Abstract
Liver cirrhosis (LC) and hepatocellular carcinoma (HCC) are life-threatening conditions frequently associated with chronic hepatitis B virus (HBV) infection in Asian countries, including Indonesia. HBV genotypes and several specific mutations are associated with disease progression. To clarify the geographical variation in viral characteristics, HBV genotypes and gene mutations were investigated in patients with advanced liver disease (ALD) in Samarinda, East Kalimantan, Indonesia. Sera were collected from 41 patients with ALD at Abdul Wahab Sjahranie Hospital and HBV carriers from Red Cross Center blood bank in Samarinda, and screened for hepatitis B surface antigen and hepatitis B e-antigen. Liver function data were obtained from the medical records from each patient. HBV genotype and gene mutations were determined by polymerase chain reaction sequencing. Analysis of HBV isolates indicated that genotype B was the most frequent genotype, at 85.4 and 97.8%, followed by C, at 14.6 and 2.2%, in patients with ALD and in HBV carriers, respectively. The C1505A mutation in X region, T1753V and A1762T/G1764A mutations in the basal core promoter region and C1858T in precore (PC) region were frequent and only detected in patients with ALD (28.9, 40, 73.5 and 17.6%, respectively), whereas the G1896A mutation in the PC region was frequently detected in HBV carriers. The presence of HBV genotype B and certain HBV gene mutations were characteristic of patients with ALD in East Kalimantan.
Keywords: genotype, hepatitis B virus, liver disease, mutation, Indonesia
Introduction
Hepatitis B virus (HBV) infection is a major health problem worldwide. Indonesia is an intermediate-to-high HBV endemic region. Despite a decrease in the incidence of acute HBV infection in Indonesia resulting from the universal newborn HBV immunization program, the number of patients with advanced liver disease (ALD) associated with chronic HBV infection is increasing (1,2). Chronic HBV infection usually progresses to ALD, including liver cirrhosis (LC) and hepatocellular carcinoma (HCC). Demographic, environmental, viral and host-associated factors may contribute to the risk of LC and HCC in chronic HBV-infected patients (3-5).
HBV is a double-stranded DNA virus that belongs to the Hepadnaviridae family. The HBV genome contains 4 partially overlapping open reading frames (ORFs) encoding the surface, precore (PC)/core, polymerase and X proteins, which have diverse mutation patterns (6). Mutations in the 4 ORFs are associated with liver disease. The A1762T/G1764A, T1753V, C1766T and T1768A mutations in the basal core promoter (BCP), C1653T in the enhancer region II and G1896A in the PC region are responsible for modulating the severity of the disease presentation. Specific mutations in the HBV genome were identified as predictors of LC and HCC in patients with chronic HBV infection (7,8). However, the specific mutations may differ depending on the HBV genotype (3,9).
A total of 10 HBV (A-J) genotypes have been identified worldwide (3,10). Genotypes B and C are predominant in Asia. HBV genotypes are associated with the clinical characteristics of patients (11). Genotype B is associated with spontaneous hepatitis B e-antigen (HBeAg) seroconversion at a younger age and a slower progression to cirrhosis with active liver disease compared with genotype C. A high viral load and genotype C are associated with HCC. HBeAg positivity is more frequent in patients infected with HBV genotype C compared with those infected with genotype B (12). Furthermore, subgenotypes have been identified for certain HBV genotypes. HBV subgenotypes are associated with geographical distribution and ethnic origin (10).
In Indonesia, genotype B is the predominant HBV genotype, particularly in the Western region, whereas genotype C is predominant in the Eastern region (13). Samarinda is the capital city of the East Kalimantan province. HBV infection is prevalent in the general population in West Kalimantan province, and genotype B is predominant among children in West Kotawaringin, in the Central Kalimantan province (2,14). However, there are limited data on the characteristics of HBV infection in Samarinda. The present study investigated the distribution of HBV genotypes and mutations in patients with ALD in Samarinda.
Materials and methods
Sample collection
Serum samples were collected from 41 patients with ALD at Abdul Wahab Sjahranie Hospital (Samarinda, Indonesia) from June 2012 to May 2013. A total of 23 patients with HCC (17 males and 6 females), with a mean [± standard deviation (SD)] age of 52.8±9.3 years, and 18 LC patients (15 male and 3 female, mean [±SD] age, 50.6±12.2 years) were included. Inclusion criteria were as follows: A diagnosis of HCC or LC, and provision of informed consent. LC was diagnosed by identification of abnormal liver morphology using ultrasonography (USG). HCC was diagnosed by USG indicating the presence of a tumor with α-fetoprotein (AFP) levels ≥200 ng/ml. The severity of the clinical manifestations was determined by the clinicians. Alanine aminotransferase (ALT), aspartate aminotransferase (AST) and AFP levels were obtained from patient medical records. Patients were not diagnosed with LC or HCC as part of the present study. Hepatitis B surface antigen (HBsAg)-positive serum samples were obtained from blood donors screened at the Red Cross Center, Samarinda from June to July 2012. A total of 46 HBsAg-positive serum samples (male 44 and 2 female, mean [±SD] age, 34.3±8.1 yeas) were randomly included in the present study. The inclusion criteria for the blood donors were: Positive for HBsAg and provision of informed consent. A total of ~1.5 ml of serum from each donor was provided at the Red Cross Center (Samarinda, Indonesia). The serum samples collected and stored at -20˚C in Abdul Wahab Sjahranie Hospital and the Red Cross Center, and were then placed in styrofoam boxes with frozen ice packs during the transportation to the Institute of Tropical Diseases, Airlangga University (Surabaya, Indonesia). The samples were stored at -80˚C until use in the Institute of Tropical Diseases, Airlangga University. Ethical approval was obtained from the Ethics Committees at Airlangga University, Mulawarman University (Samarinda, Indonesia) and Kobe University (Kobe, Japan). Informed consent for participation was obtained from each individual.
