Abstract
Background: Prostate cancer (PCa) is the most frequent cancer and the third leading cause of cancer death among German men. One option for PCa early detection is prostate-specific antigen (PSA) testing, which is still under debate regarding its risk benefits. Besides recommendations on the early PCa detection, daily practice on PSA testing varies in, for example, information communication and usage of the test. This pilot study assessed potential differences between general practitioners (GPs) and urologists in handling PSA testing and guidelines on early detection of PCa.
Methods: 172 GPs belonging to the teaching network of the University of Oldenburg in Lower Saxony and Bremen and 128 practicing urologists were included in the online survey focusing on PSA testing. The questionnaire covered 43 questions on topics as the usage of the test, information communication, handling of test results and handling of/knowledge about national and international guidelines on PCa. Wether PSA testing is used in accordance with guidelines was also explored in four standardized case scenarios. Statistical analysis was done at a descriptive level.
Results: In total, 65 doctors participated in the survey (response proportion: 21.7%, n=65; 27.9%, n=48 [GPs]; 13.2%, n=17 [urologists]). Results of 41 GPs and 14 urologists were analyzed. The PSA test was judged as useful by all urologists, while almost half of the GPs valued the test as ambivalent or not useful. Urologists showed a more proactive approach of informing men on PSA testing. Regarding guidelines and recommendations on PSA testing, GPs were less familiar with them compared to the urologists. Doctors of both specialties did not always treat men in consistence with the guidelines. This was partially in contradiction to their self-appraisal.
Conclusion: This pilot study is highlighting differences in PSA testing practices between GPs and urologists in Germany. Urologists showed a more proactive approach. For further verification, we plan a more comprehensive study covering several German states.
Keywords: survey, guidelines, recommendations, testing practice, daily routine, PSA
Background
Prostate cancer (PCa) incidence varies widely around the world, with by far the highest rates in North America and Oceania.1 In Germany, PCa is the most frequent cancer and the third leading cause of cancer death among men.2 In the USA, the implementation of prostate-specific antigen (PSA) testing for the early detection of PCa in 1986 resulted in a rapid increase in PCa incidence. In Germany and other European countries, PSA testing was implemented later and increased in the 1990s.3 Potentially caused by the introduction of the PSA testing, PCa incidence strongly increased during the following years in Germany, too.4,5 Risks and benefits of this PCa screening method are controversial. Diagnosis at earlier stage is one potential advantage of the PSA testing, while disadvantages such as overdiagnosis and -treatment of harmless tumors followed by possible complications, eg, incontinence and impotence, also have to be considered.6,7 Results of large studies such as the “European Randomized Study of Screening for Prostate Cancer“ (ERSPC) and the “Prostate, Lung, Colorectal and Ovarian“ (PLCO) trial show different findings. The ERSPC shows that the PCa mortality rates are possible to decrease by PSA testing, while in the PLCO trial they first concluded that screening was not associated with PCa mortality rates, whereas later results are in consistence with the ERSPC.8–11 Study results and recommendations based on these studies influence PSA testing practice and also are essential for policy making.12–14 In contrast to breast cancer early detection, PSA testing has not been approved as a PCa early detection service of the German statutory health insurance. Therefore, in Germany, PSA testing is an individual health service which has to be paid by the patient himself.
The 2014 version of the German S3 guideline on PCa was the first one with a “dissenting opinion” by the German College of General Practitioners and Family Physicians (Deutsche Gesellschaft für Allgemeinmedizin und Familienmedizin [DEGAM]) for the use of PSA testing in the field of general practice.15 Apart from differences in guidelines between countries and organizations, this guideline also states divergent recommendations for doctors working in different specialist disciplines, namely urology versus general practice. Urologists are advised to proactively inform men of at least 45 years of age and presumed life expectancy of at least 10 years of age about PSA testing, considering pros and cons. GPs should not actively inform men about PSA testing or only if the man asks for it. Therefore, it is expected that the daily routine in medical consultation, performance and other aspects concerning PSA testing varies. Despite the high significance of informed decision-making (for example emphasized in the German S3 guideline15), the clinical experience also shows that there is big variation in practice. The way the patient is informed has a high impact on the patient’s demand and use of medical services as well as on the satisfaction with early detection by PSA testing.16,17 A GP’s recommendation clearly affects the patient’s decision for or against a PSA test.18 Furthermore, insufficient knowledge or individual conviction of the doctor influences treatment decisions in practice.19,20 A survey among medical doctors with different specialties might highlight differences in PSA testing practice and in handling guidelines on PCa by specialist discipline.
This study was part of two affiliated pilot studies, aiming to assess attitude and practice of GPs and urologists on PSA testing. In this article, we focus on the survey based on the country-specific questionnaire covering the northwest of Lower Saxony and Bremen to investigate potential differences in daily routine practice regarding PSA testing between German GPs/internists and urologists.
Methods
Study population
A survey was set up to assess potential differences in routine PSA practice. The survey covered GPs within the teaching network of the University of Oldenburg in Lower Saxony and Bremen and urologists approached by the registry of the Professional Association of German Urologists (Berufsverband der Deutschen Urologen [BvDU]). We only considered practicing urologists for our study. Overall, 172 GPs and 128 urologists were invited to participate in the online survey.
Questionnaire
The questionnaire covered 43 questions on several topics such as consultation, usage of the PSA test, processing of PSA test results, adherence to and knowledge about guidelines on PCa as well as characteristics of the respondents. Furthermore, the survey included four case scenarios to simulate situations and assess the GPs/urologist decision. The questionnaire was tested by three GPs and one urologist. The online questionnaire was developed using the software SoSci Survey (www.soscisurvey.de).
