Abstract
Purpose: Aloin (ALO), a bioactive ingredient extracted from aloe vera, has anti-tumor effects. High Mobility Group Box 1 (HMGB1), a highly conserved nuclear DNA-binding protein, has been implicated in various cancer types. Highly expressed HMGB1 is closely associated with tumor cells apoptosis, proliferation and migration. We investigated the specific molecular mechanisms by which ALO-induced apoptosis by targeting HMGB1 in gastric cancer cells.
Materials and methods: Human gastric cancer HGC-27 cells were treated with different doses of ALO (100, 200 and 400 µg/ml) for 24 h, after which DAPI staining was used to observe the nuclear morphology, Annexin V/PI double staining assay was used to determine the rate of apoptosis; Western blotting was used to detect the levels of PARP, pro-caspase3, HMGB1 and RAGE; nuclear translocation of HMGB1 was determined by conducting a nucleoplasm separation experiment. The Enzyme linked immunosorbent assay (ELISA) assay was used to detect release of HMGB1. The HGC-27 cells, transfected with HMGB1 shRNA plasmids, were stimulated with ALO for 24 h, after which a flow cytometry assay was used to detect the rate of apoptosis. HGC-27 cells were pre-treated with or without ALO and then stimulated with rhHMGB1, the phosphorylation of Akt, mTOR, P70S6K, S6, 4EBP1, ERK, P90RSK, cAMP regulatory element binding (CREB) were detected by Western blotting.
Results: After different doses of ALO treatment, the nuclei showed morphological changes characteristic of apoptosis. Apoptotic rates were enhanced in a dose dependent manner. The level of cleaved PARP was enhanced and pro-caspase3, HMGB1 and RAGE levels were reduced, HMGB1 nuclear translocation and release were inhibited. The activation of rhHMGB1-induced Akt-mTOR-P70S6K and ERK-CREB signalling pathways was inhibited by ALO. Blocking these signalling pathways by special inhibitors and HMGB1 knockdown could enhance ALO-induced HGC-27 cell apoptosis.
Conclusion: ALO- induced HGC-27 cell apoptosis by down-regulating expressions of HMGB1 and RAGE, inhibiting HMGB1 release and then suppressing rhHMGB1-induced activation of Akt-mTOR-P70S6K and ERK-P90RSK-CREB signalling pathways.
Keywords: Aloin, gastric cancer, HMGB1, Akt, mTOR, P70S6K, ERK, P90RSK, CREB, apoptosis
Introduction
Gastric cancer is a common malignancy tumor in China, and is associated with high morbidity and mortality. At present, chemotherapy, radiotherapy and surgery are the main treatments for gastric cancer. However, due to the lack of early diagnostic markers and the characteristics of easy metastasis, the therapeutic effects against gastric cancer is remain unsatisfactory.1,2 Therefore, searching for biological targets for early diagnosis of gastric cancer will assist gastric cancer treatment and prognosis.
The High Mobility Group Box 1 (HMGB1) is a highly conserved nuclear DNA-binding protein.3 As a nuclear protein, HMGB1 plays an important role in DNA repair, transcription and differentiation.4 HMGB1 can also be released into the extracellular space by active secretion or passive release.5,6 As an extracellular signal molecule, HMGB1 induces “secondary biological effects” by binding with its receptors, such as RAGE or TLRs, after which it is involved in the onset and development of various diseases, including inflammation and cancer.7,8 HMGB1 is highly expressed in many malignant tumors and reportedly plays an important role in tumor apoptosis, proliferation and metastasis. It is also considered an early biological target of many malignant tumors.9,10 Inhibition of HMGB1 enhances hepatoma cell apoptosis induced by doxorubicin.11 In addition, the correlation of HMGB1 and gastric cancer has been reported. In gastric cancer, highly expressed HMGB1 promotes the cell proliferation and migration by activating NF-кB and ERK signal pathways,12,13 HMGB1 silencing sensitises cells to oxaliplatin and induces MGC-803 cell apoptosis.8 These studies suggest that HMGB1 may be considered a novel therapeutic target.
Aloin (ALO), is a natural compound extracted from aloe vera. It has anti-cancer abilities and can inhibit cell proliferation and induce apoptosis of lung, colorectal and breast cancers.14–16 Our previous study also showed that ALO could induce gastric cancer cell apoptosis by activating the MAPKs signalling pathway.17 However, it is unknown whether ALO induces apoptosis of gastric cancer cells by targeting HMGB1.
In this study, we mainly explored the role of HMGB1 in ALO-induced gastric cancer cells apoptosis and further investigated the underlying molecular mechanism. Our data shows that ALO induces apoptosis in gastric cancer HGC-27 cells via decreasing the expression and release of HMGB1, inhibiting the activation of rhHMGB1-induced Akt-mTOR-P70S6K and ERK-P90RSK-cAMP regulatory element binding (CREB) signalling pathways. This study will provide experimental basis for HMGB1 as a therapeutic target for gastric cancer and provide a new perspective for the effect of ALO on apoptosis of gastric cancer cells.
Materials and methods
Antibodies and reagents
ALO, Rapamycin, LY294002, U0126 all were purchased from Selleck Chemicals (Houston,TX, USA), rhHMGB1 was purchased from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany), DAPI was obtained from Thermo Fisher Scientific, Inc. (Waltham, MA, USA). Human HMGB1 ELISA kit was bought from CUSABIO (Wuhan, China); CCK-8 kit and Annexin V/PI apoptotic kit were purchased from KeyGen Biotech Co., Ltd. (Nanjing, China); Primary antibodies of β-actin, PARP, pro-caspase3, cleaved caspase3, p-Akt, Akt p-mTOR, mTOR, p-P70S6K, P70S6K, p-S6, S6, p-4EBP1, 4EBP1, p-ERK, p-P90RSK, p-CREB all were purchased from Cell Signalling Technology (Beverly, MA, USA), Secondary antibodies coupled to IRDye800 fluorophore for use with the Odyssey Infrared Imaging System were purchased from LI-COR Biosciences (Lincoln, Nebraska, USA). Image J version 1.52 software (National Institutes of Health) was used for the densitometry analysis.