Serological assay
All sera from patients with ALD were screened for HBsAg by reverse passive hemagglutination (Mycell II HBsAg1AA1; Institute of Immunology, Tokyo, Japan). Serum samples from blood donors were also confirmed for HBsAg using the aforementioned kit. HBeAg status was determined using an enzyme immunoassay method (Immunis HBeAg EIA kit1A81; Institute of Immunology) for all serum samples from patients with ALD and blood donors. In this assay, absorbance of each microwell was measured at a wavelength of 450 nm. The controls for the HBsAg and HBeAg assays were included according to the manufacturer's protocol.
Viral DNA extraction and PCR amplification
Viral DNA was extracted from 200 µl serum using a QIAamp DNA Blood Mini kit (Qiagen GmbH, Hilden, Germany) according to the manufacturer's protocol. The presence of mutations within the HBV genome was detected using polymerase chain reaction (PCR) with Taq PCR master mix kit (Qiagen GmbH) targeting the ORFs of the X gene (nt. 1374-1896) including the BCP, the PC (nt. 1814-1901), and core (nt. 1901-2045). The primers used to determine the mutations and genotypes were as follows: HB5F forward (5'-CTCTGCCGATCCATACTGCGGAA-3'; nt. 1256-1278); HB5R reverse (5'-TTAACCTAATCTCCT CCCCCA-3'; nt. 1761-1741); HB7F forward (5'-GAGACCGTG AACGCCCA-3'; nt. 1611-1630); HB7R reverse (5'-CCTGAG TGCTGTATGGTGAGG-3'; nt. 2072-2048); HB11F forward (5'-GGGTCACCATATTCTTGGGAA-3'; nt. 2814-2834); HB11R reverse (5'-GAACTGGAGCCAGCAGG-3'; nt. 75-56). The thermocycling conditions for each primer pair were as follows: 94˚C for 5 min, followed by 40 cycles of 94˚C for 1 min, 55˚C for 1 min, and 72˚C for 1 min, followed by a final extension of 72˚C for 5 min (15).
Direct sequencing and phylogenetic analysis
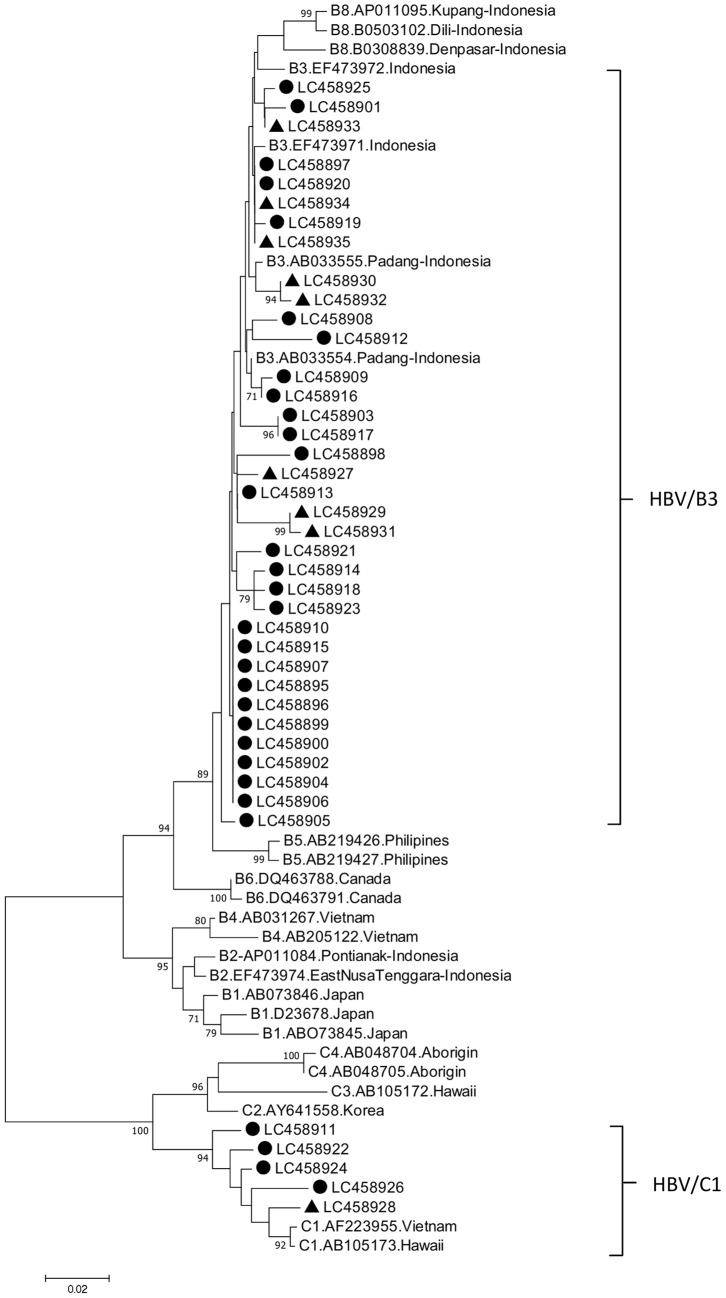
Nucleotide sequences of the amplified fragments were determined using the BigDye Deoxy Terminator v1.1 cycle sequencing kit with an ABI Prism 310 Genetic Analyzer (Applied Biosystems; Thermo Fisher Scientific, Inc., Waltham, MA, USA). All sequence data analyses were performed with Genetyx Windows v9 (Genetyx Corporation, Tokyo, Japan). The sequences obtained in the present study were aligned with those from the DNA Data Bank of Japan (DDBJ) (16), European Molecular Biology Laboratory (EMBL) (http://www.ebi.ac.uk/embl.html) and GenBank (17) DNA databases, and the variability of the X, BCP, PC and core sequences was analyzed. HBV genotypes were determined based on a percentage homology >96% between the pre-S gene and HBV isolates from the DDBJ, EMBL and GenBank databases (18) analyzed using the program Genetyx Windows v9. A phylogenetic tree was constructed by the Neighbor-Joining method to determine the HBV genotype/subgenotype, and bootstrap resampling was performed 1,000 times using Molecular Evolutionary Genetic Analysis software v7(19). Phylogenetic tree analysis with the representative samples from patients with ALD and HBV carriers was performed. The representative 32 strains from patients with ALD and 9 from the HBV carriers were included (Fig. 1). The nucleotide sequences presented in Fig. 1 have been deposited in the GenBank database under accession numbers LC4558895-LC458926 and LC458927-LC458935.
