Abstract
Background: Bupivacaine (BUP) acts as a local anesthetic, which is extensively used for clinical patients but could generate neurotoxicity in neurons. Tetramethylpyrazine (TET) exhibits strong neuron protective effects against neurotoxicity. Hence, we investigate the effect of TET on BUP-induced neurotoxicity in SH-SY5Y cells.
Methods: CCK-8 assay was used to detect cell proliferation in SH-SY5Y cells. In addition, Western blotting was used to examine Bax, Bcl-2, active caspase 3, LC3II, Beclin 1 and p-62 protein levels in cells. Moreover, ELISA assay was used to detect the levels of total glutathione (GS), superoxide dismutase (SOD) and malondialdehyde (MDA) in cells.
Results: In this study, we found that TET attenuated the neurotoxicity of BUP on SH-SY5Y cells. Meanwhile, TET alleviated BUP-induced apoptosis in SH-SY5Y cell via decreasing the expressions of active caspase-3 and Bax and increasing the expression of Bcl-2. In addition, monodansylcadaverine staining assay and Western blotting results confirmed that TET induced autophagy in SH-SY5Y cells via increasing the LC3II/I and Beclin 1 levels. Furthermore, TET attenuated BUP-induced oxidative damage in SH-SY5Y cells via upregulation of the levels of total GS and SOD and downregulation of the level of MDA. Interesting, the protective effects of TET against BUP-induced neurotoxicity in SH-SY5Y cells were reversed by autophagy inhibitor 3-methyladenine (3MA).
Conclusion: These data indicated that TET may play a neuroprotective role via inhibiting apoptosis and inducing autophagy in SH-SY5Y cells. Therefore, TET may be a potential agent for the treatment of human neurotoxicity induced by BUP.
Keywords: tetramethylpyrazine, bupivacaine, neurotoxicity, human neuroblastoma cell
Introduction
Local anesthetics (LAs) are grouped into ester and amide types due to their chemical structure, which are indispensable for regional anesthesia in surgical procedures.1,2 However, clinical studies indicated that exposure to LAs for long duration or at high dosage may cause spinal neurotoxicity or may induce nonreversible neurological complications.3–5 Moreover, LAs also may cause postsurgical nervous complications.1 For example, bupivacaine (BUP) acts as a LA and is extensively used for epidural anesthesia, nerve blockade and postoperative analgesia in clinical patients.1,6 However, the mechanism of LA toxicity remains unclear. Hence, it is indispensable to develop effective methods to prevent its neurotoxicity.
Tetramethylpyrazine (TET) is an alkaloid that originally extracted from a traditional Chinese herbal medicine Ligusticum chuanxiong.7,8 TET has been used as an effective compound to remedy patients with kidney, heart and brain diseases.9–11 In addition, TET also has been reported to provide neuroprotective effect against ischemic brain damage in rats.9 Several studies have shown that TET exhibited strong protective effects against neurotoxicity in Alzheimer’s disease and Parkinson’s disease.12,13 Hu et al studied that TET derivative could protect primary neurons against glutamate-induced excitotoxicity.12 Moreover, TET also demonstrated a potent neuroprotective role in MPP+-induced neuro cells via activating transcription factor MEF2D.13
In spite of many reports indicating that TET plays a protective effect against neurotoxicity, little is known about the effect of TET on BUP-induced neurotoxicity. Hence, the present study aimed to investigate the mechanisms underlying the protective effect of TET against BUP-induced neurotoxicity in vitro.
Materials and methods
Cell cultures and cell transfection
Human neuroblastoma cell line SH-SY5Y (ATCC) were cultured in DMEM (Thermo Fisher Scientific, Inc., Waltham, MA, USA) medium with 10% FBS (Thermo Fisher Scientific) and 1% penicillin/streptomycin in a constant temperature incubator at 37°C and at 5% CO2 concentration.
TET was dissolved with 0.1% DMSO (stock solution of 10 mM TET in DMSO). To study the role of TET in BUP-induced SH-SY5Y cell neurotoxicity, SH-SY5Y cells were pretreated with 10 μM 3-methyladenine (3MA, an autophagy inhibitor) for 1 hr, and then treated with TET for 24 hrs. Later on, cells were treated with BUP for 48 hrs. BUP (Art. No. B5274) and TET (Art. No. 95162) were purchased from Sigma Aldrich (St. Louis, MO, USA).
Cell Counting Kit-8 (CCK-8) assay
CCK-8 is metabolically reduced in viable cells to a water-soluble formazan product. The amount of formazan is proportional to the number of living cells. This characteristic can be used for cell viability analysis. Therefore, cell viability was evaluated using a CCK-8 assay (Beyotime Institute of Biotechnology, Haimen, China) according to the manufacturer’s protocol. SH-SY5Y cells were plated into a 96-well plate at 5×103 cells/well in 100 μL culture medium. BUP (0, 100, 250, 500, 1,000 μM), or TET (0, 100, 200, 400, 800 μM) was added into each well, and cells were incubated in a CO2 incubator at 37°C. In addition, BUP (0, 500 μM) and/or TET (0, 100, 150, 200, 300, 400 μM) were added into each well, and cells were incubated in a CO2 incubator at 37°C. After that, each well was added with 10 μL of CCK-8 solution and cultured at 37°C for another 3 hrs. The OD value was measured at 450 nm using a Thermo Multiskan FC microplate reader (Thermo Fisher Scientific). SH-SY5Y cells were pretreated with TET for 24 hrs, and then were treated with BUP for another 48 hrs. The recovery rate of viability was calculated by the equation: (ViabilityBUP+TET − Viability BUP)/Viability BUP. Median effect concentration (EC50) was calculated with GraphPad Prism software (version 7.0, La Jolla, CA, USA).