Cell culture
A human undifferentiated gastric cancer HGC‑27 cell line was bought from GuangZhou Cellcook Biotech Co., Ltd. (Guangzhou, China). The cells were cultured in RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) containing 10% fetal bovine serum (FBS; Lonsera, South America), 10% non‑essential amino acids (NEAA), 100 μg/ml streptomycin and 100 U/ml penicillin (Beyotime Institute of Biotechnology, Haimen, China), and incubated in a cell incubator at 37°C and 5% CO2.
Cell counting kit‑8 (CCK‑8) assay
Cell viability was detected by CCK-8 kit according to the manufacturer’s protocol. In brief, HGC‑27 cells were planted in 96 well cell culture plates, following incubation with different concentrations of ALO (25, 50, 100, 200, 300, 400 μg/ml) for 24 h. We added 10 μl/well of CCK-8 working fluid, and after incubation for another 2 h, the absorbance at 450 nm was measured using a MultiskanTM GO plate reader (Thermo Fisher Scientific, Inc.). These data are shown as mean ± SD.
Morphological examination of apoptotic nuclei using DAPI staining
HGC-27 cells were seeded in 12 well cell culture plates, after treatment with different doses of ALO (100, 200, 400 μg/ml) for 24 h, the cell medium was discarded. The cells in the 12 well plates were washed with PBS and fixed with 4% paraformaldehyde for 30 min at room temperature followed by DAPI staining in the dark. The nuclei morphology was observed and photographed under inverted fluorescence microscope (Olympus, Japan).
Annexin V-FITC/PI double staining assay
Apoptotic rate was determined by Annexin V/PI double staining assay. Briefly, after treatment, HGC‑27 cells were collected and twice washed with PBS. Cells were resuspended in 500 μl of binding buffer, then 5 μl PI and 5 μl Annexin V -fluorescein isothiocyanate (FITC) were added. After staining in the dark, apoptotic rate was detected by flow cytometry (BD Biosciences, Franklin Lakes, NJ, USA).
Nucleoplasm separation experiment
After treatment with ALO, the cytoplasmic and nuclear proteins of HGC-27 cells were extracted using a Nuclear and Cytoplasmic Protein Extraction kit (P0028; Beyotime Institute of Biotechnology) according to the instruction provided by the manufacturer.
Western blotting
After treatment, HGC-27 cells were rinsed with cold PBS three times and then lysed in RIPA buffer with added protease inhibitors (Beyotime Institute of Biotechnology, Haimen, China) for 30 min on ice. Lysates were centrifuged (12,000 rpm) at 4°C for 15 min, after which the supernatant was collected. Protein concentration was determined by bicinchoninic acid assay. Equal total protein of each sample was loaded onto 10 or 12% gels for SDS‑PAGE and transferred to nitrocellulose membranes (Pall Corporation, Port Washington, NY, USA), which were blocked with 5% skim milk powder in TBST for 1 h at room temperature, then incubated with the indicated primary antibodies overnight at 4°C. After washing three times in TBST, the membranes were incubated with IRDye800 fluorophore‑conjugated secondary antibody for 1 h at room temperature in the dark. The protein expressions were detected using the LI‑COR Odyssey Infrared Imaging System (LI‑COR Biosciences) and Image J 1.52 software was used to quantify the proteins.
Enzyme linked immunosorbent assay (ELISA)
HGC-27 cells were incubated with ALO for 24 h. After this, the cell medium was collected, and HMGB1 levels in cell culture supernatant were examined using a human HMGB1 ELISA kit (CUSABIO, Wuhan, China), as per the instructions of the manufacture. Each treatment was performed in triplicate, the resultant data are shown as mean ± SD.
HMGB1 interference plasmids and cell transfection
HMGB1 interference plasmids and negative plasmids were purchased from GeneChem Co., Ltd. (Shanghai, China). HGC-27 cells were seeded in 6 well cell culture plates. When the cell density reached 80%, HMGB1 interference and negative plasmids were transfected using the Lipofectamine® 3000 Reagent (Thermo Fisher Scientific, Inc.). Operations were performed according to the manufacturer′s instructions. The interference efficiency was detected by using Western blotting.
Statistical analysis
All data are expressed as mean ± SD. Statistical analyses were performed using SPSS Version 17.0 software (SPSS, Inc., Chicago, IL, USA). The results were compared using one-way ANOVA. P<0.05 was taken as a statistically significant difference.