Figure 1.
Neighbor-joining phylogenetic tree of nucleotide sequences from the partial pre-S gene of HBV isolates from Samarinda. Bold circles represent patients with advanced liver disease. Bold triangles represent HBV carriers. Non-labelled strains denote the reference strains. The genotypes/subgenotypes are indicated on the right. HBV, hepatitis B virus.
Quantification of HBV DNA
Viral load was assessed for all 41 samples from the patients with ALD using a TaqMan quantitative (q)PCR method for absolute quantification using an ABI 7300 real-time PCR system (Applied Biosystems; Thermo Fisher Scientific, Inc.). Briefly, 2 µl of HBV-DNA was amplified using a TaqMan Universal PCR master mix (Applied Biosystems; Thermo Fisher Scientific, Inc.), primer pairs (SF1 forward, 5'-CACATCAGGATTCCTAGGACC-3'; nt. 166-186; SR1 reverse, 5'-GGTGAGTGATTGGAGGGTTG-3'; nt. 339-321) covering the surfaceregion of HBV genome, and TaqMan probe (HBSP1:FAM-5'-CAGAGTCTAGACTCGTGGTGGACT TC-3'-TAMRA; nt. 242-267). The thermocycling conditions were as follows: Short incubation at 50˚C for 2 min, initial denaturation at 95˚C for 10 min, then 53 cycles of denaturation at 95˚C for 20 sec and annealing-extension at 60˚C for 1 min (20).
Statistical analysis
Statistical analysis was performed using SPSS v22 (IBM Corp., Armonk, NY, USA) using the χ2 test or Fisher's exact test for categorical variables. The t-test was used for continuous variables. P<0.05 was considered to indicate a statistically significant difference.
Results
Demographic and clinical characteristics of patients with ALD
The study analyzed 41 patientswith ALD incomparison with 46 HBV carriers as controls. Demographic and clinical characteristics in patients with ALD (LC and HCC) are summarized in Table I, and those of patients with ALD and HBV carriers are described in Table II. The mean age was significantly older in patients with ALD (51.9±10.6 years) compared with that in HBV carriers (34.3±8.1 years). In the male patients and control subjects, prevalence of HBV infection was significantly increased in HBV carriers compared with that in the patients with ALD, and HBV infection was more prevalent in males in the ALD and HBV carrier control groups compared with females (Table II). The majority of the patients were natives of Kalimantan (Dayak, Banjar and Kutai ethnic groups), followed by Javanese and other ethnic groups (Buton, Sasak and Bugis). AST levels in patients with HCC (243.7±237.0 U/l) were significantly increased compared with those in the patients with LC (105.0±55.7 U/l), while ALT levels in patients with HCC (104.8±99.8 U/l) were also increased compared with those in patients with LC (73.7±75.2 U/l), but this difference was not significant (Table I).
Table I.
Demographic, virological and clinical characteristics of HBV-infected patients with HCC and LC.