Immunofluorescence assay
The Ki-67 protein (also known as MKI67) is a cellular marker for proliferation.14 SH-SY5Y cells (4×105 cells/well) were plated to 24-well plates overnight, then treated with BUP (500 μM) and/or TET (200 μM), or BUP+TET+3MA. After that, cells were fixed in pre-cold methanol at −20°C for 10 mins. Next, cells were incubated with primary antibodies for anti-Ki67 (Abcam; ab15580) (1:1,000) and DAPI (ab104139) (1:1,000) at 4°C overnight. Subsequently, cells were incubated with secondary antibodies (Abcam; ab150080) (1:5,000) at 37°C for 1 hr. The samples were observed by fluorescence microscope at once (Olympus CX23 Tokyo, Japan).
Flow cytometric analysis of cell apoptosis
Apoptotic cells were detected according to a previously described method.15 Briefly, SH-SY5Y cells (5×105 cells/well) were seeded to 6-well plates overnight, then treated with BUP (500 μM) and/or TET (200 μM), or BUP+TET+3MA. Cell scraper was used to detach the cells from the culture plate. After that, apoptotic cells were stained with dual-staining Annexin V-fluorescein isothiocyante (FITC)-propidium iodide (PI) (Thermo Fisher Scientific) and measured by FCM flow cytometer (BD Bioscience, San Jose, CA, USA).
Western blot analysis
SH-SY5Y cells (5×105 cells/well) were seeded to 6-well plates overnight, then treated with BUP (500 μM) and/or TET (200 μM), or BUP+TET+3MA. BCA Protein Assay Kit (Beyotime, Shanghai, China) was used to quantify the soluble protein concentration in the supernatant. Protein samples (30 μg/lane) were separated by polyacrylamide gel electrophoresis. Following polyacrylamide gel electrophoresis, proteins were transferred onto polyvinylidene fluoride membranes (PVDF, Thermo Fisher Scientific). PVDF membranes were treated with primary antibodies overnight at 4°C. On the next day, the PVDF membrane was treated with secondary antibody at room temperature for 1 hr. The following primary antibodies were used: anti-active caspase 3 (Abcam ab2302) (1:1,000), anti-β-actin (Abcam ab8227) (1:1,000), anti-Bax (Abcam ab32503) (1:1,000), anti-Bcl-2 (Abcam ab32124) (1:1,000), anti-LC3I (Abcam ab62720) (1:1,000), anti-LC3II (Abcam ab48394) (1:1,000), anti-Beclin 1 (Abcam ab207612) (1:1,000), and anti-p62 (Abcam ab155686) (1:1,000). The second antibody was HRP-labeled anti-rabbit (1:5,000, PTG (Carlsbad, CA, USA), USA). Finally, the PVDF membranes were incubated with ECL reagent (Santa Cruz Biotechnology, Santa Cruz, CA, USA). The density of blots for targets was normalized to β-actin.
Monodansylcadaverine (MDC) staining
SH-SY5Y cells (4×105 cells/well) were seeded to 24-well plates overnight, then treated with BUP (500 μM) and/or TET (200 μM), or BUP+TET+3MA. After that, cells were stained with a 0.05 mM MDC (Sigma Aldrich, #D4008) at 37°C for 30 mins. Fluorescence of cells was instantly observed and counted with a Hitachi F-2000 fluorescence microscope (Olympus Corporation).
Measurement of cytokines by ELISA
SH-SY5Y cells (4×105 cells/well) were seeded to 24-well plates overnight, then treated with BUP (500 μM) and/or TET (200 μM), or BUP+TET+3MA. After that, the levels of total GS, MDA and SOD in SH-SY5Y cells were measured using ELISA kits in accordance with the manufacturer’s instructions (Beyotime).
Glutathione assay
Levels of intracellular reduced glutathione (GSH) and oxidized glutathione (GSSG) were measured using ELISA kits according to the manufacturer’s specifications (Beyotime). Absorbance was read at 450 nm by Thermo Multiskan FC microplate reader (Thermo Fisher Scientific). The level of total glutathione (total GS) is the sum of GSH level and GSSG level.
Superoxide dismutase (SOD) and malondialdehyde (MDA) assays
The levels of SOD and MDA were measured using ELISA kits according to the manufacturer’s specifications (Beyotime). For MDA detection, the absorbance was read at 532 nm by Thermo Multiskan FC microplate reader (Thermo Fisher Scientific). For SOD detection, the absorbance was read at 532 nm by Thermo Multiskan FC microplate reader (Thermo Fisher Scientific).
Statistical analysis
Each group executed at least three independent experiments, and all data were presented in the form of mean ± SD. Student’s t-test was used to analyze the comparison between two groups. The comparisons among multiple groups were made with one-way ANOVA followed by Dunnett’s test. P<0.05 or P<0.01 was considered to indicate a statistically significant difference (*P<0.05, **P<0.01).