Conducting the questionnaire
For conducting the questionnaire, the GPs and urologists first received an e-mail with information on the project and the upcoming questionnaire. A few weeks later (in June 2016), the survey started and the doctors received a link to the online questionnaire via e-mail. Three weeks later, the participants received a reminding e-mail. The interval for participation was about 7 weeks.
Statistical analysis
Statistical analysis was done at descriptive level. We refer to the group urologists and the group GPs, where the latter also covers internists (n=5).
Results
Response proportions and characteristics
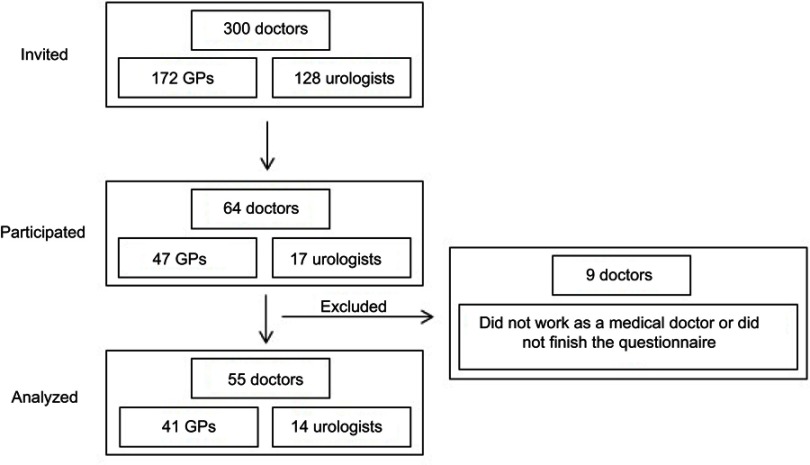
Out of 172 GPs and 128 urologists, 64 participated overall, resulting in a response proportion of 21.3% (27.3%, n=47 [GPs]; 13.3%, n=17 [urologists]). Nine participants were excluded as they either did not finish the questionnaire (n=7) or were currently not working as a medical doctor (n=2). Unfortunately, no information is available for the nonparticipating physicians. Finally, the results of 55 questionnaires were considered for the analysis (see Figure 1). Group size differs between the two specialties (41 GPs and 14 urologists).
Figure 1.
Flowchart of survey participants (general practitioners and urologists).
Abbreviation: GPs, general practitioners.
Tables 1 and 2 show the specialty and the characteristics of the respondents, respectively. The median age was 54.0 years for the GPs (IQR=10, mean=54.0, n=41) and 51.5 years for the urologists (IQR=8.75, mean=52.1, n=14). An unequal distribution was given for the sex of the respondents; there were no female urologists, while 87.8% (n=36) of the GPs were male (see Table 2).
Table 1.
Specialty of respondents
| Specialty | n | % |
|---|---|---|
| General medicine General medicine and internal medicine General medicine and surgery General medicine and anaestesia Internal medicine |
31 2 2 1 5 |
56.4 3.6 3.6 1.8 9.1 |
| Urology | 14 | 25.5 |
Table 2.
Characteristics of respondents by general practitioners and urologists, n (%)
| Variable | Categories | GPs | Urologists |
|---|---|---|---|
| Sex | Male | 36 (87.8) | 14 (100.0) |
| Female | 4 (9.8) | 0 (0.0) | |
| Others | 1 (2.4) | 0 (0.0) | |
| Specialty | Yes | 41 (100.0) | 14 (100.0) |
| No | 0 (0.0) | 0 (0.0) | |
| Type of practice | Solo | 12 (29.3) | 4 (28.6) |
| Group | 23 (56.1) | 8 (57.1) | |
| Practice sharing | 5 (12.2) | 1 (7.1) | |
| Community health center | 1 (2.4) | 1 (7.1) |
Abbreviation: GPs, general practitioners.
PSA testing practice
Questions on PSA testing are provided in Tables 3–6. More than two-third of the GPs (65.9%, n=27) and nearly 90.0% of the urologists (85.7%, n=12) stated to follow a standard procedure regarding PSA testing which is not older than 3 years for the majority (63.0%, n=17 [GPs] and 58.3%, n=7 [urologists]). All urologists indicated that they inquire if the patient wishes to do a PSA test (majority of urologists [85.7%, n=12] orally). In the GP group, 24.4% (n=10) stated that they do not ask the patient if he wishes to do a PSA test, while 73.2% (n=30) do this orally (see Table 3).
Table 3.