| Characteristics | All | HCC | LC | P-value |
|---|---|---|---|---|
| Age, years | 51.9±10.6 | 52.8±9.3 | 50.6±12.2 | NS |
| Sex, male/female (%) | 32/41 (78.0%) | 17/23 (73.9) | 15/18 (83.3) | NS |
| Ethnic group (%) | NS | |||
| Kalimantan (Dayak, Banjar, Kutai) | 15.41 (36.6) | 9/23 (39.1) | 6/18 (33.3) | |
| Java | 11/41 (26.8) | 6/23 (8.7) | 5/18 (27.8) | |
| Others (Buton, Sasak, Bugis) | 13/41 (31.7) | 6/23 (26.1) | 7/18 (38.9) | |
| Unknown | 2/41 (4.87) | 2/23 (8.7) | 0/18 (0.0) | |
| Liver function | ||||
| ALT (U/l) | 90.5±89.5 | 104.8±99.8 | 73.7±75.2 | NS |
| AST (U/l) | 178.0±189.6 | 243.7±237.0 | 105.0±55.7 | <0.05 |
| AFP, ng/ml (%) | NS | |||
| ≥200 | 16/41 (39.0) | 11/23 (47.8) | 5/18 (27.8) | |
| ≤200 | 10/41 (24.4) | 3/23 (13.0) | 7/18 (38.9) | |
| Unknown | 15/41 (36.6) | 9/23 (39.1) | 6/18 (33.3) | |
| HBsAg (R-PHA) (%) | NS | |||
| Positive | 33/41 (80.5) | 18/23 (78.3) | 15/18 (83.3) | |
| Negative | 5/41 (12.2) | 3/23 (13.0) | 2/18 (11.1) | |
| Unknown | 3/41 (7.3) | 2/23 (8.7) | 1/18 (5.5) | |
| HBeAg (EIA) (%) | NS | |||
| Positive | 3/41 (7.3) | 2/23 (8.7) | 1/18 (5.6) | |
| Negative | 38/41 (92.7) | 21/23 (91.3) | 17/18 (94.4) | |
| Quantitative HBV DNA (log copies/ml) | 6.1±2.4 | 5.2±2.6 | 7.3±1.4 | NS |
| HBV DNA, positive samples (%) | ||||
| Pre-S region | 41/41 (100.0) | 23/23 (100.0) | 18/18 (100.0) | NS |
| X region | 38/41 (92.7) | 21/23 (91.3) | 17/18 (94.4) | NS |
| Core region | 40/41 (97.6) | 22/23 (95.6) | 18/18 (100.0) | NS |
| Subgenotype (Pre-S) (%) | NS | |||
| B3 | 35/41 (85.4) | 21/23 (91.3) | 14/18 (77.8) | |
| C1 | 6/41 (14.6) | 2/23 (8.7) | 4/18 (22.2) |
Data concerning age, ALT and AST levels are presented as mean ± standard deviation. HBV, hepatitis B virus; HCC, hepatocellular carcinoma; LC, liver cirrhosis; AFP, α-fetoprotein; ALT, alanine transferase; AST, aspartate transaminase; HBsAg, Hepatitis B surface antigen; R-PHA, reversed passive hemagglutination; HBeAg, hepatitis B e-antigen; EIA, enzyme immunoassay; NS, not significant. Statistical analysis was performed using χ2 test or Fisher's exact test for categorical variables. t-tests were used for continuous variables.
Table II.
Demographic and virological characteristics of patients with ALD and HBV carriers.
| Characteristics | ALD | HBV carriers | P-value |
|---|---|---|---|
| Age, years | 52.8±9.3 | 50.6±12.2 | <0.05 |
| Sex, male/female (%) | 17/23 (73.9) | 15/18 (83.3) | <0.05 |
| Ethnic group (%) | NS | ||
| Kalimantan (Dayak, Banjar, Kutai) | 15/41 (36.6) | 18/46 (39.1) | |
| Java | 11/41 (26.8) | 15/46 (32.6) | |
| Others (Buton, Sasak, Bugis) | 13/41 (31.7) | 11/46 (23.9) | |
| Unknown | 2/41 (4.9) | 2/46 (4.3) | |
| HBeAg (EIA) (%) | NS | ||
| Positive | 3/41 (7.3) | 9/45 (5.6) | |
| Negative | 38/41 (92.7) | 36/45 (94.4) | |
| HBV DNA, positive samples (%) | NS | ||
| Pre-S region | 41/41 (100.0) | 46/46 (100.0) | |
| X region | 38/41 (91.3) | 46/46 (100.0) | |
| Core region | 40/41 (97.6) | 46/46 (100.0) | |
| Subgenotype (Pre-S) (%) | <0.05 | ||
| B3 | 35/41 (85.4) | 45/46 (97.8) | |
| C1 | 6/41 (14.6) | 1/46 (2.2) |
Data concerning age are presented as mean ± standard deviation. HBV, hepatitis B virus; ALD, advanced liver disease; HBeAg, hepatitis B e-antigen; EIA, enzyme immunoassay; NS, not significant; R-PHA, reversed passive hemagglutination. Statistical analysis was performed using χ2 test or Fisher's exact test for categorical variables. t-tests were used for continuous variables.
Phylogenetic analysis
Phylogenetic analysis in Pre-S1 gene of HBV strains was performed. The HBV/B3 (85.4%) and HBV/C1 (14.6%) subgenotypes were identified in patients with ALD, and HBV/B3 (97.8%) and HBV/C1 (2.2%) subgenotypes were identified in HBV carriers. The HBV/C1 subgenotype was significantly more prevalent in patients with ALD than that in HBV carriers in East Kalimantan, Indonesia.
Mutations in the X, BCP, PC, and core regions of patients with ALD
The following mutations were detected in the HBV isolates from patients with ALD: C1505A, T1631C, C1638T and C1726A in the X region; T1753V, A1762T/G1764A double mutation and T1768A in the BCP; C1858T, G1896A and G1899A in the PC; and G1915T in the core region. Compared with HBV carriers, patients with ALD exhibited significantly increased incidence rates of HBV mutation in the BCP region: double mutation A1762T/G1764A and T1753V, in PC region: C1858T and in X region: C1505A. The G1896A mutation was significantly more prevalent in HBV carriers compared with patients with ALD (Table III).
Table III.