Results
Aloin reduced HGC-27 cells viability and induced cellular apoptosis or death
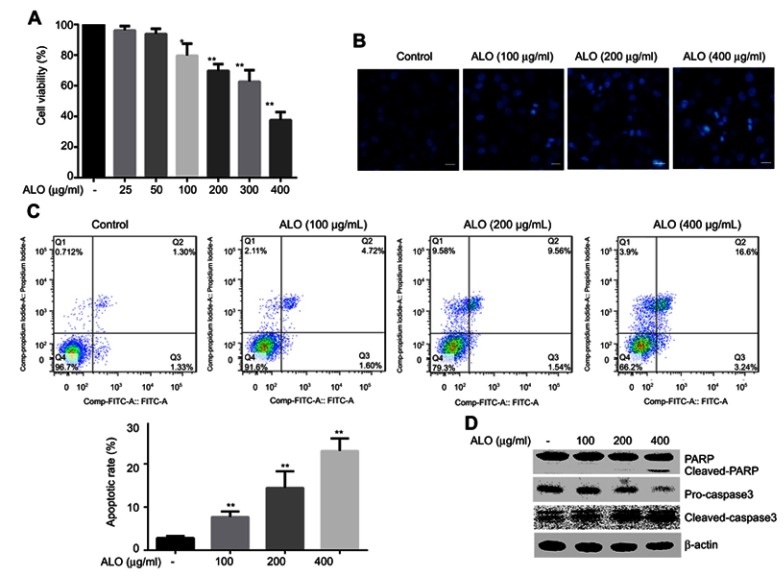
We first detected the effect of ALO on HGC-27 cell viability. HGC-27 cells viability was clearly inhibited after ALO treatment, and the inhibitory effect was concentration dependent (Figure 1A). According to this result, we selected 100, 200 and 400 μg/ml of ALO for the subsequent experiments. Secondly, the effects of ALO on HGC-27 cell apoptosis were detected through DAPI staining and Annexin V/PI double staining assays. These results showed that the nuclear sizes were uniform, and the cells of the control group had a light blue, round nuclear morphology. However, ALO treated cells showed different nuclear sizes and clear nuclear condensation (Figure 1B). Flow cytometry assay data showed that the apoptotic rate was 2.83% in the control group, whereas it was 7.73 after 100 μg/ml ALO,14.49 after 200 μg/ml ALO and 23.04% 400 μg/ml ALO treatments (Figure 1C). Additionally, ALO-induced expression of cleaved PARP increased, while pro-caspase3 levels of decreased in a dose dependent manner (Figure 1D). Taken together, these results suggested that ALO could induce apoptosis of gastric cancer HGC-27 cells.
Figure 1.
ALO reduced HGC-27 cell viability and induced cell apoptosis or death. HGC-27 cells were treated with different doses of ALO for 24 h, after which the cell viability was determined by CCK-8 assay (A). Nuclear morphology was observed using DAPI staining, magnification ×100; Scale bar, 100 µm (B).The apoptotic rate of HGC-27 cells was detected using a flow cytometry assay. The experiment was independently repeated for three times, and the representative results are shown (C). The expressions of PARP and pro-caspase3 were determined by Western blotting, β-actin was used as a loading control (D). Data are presented as mean ± SD of three independent experiments. * p<0.05 and ** p<0.01 vs the control group.
Abbreviation: ALO, aloin.
Aloin reduced the expression and release of HMGB1 in HGC-27 cells
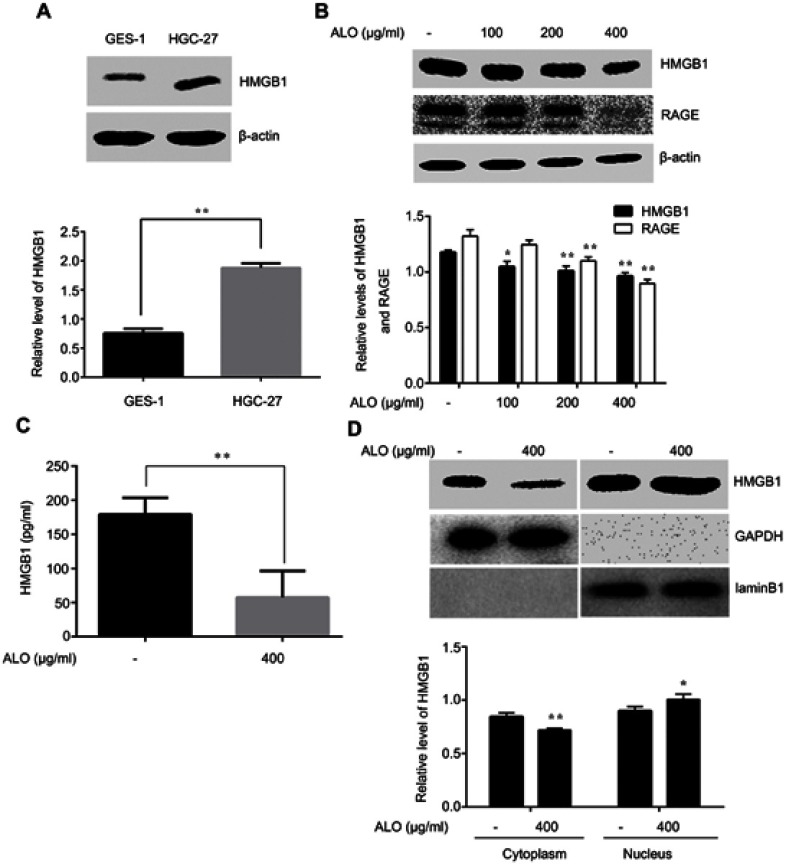
During tumor development, HMGB1 acts as both a pro- and anti-tumor protein by either promoting or suppressing tumor growth, proliferation, angiogenesis, invasion and metastasis.18 In order to prove the correlation between HMGB1 and gastric cancer, we detected the total level of HMGB1 in normal gastric epithelial cells GES-1 and gastric cancer cells HGC-27 using Western blotting. Our results showed that HMGB1 expression in HGC-27 cells was higher than that in GES-1 cells (Figure 2A). This result implied that HMGB1 might act as a target for gastric cancer treatment.
Figure 2.
