Abstract
Objective:
Drug craving serves as the major motivator to propagate drug use and is thought to elicit relapse in abstinent individuals. Although craving for methamphetamine has been investigated using both laboratory and neuroimaging methodologies, the relationship between drug-induced craving and neural responses to methamphetamine cues has yet to be explored. Therefore, the present study investigated whether methamphetamine-induced craving responses in the laboratory were associated with neural response to methamphetamine cues.
Method:
Non–treatment-seeking individuals with methamphetamine use disorder (n = 15) completed two sessions, one in the laboratory where they underwent a methamphetamine infusion, and one in the magnetic resonance imaging scanner where they viewed methamphetamine cues.
Participants
reported their craving for methamphetamine over the course of the laboratory session. Analyses examined the association between peak ratings of methamphetamine-induced craving and neural activation to methamphetamine cues.
Results:
In individuals with a methamphetamine use disorder, methamphetamine-induced craving was positively associated with neural methamphetamine cue reactivity in the precuneus, putamen, and ventromedial prefrontal cortex (Z > 2.3, p < .05).
Conclusions:
There is a shared neurobiology underlying cue- and drug-induced craving in individuals with methamphetamine use disorder. Treatments that disrupt this circuitry may decrease craving and help prevent relapse.
Drug craving, the subjective experience of wanting a drug, is a core feature of substance use disorders. Craving serves as the major motivator to propagate drug use and is thought to elicit relapse in abstinent individuals (Robinson & Berridge, 1993; Sinha & Li, 2007; Weiss, 2005). Recently, the criteria for substance use disorder according to the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5; American Psychiatric Association [APA], 2013), were revised to include craving as a symptom, whereby craving is defined as a strong desire or urge to use drugs (APA, 2013; Hasin et al., 2013). This addition increases consistency between the DSM-5 and the International Statistical Classification of Diseases and Related Health Problems, 10th Revision (ICD-10; World Health Organization, 1992), which includes craving in its substance dependence criteria. Further, the addition of the craving symptom to DSM-5 is supported by evidence from behavioral, neuroimaging, pharmacology, and genetics studies, which highlight the importance of craving in maintaining drug addiction (Olney et al., 2018; Robinson & Berridge, 1993). Importantly for methamphetamine use disorder (MUD), craving is persistent, reported to last at least 5 weeks into abstinence (Galloway et al., 2009; Hartz et al., 2001; Zorick et al., 2011), which renders methamphetamine users particularly vulnerable to relapse during this period of early recovery. Furthermore, thoughts and beliefs about craving are predictive of relapse in methamphetamine users, such that individuals who endorse more negative thoughts and beliefs about craving are at an increased risk of relapsing (Lee et al., 2010). Thus, reduction of craving has been highlighted as a common goal in the treatment of substance use disorders (Tiffany et al., 2012).
The craving state has been linked to mesolimbic and glutamatergic neuroadaptations associated with chronic drug use (Kalivas & Volkow, 2005). In individuals with MUD, methamphetamine cue exposure is associated with increased activity in mesocorticolimbic circuitry (Courtney et al., 2016; Huang et al., 2018; Malcolm et al., 2016), and self-reported levels of craving are positively correlated with precuneus and medial prefrontal cortex activation during exposure to methamphetamine cues (Courtney et al., 2016; Huang et al., 2018). Although no studies have examined neural response during methamphetamine administration in a methamphetamine-using population, similar studies have been conducted with other stimulant-using populations. Cocaine infusions administered in cocaine-dependent individuals also result in increased mesocorticolimbic activation, which positively correlates with subjective craving ratings (Breiter et al., 1997; Risinger et al., 2005). Intriguingly, cue-induced craving can be non-invasively modulated by modifying excitability in the prefrontal cortex through brain stimulation techniques, further implicating this circuitry in the processing of subjective craving (Li et al., 2013; Shahbabaie et al., 2014).
In the laboratory, exposure to both visual and tactile methamphetamine cues and intravenous (IV) methamphetamine induces craving responses in methamphetamine users (Newton et al., 2005, 2008; Tolliver et al., 2010; Wang et al., 2013). Methamphetamine craving induced by exposure to audio-script and physical paraphernalia cues is positively associated with ratings of subjective craving after an IV methamphetamine challenge, which suggests that there may be shared neurocircuitry underlying cue-induced and drug-induced responses to methamphetamine (Roche et al., 2017). However, the relationship between methamphetamineinduced craving responses in the laboratory setting and neural response to methamphetamine cues has yet to be explored. Therefore, the primary objective of this secondary analysis was to integrate human laboratory and neuroimaging methodologies to assess if methamphetamine-induced craving responses were associated with neural responses to methamphetamine cues. We also sought to assess the specificity of this association by evaluating the association of the subjective responses to methamphetamine with neural methamphetamine cue-reactivity.