Questions on PSA testing I by general practitioners and urologists, n (%)
| Question | Categories | GPs | Urologists |
|---|---|---|---|
| Is there a standard procedure regarding PSA testing (in your practice)? | Yes | 27 (65.9) | 12 (85.7) |
| No | 14 (34.1) | 2 (14.3) | |
| If yes, how old is this standard? | ≤3 years | 17 (63.0) | 7 (58.3) |
| 4–9 years | 6 (22.2) | 5 (41.7) | |
| ≥10 years | 4 (14.8) | 0 (0.0) | |
| Who is responsible for medical consultation on PSA testing (in your practice)? | Doctor | 11 (26.8) | 2 (14.3) |
| Doctor, medical assistant | 3 (7.3) | 0 (0.0) | |
| No reply | 27 (65.9) | 12 (85.7) | |
| How do you ask the patient if there is a wish to do a PSA test? | Not at all | 10 (24.4) | 0 (0.0) |
| Oral | 30 (73.2) | 12 (85.7) | |
| Standardized written form | 0 (0.0) | 1 (7.1) | |
| Oral, standardized written form | 1 (2.4) | 1 (7.1) | |
| How is the consultation on PSA testing done? | Oral | 33 (80.5) | 3 (21.4) |
| Give away info material | 0 (0.0) | 1 (7.1) | |
| Oral, info material in waiting room | 1 (2.4) | 5 (35.7) | |
| Oral, give away info material | 7 (17.1) | 3 (21.4) | |
| Oral, info material in waiting room and give away | 0 (0.0) | 2 (14.3) | |
| Others namely… | |||
| - Online decision aid PSA (of the AOK) | 1 (2.4) | 0 (0.0) | |
| - recommendations on info material regarding PSA testing | 1 (2.4) | 0 (0.0) | |
| - internet links | 1 (2.4) | 0 (0.0) |
Abbreviations: AOK, Allgemeine Ortskrankenkasse, one of the German statutory health insurances; GPs, general practitioners; PSA, prostate-specific antigen.
Table 6.
Questions on PSA testing IV, n (%)
| Question | Categories | GPs | Urologists | |
|---|---|---|---|---|
| Which further actions did you take the last time having an asymptomatic patient with an increased PSA level (according to your definition of increased)? Did you… | …check the PSA level within a certain interval? | Yes | 22 (53.7) | 14 (100) |
| No | 19 (46.3) | 0 (0.0) | ||
| …directly refer the patient to a urologist? (only GPs’ replies) | Yes | 28 (68.3) | n/a | |
| No | 13 (31.7) | n/a | ||
| Others, namely | Sonography | 1 (2.4) | 1 (7.1) | |
| Rectal examination and sonography | 1 (2.4) | - | ||
| Asked if the patient smokes | 1 (2.4) | - | ||
| Consultation and wait-and-see attitude | 1 (2.4) | - | ||
| PSA control after 6 months | - | 1 (7.1) | ||
| PSA control after antibiosis | - | 1 (7.1) | ||
| Assuming you decided to check the PSA level again which, again, is conspicuous. How did you proceed with your last patient, again having an increased PSA level? Did you… | …perform a third PSA test? | Yes | 3 (21.3) | 5 (35.7) |
| No | 10 (76.9) | 9 (64.3) | ||
| …directly refer the patient to a urologist? (only GPs’ replies) | Yes | 8 (61.5) | n/a | |
| No | 5 (38.5) | n/a | ||
| Other, namely | Biopsy | - | 5 (35.5) | |
| Biopsy or MRI of the prostate | - | 1 (7.1) | ||
| Transrectal ultrasonography, if necessary biopsy | - | 1 (7.1) | ||
| Depending on the PSA level, if necessary biopsy | - | 1 (7.1) | ||
| Preclusion/treatment of infection | - | 1 (7.1) |
Abbreviations: GPs, general practitioners; PSA, prostate-specific antigen; n/a, not applicable.
Three-fourths of the GPs and all urologists always or often inform men on PSA testing during an early detection of cancer examination (75.6%, n=31 [GPs]; 100.0%, n=14 [urologists]). In case of discomfort in the lower urinary tract and unclear discomfort, GPs replies indicated a heterogeneous picture, whereas urologists showed a more proactive approach of informing men on PSA testing. Similar results can be found for the frequency of discussing certain factors with men before testing. The majority of all respondents stated to always or often discuss the listed factors (see Table 4). A more detailed table on the information communication of PSA testing can be found in Table S1.
Table 4.
Questions on PSA testing II by general practitioners and urologists, n (%)
| Question | Categories | GPs | Urologists | ||||
|---|---|---|---|---|---|---|---|
| Never + Rarely | Sometimes | Always + Often | Never + Rarely | Sometimes | Always + Often | ||
| On which occasions do you inform your patient on PSA testing? | Within an early cancer detection examination | 6 (14.6) | 4 (9.8) | 31 (75.6) | 0 (0.0) | 0 (0.0) | 14 (100.0) |
| In case of a positive family anamnesis | 6 (14.7) | 4 (9.8) | 31 (75.7) | 0 (0.0) | 1 (7.1) | 13 (92.9) | |
| In case of discomfort in the lower urinary tract | 16 (39.1) | 8 (19.5) | 17 (41.5) | 1 (7.1) | 2 (14.3) | 11 (78.5) | |
| In case of unclear discomfort | 17 (41.5) | 10 (24.4) | 14 (34.2) | 1 (7.1) | 3 (21.4) | 10 (71.4) | |
| How often do you discuss the following aspects with your patients before performing a PSA test? | Impact on overall mortality | 14 (34.2) | 4 (9.8) | 23 (56.1) | 2 (14.2) | 3 (21.4) | 9 (64.3) |
| Impact on disease-specific mortality | 7 (17.1) | 3 (7.3) | 31 (75.6) | 2 (14.2) | 1 (7.1) | 11 (78.6) | |
| Impact on risk of metastasis | 9 (22.0) | 8 (19.5) | 24 (58.6) | 1 (7.1) | 1 (7.1) | 12 (85.7) | |
| Potential overdiagnosis | 3 (7.3) | 5 (12.2) | 33 (80.5) | 2 (14.2) | 1 (7.1) | 11 (78.6) | |
| Issue of false positives | 0 (0.0) | 6 (14.6) | 35 (85.4) | 0.0 (0) | 0 (0.0) | 14 (100.0) | |
| Potential anxiety during waiting on test result | 13 (31.7) | 6 (14.6) | 22 (53.7) | 6 (42.9) | 1 (7.1) | 7 (50.0) | |
| Potential follow-up examinations if the test result is conspicuous | 1 (2.4) | 8 (19.5) | 32 (78.0) | 0 (0.0) | 1 (7.1) | 13 (92.8) | |
| Adverse effects of the treatment | 5 (12.2) | 12 (29.3) | 24 (58.5) | 0 (0.0) | 6 (42.9) | 8 (57.1) | |
Abbreviations: GPs, general practitioners; PSA, prostate-specific antigen.