Results
TET attenuated BUP-induced neurotoxicity in SH-SY5Y cells
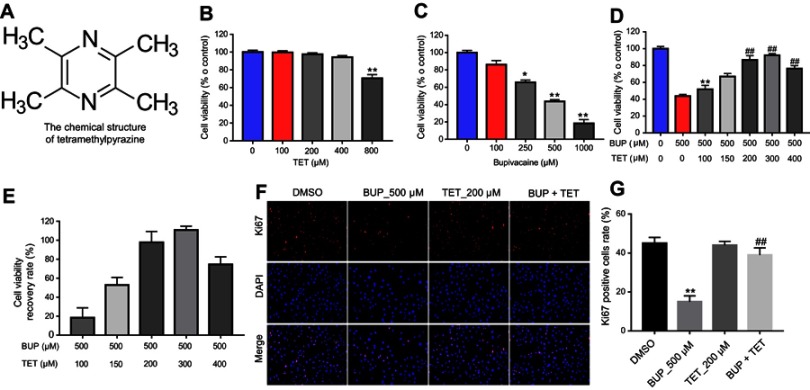
The chemical structure of TET is indicated in Figure 1A. CCK-8 assay was used to evaluate the effects of TET on the viability of SH-SY5Y cells. As shown in Figure 1B, TET (100, 150, 200, 300 or 400 μM) had no effect on SH-SY5Y cell viability. In contrast, BUP dose dependently inhibited SH-SY5Y cell viability and 500 μM BUP induced about 50% cell growth inhibition (Figure 1C). Therefore, BUP (500 μM) was utilized in the following experiments. In addition, BUP inhibited proliferation in SH-SY5Y cells, which was significantly reversed by 200 μM TET treatment (Figure 1D and E). The EC50 for TET was 146 μM. Ki67 is normally used as a detector of cells in a proliferative state.16 In immunofluorescence assays, TET exhibited markedly protective effects against BUP‐induced cell death (Figure 1F and G). These results suggested that TET could attenuate BUP-induced cytotoxicity in SH-SY5Y cells.
Figure 1.
TET attenuated BUP-induced cytotoxicity in SH-SY5Y cells. (A) The chemical structure of TET. (B) Cell viability of SH-SY5Y cells treated with TET (0, 100, 200, 400, 800 μM) for 24 hrs was determined using CCK-8 assay. (C) Cell viability of SH-SY5Y cells treated with BUP (0, 100, 250, 500, 1,000 μM) for 48 hrs was determined using CCK-8 assay. (D) Cell viability of SH-SY5Y cells treated with TET (0, 100, 150, 200, 300, 400 μM) and/or BUP (500 μM) was determined using CCK-8 assay. *P<0.05, **P<0.01 compared with 0 μM group; ##P<0.01 compared with 500 μM BUP group. (E) The recovery rate of cell viability. (F) SH-SY5Y cells were exposed to 200 μM TET with or without 500 μM BUP. Relative fluorescence expression levels were quantified by Ki67 and DAPI staining. (G) The number of Ki67 positive cells was counted. **P<0.01, compared with DMSO group; ##P<0.01 compared with 500 μM BUP group
Abbreviations: BUP, bupivacaine; TET, tetramethylpyrazine.
TET attenuated BUP-induced cytotoxicity via inhibition of apoptosis in SH-SY5Y cells
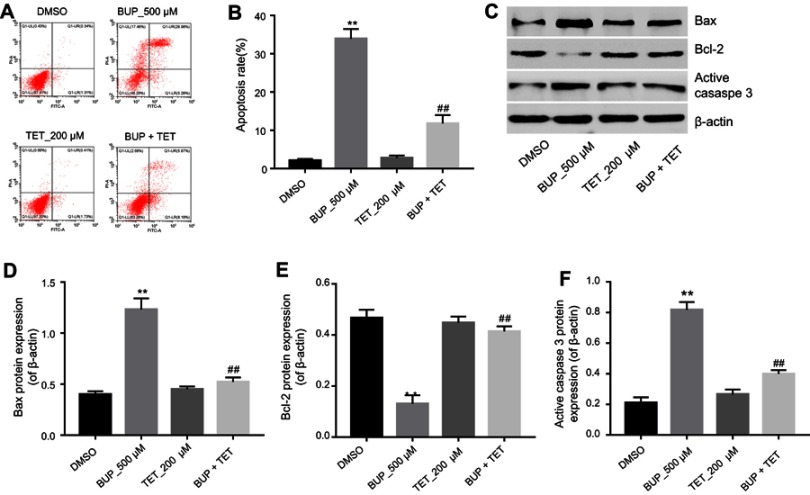
To further explore the effects of TET on BUP-induced apoptosis in SH-SY5Y cells, flow cytometry was applied. As indicated in Figure 2A and B, BUP significantly induced apoptosis in SH-SY5Y cells, which was markedly attenuated by TET. In addition, Western blotting was used to detect the expression of apoptosis-related proteins Bax, Bcl-2 and active caspase 3. The data indicated that the expressions of Bax and active caspase 3 were significantly increased, while the level of Bcl-2 was decreased in the BUP-treated group compared with the DMSO group (Figure 2C–F). However, these effects were significantly reversed by TET in BUP-treated SH-SY5Y cells (Figure 2C–F). These results suggested that TET attenuated BUP-induced cytotoxicity via inhibition of apoptosis in SH-SY5Y cells.
Figure 2.
TET attenuated BUP-induced cytotoxicity via inhibition of apoptosis in SH-SY5Y cells. SH-SY5Y cells were exposed to 200 μM TET with or without 500 μM BUP. (A) Apoptotic cells were detected with Annexin V and PI double staining. (B) The apoptosis cell rates were calculated. (C) Expressions of Bax, Bcl-2 and active caspase-3 were analyzed by Western blotting in SH-SY5Y cells. (D) Bax relative expression was quantified by normalizing to β-actin. (E) Bcl-2 relative expression was quantified by normalizing to β-actin. (F) Active caspase-3 relative expression was quantified by normalizing to β-actin. **P<0.01, compared with DMSO group; ##P<0.01 compared with 500 μM BUP group.