Method
The study protocol and all procedures were approved by the Institutional Review Board of the University of California, Los Angeles. Detailed methodology of the general screening and experimental procedures has been published elsewhere (Courtney et al., 2016; Ray et al., 2015) and is summarized here. Interested participants completed a telephone screen and eligible participants were scheduled for an in-person screening session, during which participants gave informed consent. During the screen, participants completed a psychiatric diagnostic interview and a battery of individual difference measures, including demographic questionnaires and drug use assessments. A physical examination was performed to ensure medical eligibility.
Sixteen medically eligible, non–treatment-seeking individuals with current methamphetamine abuse or dependence completed two, 5-day inpatient protocols investigating the effects of a medication (naltrexone) on subjective response to methamphetamine administration (Ray et al., 2015) and on neural substrates of methamphetamine cue-reactivity (Courtney et al., 2016). Data collected while participants were under placebo were used for the present study (see Supplemental Material for analyses from the naltrexone condition). (Supplemental material appears as an online-only addendum to the article on the journal’s website.) Participants were required to test negative on a urine drug test (except for marijuana, which was allowed to be positive). Participants were scanned on Day 3 of the 5-day inpatient protocol. On Day 4, participants completed an IV methamphetamine challenge.
Neuroimaging procedures
Neuroimaging data were acquired on a 3 Tesla Siemens Trio scanner at the UCLA Staglin Center for Cognitive Neuroscience. Detailed neuroimaging parameters can be found in Courtney et al. (2016). Smokers were offered time to smoke a cigarette before the scan. Briefly, the protocol consisted of a high-resolution, matched-bandwidth (MBW) scan and a structural magnetization-prepared rapid-acquisition gradient echo (MPRAGE) scan. This was followed by two runs of the Methamphetamine Cues Task, which included four blocks of methamphetamine cue pictures, consisting of pictures of the drug, drug pipes, and drug use, and four blocks of control cue pictures, consisting of matched neutral pictures, presented pseudorandomly. Four pictures were presented during each block for 5 seconds each, resulting in a total of 32 pictures for each condition. Participants were instructed to press a button during each picture presentation to ensure attention during the task.
One subject was excluded from all analyses because of excessive motion during the functional magnetic resonance imaging (fMRI; exceeding 3 mm translation), leaving a final sample of 15 individuals who completed both neuroimaging and the IV methamphetamine challenge. fMRI data processing was carried out using FEAT (FMRI Expert Analysis Tool) Version 6.00, part of FSL (FMRIB’s Software Library, www.fmrib.ox.ac.uk/fsl). fMRI data were motion corrected, brain extracted, smoothed at a FWHM Gaussian kernel of 5 mm, and high-pass filtered using a 100-second cutoff. Echoplanar imaging images were first registered to the MBW, then to the MPRAGE using affine transformations, and then to standard Montreal Neurological Institute space.
Methamphetamine administration procedures
Participants completed an IV methamphetamine challenge consisting of an infusion of 30-mg methamphetamine, which was administered in two 15-mg doses, separated by 30 minutes (Ray et al., 2015). IV administration was used to provide increased dosing precision and to reduce intersubject variability in subjective responses to methamphetamine. Assessments of subjective responses to methamphetamine were collected at 5, 10, 15, 20, 30, 60, 90, and 120 minutes after the second 15-mg dose. Subjective response was measured using the Drug Effects Questionnaire, an 11-item questionnaire that captures subjective drug effects (Morean et al., 2013). Participants were asked to rate their current feelings on a Likert scale ranging from 0 (none at all) to 10 (a lot) to questions such as, “How much do you crave more of the drug right now?” (later referred to as “craving”), “How high are you?” (later referred to as “high”), and “How bad are the drug effects you are feeling now?” (later referred to as “bad”). The primary subjective response of interest was craving, collected at the peak response to methamphetamine, averaged across subjects and baseline corrected. This is consistent with previous work demonstrating a sharp peak response to methamphetamine, typically around 15 minutes after infusion (Harris et al., 2003). Subjective responses of “high” and “bad” were included to evaluate the specificity of effects to craving, as informed by our previous work demonstrating that these dimensions of subjective response were relatively insensitive to methamphetamine craving (Roche et al., 2017). These subjective responses were also selected because we hypothesized that they would not be strongly intercorrelated, allowing for the evaluation of different components of the subjective response to methamphetamine.