HBV mutations in patients with ALD and HBV carriers.
| Mutations | ALD (%) | HBV carriers (%) | P-value |
|---|---|---|---|
| X region | |||
| C1495T | 0/38 (0.0) | 0/46 (0.0) | NS |
| C1505A | 11/38 (28.9) | 0/46 (0.0) | <0.05 |
| T1631C | 21/38 (55.3) | 22/46 (47.8) | NS |
| C1638T | 17/38 (44.7) | 13/46 (28.3) | NS |
| C1726A | 5/36 (13.9) | 11/46 (23.9) | NS |
| BCP region | |||
| T1753V | 14/35(40) | 0/46 (0.0) | <0.05 |
| A1762T/G1764A | 25/34 (73.5) | 0/46 (0.0) | <0.05 |
| T1768A | 0/34 (0.0) | 5/46 (10.9) | NS |
| PC region | |||
| C1858T | 6/34 (17.6) | 0/46 (0.0) | <0.05 |
| G1896A | 12/34 (35.3) | 29/46 (63.0) | <0.05 |
| G1899A | 11/34 (32.3) | 6/46 (13.0) | NS |
| Core region | |||
| G1915T | 1/33 (3.0) | 0/46 (0.0) | NS |
NS, not significant; BCP, basal core promoter; PC, precore. Statistical analysis was performed using χ2 test or Fisher's exact test for categorical variables. Out of 41 patients with advanced liver disease, the total number in each gene region indicates the number of patients whose strains were successfully sequenced.
Association between mutations and HBV genotype in patients with ALD and HBV carriers
The association between mutations in the X, BCP, PC and core regions and genotypes of patients with ALD was analyzed (Table IV). Mutations in the X region were detected only in HBV/B subgenotypes. In particular, T1631C and C1638T mutations were significantly more prevalent in the HBV/B genotypes compared with the HBV/C genotypes. The prevalence of C1858T mutation was significantly increased in the HBV/C genotypes compared with the HBV/B genotypes. A1762T/G1764A mutations in the BCP region were frequently detected in HBV/B (67.8%) and HBV/C (100%) genotypes.
Table IV.
HBV mutations in patients with advanced liver disease according to genotype.
| Mutations | Genotype B (%) | Genotype C (%) | P-value |
|---|---|---|---|
| X region | |||
| C1495T | 0/32 (0.0) | 0/6 (0.0) | NS |
| C1505A | 11/32 (34.4) | 0/6 (0.0) | NS |
| T1631C | 21/32 (65.6) | 0/6 (0.0) | <0.05 |
| C1638T | 17/32 (53.1) | 0/6 (0.0) | <0.05 |
| C1726A | 5/30 (16.7) | 0/6 (0.0) | NS |
| BCP region | |||
| T1753CV | 11/29 (37.9) | 3/6 (50.0) | NS |
| A1762T/G1764A | 19/28 (67.8) | 6/6 (100.0) | NS |
| T1768A | 0/28 (0.0) | 0/6 (0.0) | NS |
| PC region | |||
| C1858T | 0/28 (0.0) | 6/6 (100.0) | <0.05 |
| G1896A | 10/28 (35.7) | 2/6 (33.3) | NS |
| G1899A | 6/28 (21.4) | 5/6 (83.3) | NS |
| Core region | |||
| G1915T | 1/27 (3.7) | 0/6 (0.0) | NS |
HBV, hepatitis B virus; NS, not significant; BCP, basal core promoter; PC, precore. Statistical analysis was performed using χ2 test or Fisher's exact test for categorical variables. Out of the 35 patients with Genotype B, the total number in each region indicates the number of patients whose strains were successfully sequenced.
Discussion
The present study enrolled 41 patients from East Kalimantan, including patients with LC and HCC, and 46 HBV carriers as controls. The transmigration problem in Indonesia, which involves the migration of millions of people from the overcrowded islands, primarily Java, to settlement areas (21), has resulted in a number of transmigrants living in the outer islands, including Kalimantan. Therefore, among the 41 patients with ALD and 46 HBV carriers, only 36.6 and 39.1%, respectively, belonged to the native population of Kalimantan. The remaining patients belonged to other ethnic groups, including Javanese, Buton, Sasak and Bugis. The average age among patients with ALD was significantly older compared with the HBV carriers. This observation is consistent with our previous study conducted in Yogyakarta, which demonstrated that mean age among patients with ALD was older (54 years) compared with patients with chronic HBV infection (38 years) (7). An additional study in Vietnam also revealed similar results, in that the average age of patients with HCC (49.5 years) and LC (50.9 years) was older compared with HBV carriers (22.3 years). Older age was one of independent risk factor for the development of HCC (22).
Viral hepatitis infection may account for a marked increase in aminotransferase levels. The increase in ALT associated with hepatitis C infection is generally more marked compared with that associated with hepatitis B (23). Despite the association between elevated ALT levels and hepatocellular disease, the absolute peak of the ALT increase is not correlated with the extent of liver cell damage (24). In the present study, AST levels were high in patients with LC and HCC, and no significant difference between the two groups of patients was observed, whereas AST levels were increased in patients with HCC compared with patients with LC. AST and ALT are hepatocyte-predominant enzymes (25); it has been established that AST is more abundant in the liver and that increases in AST levels are more sensitive to rates of enzymatic clearance and mitochondrial injury compared with ALT (26). A number of previous studies revealed that the AST/ALT ratio is frequently used for the assessment of hepatic fibrosis and the detection for HCC recurrence (27-29), which supports the results of the present study.