ALO suppressed the expression and release of HMGB1 in HGC-27 cells. HMGB1 levels in normal gastric epithelial GES-1 and gastric cancer HGC-27 cells were detected by Western blotting (A). Data are presented as mean ± SD of three independent experiments. ** p<0.01 vs GES-1 cells. HGC-27 cells were treated with different doses of ALO (100, 200 and 400 μg/ml) for 24 h, total proteins and cell culture supernatant were extracted and the expressions of HMGB1 and RAGE were determined by using Western blotting (B). HMGB1 release was determined by ELISA assay (C). Nuclear translocation of HMGB1 was detected by completion of a nucleoplasm separation experiment (D). Data are presented as mean ± SD of three independent experiments. *p<0.05 and ** p<0.01 vs control group.
Abbreviation: ALO, aloin.
We further examined the effect of ALO on HMGB1 expression and release. The levels of total HMGB1 and RAGE were detected by Western blotting, the release of HMGB1 was determined by using ELISA assay, and the nuclear translocation of HMGB1 was examined usig a nucleoplasm separation experiment. Figure 2B shows that ALO reduced the expression of HMGB1 in a dose dependent manner. In addition, our results also indicated that the HMGB1 level in cell culture supernatant was decreased after ALO treatment (Figure 2C). In the control group, the cytoplasmic HMGB1 level was higher than that in the ALO group, while the expression of HMGB1 in nuclear proteins showed the opposite change (Figure 2D). Collectively, these results suggested that ALO not only inhibited HMGB1 expression, but also blocked HMGB1 release.
Down-regulation of HMGB1 expression enhanced Aloin induced HGC-27 cells apoptosis
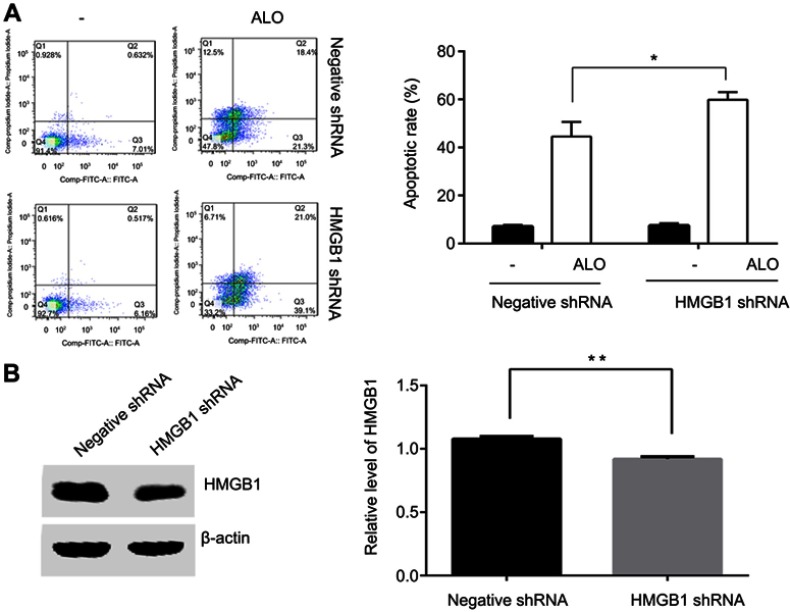
It has been reported that knockdown HMGB1 by shRNA transfection or inhibition of HMGB1 release enhanced melphalan-induced the apoptosis of human pancreatic cancer Panc2.03 cells and colon cancer HCT116 cells.19 In gastric cancer HGC-27 cells, we sought to determine whether HMGB1 affected ALO-induced apoptosis. HGC-27 cells were transfected with HMGB1 shRNA and negative plasmids respectively. After 48 h transfection, the cells were stimulated with 400 μg/ml of ALO for 24 h. The apoptotic rate was detected by flow cytometry assay. As shown in Figure 3A, in cells transfected with negative plasmids, the apoptotic rate was 44.58% after ALO treatment. However, the rate of apoptosis increased to 59.86% in HMGB1 shRNA transfected cells. The interference efficiency of HMGB1 shRNA is shown in Figure 3B. This result signified that down-regulation of HMGB1 enhanced ALO-induced HGC-27 apoptosis.
Figure 3.
Down-regulation of HMGB1 enhanced ALO-induced HGC-27 cell apoptosis. HGC-27 cells were transfected with negative and HMGB1 shRNA plasmids respectively. After 48 h transfection, the cells were treated with 400 μg/ml of ALO for 24 h, after which the apoptotic rates were detected by flow cytometry (A). After transfection, total proteins were extracted, and HMGB1 interference efficiency was examined by Western blotting (B). The experiment was repeated three times and data are shown in mean ± SD.*p<0.05 and ** p<0.01 vs the negative plasmids transfection group.
Abbreviation: ALO, aloin.