Statistical analysis
Whole-brain statistical analysis was performed in FSL. The primary contrast of interest, the Methamphetamine Cue > Control Cue contrast, was defined in the first-level models. The second-level model combined the contrast images across the two runs, within subjects. The third-level model combined the contrast images between subjects. The subjective response correlational analyses (“craving,” “high,” “bad”) were conducted within a single analysis on the Methamphetamine Cue > Control Cue contrast images. Age, sex, and smoking status were included as covariates of no interest. Z-statistic images were thresholded using a cluster threshold of Z > 2.3 and a (corrected) cluster significance threshold of p = .05 (Worsley, 2001).
Results
Fifteen individuals (80% male; Mage = 36.6 [SD = 8.82]; Myears of education = 13.07 [SD = 3.70]; ethnicity = 4 White, 4 African American, 6 Latino, 1 Asian) completed the IV methamphetamine challenge and neuroimaging protocol, and provided usable subjective data for analysis. All participants met DSM-IV (dependence/abuse: 13/2) and DSM-5 criteria for current MUD (mild/moderate/severe: 5/4/6). Participants had used methamphetamine for an average of 10.33 years (SD = 8.39; range: 1–31) and were abstinent for an average of 9.58 days (SD = 6.58 days; range: 1–19) before the inpatient visit. Fourteen individuals reported smoking methamphetamine as their primary route of administration; one individual reported snorting as his/her primary route of administration. Ten individuals (66.67%) were current smokers. Smoking participants smoked their last cigarette on average 7.73 hours (SD = 21.42 hours; range: 10–4,320 minutes, Mdn = 32.5 minutes) before the scan.
Subjective response to methamphetamine
Participants’ average “craving” rating was 3.53 (SD = 3.52, range: 0–10); the average rating of “high” was 5.8 (SD = 3.53, range: 0–10); and average rating of “bad” was 0.53 (SD = 1.81, range: 0–7; all scores corrected for baseline ratings). As we hypothesized, responses of “craving,” “high,” and “bad” were not correlated with each other (all rs < .19, ps > .51).
fMRI results
Consistent with previous studies evaluating neural methamphetamine cue-reactivity (Courtney et al., 2016; Malcolm et al., 2016), the Methamphetamine Cue > Control Cue contrast activated a widespread set of regions, including mesocorticolimbic regions, such as the ventral and dorsal striatum, and ventromedial prefrontal cortex (vmPFC). Additional areas showing higher activation to methamphetamine cues include the precuneus, insula, anterior and posterior cingulate, and occipital lobe (all Zs > 2.3, p < .05).
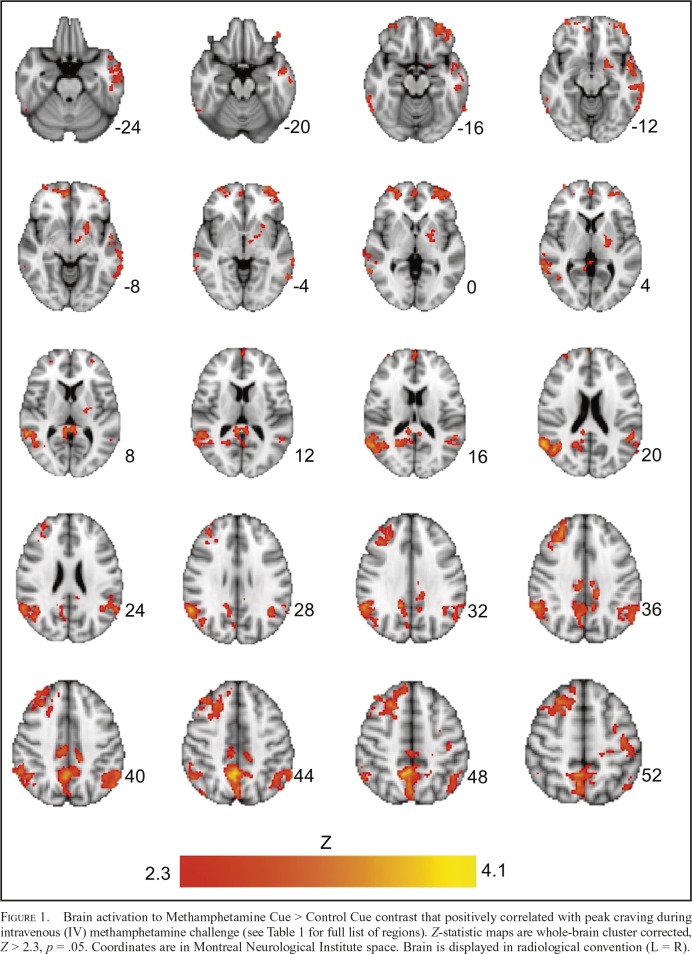
To test the primary hypothesis that methamphetamineinduced craving is associated with neural methamphetamine cue-reactivity, we examined the correlation between peak ratings of craving with in-scanner cue-reactivity. Baselinecorrected peak ratings of craving were found to positively correlate with neural methamphetamine cue reactivity in several regions, including the precuneus, vmPFC, and the putamen (Figure 1, Table 1). There were no significant negative associations between craving ratings and neural cuereactivity. There were also no significant positive or negative associations between “high” or “bad” responses and neural cue reactivity.