The present study analyzed patients with ALD infected with HBV and HBV carriers to investigate HBV genotypes and mutations associated the severity of the liver disease. Consistent with previous studies (8,22,30,31), mutations in the BCP region, particularly the double mutation A1762T/G1764A and T1753V, were associated with the risk of ALD in the present study. Similar results were described in a previous study in Brazil, in which the 2 mutations in the BCP region (combination double mutation A1762T/G1764A and T1753V) exhibited higher frequencies in patients with HCC compared with patients without HCC, even though difference was not significant (32). The mutation C1505A in the X region was more frequently detected in ALD compared with chronic hepatitis B, even though this comparison was not significantly different in our study, and as previously demonstrated (7). In addition, the A1762T/G1764A double mutation, T1753V, C1505A, and C1858T were not identified in HBV carriers in the present study. The C1858T mutation is commonly identified in Asia (9), and more frequent in patients with HCC than in patients with chronic hepatitis B in Vietnam (22). However, a previous study conducted in China indicated that there was no significant difference in the C1858T mutation between HBV carriers and patients with HBV liver diseases (22,33). The G1896A mutation was more frequent in HBV carriers compared with patients with ALD in the present study, which is consistent with a previous study conducted in Malaysia (9). A similar result was also revealed in a study from Korea, in which the rates of G1896A mutation were not significantly different between HCC and non-HCC groups (34). However, a Brazilian study suggested that the rate of G1896A mutation was identified to be increased in patients without HCC compared with patients with HCC, although this difference was not significant (31). By contrast, the G1896A mutation in the PC region is one of the most commonly identified mutation in ALD and chronic HBV infection (7,9,34).
The A1762T/G1764A double mutation in the BCP region, which is responsible for decreased PC mRNA synthesis, results in the decreased secretion of HBeAg and serves a significant role in hepatocarcinogenesis, primarily in patients with HBeAg-negative hepatitis (34). Previous studies have demonstrated that A1762T/G1764A mutations decrease HBeAg production to ~70% (30,31). The C1858T mutation decreases the synthesis of HBeAg and is a precursor of the G1896A mutation. The C1858T and G1896A mutations stabilize the structure of the epsilon loop, which may result in increased viral replication (35). In the present study, the majority of patients with ALD were HBeAg-negative, which may be associated with the predominance of double mutations in the BCP region. However, this was not confirmed as the majority of HBV carriers were also HBeAg-negative in the study cohort. The G1896A mutation produces a premature stop codon and decreases the synthesis of HBeAg. This mutation serves a role in preventing the translation of HBeAg and inhibits the production of HBeAg. The presence of a stop codon mutation maybe a mechanism of immune evasion (9,23). The predominance of the G1896A mutation in the PC region observed in the present study may also be associated with the high prevalence of patients who were HBeAg-negative.
In the present study, the two patient groups demonstrated that the predominant HBV genotype/subgenotype was B3, followed by C1, which was consistent with previous studies from Indonesia revealing that the B and C genotypes are predominant in this nation (13,18). The G1896A mutation was more frequent in genotype B compared with genotype C among patients with asymptomatic chronic HBV infection (9). A previous study from China indicated that the double mutation in BCP region was detected with ALD in genotypes B and C (30). The risk of ALD is associated with certain HBV genotypes, and the prevalence of HBV mutations in certain HBV genotypes (31).
In conclusion, the analysis of patients from East Kalimantan demonstrated that the HBV genotype B was predominant, followed by the genotype C. Several mutations were detected in patients with ALD, including C1505A in the X region, T1753V and A1762T/G1764A in the BCP region and C1858T in the PC region. Mutations in the X gene were only detected in patients with genotype B, and C1858T mutation in the PC was only detected in patients with genotype C. A limitation of the present study was the small number of samples analysed; additional studies with a larger sample cohort are required to confirm the results suggesting that viral factors affect the development of ALD.
Acknowledgements
Not applicable.
Funding
The present study was supported by a Grant-in-Aid from the Ministry of Education, Culture, Sports, Science and Technology, Japan (grant no. 16H05826) and a Grant-in-Aid from the Japan Initiative for Global Research Network on Infectious Disease supported by The Ministry of Education, Culture, Sports, Science and Technology, Japan, and in part by a Grant-in-Aid from Professor Dato' Sri Tahir through Tahir professorship, Indonesia.
Availability of data and materials
All data generated or analyzed in the present study are included in this published article.
Authors' contributions
The study was conducted and designed by RMW and TU. ISM, RMW, PBP, AI and MA performed the sample collection and laboratory experiments. RMW, TU, J, and LNY were responsible for the analysis and interpretation of the data. RMW and J wrote the manuscript. MIL, S, YY, YH, TU, and J reviewed and edited the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Ethical approval was obtained from the Ethics Committees at Airlangga University (Samarinda, Indonesia), Mulawarman University (Samarinda, Indonesia) and Kobe University (Kobe, Japan). Informed consent for participation was obtained from each individual.
Patient consent for publication
All patients agreed to the publication of any relevant data on the basis of anonymization of all personal data.
Competing interest
The authors declare that they have no competing interests.