Aloin inhibited rhHMGB1-induced activation of Akt-mTOR-P70S6K and ERK-P90RSK-CREB signalling pathways
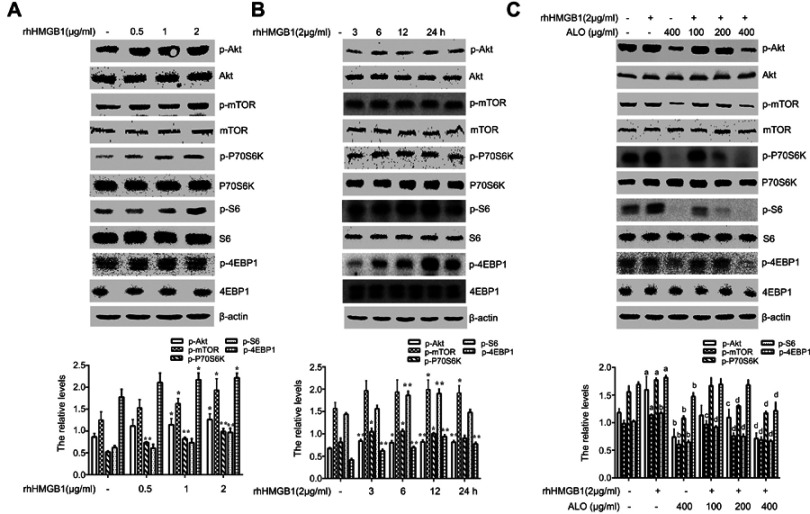
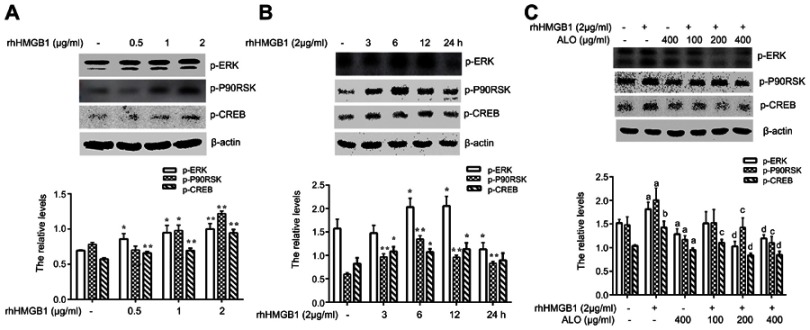
Our above results showed that ALO could reduce the expression levels of HMGB1 and its receptor RAGE and inhibit the release of HMGB1. Additionally, HMGB1 acts as an extracellular signal molecule can induce many signal pathways including MAPKs, Akt and NF-кB activation through binding with receptors.8,20 Among the important signal molecules, Akt and ERK have an important relationship with cancer initiation and progression.21 Thus, we further investigated whether ALO affected HMGB1-induced Akt and ERK signalling pathways activation. HGC-27 cells were first treated with different doses of rhHMGB1 (0.5, 1 and 2 μg/ml) for 12 h, the phosphorylation of Akt and ERK signaling pathways were detected by Western blotting. Figures 4A and 5A show that rhHMGB1 enhanced the phosphorylation of Akt-mTOR-P70RSK and ERK-P90RSK-CREB in a dose dependent manner. Secondly, HGC-27 cells were treated with 2 μg/ml of rhHMGB1 for the different times (3, 6, 12 and 24 h), the activation of these signal pathways was also detected by using Western blot. As shown in Figure 4B, the phosphorylation of Akt, mTOR and P70S6K began to increase around 3 h, peaking at 6~12 h and then decreased around 24 h. The activation of ERK, P90RSK and CREB also showed the same change (Figure 5B). Lastly, HGC-27 cells were pre-treated with different doses of ALO for 2 h, and then stimulated with rhHMGB1 for 12 h, the phosphorylation of Akt-mTOR-P70RSK and ERK-P90RSK-CREB signal pathways were determined by Western blot. Figures 4C and 5C showed that aloin could inhibit rhHMGB1-induced the phosphorylation of the above signalling pathways.
Figure 4.
Aloin (ALO) inhibited the activation of the Akt-mTOR-P70S6K signalling pathway induced by rhHMGB1. HGC-27 cells were treated with different doses of rhHMGB1 for 12 h (A), or with 2 µg/ml of rhHMGB1 at different times (3, 6, 12, 24 h) (B), the phosphorylatio and total amount of Akt, mTOR, P70S6K, S6, 4EBP1 were detected by Western blotting. The relative levels were normalized by β-actin. Data are presented as mean ± SD of three independent experiments. *p<0.05 and ** p<0.01 vs control group. HGC-27 cells were pre-treated with different doses of ALO for 2 h, and then stimulated with 2 µg/ml of rhHMGB1 for 12 h (C). The phosphorylation and total amount of Akt, mTOR, P70S6K, S6, 4EBP1 were detected by Western blotting. The relative levels were normalized by β-actin. Data are presented as mean ± SD of three independent experiments. ap<0.05 and bp<0.01 vs control group, cp<0.05 and dp<0.01 vs rhHMGB1 group.
Figure 5.
ALO inhibited the activation of the ERK-P90RSK-CREB signalling pathway induced by rhHMGB1 in HGC-27 cells. HGC-27 cells were treated with different doses of rhHMGB1 for 12 h (A), or with 2 µg/ml of rhHMGB1 over different time points (B). The phosphorylation of ERK, P90RSK and CREB were detected by Western blotting. Data are presented as mean ± SD of three independent experiments. *p<0.05 and ** p<0.01 vs control group. HGC-27 cells were pre-treated with different doses of ALO for 2 h, and then stimulated with 2 µg/ml of rhHMGB1 for 12 h (C). The phosphorylation of ERK, P90RSK and CREB were detected by Western blotting. The relative levels were normalized by β-actin. Data are presented as mean ± SD of three independent experiments. ap<0.05 and bp<0.01 vs control group, cp<0.05 and dp<0.01 vs rhHMGB1 group.
Abbreviation: ALO, aloin.