Table S1.
Questions on PSA testing V by general practitioners and urologists, n (%)
| Question | GPs | Urologists | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Never | Rarely | Sometimes | Often | Always | Never | Rarely | Sometimes | Often | Always | ||
| On which occasions do you inform your patient on PSA testing? | Within an early cancer detection examination | 7.3 (3) | 7.3 (3) | 9.8 (4) | 19.5 (8) | 56.1 (23) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (7.1) | 13 (92.9) |
| In case of a positive family anamnesis | 4 (9.8) | 2 (4.9) | 4 (9.8) | 9 (22.0) | 22 (53.7) | 0 (0.0) | 0 (0.0) | 1 (7.1) | 0 (0.0) | 13 (92.9) | |
| In case of discomfort in the lower urinary tract | 7 (17.1) | 9 (22.0) | 8 (19.5) | 8 (19.5) | 9 (22.0) | 0 (0.0) | 1 (7.1) | 2 (14.3) | 8 (57.1) | 3 (21.4) | |
| In case of unclear discomfort | 5 (12.2) | 12 (29.3) | 10 (24.4) | 10 (24.4) | 4 (9.8) | 0 (0.0) | 1 (7.1) | 3 (21.4) | 7 (50.0) | 3 (21.4) | |
| How often do you discuss the following aspects with your patients before performing a PSA test? | Impact on overall mortality | 7 (17.1) | 7 (17.1) | 4 (9.8) | 9 (22.0) | 14 (34.1) | 1 (7.1) | 1 (7.1) | 3 (21.4) | 5 (35.6) | 4 (28.6) |
| Impact on disease-specific mortality | 3 (7.3) | 4 (9.8) | 3 (7.3) | 14 (34.1) | 17 (41.5) | 1 (7.1) | 1 (7.1) | 1 (7.1) | 6 (42.9) | 5 (35.7) | |
| Impact on risk of metastasis | 5 (12.2) | 4 (9.8) | 8 (19.5) | 9 (22.0) | 15 (36.6) | 0 (0.0) | 1 (7.1) | 1 (7.1) | 9 (64.3) | 3 (21.4) | |
| Potential overdiagnosis | 1 (2.4) | 2 (4.9) | 5 (12.2) | 10 (24.4) | 23 (56.1) | 1 (7.1) | 1 (7.1) | 1 (7.1) | 5 (35.7) | 6 (42.9) | |
| Issue of false positives | 0 (0.0) | 0 (0.0) | 6 (14.6) | 12 (29.3) | 23 (56.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 4 (28.6) | 10 (71.4) | |
| Potential anxiety during waiting on test result | 6 (14.6) | 7 (17.1) | 6 (14.6) | 7 (17.1) | 15 (36.6) | 2 (14.3) | 4 (28.6) | 1 (7.1) | 6 (42.9) | 1 (7.1) | |
| Potential follow-up examinations if the test result is conspicuous | 1 (2.4) | 0 (0.0) | 8 (19.5) | 11 (26.8) | 21 (51.2) | 0 (0.0) | 0 (0.0) | 1 (7.1) | 3 (21.4) | 10 (71.4) | |
| Adverse effects of the treatment | 1 (2.4) | 4 (9.8) | 12 (29.3) | 11 (26.8) | 13 (31.7) | 0.0 (0) | 0.0 (0) | 6 (42.9) | 5 (35.7) | 3 (21.4) | |
Abbreviations: GPs, general practitioners; PSA, prostate-specific antigen.
One-third of the GPs and a proportion of 50% of the urologists answered on when a test is performed if a patient asks for it, that they either test the same day (36.6%, n=15 [GPs]; 57.1%, n=8 [urologists]) or cannot give a generalized answer (“depends on the patient”) (34.1%, n=14 [GPs]; 42.9%, n=6 [urologists]).
More than half of the GPs (53.7%, n=22) replied that the proportion of men aged 45 years and older that finally receives (at least) one PSA test (irrespective of where the test is performed) is almost none or considerably less than half. On the other hand, almost 80% of the urologists (78.5%, n=11) stated that almost all or considerably more than half finally receive (at least) one PSA test, while none of them chose almost none or considerably less than half. Almost all GPs indicated that the blood sample is analyzed in an external laboratory (97.6%, n=40), while half of the urologists (50.0%, n=7) conduct the analysis in their own practice. Although almost 40.0% of the GPs (39.0%, n=15) would not recommend a test at all, the majority of the urologists (57.1%, n=8) chose 10–14 years of life expectancy for an asymptomatic patient to recommend a PSA test (see Table 5).
Table 5.