Figure 1.
Brain activation to Methamphetamine Cue > Control Cue contrast that positively correlated with peak craving during intravenous (IV) methamphetamine challenge (see Table 1 for full list of regions). Z-statistic maps are whole-brain cluster corrected, Z > 2.3, p = .05. Coordinates are in Montreal Neurological Institute space. Brain is displayed in radiological convention (L = R).
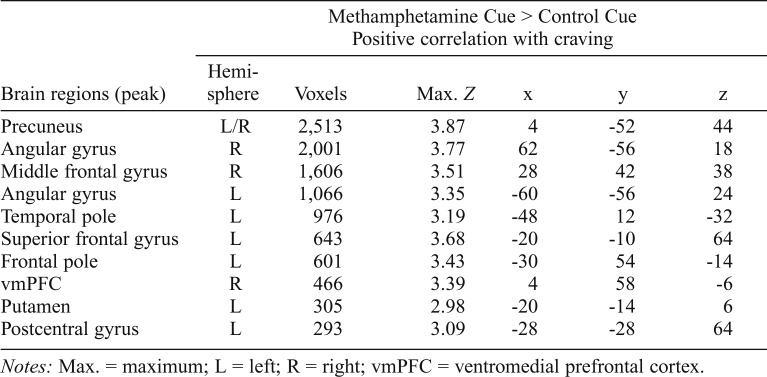
Table 1.
Locations of brain activation where methamphetamine-induced craving positively correlates with the Methamphetamine Cue > Control Cue contrast (whole-brain cluster corrected at Z > 2.3, p < .05)
| Brain regions (peak) | Methamphetamine Cue > Control Cue Positive correlation with craving |
|||||
| Hemi- sphere | Voxels | Max. Z | x | y | z | |
| Precuneus | L/R | 2,513 | 3.87 | 4 | -52 | 44 |
| Angular gyrus | R | 2,001 | 3.77 | 62 | -56 | 18 |
| Middle frontal gyrus | R | 1,606 | 3.51 | 28 | 42 | 38 |
| Angular gyrus | L | 1,066 | 3.35 | -60 | -56 | 24 |
| Temporal pole | L | 976 | 3.19 | -48 | 12 | -32 |
| Superior frontal gyrus | L | 643 | 3.68 | -20 | -10 | 64 |
| Frontal pole | L | 601 | 3.43 | -30 | 54 | -14 |
| vmPFC | R | 466 | 3.39 | 4 | 58 | -6 |
| Putamen | L | 305 | 2.98 | -20 | -14 | 6 |
| Postcentral gyrus | L | 293 | 3.09 | -28 | -28 | 64 |
Notes: Max. = maximum; L = left; R = right; vmPFC = ventromedial prefrontal cortex.
Discussion
This study examined whether methamphetamineinduced craving responses in the laboratory were associated with neural response to methamphetamine cues. The results suggest that methamphetamine-induced craving is positively correlated with patterns of brain activation during methamphetamine cue-exposure. Specifically, craving for methamphetamine was positively correlated with activation in the precuneus, as well as in mesocorticolimbic circuitry, including the vmPFC and the putamen. These findings were specific to subjective responses of craving, as subjective responses of “high” and “bad” to methamphetamine were not significantly associated with neural response to methamphetamine cues.The precuneus has emerged as a key substrate of neural cue-reactivity across classes of drugs (Courtney & Ray, 2014; Courtney et al., 2014). Positive associations between cue-elicited ratings of craving and precuneus activation to drug cues have been reported in methamphetamine (Courtney et al., 2016), alcohol (Park et al., 2007), and nicotine users (Brody et al., 2007). We extended this work by identifying a similar relationship between methamphetamine-induced craving and precuneus activation to methamphetamine cues. The precuneus has dense projections to both cortical and subcortical regions and is thought to play a role in the integration of external and self-generated information, including self-related episodic memory retrieval and mental imagery strategies (Cavanna & Trimble, 2006), processes that are likely to be involved in the subjective experience of craving.