Abbreviations: BUP, bupivacaine; TET, tetramethylpyrazine.
TET attenuated BUP-induced cytotoxicity via stimulation of autophagy in SH-SY5Y cells
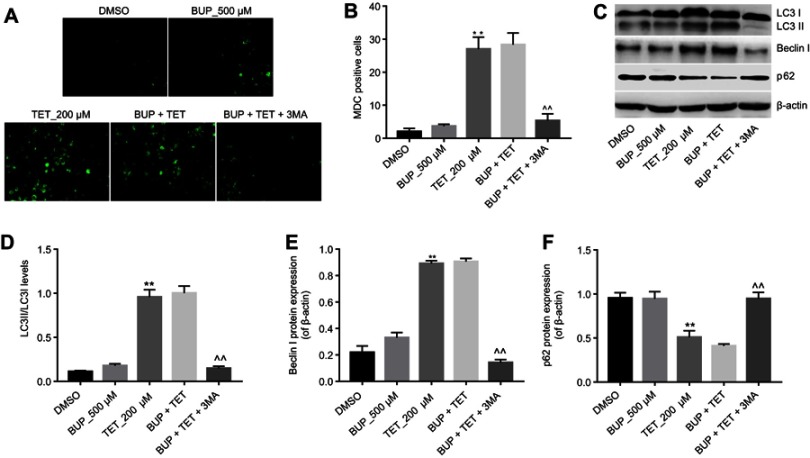
To investigate whether TET attenuated BUP-induced cytotoxicity via regulation of autophagy, MDC assay was performed in this study. As shown in Figure 3A and B, the autophagic vacuoles and autophagosome were dramatically increased in the TET-treated group compared with the DMSO group or BUP group. As expected, the autophagic vacuoles and autophagosome were significantly decreased in the presence of 3MA treatment (Figure 3A and B).
Figure 3.
TET attenuated BUP-induced cytotoxicity via stimulation of autophagy in SH-SY5Y cells. SH-SY5Y cells were exposed to 200 μM TET or/and 500 μM BUP or BUP+TET+3MA. (A) MDC staining was used to observe the autophagosomes formatting (×200 magnification). (B) The number of autophagosomes in cells was counted. (C) Expressions of LC3II, Beclin1 and p62 were analyzed by Western blotting in SH-SY5Y cells. (D) LC3II relative expression was quantified by normalizing to LC3I. (E) Beclin1 relative expression was quantified by normalizing to β-actin. (F) p-62 relative expression was quantified by normalizing to β-actin. **P<0.01, compared with DMSO group; ^^P<0.01 compared with BUP+TET group.
Abbreviations: 3MA, 3-methyladenine; BUP, bupivacaine; monodansylcadaverine; TET, tetramethylpyrazine.
Western blotting was next utilized to detect the expression of autophagy-related proteins LC3II, Beclin 1 and p-62. As shown in Figure 3C–F, the levels of LC3II and Beclin 1 were dramatically increased, while the expression of p-62 was decreased in the TET-treated group. However, TET-induced LC3II and Beclin 1 protein increases were significantly reversed by 3MA treatment. All these results suggested that TET attenuated BUP-induced cytotoxicity via stimulating autophagy in SH-SY5Y cells.
TET attenuated BUP-induced cytotoxicity via inhibition of oxidative damage in SH-SY5Y cells
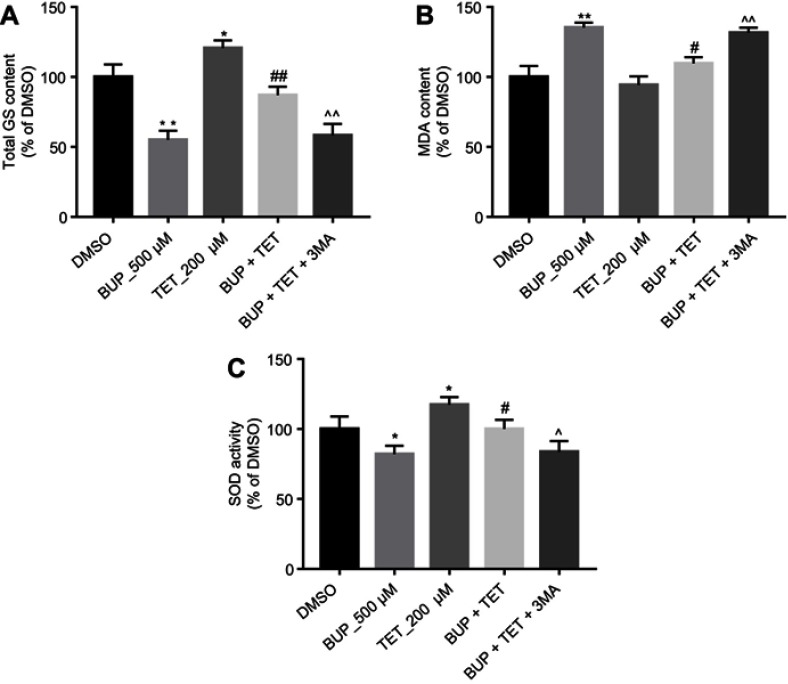
Previous studies have indicated that TET exhibited protective effects on oxidative damage of vascular endothelial cells in response to hydrogen peroxide.17 As indicated in Figure 4A–C, TET significantly increased the levels of total GS and SOD in SH-SY5Y cells, compared with the DMSO group. Meanwhile, the results of ELISA revealed that BUP-induced total GS and SOD downregulation and MDA upregulation were markedly reversed by TET treatment in SH-SY5Y cells. However, when 3MA was present, the protective effect of TET was abolished (Figure 4A–C). These results suggested that TET attenuated BUP-induced cytotoxicity via inhibition of cell oxidative damage in SH-SY5Y cells.