Questions on PSA testing III, n (%)
| Question | Categories | GPs | Urologists |
|---|---|---|---|
| When do you usually perform a PSA test if a patient asks for it? | Same day | 15 (36.6) | 8 (57.1) |
| New appointment | 9 (22.0) | 0 (0.0) | |
| Depends on the patient | 14 (34.1) | 6 (42.9) | |
| Others, namely… | 0 (0.0) | ||
| - after informing about benefit and risk | 1 (2.4) | - | |
| - test is only performed in justified exceptional cases | 1 (2.4) | - | |
| - sex, cycle | 1 (2.4) | - | |
| Which proportion of men aged 45 years and older in your practice finally receives (at least) one PSA test (irrespective of where the test is performed)? | Almost none | 7 (17.1) | 0 (0.0) |
| Considerably less than half | 15 (36.6) | 0 (0.0) | |
| Approximately half | 8 (19.5) | 3 (21.4) | |
| Considerably more than half | 7 (17.1) | 8 (57.1) | |
| Almost all | 4 (9.8) | 3 (21.4) | |
| Where is the blood sample (of the PSA test) analyzed? | In own practice | 1 (2.4) | 7 (50.0) |
| External laboratory | 40 (97.6) | 6 (42.9) | |
| Others (eg, at a community health center) | 0 (0.0) | 1 (7.1) | |
| How many years of life expectancy does an asymptomatic patient need to have at least for you to recommend a PSA test? | Irrespective of the life expectancy (meaning also for patients with life expectancy of <5 years) | 6 (14.6) | 2 (14.3) |
| 5–9 years | 5 (12.2) | 4 (28.6) | |
| 10–14 years | 10 (24.4) | 8 (57.1) | |
| ≥ 15 years | 4 (9.8) | 0 (0.0) | |
| Not at all | 16 (39.0) | 0 (0.0) |
Abbreviations: GPs, general practitioners; PSA, prostate-specific antigen.
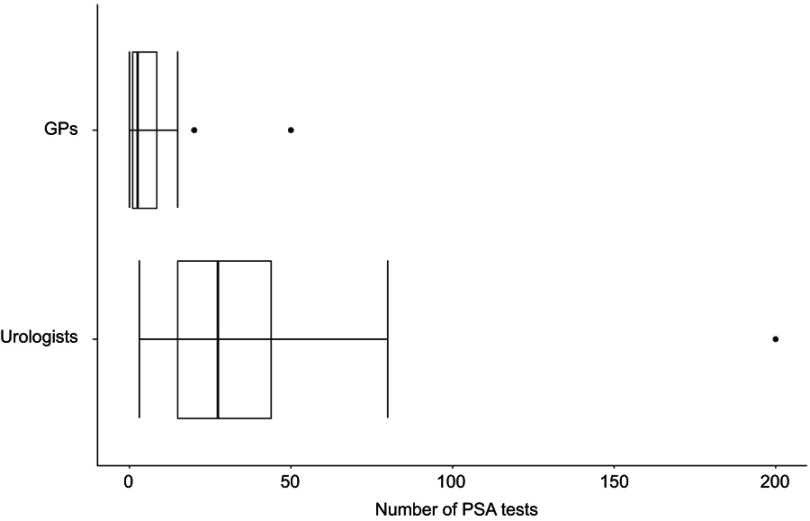
Figure 2 shows the boxplots of the number of PSA tests performed during the last 2 weeks by GPs and urologists. As expected, the median number of tests performed by urologists is higher than the corresponding one of the GPs (27.5, n=41 and 2.0, n=14, respectively).
Figure 2.
Number of PSA test performed during the last 2 weeks by general practitioners (n=41) and urologists (n=14).
Abbreviations: GPs, general practitioners; PSA, prostate-specific antigen.
Being asked which further actions were taken the last time seeing an asymptomatic patient with an increased PSA level, more than half of the GPs (53.7%, n=22) indicated to have checked the PSA level within a certain interval, while 68.3% (n=28) directly referred the patient to a urologist (see Table 6). All 14 urologists stated that they checked the PSA level within a certain interval the last time they saw an asymptomatic patient with an increased PSA level.
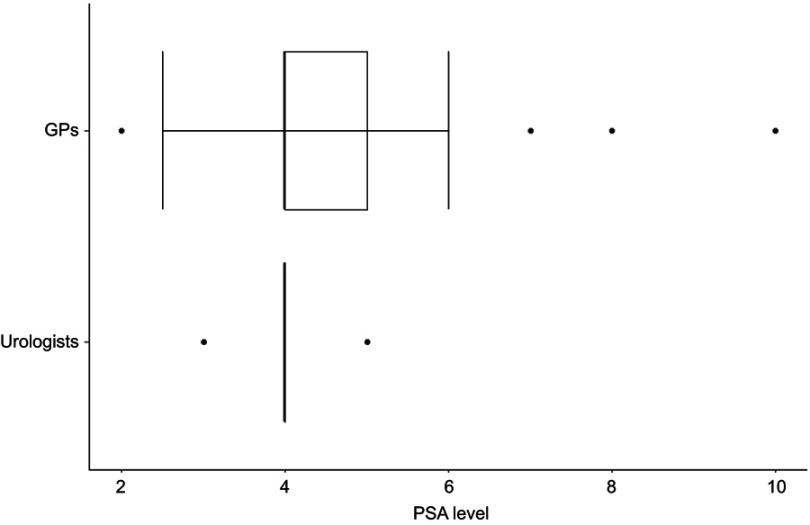
Figure 3 shows the boxplots of the PSA level at which the participants would take further actions for an asymptomatic patient who received a PSA test within an early detection examination. For both groups the median was 4.0 (n=41 [GPs], n=14 [urologists]).
Figure 3.
PSA level at which general practitioners (n=41) and urologists (n=14) would take further actions for an asymptomatic patient who received a PSA test within an early detection examination.
Abbreviations: GPs, general practitioners; PSA, prostate-specific antigen.