Methamphetamine-induced craving was also associated with activation in the putamen and vmPFC, which are part of the mesocorticolimbic reward circuit. Previous studies have reported associations between dorsal striatal (i.e., putamen) activation and craving in stimulant-using populations. In cocaine-dependent individuals undergoing a self-paced IV infusion of cocaine, activation of the putamen is positively correlated with subjective ratings of cocaine craving (Risinger et al., 2005). Further, positron emission tomography studies have shown that in cocaine-dependent individuals, dorsal striatal dopamine is released during the presentation of cocaine cues, and the magnitude of dopamine released is correlated with self-reported craving (Volkow et al., 2006; Wong et al., 2006), suggesting that dopamine is involved in the conditioned responses that trigger craving, particularly in regions implicated in habit learning (Volkow et al., 2009). Activation of the vmPFC has also been previously implicated in craving in stimulant users. In methamphetamine users, medial prefrontal cortex (mPFC) activity to methamphetamine cues is positively associated with craving and frequency of drug use (Huang et al., 2018). Further, increased activation of the mPFC has been reported in a subset of cocaine users who report stimulant drug-induced craving (Volkow et al., 1999). Intriguingly, drug cue reactivity in frontal-striatal circuits (containing the vmPFC and putamen) can be attenuated through continuous theta burst stimulation to the vmPFC (Kearney-Ramos et al., 2018), which suggests that noninvasive brain stimulation may be a viable treatment to target craving circuitry and reduce relapse risk.
There were no significant associations with methamphetamine-induced subjective responses of “high” or “bad” with neural methamphetamine cue-reactivity, suggesting that these effects were specific to the construct of craving. Of note, participants were required to be abstinent from substances (including methamphetamine) before the fMRI session, verified by a negative urine drug screen. In smoking populations, nicotine-deprived individuals (i.e., short-term abstinent individuals) demonstrate more prefrontal activation to smoking cues and report higher craving urges than nondeprived smokers (Wilson & Sayette, 2015). Therefore, the participants’ state of acute abstinence may have increased their sensitivity toward craving during methamphetamine cue-exposure, which shares neurobiological substrates with drug-induced craving. Furthermore, the majority of participants in this study were current smokers and may have been experiencing nicotine withdrawal, which may have contributed to heightened prefrontal activation. However, most of these individuals smoked within an hour before scan (80%). Therefore, it is unlikely that these individuals were experiencing high levels of nicotine withdrawal.
It should also be noted that the average baseline-corrected levels of drug-induced craving were mild, perhaps because participants were no longer deprived of the drug. Baselinecorrected ratings of craving spanned the entire assessment range, from 0 to 10, indicating considerable variability in the participants’ response to IV methamphetamine. The route of administration may be responsible for some of this variability, as none of the participants reported IV use as their primary route of administration. Therefore, IV administration, although providing methodological advantages, may have reduced subjective responses and potentially weakened the associations between craving ratings and neural cue-reactivity.
This preliminary study has several limitations that should be considered when interpreting the results. First, this study has a small sample size of 15. Second, this study lacks a control group, which limits our ability to draw conclusions about how craving neurobiology is altered in individuals with MUD. Third, both neuroimaging and laboratory sessions occurred while participants were given a placebo medication, as part of a larger medication study. Therefore, it is possible that self-report and neural signals may be modulated by participants’ expectations of medication effects. Future research should validate these findings in a larger sample, without the potential impact from medication interactions. Fourth, subjective responses were induced by an IV methamphetamine administration, which differed from the participants’ primary route of administration. The cues in the fMRI task included pictures of methamphetamine paraphernalia used for smoking as well as IV use. Future research should pair the route of methamphetamine challenge administration and methamphetamine cue images. Finally, we did not control for menstrual phase in female participants. There is some evidence for a role in gonadal hormones in methamphetamine administration in animal models (Kucerova et al., 2009). Therefore, the female methamphetamine users may have had different subjective responses or cue-reactivity depending on their menstrual phase. However, as there were only three female participants in this study, it is unlikely that this would have a large impact on our findings.
In conclusion, this study provides preliminary evidence for shared neurobiology underlying cue-induced and druginduced craving in individuals with methamphetamine use disorder. This finding indicates that craving is a transdiagnostic phenotype that can be assessed using multiple methodologies, including through experimental manipulations of intravenous drug infusion and through cue-exposure during neuroimaging.