Figure 4.
TET attenuated BUP-induced cytotoxicity via inhibition of oxidative damage in SH-SY5Y cells. SH-SY5Y cells were exposed to 200 μM TET or/and 500 μM BUP or BUP+TET+3MA. (A) The production of total GS was assayed in the culture media. (C) The production of MDA was assayed in the culture media. (D) The production of SOD was assayed in the culture media. *P<0.05, **P<0.01 compared with DMSO group; #P<0.05, ##P<0.01 compared with 500 μM BUP group; ^P<0.05, ^^P<0.01 compared with BUP+TET group.
Abbreviations: 3MA, 3-methyladenine; BUP, bupivacaine; monodansylcadaverine; GS, glutathione; MDA, malondialdehyde; SOD, superoxide dismutase; TET, tetramethylpyrazine.
Inhibition of autophagy abolished the protective effect of TET against BUP in SH-SY5Y cell
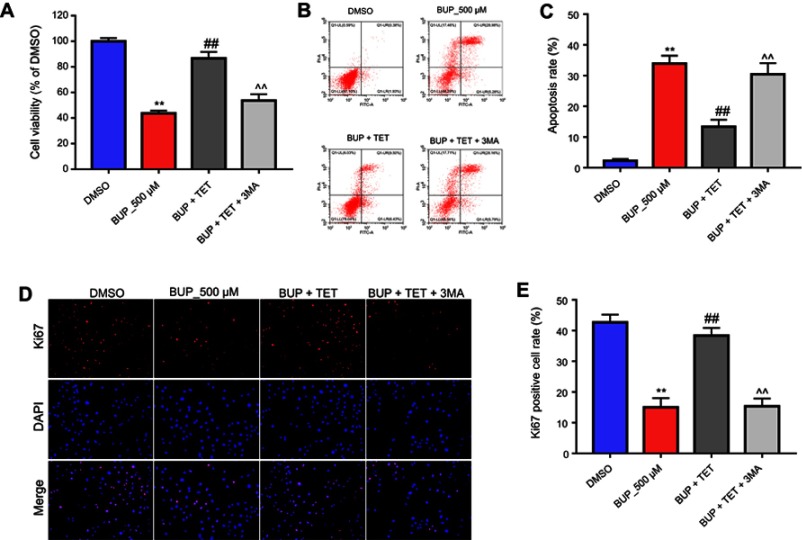
To confirm the protective effect of TET against BUP in SH-SY5Y cell via stimulation of autophagy, 3MA was used in this study. As indicated in Figure 5A, D, and E, the protective effect of TET in BUP-treated SH-SY5Y cells was reversed by 3MA. Meanwhile, the antiapoptotic effects of TET in BUP-induced SH-SY5Y cells were also alleviated by 3MA (Figure 5B and C). All these results confirmed that TET attenuated BUP-induced cytotoxicity in SH-SY5Y cells via stimulation of autophagy.
Figure 5.
Inhibition of autophagy abolished the protective effect of TET against BUP in SH-SY5Y cell. SH-SY5Y cells were exposed to 200 μM TET with 500 μM BUP and BUP+TET+3MA. (A) Cell viability was determined using CCK-8 assay in SH-SY5Y cells in 72 hrs. (B) Apoptotic cells were detected with Annexin V and PI double staining. (C) The apoptosis cell rates were calculated. (D) Relative fluorescence expression levels were quantified by Ki67 and DAPI staining. (E) The number of Ki67 positive cells was counted. **P<0.01 compared with DMSO group; ##P<0.01 compared with 500 μM BUP group; ^^P<0.01 compared with BUP+TET group.
Abbreviations: 3MA, 3-methyladenine; BUP, bupivacaine; monodansylcadaverine; TET, tetramethylpyrazine.
Discussion
In the present study, we demonstrated the protective effect of TET in BUP-induced neurotoxicity in SH-SY5Y cells. TET (200 μM) pretreatment suppressed BUP-induced neurotoxicity, apoptosis and cellular redox indicators accumulation in SH-SY5Y cells. Our results suggested that TET attenuated BUP-induced neuronal injury through inhibiting apoptosis and inducing autophagy.
SH-SY5Y cells are widely used for studying the neurotoxicity of BUP, because they can simulate the biological characteristics of neurons. In this study, 500 μM BUP significantly inhibited SH-SY5Y cell proliferation, as evidenced by the CCK-8 assay results. In addition, we observed that 200 μM of TET had no effect on SH-SY5Y cell proliferation, but could prevent BUP-induced SH-SY5Y cells toxicity. Dangduga et al found that TET performed neuroprotective activity in 3-nitropropionic acid-induced Huntington’s disease-like symptoms.18 Meanwhile, Li et al found that TET could protect rat from alcoholic-induced chronic alcoholic encephalopathy.7 These results were consistent with our finding, and confirmed TET may serve as a neuroprotective role in BUP-treated SH-SY5Y cells.
Apoptosis is a basic process which is participated in a variety of physiological conditions. We also observed that TET reduced the expression of Bax and active caspase 3 and increased the level of Bcl-2 in BUP-treated SH-SY5Y cells. Chen et al found that paeoniflorin attenuated neurotoxicity in BUP-treated SH-SY5Y cells via suppressing the level of Bax and caspase 3 and increasing the level of Bcl-2.19 Similarly, Wang et al demonstrated neuroprotection by epigallo catechin gallate against BUP-induced cell toxicity via regulating Bax, Bcl-2 and caspase 3 in SH-SY5Y cells.20 Consistent with these findings, we indicated that TET attenuated BUP-induced cytotoxicity via reducing the expression of Bax and active caspase 3 and increasing the level of Bcl-2.