Inhibitory the activation of Akt、mTOR and ERK enhanced Aloin induced HGC-27 cells apoptosis
To prove Akt and ERK pathways involved in ALO-induced HGC-27 cell apoptosis, LY294002,Rapamycin and U0126, the special inhibitor of Akt, mTOR and ERK were used. Firstly, the inhibitory effect of Rapamycin was investigated. HGC-27 cells were treated with different doses of Rapamycin (25, 50, 100 nM) for 30 or 60 min, the phosphorylation of mTOR was detected by Western blot. As shown in Figure 6A, Rapamycin suppressed phosphorylation of mTOR, In particular, 25 nM of Rapamycin for 1 hr exerted a significant inhibitory effect. Secondly, the inhibitory effect of LY294002, Rapamycin and U0126 on phosphorylation of Akt, mTOR and ERK respectively was tested by using Western blot. Figure 6B shows that treatment of HGC-27 cells with LY294002 (15 μM), Rapamycin (25 nM) and U0126 (20 μM) decreased the phosphorylation of Akt, mTOR and ERK respectively. Finally, we examined ALO-induced inhibition of gastric cancer cell apoptosis. HGC-27 cells were pre-treated with inhibitor respectively for 1 h, and then stimulated with ALO for 24 h, DAPI staining and flow cytometry assay were used to detect gastric cancer cells apoptosis. Here, the inhibitor enhanced nuclear condensation and fragmentation induced byALO in HGC-27 cells (Figure 6C). The apoptotic rate in ALO treated cells was 22.31%. However, LY294002, Rapamycin and U0126 pre-treatment all increased ALO-induced apoptosis to 36.31%, 32.99%and 30.32% respectively (Figure 6D).
Figure 6.
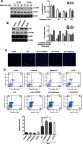
Inhibition of Akt, mTOR and ERK activation enhanced ALO-induced HGC-27 cells apoptosis. HGC-27 cells were treated with different doses of Rapamycin (25, 50, 100 nM) for 30 or 60 min, p-mTOR and mTOR expressions were detected by Western blot (A). HGC-27 cells were treated with LY294002 (15 μM), Rampmycin (25 nM) or U0126 (20 μM) for 1 h,the phosphorylation of Akt, mTOR and ERK was determined by Western blotting respectively (B). Data are presented as mean ± SD of three independent experiments. *p<0.05 and **p<0.01 vs control group. HGC-27 cells were pre-treated with LY294002 (15 μM), Rampmycin (25 nM) or U0126 (20 μM) for 1 h,and then 400 μg/ml of ALO and stimulated for another 24 h. Nuclear morphology was observed by using DAPI staining (C). The apoptotic rate of HGC-27 cells was detected by flow cytometry (D).
Abbreviation: ALO, aloin.
In conclusion, ALO-induced apoptosis of gastric cancer HGC-27 cells by inhibiting the expression and release of HMGB1. ALO also suppressed HMGB1-induced activation of Akt-mTOR-P70S6K and ERK-P90RSK-CREB signalling pathways. These results provide new insight into the anti-tumor effects of ALO on gastric cancer cells.
Discussion
We investigated the molecular mechanisms of ALO- induced gastric cancer apoptosis, achieved by targeting HMGB1. ALO inhibited HGC-27 cell viability, and induced their apoptosis (Figure 1). Although we have found that ALO could induce apoptosis of gastric cancer cells by regulating MAPKs signal pathway,17 the underlying mechanisms require further elucidation.
HMGB1 is highly expressed in cancer tissues and is closely related to the apoptosis, proliferation and migration of cancer cells. Anti-cancer therapies that target HMGB1 have attracted more and more attention.12,22 HMGB1 was more highly expressed in HGC-27 cells, compared to GES-1 cells (Figure 2A). These data suggest that HMGB1 may act as a biomarker for gastric cancer. Given this, we further explored the effects of ALO on HMGB1 expression. As shown in Figure 2B, ALO reduced HMGB1 expression in a concentration dependent manner. RAGE, a transmembrane protein, is also a receptor of HMGB1, and the expression of RAGE is closely associated with gastric and colorectal cancer invasion and metastasis of cancer.23,24 The HMGB1-RAGE signalling pathway plays an important role in the occurrence and development of tumor,25–27 and the accumulation of HMGB1 and RAGE activates a complex signalling network for cell survival and apoptosis evasion.28 Therefore, targeting of HMGB1-RAGE signalling could be a promising therapy for these types of cancer. As shown in Figure 2B, the expression level of RAGE also decreased after ALO treatment. Collectively, our results imply that ALO induces gastric cancer apoptosis by partially regulating the expressions of HMGB1 and RAGE partly.
Although at baseline HMGB1 was mainly localised to the nucleus, it can transfer to the cytoplasm and extracellular spaces. Released HMGB1 mediates diverse responses by binding to its receptors, then triggering various biological effects, including cell proliferation, differentiation and apoptosis.29 Therefore, we also explored the role of ALO in the release of HMGB1 in HGC-27 cells. As displayed in Figure 2C and D ALO clearly decreased the release and nuclear transposition of HMGB1. To further clarify the effect of HMGB1 on ALO-induced apoptosis, we down regulated HMGB1 expression by transfection of HMGB1 shRNA plasmids into HGC-27 cells. As shown in Figure 3, ALO clearly enhanced HGC-27 cell apoptosis in HMGB1 shRNA transfected cells compared with negative plasmid transfected cells. The above results guided us to explore whether ALO further activated the HMGB1-triggered intracellular signalling pathway, thereby inducing gastric cancer cell apoptosis.
The Akt-mTOR signalling pathway plays an important role in cell proliferation, survival, growth and apoptosis.30 The signalling pathway of the CREB is over-expressed and phosphorylated in many tumors, and is involved in cell proliferation, differentiation and survival. Many kinases such as Akt, ERK, P90RSK, CaMK can induce phosphorylation of CREB.31 HMGB1-RAGE interaction regulates the proliferation, migration and apoptosis of cancer cells by activating a complex signalling network that includes Akt, ERK and NF-кB.8,20 Thus, inhibition of these signalling pathways could be potential target for many cancer therapies.