Case scenarios
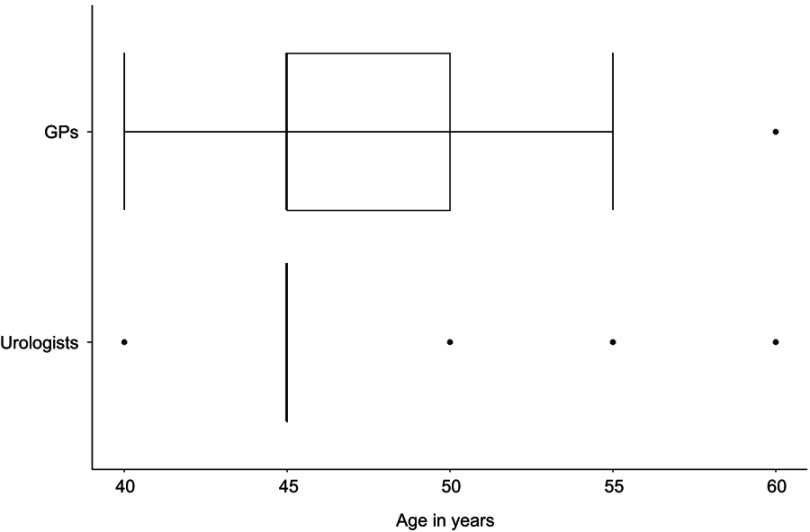
All urologists (100%, n=14) would recommend a PSA test to an asymptomatic patient without risk factors at a certain age. The corresponding figure for the GPs is 51.2% (n=21) (see Table 7, case scenario 1). Following up on case scenario 1, the minimum age is illustrated in Figure 4. All urologists (100%, n=14) stated to actively address a PSA test to a 45-year old patient with at least 10 years of life expectancy who does not ask for an early detection examination based on PSA testing. This statement was given by less than half of the GPs (41.5%, n=17) (see Table 7, case scenario 2). Almost one-third of the GPs (29.3%, n=12) would not recommend a second PSA test at all to a 45-year-old patient with a PSA level of 1–2 ng/mL (see Table 7, case scenario 3). More than one-third of the GPs (39.1%, n=16) would never or rarely perform a PSA test in a patient older than 45 years having an obstructive voiding disorder. All urologists would at least sometimes perform a test in such a situation, whereas the majority (50.0%, n=7) stated often (see Table 7, case scenario 4).
Table 7.
Case scenarios, n (%)
| Question | Categories | GPs | Urologists |
|---|---|---|---|
| Case scenario 1: Imagine you see an asymptomatic patient without risk factors. Would you recommend him a PSA test at a certain age? | Yes | 21 (51.2) | 14 (100.0) |
| No | 19 (46.3) | 0 (0.0) | |
| Cannot reply to that question | 1 (2.4) | 0 (0.0) | |
| Case scenario 2: Imagine you see a 45-year old patient with life expectancy of at least 10 years who does not ask for an early detection examination based on PSA testing in your practice. Would you actively address a PSA test? | Yes | 17 (41.5) | 14 (100.0) |
| No | 24 (58.5) | 0 (0.0) | |
| Case scenario 3: Imagine a 45-year old patient with life expectancy of at least 10 years, having a PSA level of 1–2ng/mL. Which interval would you recommend for a PSA test? | Interval every year or more often | 5 (12.2) | 5 (35.7) |
| Interval every 2 years | 15 (36.6) | 6 (42.9) | |
| Interval every 3 years | 1 (2.4) | 2 (14.3) | |
| Interval every 4 years | 2 (4.9) | 1 (7.1) | |
| Interval less than every 4 years | 6 (14.6) | 0 (0.0) | |
| Not at all | 12 (29.3) | 0 (0.0) | |
| Case scenario 4: How often do you perform a PSA test in a patient older than 45 years having an obstructive voiding disorder? | Never | 7 (17.1) | 0 (0.0) |
| Rarely | 9 (22.0) | 0 (0.0) | |
| Sometimes | 13 (31.7) | 4 (28.6) | |
| Often | 8 (19.5) | 7 (50.0) | |
| Always | 4 (9.8) | 3 (21.4) |
Abbreviations: GPs, general practitioners; PSA, prostate-specific antigen.
Figure 4.
Case scenario 1a: Minimum age at which general practitioners (n=41) and urologists (n=14) would recommend a PSA test to an asymptomatic patient of a certain age without risk factors, if they would recommend one.
Abbreviation: GPs, general practitioners.
Figure 4 shows the boxplots of the minimum age at which GPs and urologists would recommend a PSA test to an asymptomatic patient of a certain age without risk factors. For both groups, the median was 45 years of age (n=41 [GPs], n=14 [urologists]).
Guidelines and recommendations
Knowledge about national/international guidelines and studies on PSA testing was related to the specialist discipline of the doctors. Most GPs indicated to be aware of and consider the practice recommendations on PSA screening of the German College of General Practice and Family Medicine,21 while all urologists indicated to know the German S3 guideline on PCa15 in detail (see Table 8). Half of the GPs (48.8%, n=20) never heard about the European Association of Urology (EAU) guidelines on PCa.22 International recommendations and guidelines such as the American Urological Association (AUA) guidelines on PCa,23 the National Comprehensive Cancer Network (NCCN) guidelines on PCa early detection,24 the American Cancer Society (ACS) guidelines on PCa early detection25 and the recommendation statements of the US Preventive Services Task Force (USPSTF)26 were rarely known in detail. Further, they were less known among GPs than among urologists. The same picture was given for international studies such as the ERSPC and PLCO study.8–11 More than half of the GPs (58.5%, n=24) pointed out that they have never heard about the ERSPC.