Autophagy also plays important roles in neuro-cell survival and death, and is receiving increasing focus in neurotoxicity research.21 A previous study indicated that small molecules demonstrate a neuroprotective effect by enhancing autophagy and inhibiting apoptosis, such as flubendazole, flupirtine and aromatic carbamates.22–24 In addition, dexmedetomidine protects neurons from the toxicity of the anesthetic sevoflurane by enhancing autophagy and inhibiting apoptosis.25 Thus, to further examine the neuroprotective effect of TET, we also investigated whether TET induced autophagy in SH-SY5Y cells. MDC staining and Western blotting results revealed that TET could induce autophagy in SH-SY5Y cells. Previous studies demonstrated that autophagy act as a pro-survival action via increasing the expression of LC3II/I and Beclin 1.26,27 Li et al found that Tris(1, 3-dichloro-2-propyl) phosphate induced autophagy in SH-SY5Y cells via increasing the level of LC3II/I and Beclin 1 and decreasing the level of p62,28 which was in accordance with our findings. In addition, a previous study also indicated that BUP could mediate the upregulation of LC3II and the downregulation of P62.21 Differently, our study showed that BUP (500 μM) could not induce autophagy in SH-SY5Y cells. The difference between our result and previous study might reflect differences in the dose of BUP exposure (500 μM vs 900 μM).21
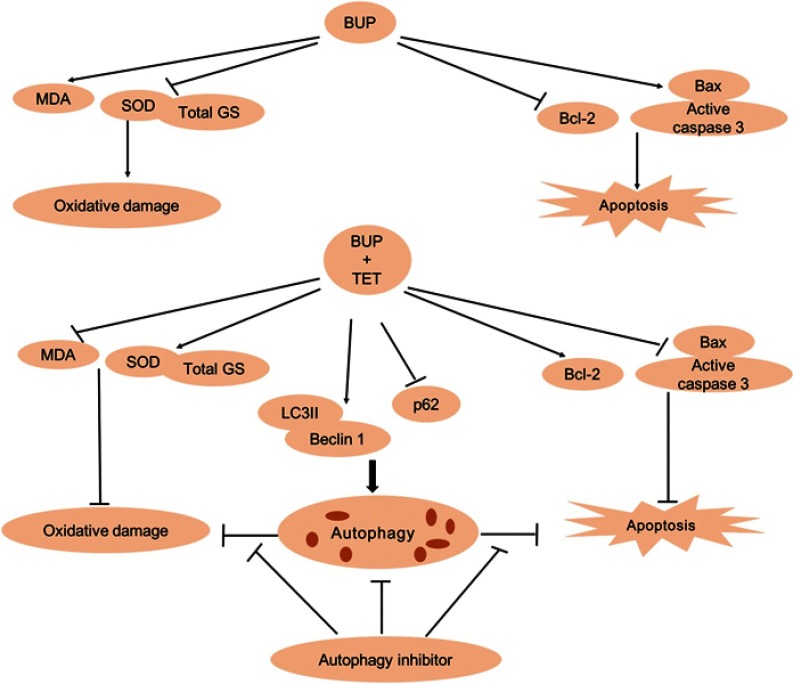
In order to confirm whether TET-induced autophagy is pro-proliferative or proapoptotic, we used autophagy inhibitor 3MA to suppress SH-SY5Y cell autophagy. Our results indicated that 3MA significantly inhibited TET-induced autophagy. Furthermore, inhibition of autophagy by 3MA further increased cell proliferation inhibition and apoptosis. All these reports further confirm our findings that autophagy inhibition could enhance cell apoptosis. These results indicated that autophagy may exhibit a prosurvival effect (Figure 6).
Figure 6.
The overview of TET attenuated BUP-induced neurotoxicity in SH-SY5Y cells. BUP-induced SH-SY5Y cell apoptosis and oxidative damage were reversed by TET treatment. In addition, TET induced SH-SY5Y cell autophagy, and autophagy exerts prosurvival and antioxidant roles.
Abbreviations: BUP, bupivacaine; monodansylcadaverine; TET, tetramethylpyrazine.
GSH and SOD are intracellular antioxidants, while MDA serves as an indicator of lipid peroxidation, which all play a vital role in the action of apoptosis and autophagy.29,30 In this study, TET treatment increased the total GS and SOD levels. Huang et al indicated that Xiangxi flavor vinegar exhibits the effect of antioxidant via upregulatiton of the levels of GSH and SOD in C. elegans.31 This result verified that TET may act as an antioxidant property. Meanwhile, BUP-induced total GS and SOD downregulation and MDA upregulation were reversed by TET treatment. Consistent with our findings, Guo et al also found that TET could attenuate cardiac function from myocardial injury via increasing the level of GSH and SOD and decreasing MDA.32 These data indicated that TET could reverse BUP-induced oxidative damage in SH-SY5Y cells. However, when 3‐MA was present, the level of total GS, SOD and MDA returned to the level of the BUP group. All these reports confirm our findings that inhibition of autophagy could enhance oxidative damage in SH-SY5Y cells. These results indicated that autophagy may exert an antioxidant role (Figure 6). A previous study found that SOD could suppress BUP-provoked apoptosis of human monocytic cells.33 Li et al indicated that BUP induces neurotoxicity through inducing excessive ROS, but the underlying mechanism remains unclear.34 Similarly, limitation of this study should be noted. We did not investigate the mechanisms by which TET protects BUP-induced cytotoxicity on SH-SY5Y cells from the perspective of oxidative stress.
