL-27 by IL-17 in rheumatoid arthritis. Inflamm Res. 2012;61:1339–45. doi: 10.1007/s00011-012-0534-7. [DOI] [PubMed] [Google Scholar]
- 31.Lee SY, Yoon BY, Kim JI, et al. Interleukin-17 increases the expression of Toll-like receptor 3 via the STAT 3 pathway in rheumatoid arthritis fibroblast-like synoviocytes. Immunology. 2014;141:353–61. doi: 10.1111/imm.12196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shoda H, Nagafuchi Y, Tsuchida Y, et al. Increased serum concentrations of IL-1 beta, IL-21 and Th17 cells in overweight patients with rheumatoid arthritis. Arthritis Res Ther. 2017;19:111. doi: 10.1186/s13075-017-1308-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gonzálezalvaro I, Ortiz AM, Tomero EG, et al. Baseline serum RANKL levels may serve to predict remission in rheumatoid arthritis patients treated with TNF antagonists. Ann Rheum Dis. 2007;66:1675. doi: 10.1136/ard.2007.071910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lepperdinger G. Inflammation and mesenchymal stem cell aging. Curr Opin Immunol. 2011;23:518–24. doi: 10.1016/j.coi.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lan W, Petznick A, Heryati S, et al. Nuclear factor-κB: Central regulator in ocular surface inflammation and diseases. Ocular Surface. 2012;10:137–48. doi: 10.1016/j.jtos.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 36.Zheng C, Yin Q, Wu H. Structural studies of NF-κB signaling. Cell Res. 2011;21:183–95. doi: 10.1038/cr.2010.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thakur BK, Chandra A, Dittrich T, et al. Inhibition of SIRT1 by HIV-1 viral protein Tat results in activation of p53 pathway. Biochem Biophys Res Commun. 2012;424:245–50. doi: 10.1016/j.bbrc.2012.06.084. [DOI] [PubMed] [Google Scholar]
- 38.Zhao W, Kruse JP, Tang Y, et al. Negative regulation of the deacetylase SIRT1 by DBC1. Nature. 2008;451:587–90. doi: 10.1038/nature06515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhao RC. Immunosuppressive properties of cloned bone marrow mesenchymal stem cells. Cell Res. 2007;17:240–48. doi: 10.1038/cr.2007.4. [DOI] [PubMed] [Google Scholar]
- 40.Lin R, Ma H, Ding Z, et al. Bone marrow-derived mesenchymal stem cells favor the immunosuppressive T cells skewing in a Helicobacter pylori model of gastric cancer. Stem Cells Dev. 2013;22:2836–48. doi: 10.1089/scd.2013.0166. [DOI] [PubMed] [Google Scholar]
- 41.Fife BT, Pauken KE, Eagar TN, et al. Interactions between PD-1 and PD-L1 promote tolerance by blocking the TCR-induced stop signal. Nat Immunol. 2009;10:1185–92. doi: 10.1038/ni.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Anna P, Gros A, Robbins PF, et al. High frequency CD8+PD-1+ TCR clonotypes isolated from fresh melanomas display anti-tumor activity. J Immunother Cancer. 2015;3(Suppl 2):P42. [Google Scholar]
- 43.Li S, Liao W, Chen M, et al. Expression of programmed death-1 (PD-1) on CD4+ and CD8+ T cells in rheumatoid arthritis. Inflammation. 2014;37:116–21. doi: 10.1007/s10753-013-9718-8. [DOI] [PubMed] [Google Scholar]
- 44.Wilder RL, Lafyatis R, Roberts AB, et al. Transforming growth factor-β in rheumatoid arthritis. Ann NY Acad Sci. 2010;593:197–207. doi: 10.1111/j.1749-6632.1990.tb16112.x. [DOI] [PubMed] [Google Scholar]
- 45.Chemel M, Brion R, Segaliny A, et al. BMP-2 and TGF-β1 inhibit the expression of the pro-inflammatory cytokine IL-34 in rheumatoid arthritis synovial fibroblasts. Am J Pathol. 2017;187(1):156–62. doi: 10.1016/j.ajpath.2016.09.015. [DOI] [PubMed] [Google Scholar]
- 46.Nie H, Zheng Y, Li R, et al. Phosphorylation of FOXP3 controls regulatory T cell function and is inhibited by TNF-α in rheumatoid arthritis. Nat Med. 2013;19:322–28. doi: 10.1038/nm.3085. [DOI] [PubMed] [Google Scholar]
- 47.Lin SC, Chen KH, Lin CH, et al. The quantitative analysis of peripheral blood FOXP3-expressing T cells in systemic lupus erythematosus and rheumatoid arthritis patients. Eur J Clin Invest. 2010;37:987–96. doi: 10.1111/j.1365-2362.2007.01882.x. [DOI] [PubMed] [Google Scholar]