References
- 1.Khan M, Dong JJ, Acharya SK, Dhagwahdorj Y, Abbas Z, Jafri SMW, Mulyono DH, Tozun N, Sarin SK. Hepatology issues in Asia: Perspective from regional leaders. J Gastroenterol Hepatol. 2004;19 (Suppl 7):S419–S430. doi: 10.1111/j.1440-1746.2004.03728.x. [DOI] [Google Scholar]
- 2.Purwono PB, Juniastuti Amin M, Bramanthi R, Nursidah Resi EM, Wahyuni RM, Yano Y, Soetjipto Hotta H, et al. Hepatitis B virus infection in Indonesia 15 years after adoption of a universal infant vaccination program: Possible impacts of low birth dose coverage and a vaccine-escape mutant. Am J Trop Med Hyg. 2016;95:674–679. doi: 10.4269/ajtmh.15-0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lindh M, Andersson AS, Gusdal A. Genotypes, nt 1858 variants, and geographic origin of hepatitis B virus - large-scale analysis using a new genotyping method. J Infect Dis. 1997;175:1285–1293. doi: 10.1086/516458. [DOI] [PubMed] [Google Scholar]
- 4.Fattovich G, Bortolotti F, Donato F. Natural history of chronic hepatitis B: Special emphasis on disease progression and prognostic factors. J Hepatol. 2008;48:335–352. doi: 10.1016/j.jhep.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 5.Nishida N, Tokunaga K, Mizokami M. Genome-Wide Association Study Reveals Host Genetic Factors for Liver Diseases. J Clin Transl Hepatol. 2013;1:45–50. doi: 10.14218/JCTH.2013.010XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mizokami M, Orito E, Ohba K, Ikeo K, Lau JY, Gojobori T. Constrained evolution with respect to gene overlap of hepatitis B virus. J Mol Evol. 1997;44 (Suppl 1):S83–S90. doi: 10.1007/pl00000061. [DOI] [PubMed] [Google Scholar]
- 7.Heriyanto DS, Yano Y, Utsumi T, Anggorowati N, Rinonce HT, Lusida MI, et al. Mutations Within Enhancer II and BCP Regions of Hepatitis B Virus in Relation to ALDs in Patients Infected With Subgenotype B3 in Indonesia. J Med Virol. 2012;84:44–51. doi: 10.1002/jmv.22266. [DOI] [PubMed] [Google Scholar]
- 8.Zhang X, Ding HG. Key role of hepatitis B virus mutation in chronic hepatitis B development to hepatocellular carcinoma. World J Hepatol. 2015;7:1282–1286. doi: 10.4254/wjh.v7.i9.1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suppiah J, Mohd Zain R, Bahari N, Haji Nawi S, Saat Z. G1896A Precore mutation and association with HBeAg status, genotype and clinical status in patients with chronic hepatitis B. Hepat Mon. 2015;15e31490 doi: 10.5812/hepatmon.31490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Utsumi T, Yano Y, Hotta H. Molecular epidemiology of hepatitis B virus in Asia. World J Med Genet. 2014;4:19–26. doi: 10.1046/j.1442-200x.2004.01867.x. [DOI] [PubMed] [Google Scholar]
- 11.Sunbul M. Hepatitis B virus genotypes: Global distribution and clinical importance. World J Gastroenterol. 2014;20:5427–5434. doi: 10.3748/wjg.v20.i18.5427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang Y, Lok AS. Viral factors and outcomes of chronic HBV infection. Am J Gastroenterol. 2011;106:93–95. doi: 10.1038/ajg.2010.404. [DOI] [PubMed] [Google Scholar]
- 13.Mulyanto Depamede SN, Surayah K, Tsuda F, Ichiyama K, Takahashi M, Okamoto H. A nationwide molecular epidemiological study on hepatitis B virus in Indonesia: Identification of two novel subgenotypes, B8 and C7. Arch Virol. 2009;154:1047–1059. doi: 10.1007/s00705-009-0406-9. [DOI] [PubMed] [Google Scholar]
- 14.Darmawan E, Turyadi KE, El-Khobar KE, Nursanty NK, Thedja MD, Muljono DH. Seroepidemiology and occult hepatitis B virus infection in young adults in Banjarmasin, Indonesia. J Med Virol. 2015;87:199–207. doi: 10.1002/jmv.24045. [DOI] [PubMed] [Google Scholar]
- 15.Sugauchi F, Mizokami M, Orito E, Ohno T, Kato H, Suzuki S, Kimura Y, Ueda R, Butterworth LA, Cooksley WG. A novel variant genotype C of hepatitis B virus identified in isolates from Australian Aborigines: Complete genome sequence and phylogenetic relatedness. J Gen Virol. 2001;82:883–892. doi: 10.1099/0022-1317-82-4-883. [DOI] [PubMed] [Google Scholar]
- 16.Tateno Y, Imanishi T, Miyazaki S, Fukami-Kobayashi K, Saitou N, Sugawara H, Gojobori T. DNA Data Bank of Japan (DDBJ) for genome scale research in life science. Nucleic Acids Res. 2002;30:27–30. doi: 10.1093/nar/30.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Benson DA, Cavanaugh M, Clark K, Karsch-Mizrachi I, Lipman DJ, Ostell J, Sayers EW. GenBank. Nucleic Acids Res. 2013;41 (D1):D36–D42. doi: 10.1093/nar/gks1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lusida MI, Nugrahaputra VE, Soetjipto Handajani R, Nagano-Fujii M, Sasayama M, Utsumi T, Hotta H. Novel subgenotypes of hepatitis B virus genotypes C and D in Papua, Indonesia. J Clin Microbiol. 2008;46:2160–2166. doi: 10.1128/JCM.01681-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumar S, Stecher G, Tamura K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol Biol Evol. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abe A, Inoue K, Tanaka T, Kato J, Kajiyama N, Kawaguchi R, Tanaka S, Yoshiba M, Kohara M. Quantitation of hepatitis B virus genomic DNA by real-time detection PCR. J Clin Microbiol. 1999;37:2899–2903. doi: 10.1128/jcm.37.9.2899-2903.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fearnside PM. Transmigration in Indonesia: Lessons from its environmental and social impacts. Environ Manage. 1997;21:553–570. doi: 10.1007/s002679900049. [DOI] [Google Scholar]