Conclusion
The present study indicated for the first time that TET induced apoptosis, autophagy and antioxidant activity in SH-SY5Y cells. These results also demonstrated that autophagy may play prosurvival and antioxidant effects in BUP-treated SH-SY5Y cells. Therefore, these findings indicated that TET may serve as a potential agent for the treatment of human neurotoxicity induced by BUP.
Author contributions
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work .
References
- 1.Fan Y-L, Li H-C, Zhao W, et al. Curcumin attenuated bupivacaine-induced neurotoxicity in SH-SY5Y cells via activation of the Akt signaling pathway. Neurochem Res. 2016;41(9):2425–2432. doi: 10.1007/s11064-016-1955-4 [DOI] [PubMed] [Google Scholar]
- 2.Yu X-J, Zhao W, Li Y-J, et al. Neurotoxicity comparison of two types of local anaesthetics: amide-bupivacaine versus ester-procaine. Sci Rep. 2017;7:45316. doi: 10.1038/srep45316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hampl K, Steinfeldt T, Wulf H. Spinal anesthesia revisited: toxicity of new and old drugs and compounds. Curr Opin Anaesthesiol. 2014;27(5):549–555. doi: 10.1097/ACO.0000000000000108 [DOI] [PubMed] [Google Scholar]
- 4.Guo J, Wang H, Tao Q, et al. Antidepressant imipramine protects bupivacaine-induced neurotoxicity in dorsal root ganglion neurons through coactivation of TrkA and TrkB. J Cell Biochem. 2017;118(11):3960–3967. doi: 10.1002/jcb.26051 [DOI] [PubMed] [Google Scholar]
- 5.Chen L, Wang X, Huang W, et al. MicroRNA-137 and its downstream target LSD1 inversely regulate anesthetics-induced neurotoxicity in dorsal root ganglion neurons. Brain Res Bull. 2017;135:1–7. doi: 10.1016/j.brainresbull.2017.09.004 [DOI] [PubMed] [Google Scholar]
- 6.Kendall MC, Castro Alves LJ, De Oliveira G Jr. Liposome bupivacaine compared to plain local anesthetics to reduce postsurgical pain: an updated meta-analysis of randomized controlled trials. Pain Res Treat. 2018;2018:5710169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li H, Yang X, Shi W, et al. Protective effects of tetramethylpyrazine on cerebrovascular regulations in rats with chronic alcoholic encephalopathy. Biomed Environ Sci. 2015;28(9):691–695. doi: 10.3967/bes2015.010 [DOI] [PubMed] [Google Scholar]
- 8.Bai X-Y, Wang X-F, Zhang L-S, Du P-C, Cao Z, Hou Y. Tetramethylpyrazine ameliorates experimental autoimmune encephalomyelitis by modulating the inflammatory response. Biochem Biophys Res Commun. 2018;503:1968–1972. doi: 10.1016/j.bbrc.2018.07.143 [DOI] [PubMed] [Google Scholar]
- 9.Kao T-K, Ou Y-C, Kuo J-S, et al. Neuroprotection by tetramethylpyrazine against ischemic brain injury in rats. Neurochem Int. 2006;48(3):166–176. doi: 10.1016/j.neuint.2005.10.008 [DOI] [PubMed] [Google Scholar]
- 10.Feng L, Ke N, Cheng F, et al. The protective mechanism of ligustrazine against renal ischemia/reperfusion injury. J Surg Res. 2011;166(2):298–305. doi: 10.1016/j.jss.2009.04.005 [DOI] [PubMed] [Google Scholar]
- 11.Lv L, Jiang S-S, Xu J, Gong J-B, Cheng Y. Protective effect of ligustrazine against myocardial ischaemia reperfusion in rats: the role of endothelial nitric oxide synthase. Clin Exp Pharmacol Physiol. 2012;39(1):20–27. doi: 10.1111/j.1440-1681.2011.05628.x [DOI] [PubMed] [Google Scholar]
- 12.Hu S, Hu H, Mak S, et al. A novel tetramethylpyrazine derivative prophylactically protects against glutamate-induced excitotoxicity in primary neurons through the blockage of N-methyl-D-aspartate receptor. Front Pharmacol. 2018;9:73. doi: 10.3389/fphar.2018.00073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu S, Wang L, Mak S, et al. Potent protection against MPP(+)-induced neurotoxicity via activating transcription factor MEF2D by a novel derivative of naturally occurring danshensu/tetramethylpyrazine. Neuromolecular Med. 2016;18(4):561–572. doi: 10.1007/s12017-016-8399-5 [DOI] [PubMed] [Google Scholar]
- 14.Scholzen T, Gerdes J. The Ki-67 protein: from the known and the unknown. J Cell Physiol. 2000;182(3):311–322. doi: [DOI] [PubMed] [Google Scholar]
- 15.Liu X, Xu Y, Zhang L, Liu T, Zhang H. Prucalopride inhibits proliferation of ovarian cancer cells via phosphatidylinositol 3-kinase (PI3K) signaling pathway. Med Sci Monit. 2018;24:4137–4145. doi: 10.12659/MSM.907853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang B, Liu Y, Zhang J. Silencing of miR-19a-3p enhances osteosarcoma cells chemosensitivity by elevating the expression of tumor suppressor PTEN. Oncol Lett. 2019;17(1):414–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ou Y, Guo XL, Zhai L, Liu XY, Cheng YN. TMPDP, a tetramethylpyrazine derivative, protects vascular endothelial cells from oxidation damage by hydrogen peroxide. Pharmazie. 2010;65(10):755–759. [PubMed] [Google Scholar]