Table 8.
Questions on guidelines and recommendations I by general practitioners and urologists I, n (%)
| Question | Categories | GPs | Urologists | ||||
|---|---|---|---|---|---|---|---|
| Yes, I know it in detail | Yes, I heard about it | No, I never heard about it | Yes, I know it in detail | Yes, I heard about it | No, I never heard about it | ||
| Are you aware of the following guidelines and study recommendations/results regarding PSA testing (irrespective of the version)? | DEGAM recommendations | 21 (51.2) | 17 (41.5) | 3 (7.3) | 2 (14.3) | 9 (64.3) | 3 (21.4) |
| German S3 guideline | 10 (24.4) | 28 (68.3) | 3 (7.3) | 14 (100.0) | 0 (0.0) | 0 (0.0) | |
| EAU guidelines | 3 (7.3) | 18 (43.9) | 20 (48.8) | 10 (71.4) | 4 (28.6) | 0 (0.0) | |
| AUA recommendations | 2 (4.9) | 9 (22.0) | 30 (73.2) | 3 (21.4) | 11 (78.6) | 0 (0.0) | |
| NCCN guidelines | 1 (2.4) | 7 (17.1) | 33 (80.5) | 1 (7.1) | 10 (71.4) | 3 (21.4) | |
| ACS guidelines | 1 (2.4) | 11 (26.8) | 29 (70.7) | 3 (21.4) | 7 (50.0) | 4 (28.6) | |
| USPSTF recommendations | 1 (2.4) | 7 (17.1) | 33 (80.5) | 4 (28.6) | 6 (42.9) | 4 (28.6) | |
| ERSPC | 2 (4.9) | 15 (36.6) | 24 (58.5) | 7 (50.0) | 5 (35.7) | 2 (14.3) | |
| PLCO | 2 (4.9) | 7 (17.1) | 32 (78.0) | 4 (28.6) | 5 (35.7) | 5 (35.7) | |
Abbreviations: ACS, American Cancer Society; AUA, American Urological Association; EAU, European Association of Urology; DEGAM, German College of General Practice and Family Medicine; ERSPC, European Randomized Study of Screening for Prostate Cancer; GPs, general practitioners; PLCO, Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial; PSA, prostate-specific antigen; NCCN, National Comprehensive Cancer Network; USPSTF, United States Preventive Services Task Force.
Urologists mostly consider the German S3 and the EAU guideline(s) in their daily practice, while GPs indicated a rather moderate consideration of the DEGAM recommendations (see Table 9). None of the urologists and 5 (12.2%) of the GPs stated that their daily practice regarding PSA testing is less than moderately influenced by national/international studies and guidelines/recommendations (see Table 10).
Table 9.
Questions on guidelines and recommendations II by general practitioners and urologists
| Question | Categories | GPs | Urologists | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) | Median | 1st Quartile | 3rd Quartile | n | Mean (SD) | Median | 1st Quartile | 3rd Quartile | n | ||
| If yes (you are aware of the guidelines and study recommendations/results), do you consider them in your daily practice regarding PSA testing? | DEGAM recommendations | 56.6 (31.4) | 57.0 | 40.0 | 83.8 | 40 | 11.8 (15.6) | 5.0 | 2.0 | 26.0 | 11 |
| German S3 guideline | 38.7 (28.9) | 37.5 | 13.3 | 57.0 | 40 | 94.9 (7.7) | 96.0 | 91.8 | 101.0 | 14 | |
| EAU guidelines | 20.3 (22.5) | 7.0 | 2.0 | 46.0 | 23 | 64.8 (35.9) | 73.5 | 26.5 | 101.0 | 14 | |
| AUA recommendations | 12.3 (16.0) | 5.0 | 1.0 | 20.0 | 13 | 26.8 (28.5) | 20.5 | 1.8 | 47.5 | 14 | |
| NCCNguidelines | 12.6 (17.7) | 4.5 | 1.0 | 19.8 | 10 | 13.1 (12.3) | 6.0 | 1.0 | 27.0 | 11 | |
| ACS guidelines | 14.5 (17.7) | 6.5 | 5.0 | 18.5 | 14 | 21.3 (25.8) | 19.0 | 1.8 | 27.8 | 10 | |
| USPSTF recommendations | 14.8 (18.8) | 8.5 | 1.8 | 21.5 | 10 | 13.4 (12.6) | 12.5 | 1.0 | 24.3 | 10 | |
| ERSPC | 14.7 (17.4) | 6.0 | 1.0 | 29.0 | 19 | 42.8 (35.0) | 34.5 | 12.8 | 73.8 | 12 | |
| PLCO | 14.9 (19.1) | 1.0 | 1.0 | 37.0 | 11 | 10.8 (20.5) | 2.0 | 1.0 | 11.5 | 9 | |
Abbreviations: ACS, American Cancer Society; AUA, American Urological Association; EAU, European Association of Urology; DEGAM, German College of General Practice and Family Medicine; ERSPC, European Randomized Study of Screening for Prostate Cancer; GPs, general practitioners; PLCO, Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial; PSA, prostate-specific antigen; NCCN, National Comprehensive Cancer Network; USPSTF, United States Preventive Services Task Force.
Table 10.