Our results showed that rhHMGB1 stimulated HGC-27 cells, enhanced the phosphorylation of the Akt-mTOR-P70S6K and ERK-P90RSK-CREB signalling pathways. ALO pre-treatment clearly inhibited rhHMGB1–induced these signalling pathways activation (Figures 4 and 5). In order to test Akt-mTOR and ERK pathways involved in ALO-induced HGC-27 cells apoptosis, we used their special inhibitors to block the activation of these signalling pathways. Blocking of Akt or mTOR activation obviously enhanced ALO-induced HGC-27 cell apoptosis. Inhibition of the ERK signalling pathway also enhanced gastric cancer cell apoptosis induced by ALO, but the effect was not significant (Figure 6). The results might suggest that HMGB1 triggers Akt-mTOR-P70S6K activation and may play a main role in ALO-induced HGC-27 cell apoptosis.
ALO can inhibit the nuclear translocation and release of HMGB1, but how does ALO affect the nuclear translocation of HMGB1? Post-translational modifications of HMGB1, such as phosphorylation, acetylation and methylation, are closely related to their nuclear translocation and release.10 Is ALO-induced inhibition of HMGB1 nuclear translocation related to its post-translational modification? How does ALO affect the expressions of HMGB1 and RAGE? Does other molecular mechanisms involved in ALO-induced HGC-27 cells death? These are the problems that require future exploration.
In conclusion, ALO promoted gastric cancer cell apoptosis by inhibiting the expression and release of HMGB1, and phosphorylation of ATK-mTOR-P70S6K and ERK-P90RSK-CREB pathways. These results provide a new perspective for understanding the anti-tumor effect of ALO, as well as an experimental basis for HMGB1 as a potential target for the treatment of gastric cancer.
Acknowledgments
This work was supported by Natural Science Foundation of China (grant no. 81601380), Natural Science Research Project of Anhui Colleges and Universities (grant no. KJ2016SD59); Outstanding Young Talent Support Programme Key Projects of Anhui Colleges and Universities (grant no. gxyqZD2016173), Active Biological Macromolecules Research Provincial Key Laboratory Project (grant no. 1306C083008), National college Students‘ innovation and Entrepreneurship training programme project (grant nos. 201810368024 and 201710368002) and the Anhui Province College Students‘ Innovation and Entrepreneurship Training Programme project (grant no. 201710368166).
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Guo D, Zhang B, Liu S, et al. Xanthohumol induces apoptosis via caspase activation, regulation of Bcl-2, and inhibition of PI3K/Akt/mTOR-kinase in human gastric cancer cells. Biomed Pharmacother. 2018;106:1300–1306. [DOI] [PubMed] [Google Scholar]
- 2.Suren D, Arda Gokay A, Sayiner A. High mobility group box 1 (HMGB1) expression in gastric adenocarcinomas. J B.U.On.. 2018;23(2):422–427. [PubMed] [Google Scholar]
- 3.Tsung A, Klune JR, Zhang X, et al. HMGB1 release induced by liver ischemia involves Toll-like receptor 4 dependent reactive oxygenspecies production and calcium-mediated signaling. J Exp Med. 2007;204(12):2913–2923. doi: 10.1084/jem.20070247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu X, Wang W, Chen Y, et al. High mobility group box protein 1 serves as a potential prognostic marker of lung cancer and promotes its invasion and metastasis by matrix metalloproteinase-2 in a nuclear factor-kappaB-dependent manner. Biomed Res Int. 2018;2018:3453706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Evankovich J, Cho SW, Zhang R, et al. High mobility group box 1 release from hepatocytes during ischemia and reperfusion injury is mediated by decreased histone deacetylase activity. J Biol Chem. 2010;285(51):39888–39897. doi: 10.1074/jbc.M110.128348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zeng W, Shan W, Gao L, et al. Inhibition of HMGB1 release via salvianolic acid B-mediated SIRT1 up-regulation protects rats against non-alcoholic fatty liver disease. Sci Rep. 2015;5:16013. doi: 10.1038/srep16013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang H, Tracey KJ. Targeting HMGB1 in inflammation. Biochim Biophys Acta. 2010;1799(1–2):149–156. doi: 10.1016/j.bbagrm.2009.11.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Song B, Song WG, Li ZJ, et al. Effect of HMGB1 silencing on cell proliferation, invasion and apoptosis of MGC-803 gastric cancer cells. Cell Biochem Funct. 2012;30(1):11–17. doi: 10.1002/cbf.1811 [DOI] [PubMed] [Google Scholar]
- 9.Li Y, Tian J, Fu X, et al. Serum high mobility group box protein 1 as aclinical marker for ovarian cancer. Neoplasma. 2014;61(5):579–584. doi: 10.4149/neo_2014_070 [DOI] [PubMed] [Google Scholar]
- 10.Richard SA, Jiang Y, Xiang LH, et al. Post-translational modifications of high mobility group box 1 and cancer. Am J Transl Res. 2017;9(12):5181–5196. [PMC free article] [PubMed] [Google Scholar]
- 11.Lu L, Zhang D, Xu Y, Bai G, Lv Y, Liang J. miR-505 enhances doxorubicin-induced cytotoxicity in hepatocellular carcinoma through repressing the Akt pathway by directly targeting HMGB1. Biomed Pharmacother. 2018;104:613–621. doi: 10.1016/j.biopha.2018.05.087 [DOI] [PubMed] [Google Scholar]