- 18.Danduga R, Dondapati SR, Kola PK, et al. Neuroprotective activity of tetramethylpyrazine against 3-nitropropionic acid induced Huntington’s disease-like symptoms in rats. Biomed Pharmacother. 2018;105:1254–1268. doi: 10.1016/j.biopha.2018.06.079 [DOI] [PubMed] [Google Scholar]
- 19.Chen L, Li Q, Wang H, et al. Paeoniflorin attenuated bupivacaine-induced neurotoxicity in SH-SY5Y cells via suppression of the p38 MAPK pathway. J Cell Biochem. 2018. doi: 10.1002/jcb.27964. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 20.Wang LY, Li X, Han YZ. Neuroprotection by epigallo catechin gallate against bupivacaine anesthesia induced toxicity involves modulation of PI3/Akt/PTEN signalling in N2a and SH-SY5Y cells. Int J Clin Exp Med. 2015;8(9):15065–15075. [PMC free article] [PubMed] [Google Scholar]
- 21.Xiong J, Kong Q, Dai L, et al. Autophagy activated by tuberin/mTOR/p70S6K suppression is a protective mechanism against local anaesthetics neurotoxicity. J Cell Mol Med. 2017;21(3):579–587. doi: 10.1111/jcmm.13003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chauhan S, Ahmed Z, Bradfute SB, et al. Pharmaceutical screen identifies novel target processes for activation of autophagy with a broad translational potential. Nat Commun. 2015;6:8620. doi: 10.1038/ncomms9620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Makoukji J, Saadeh F, Mansour KA, et al. Flupirtine derivatives as potential treatment for the neuronal ceroid lipofuscinoses. Ann Clin Transl Neurol. 2018;5(9):1089–1103. doi: 10.1002/acn3.625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kinarivala N, Patel R, Boustany RM, Al-Ahmad A, Trippier PC. Discovery of aromatic carbamates that confer neuroprotective activity by enhancing autophagy and inducing the anti-apoptotic protein B-cell lymphoma 2 (Bcl-2). J Med Chem. 2017;60(23):9739–9756. doi: 10.1021/acs.jmedchem.7b00407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shan Y, Sun S, Yang F, Shang N, Liu H. Dexmedetomidine protects the developing rat brain against the neurotoxicity wrought by sevoflurane: role of autophagy and Drp1-Bax signaling. Drug Des Devel Ther. 2018;12:3617–123624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu B, Ruan M, Liang T, et al. Tetramethylpyrazine phosphate and borneol combination therapy synergistically attenuated ischemia-reperfusion injury of the hypothalamus and striatum via regulation of apoptosis and autophagy in a rat model. Am J Transl Res. 2017;9(11):4807–4820. [PMC free article] [PubMed] [Google Scholar]
- 27.Wang L, Zhang H-Y, Gao B, et al. Tetramethylpyrazine protects against glucocorticoid-induced apoptosis by promoting autophagy in mesenchymal stem cells and improves bone mass in glucocorticoid-induced osteoporosis rats. Stem Cells Dev. 2017;26(6):419–430. doi: 10.1089/scd.2016.0233 [DOI] [PubMed] [Google Scholar]
- 28.Li R, Zhou P, Guo Y, Lee JS, Zhou B. Tris (1, 3-dichloro-2-propyl) phosphate induces apoptosis and autophagy in SH-SY5Y cells: involvement of ROS-mediated AMPK/mTOR/ULK1 pathways. Food Chem Toxicol. 2017;100:183–196. doi: 10.1016/j.fct.2016.12.037 [DOI] [PubMed] [Google Scholar]
- 29.Cao J, Miao Q, Miao S, et al. Tetramethylpyrazine (TMP) exerts antitumor effects by inducing apoptosis and autophagy in hepatocellular carcinoma. Int Immunopharmacol. 2015;26(1):212–220. doi: 10.1016/j.intimp.2015.04.009 [DOI] [PubMed] [Google Scholar]
- 30.Sun Z-W, Zhang L, Zhu S-J, Chen W-C, Mei B. Excitotoxicity effects of glutamate on human neuroblastoma SH-SY5Y cells via oxidative damage. Neurosci Bull. 2010;26(1):8–16. doi: 10.1007/s12264-010-0813-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang RT, Huang Q, Wu GL, Chen CG, Li ZJ. Evaluation of the antioxidant property and effects in Caenorhabditis elegans of Xiangxi flavor vinegar, a Hunan local traditional vinegar. J Zhejiang Univ Sci B. 2017;18(4):324–333. doi: 10.1631/jzus.B1600293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guo L, Wang A, Sun Y, Xu C. Evaluation of antioxidant and immunity function of tetramethylpyrazine phosphate tablets in vivo. Molecules (Basel, Switzerland). 2012;17(5):5412–5421. doi: 10.3390/molecules17055412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Azma T, Ogawa S, Nishioka A, et al. Involvement of superoxide generated by NADPH oxidase in the shedding of procoagulant vesicles from human monocytic cells exposed to bupivacaine. J Thromb Thrombolysis. 2017;44(3):341–354. doi: 10.1007/s11239-017-1531-z [DOI] [PubMed] [Google Scholar]
- 34.Li YJ, Zhao W, Yu XJ, et al. Activation of p47phox as a mechanism of bupivacaine-induced burst production of reactive oxygen species and neural toxicity. Oxid Med Cell Longev. 2017;2017:8539026. doi: 10.1155/2017/8539026 [DOI] [PMC free article] [PubMed] [Google Scholar]