Footnotes
This study was supported by grants from the University of California, Los Angeles, Training Program in Translational Neuroscience of Drug Abuse (T32 DA024635), the National Institute on Drug Abuse (F31DA035604), Staglin IMHRO Center for Cognitive Neuroscience, and the University of California, Los Angeles, Clinical & Translational Research Center (CTSI Grant UL1TR000124). Kelly E. Courtney was supported by training grants from the National Institute on Alcohol Abuse and Alcoholism (T32 AA013525) and National Institute of Mental Health (T32 MH018399).
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington, DC: Author; 1994. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Arlington, VA: Author; 2013. [Google Scholar]
- Breiter H. C., Gollub R. L., Weisskoff R. M., Kennedy D. N., Makris N., Berke J. D., Hyman S. E. Acute effects of cocaine on human brain activity and emotion. Neuron. 1997;19:591–611. doi: 10.1016/s0896-6273(00)80374-8. doi:10.1016/S0896-6273(00)80374-8. [DOI] [PubMed] [Google Scholar]
- Brody A. L., Mandelkern M. A., Olmstead R. E., Jou J., Tiongson E., Allen V., Cohen M. S. Neural substrates of resisting craving during cigarette cue exposure. Biological Psychiatry. 2007;62:642–651. doi: 10.1016/j.biopsych.2006.10.026. doi:10.1016/j.biopsych.2006.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanna A. E., Trimble M. R. The precuneus: A review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–583. doi: 10.1093/brain/awl004. doi:10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Courtney K. E., Ghahremani D. G., London E. D., Ray L. A. The association between cue-reactivity in the precuneus and level of dependence on nicotine and alcohol. Drug and Alcohol Dependence. 2014;141:21–26. doi: 10.1016/j.drugalcdep.2014.04.026. doi:10.1016/j.drugalcdep.2014.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney K. E., Ghahremani D. G., Ray L. A. The effects of pharmacological opioid blockade on neural measures of drug cuereactivity in humans. Neuropsychopharmacology. 2016;41:2872–2881. doi: 10.1038/npp.2016.99. doi:10.1038/npp.2016.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney K. E., Ray L. A. Subjective responses to alcohol in the lab predict neural responses to alcohol cues. Journal of Studies on Alcohol and Drugs. 2014;75:124–135. doi: 10.15288/jsad.2014.75.124. doi:10.15288/jsad.2014.75.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galloway G. P., Singleton E. G. & the Methamphetamine Treatment Project Corporate Authors. How long does craving predict use of methamphetamine? Assessment of use one to seven weeks after the assessment of craving: Craving and ongoing methamphetamine use. Substance Abuse: Research and Treatment. 2009;1:63–79. [PMC free article] [PubMed] [Google Scholar]
- Harris D. S., Reus V. I., Wolkowitz O. M., Mendelson J. E., Jones R. T. Altering cortisol level does not change the pleasurable effects of methamphetamine in humans. Neuropsychopharmacology. 2003;28:1677–1684. doi: 10.1038/sj.npp.1300223. doi:10.1038/sj.npp.1300223. [DOI] [PubMed] [Google Scholar]
- Hartz D. T., Frederick-Osborne S. L., Galloway G. P. Craving predicts use during treatment for methamphetamine dependence: A prospective, repeated-measures, within-subject analysis. Drug and Alcohol Dependence. 2001;63:269–276. doi: 10.1016/s0376-8716(00)00217-9. doi:10.1016/S0376-8716(00)00217-9. [DOI] [PubMed] [Google Scholar]
- Hasin D. S., O’Brien C. P., Auriacombe M., Borges G., Bucholz K., Budney A., Grant B. F. DSM-5 criteria for substance use disorders: Recommendations and rationale. American Journal of Psychiatry. 2013;170:834–851. doi: 10.1176/appi.ajp.2013.12060782. doi:10.1176/appi.ajp.2013.12060782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S., Zhang Z., Dai Y., Zhang C., Yang C., Fan L., Chen H. Craving responses to methamphetamine and sexual visual cues in individuals with methamphetamine use disorder after longterm drug rehabilitation. Frontiers in Psychiatry. 2018;9:145. doi: 10.3389/fpsyt.2018.00145. doi:10.3389/fpsyt.2018.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas P. W., Volkow N. D. The neural basis of addiction: A pathology of motivation and choice. American Journal of Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. doi:10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Kearney-Ramos T. E., Dowdle L. T., Lench D. H., Mithoefer O. J., Devries W. H., George M. S., Hanlon C. A. Transdiagnostic effects of ventromedial prefrontal cortex transcranial magnetic stimulation on cue reactivity. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging. 2018;3:599–609. doi: 10.1016/j.bpsc.2018.03.016. doi:10.1016/j.bpsc.2018.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucerova J., Vrskova D., Sulcova A. Impact of repeated methamphetamine pretreatment on intravenous self-administration of the drug in males and estrogenized or non-estrogenized ovariectomized female rats. Neuro Endocrinology Letters. 2009;30:663–670. [PubMed] [Google Scholar]