- 12.Zhang J, Kou YB, Zhu JS, Chen W-X, Li S. Knockdown of HMGB1 inhibits growth and invasion of gastric cancer cells through the NF-kappaB pathway in vitro and in vivo. Int J Oncol. 2014;44(4):1268–1276. doi: 10.3892/ijo.2014.2285 [DOI] [PubMed] [Google Scholar]
- 13.Zhang QY, Wu LQ, Zhang T, Han Y-F, Lin X. Autophagy-mediated HMGB1 release promotes gastric cancer cell survival via RAGE activation of extracellular signal-regulated kinases 1/2. Oncol Rep. 2015;33(4):1630–1638. doi: 10.3892/or.2015.3782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wan L, Zhang L, Fan K, Wang J. Aloin promotes A549 cell apoptosis via the reactive oxygen speciesmitogen activated protein kinase signaling pathway and p53 phosphorylation. Mol Med Rep. 2017;16(5):5759–5768. doi: 10.3892/mmr.2017.7379 [DOI] [PubMed] [Google Scholar]
- 15.Pan Q, Pan H, Lou H, Xu Y, Tian L. Inhibition of the angiogenesis and growth of Aloin in human colorectal cancer in vitro and in vivo. Cancer Cell Int. 2013;13(1):69. doi: 10.1186/1475-2867-13-69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Esmat AY, Tomasetto C, Rio M-C. Cytotoxicity of a natural anthraquinone (Aloin) against human breast cancer cell lines with and without ErbB-2: topoisomerase IIalpha coamplification. Cancer Biol Ther. 2006;5(1):97–103. doi: 10.4161/cbt.5.1.2347 [DOI] [PubMed] [Google Scholar]
- 17.Wang ZQ, Tao H, Ma YF, et al. Aloin induces apoptosis via regulating the activation of MAPKs signaling pathway in human gastric cancer cells in vitro. Nan Fang Yi Ke Da Xue Xue Bao. 2018;38(9):1025–1031. doi: 10.12122/j.issn.1673-4254.2018.09.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He S, Cheng J, Feng X, Yu Y, Tian L, Huang Q. The dual role and therapeutic potential of high-mobility group box 1 in cancer. Oncotarget. 2017;8(38):64534–64550. doi: 10.18632/oncotarget.17885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang D, Kang R, Cheh CW, et al. HMGB1 release and redox regulates autophagy and apoptosis in cancer cells. Oncogene. 2010;29(38):5299–5310. doi: 10.1038/onc.2010.261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang Z, Zhong Z, Zhang L, et al. Down-regulation of HMGB1 expression by shRNA constructs inhibits the bioactivity of urothelial carcinoma cell lines via the NF-kappaB pathway. Sci Rep. 2015;5:12807. doi: 10.1038/srep12807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao S, Jiang Y, Zhao J, et al. Quercetin-3-methyl ether inhibits esophageal carcinogenesis by targeting the AKT/mTOR/p70S6K and MAPK pathways. Mol Carcinog. 2018;57(11):1540–1552. doi: 10.1002/mc.22876 [DOI] [PubMed] [Google Scholar]
- 22.Shen X, Hong L, Sun H, Shi M, Song Y. The expression of high-mobility group protein box 1 correlates with the progression of non-small cell lung cancer. Oncol Rep. 2009;22(3):535–539. [DOI] [PubMed] [Google Scholar]
- 23.Kuniyasu H, Oue N, Wakikawa A, et al. Expression of receptors for advanced glycation end-products (RAGE) is closely associated with the invasive and metastatic activity of gastric cancer. J Pathol. 2002;196(2):163–170. doi: 10.1002/path.1031 [DOI] [PubMed] [Google Scholar]
- 24.Sasahira T, Akama Y, Fujii K, Kuniyasu H. Expression of receptor for advanced glycation end products and HMGB1/amphoterin in colorectal adenomas. Virchows Arch. 2005;446(4):411–415. doi: 10.1007/s00428-005-1210-x [DOI] [PubMed] [Google Scholar]
- 25.Fei Q, Xiao J, Gai L, et al. HMGB1-RAGE signaling facilitates Ras-dependent Yap1 expression to drive colorectal cancer stemness and development. Mol Carcinog. 2019;58(4):500–510. [DOI] [PubMed] [Google Scholar]
- 26.Wang L, Kang FB, Wang J, et al. Downregulation of miR-205 contributes to epithelial-mesenchymal transition and invasion in triple-negative breast cancer by targeting HMGB1-RAGE signaling pathway. Anticancer Drugs. 2019;30(3):225–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li S, Gu H, Huang Y, et al. Circular RNA 101368/miR-200a axis modulates the migration of hepatocellular carcinoma through HMGB1/RAGE signaling. Cell Cycle. 2018;17(19–20):2349–2359. doi: 10.1080/15384101.2018.1526599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dhumale SS, Waghela BN, Pathak C. Quercetin protects necrotic insult and promotes apoptosis by attenuating the expression of RAGE and its ligand HMGB1 in human breast adenocarcinoma cells. IUBMB Life. 2015;67(5):361–373. doi: 10.1002/iub.1379 [DOI] [PubMed] [Google Scholar]
- 29.Huang CY, Chiang SF, Chen WT, et al. HMGB1 promotes ERK-mediated mitochondrial Drp1 phosphorylation for chemoresistance through RAGE in colorectal cancer. Cell Death Dis. 2018;9(10):1004. doi: 10.1038/s41419-018-1019-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qin G, Li P, Xue Z. Triptolide induces protective autophagy and apoptosis in human cervical cancer cells by downregulating Akt/mTOR activation. Oncol Lett. 2018;16(3):3929–3934. doi: 10.3892/ol.2018.9074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sakamoto KM, Frank DA. CREB in the pathophysiology of cancer: implications for targeting transcription factors for cancer therapy. Clin Cancer Res. 2009;15(8):2583–2587. doi: 10.1158/1078-0432.CCR-08-1137 [DOI] [PMC free article] [PubMed] [Google Scholar]