- 22.Truong BX, Seo Y, Yano Y, Ho PTT, Phuong TM, Long DV, Son NT, Long NC, Kato H, Hayashi Y, et al. Genotype and variations in core promoter and pre-core regions are related to progression of disease in HBV-infected patients from Northern Vietnam. Int J Mol Med. 2007;19:293–299. [PubMed] [Google Scholar]
- 23.Marcellin P. Hepatitis C: The clinical spectrum of the disease. J Hepatol. 1999;31 (Suppl 1):9–16. doi: 10.1016/s0168-8278(99)80368-7. [DOI] [PubMed] [Google Scholar]
- 24.Gowda S, Desai PB, Hull VV, Math AAK, Vernekar SN, Kulkarni SS. A review on laboratory liver function tests. Pan Afr Med J. 2009;3(17) doi: 10.11604/pamj.2009.3.17.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giannini EG, Testa R, Savarino V. Liver enzyme alteration: A guide for clinicians. CMAJ. 2005;172:367–379. doi: 10.1503/cmaj.1040752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Okuda M, Li K, Beard MR, Showalter LA, Scholle F, Lemon SM, Weinman SA. Mitochondrial injury, oxidative stress, and antioxidant gene expression are induced by hepatitis C virus core protein. Gastroenterology. 2002;122:366–375. doi: 10.1053/gast.2002.30983. [DOI] [PubMed] [Google Scholar]
- 27.Giannini E, Botta F, Fasoli A, Ceppa P, Risso D, Lantieri PB, Celle G, Testa R. Progressive liver functional impairment is associated with an increase in AST/ALT ratio. Dig Dis Sci. 1999;44:1249–1253. doi: 10.1023/a:1026609231094. [DOI] [PubMed] [Google Scholar]
- 28.Giannini E, Risso D, Botta F, Chiarbonello B, Fasoli A, Malfatti F, Romagnoli P, Testa E, Ceppa P, Testa R. Validity and clinical utility of the aspartate aminotransferase-alanine aminotransferase ratio in assessing disease severity and prognosis in patients with hepatitis C virus-related chronic liver disease. Arch Intern Med. 2003;163:218–224. doi: 10.1001/archinte.163.2.218. [DOI] [PubMed] [Google Scholar]
- 29.Wang ZX, Jiang CP, Cao Y, Zhang G, Chen WB, Ding YT. Preoperative serum liver enzyme markers for predicting early recurrence after curative resection of hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 2015;14:178–185. doi: 10.1016/S1499-3872(15)60353-8. [DOI] [PubMed] [Google Scholar]
- 30.Chen CH, Lee CM, Lu SN, Changchien CS, Eng HL, Huang CM, Wang JH, Hung CH, Hu TH. Clinical significance of hepatitis B virus (HBV) genotypes and precore and core promoter mutations affecting HBV e antigen expression in Taiwan. J Clin Microbiol. 2005;43:6000–6006. doi: 10.1128/JCM.43.12.6000-6006.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim JK, Chang HY, Lee JM, Baatarkhuu O, Yoon YJ, Park JY, Kim DY, Han KH, Chon CY, Ahn SH. Specific mutations in the enhancer II/core promoter/precore regions of hepatitis B virus subgenotype C2 in Korean patients with hepatocellular carcinoma. J Med Virol. 2009;81:1002–1008. doi: 10.1002/jmv.21501. [DOI] [PubMed] [Google Scholar]
- 32.Araujo OC, Barros JJ, do Ó KM, Nabuco LC, Luz CA, Perez RM, Niel C, Villela-Nogueira CA, Araujo NM. Genetic variability of hepatitis B and C viruses in Brazilian patients with and without hepatocelullar carcinoma. J Med Virol. 2014;86:217–223. doi: 10.1002/jmv.23837. [DOI] [PubMed] [Google Scholar]
- 33.Yuan J, Zhou B, Tanaka Y, Kurbanov F, Orito E, Gong Z, Xu L, Lu J, Jiang X, Lai W, et al. Hepatitis B virus (HBV) genotypes/subgenotypes in China: Mutations in core promoter and precore/core and their clinical implications. J Clin Virol. 2007;39:87–93. doi: 10.1016/j.jcv.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 34.Lee JH, Hong SP, Jang ES, Park SJ, Hwang SG, Kang SK, Jeong SH. Analysis of HBV genotype, drug resistant mutations, and pre-core/basal core promoter mutations in Korean patients with acute hepatitis B. J Med Virol. 2015;87:993–998. doi: 10.1002/jmv.24148. [DOI] [PubMed] [Google Scholar]
- 35.Lok AS, Akarca U, Greene S. Mutations in the pre-core region of hepatitis B virus serve to enhance the stability of the secondary structure of the pre-genome encapsidation signal. Proc Natl Acad Sci USA. 1994;91:4077–4081. doi: 10.1073/pnas.91.9.4077. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed in the present study are included in this published article.