- Lee N. K., Pohlman S., Baker A., Ferris J., Kay-Lambkin F. It’s the thought that counts: Craving metacognitions and their role in abstinence from methamphetamine use. Journal of Substance Abuse Treatment. 2010;38:245–250. doi: 10.1016/j.jsat.2009.12.006. doi:10.1016/j.jsat.2009.12.006. [DOI] [PubMed] [Google Scholar]
- Li X., Malcolm R. J., Huebner K., Hanlon C. A., Taylor J. J., Brady K. T., See R. E. Low frequency repetitive transcranial magnetic stimulation of the left dorsolateral prefrontal cortex transiently increases cue-induced craving for methamphetamine: A preliminary study. Drug and Alcohol Dependence. 2013;133:641–646. doi: 10.1016/j.drugalcdep.2013.08.012. doi:10.1016/j. drugalcdep.2013.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malcolm R., Myrick H., Li X., Henderson S., Brady K. T., George M. S., See R. E. Regional brain activity in abstinent methamphetamine dependent males following cue exposure. Journal of Drug Abuse. 2016;2:1–7. doi: 10.21767/2471-853x.100016. doi:10.21767/2471-853X.100016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morean M. E., de Wit H., King A. C., Sofuoglu M., Rueger S. Y., O’Malley S. S. The Drug Effects Questionnaire: Psychometric support across three drug types. Psychopharmacology. 2013;227:177–192. doi: 10.1007/s00213-012-2954-z. doi:10.1007/s00213-012-2954-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton T. F., Reid M. S., De La Garza R., Mahoney J. J., Abad A., Condos R., Elkashef A. Evaluation of subjective effects of aripiprazole and methamphetamine in methamphetamine-dependent volunteers. International Journal of Neuropsychopharmacology. 2008;11:1037–1045. doi: 10.1017/S1461145708009097. doi:10.1017/S1461145708009097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton T. F., Roache J. D., De La Garza R., II, Fong T., Wallace C. L., Li S. H., Kahn R. Safety of intravenous methamphetamine administration during treatment with bupropion. Psychopharmacology. 2005;182:426–435. doi: 10.1007/s00213-005-0102-8. doi:10.1007/s00213-005-0102-8. [DOI] [PubMed] [Google Scholar]
- Olney J. J., Warlow S. M., Naffziger E. E., Berridge K. C. Current perspectives on incentive salience and applications to clinical disorders. Current Opinion in Behavioral Sciences. 2018;22:59–69. doi: 10.1016/j.cobeha.2018.01.007. doi:10.1016/j.cobeha.2018.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park M. S., Sohn J. H., Suk J. A., Kim S. H., Sohn S., Sparacio R. Brain substrates of craving to alcohol cues in subjects with alcohol use disorder. Alcohol and Alcoholism. 2007;42:417–422. doi: 10.1093/alcalc/agl117. doi:10.1093/alcalc/agl117. [DOI] [PubMed] [Google Scholar]
- Ray L. A., Bujarski S., Courtney K. E., Moallem N. R., Lunny K., Roche D., Miotto K. The effects of naltrexone on subjective response to methamphetamine in a clinical sample: A double-blind, placebo-controlled laboratory study. Neuropsychopharmacology. 2015;40:2347–2356. doi: 10.1038/npp.2015.83. doi:10.1038/npp.2015.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risinger R. C., Salmeron B. J., Ross T. J., Amen S. L., Sanfilipo M., Hoffmann R. G., Stein E. A. Neural correlates of high and craving during cocaine self-administration using BOLD fMRI. NeuroImage. 2005;26:1097–1108. doi: 10.1016/j.neuroimage.2005.03.030. doi:10.1016/j.neuroimage.2005.03.030. [DOI] [PubMed] [Google Scholar]
- Robinson T. E., Berridge K. C. The neural basis of drug craving: An incentive-sensitization theory of addiction. Brain Research Reviews. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. doi:10.1016/0165-0173(93)90013-P. [DOI] [PubMed] [Google Scholar]
- Roche D. J. O., Worley M. J., Courtney K. E., Bujarski S., London E. D., Shoptaw S., Ray L. A.2017Naltrexone moderates the relationship between cue-induced craving and subjective response to methamphetamine in individuals with methamphetamine use disorder Psychopharmacology 2341997–2007.. doi:10.1007/s00213-017-4607-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahbabaie A., Golesorkhi M., Zamanian B., Ebrahimpoor M., Keshvari F., Nejati V., Ekhtiari H. State dependent effect of transcranial direct current stimulation (tDCS) on methamphetamine craving. International Journal of Neuropsychopharmacology. 2014;17:1591–1598. doi: 10.1017/S1461145714000686. doi:10.1017/S1461145714000686. [DOI] [PubMed] [Google Scholar]
- Sinha R., Li C. S. Imaging stress- and cue-induced drug and alcohol craving: Association with relapse and clinical implications. Drug and Alcohol Review. 2007;26:25–31. doi: 10.1080/09595230601036960. doi:10.1080/09595230601036960. [DOI] [PubMed] [Google Scholar]
- Tiffany S. T., Friedman L., Greenfield S. F., Hasin D. S., Jackson R. Beyond drug use: A systematic consideration of other outcomes in evaluations of treatments for substance use disorders. Addiction. 2012;107:709–718. doi: 10.1111/j.1360-0443.2011.03581.x. doi:10.1111/j.1360-0443.2011.03581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolliver B. K., McRae-Clark A. L., Saladin M., Price K. L., Simpson A. N., DeSantis S. M., Brady K. T. Determinants of cue-elicited craving and physiologic reactivity in methamphetamine-dependent subjects in the laboratory. American Journal of Drug and Alcohol Abuse. 2010;36:106–113. doi: 10.3109/00952991003686402. doi:10.3109/00952991003686402. [DOI] [PubMed] [Google Scholar]
- Volkow N. D., Fowler J. S., Wang G. J., Baler R., Telang F. Imaging dopamine’s role in drug abuse and addiction. Neuropharmacology, 56, Supplement 1. 2009:3–8. doi: 10.1016/j.neuropharm.2008.05.022. doi:10.1016/j.neuropharm.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow N. D., Wang G. J., Fowler J. S., Hitzemann R., Angrist B., Gatley S. J., Pappas N. Association of methylphenidate-induced craving with changes in right striato-orbitofrontal metabolism in cocaine abusers: Implications in addiction. American Journal of Psychiatry. 1999;156:19–26. doi: 10.1176/ajp.156.1.19. doi:10.1176/ajp.156.1.19. [DOI] [PubMed] [Google Scholar]
- Volkow N. D., Wang G.-J., Telang F., Fowler J. S., Logan J., Childress A. R., Wong C. Cocaine cues and dopamine in dorsal striatum: Mechanism of craving in cocaine addiction. Journal of Neuroscience. 2006;26:6583–6588. doi: 10.1523/JNEUROSCI.1544-06.2006. doi:10.1523/JNEUROSCI.1544-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G., Shi J., Chen N., Xu L., Li J., Li P., Lu L. Effects of length of abstinence on decision-making and craving in methamphetamine abusers. PLoS One. 2013;8(7):e68791. doi: 10.1371/journal.pone.0068791. doi:10.1371/journal. pone.0068791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss F. Neurobiology of craving, conditioned reward and relapse. Current Opinion in Pharmacology. 2005;5:9–19. doi: 10.1016/j.coph.2004.11.001. doi:10.1016/j. coph.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Wilson S. J., Sayette M. A. Neuroimaging craving: Urge intensity matters. Addiction. 2015;110:195–203. doi: 10.1111/add.12676. doi:10.1111/add.12676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong D. F., Kuwabara H., Schretlen D. J., Bonson K. R., Zhou Y., Nandi A., London E. D. Increased occupancy of dopamine receptors in human striatum during cue-elicited cocaine craving. Neuropsychopharmacology. 2006;31:2716–2727. doi: 10.1038/sj.npp.1301194. doi:10.1038/sj.npp.1301194. [DOI] [PubMed] [Google Scholar]
- World Health Organization. International statistical classification of diseases and related health problems, 10th Revision. Geneva, Switzerland: Author; 1992. [Google Scholar]
- Worsley K. Statistical analysis of activation images. In: Jezzard P., Matthews P. M., Smith S. M., editors. Functional MRI: An introduction to methods (pp. 251–270) Oxford, England: Oxford University Press; 2001. [Google Scholar]
- Zorick T., Sugar C. A., Hellemann G., Shoptaw S., London E. D. Poor response to sertraline in methamphetamine dependence is associated with sustained craving for methamphetamine. Drug and Alcohol Dependence. 2011;118:500–503. doi: 10.1016/j.drugalcdep.2011.04.015. doi:10.1016/j.drugalcdep.2011.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]