Questions on guidelines and recommendations III by general practitioners and urologists, n (%)
| Question | Categories | GPs | Urologists |
|---|---|---|---|
| To which extent do results of national and international studies and national and international guidelines/recommendations influence your daily practice regarding PSA testing? | Not at all | 2 (4.9) | 0 (0.0) |
| Very weak | 2 (4.9) | 0 (0.0) | |
| Weak | 1 (2.4) | 0 (0.0) | |
| Moderate | 21 (51.2) | 2 (12.5) | |
| Strong | 11 (26.8) | 12 (85.7) | |
| Very strong | 4 (9.8) | 0 (0.0) |
Abbreviations: GPs, general practitioners; PSA, prostate-specific antigen.
Comparing the results of the questionnaire to the content of the guidelines, it can be noticed that urologists as well as GPs partly did not treat men in consistence with the guidelines. For example, >40% of the GPs (41.5%, n=17) would recommend a PSA test to an asymptomatic patient without risk factors, though both German guidelines stated for GPs not to recommend a PSA test to a patient described here (see Table 7, case scenario 2).21, On the question which interval doctors would recommend for a PSA test, seeing a 45-year-old patient with a life expectancy of at least 10 years, having a PSA level of 1–2ng/mL, less than half of the doctors chose the right answer according to the German S3 guideline (interval every 2 years; 36.6%, n=15 [GPs]; 42.9%, n=6 [urologists]) (see Table 7, case scenario 3).
Daily practice
More than 80% of both groups always examine digito rectally during an early cancer detection examination (80.5%, n=33 [GPs] and 85.7%, n=12 [urologists]). A rather heterogeneous result was observed for patients with a voiding order – while all urologists (100%, n=14) always or often examine digito rectally, more than 40% of the GPs (41.5%, n=17) stated sometimes (see Table 11).
Table 11.
Questions on daily practice by general practitioners and urologists, n (%)
| Question | Categories | GPs | Urologists | ||||
|---|---|---|---|---|---|---|---|
| Never + Rarely | Sometimes | Always + Often | Never + Rarely | Sometimes | Always + Often | ||
| How often do you examine digito rectally in the following situations? | During an early cancer detection examination | 0 (0.0) | 4 (9.8) | 37 (90.3) | 0 (0.0) | 0 (0.0) | 14 (100.0) |
| If there is blood in the patient´s stool | 0 (0.0) | 3 (7.3) | 38 (92.7) | (0.0) | 0 (0.0) | 14 (100.0) | |
| If the patient has a voiding disorder | 4 (9.7) | 17 (41.5) | 20 (48.8) | 0 (0.0) | 0 (0.0) | 14 (100.0) | |
| If the patient is asymptomatic | 29 (70.3) | 8 (19.5) | 4 (9.8) | 0 (0.0) | 4 (28.6) | 10 (71.5) | |
Abbreviation: GPs, general practitioners.
PSA test and PCa risk
The general opinion on the PSA test differed between the groups (see Table 12). While two-third (64.3%, n=9) and one-third (35.7%, n=5) of the urologists judged the test as very useful and useful, respectively, the replies of the GPs were less homogeneous. More than one-third of the GPs (36.6%, n=15) judged the PSA test not to be useful. For a more detailed table on daily practice of digito rectal examining see Table S2.
Table 12.
Questions on PSA testing and prostate cancer risk, n (%)
| Question | Categories | GPs | Urologists |
|---|---|---|---|
| How do you judge the PSA test in general? | Not useful at all | 6 (14.6) | 0 (0.0) |
| Not useful | 9 (22.0) | 0 (0.0) | |
| Neither/nor | 4 (9.8) | 0 (0.0) | |
| Useful | 18 (43.9) | 5 (35.7) | |
| Very useful | 4 (9.8) | 9 (64.3) | |
| Please state your opinion. Which of the following factors have an impact on the risk to develop PCa? | Higher age (45 years and older) | 41 (100.0) | 14 (100.0) |
| Smoking | 24 (58.5) | 3 (21.4) | |
| Primary relative having PCa | 32 (78.0) | 13 (92.9) | |
| BPH | 5 (12.2) | 2 (14.3) | |
| Afro-American ethnicity | 2 (4.9) | 9 (64.3) | |
| Did you ever discover a PCa in an asymptomatic patient younger than 60 years based on a PSA test that you performed? | Yes | 29 (70.7) | 14 (100.0) |
| No | 12 (29.3) | 0 (0.0) | |
| Did you ever undergo a PSA test? (only men replies) | Yes | 20 (55.6) | 14 (100.0) |
| No | 16 (44.4) | 0 (0.0) |
Abbreviations: BPH, benign prostatic hyperplasia; GPs, general practitioners; PCa, prostate cancer, PSA, prostate-specific antigen.
Table S2.
Questions on digital rectal examining by general practitioners and urologists, n (%)
| Question | GPs | Urologists | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Never | Rarely | Sometimes | Often | Always | Never | Rarely | Sometimes | Often | Always | ||
| How often do you examine digito rectally in the following situations? | During an early cancer detection examination | 0 (0.0) | 0 (0.0) | 4 (9.8) | 4 (9.8) | 33 (80.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (14.3) | 12 (85.7) |
| If there is blood in the patient´s stool | 0 (0.0) | 0 (0.0) | 3 (7.3) | 13 (31.7) | 25 (61.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (21.4) | 11 (78.6) | |
| If the patient has a voiding disorder | 1 (2.4) | 3 (7.3) | 17 (41.5) | 12 (29.3) | 8 (19.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 4 (28.6) | 10 (71.4) | |
| If the patient is asymptomatic | 17 (41.5) | 12 (29.3) | 8 (19.5) | 4 (9.8) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 4 (28.6) | 6 (42.9) | 4 (28.6) | |
Abbreviation: GPs, general practitioners.
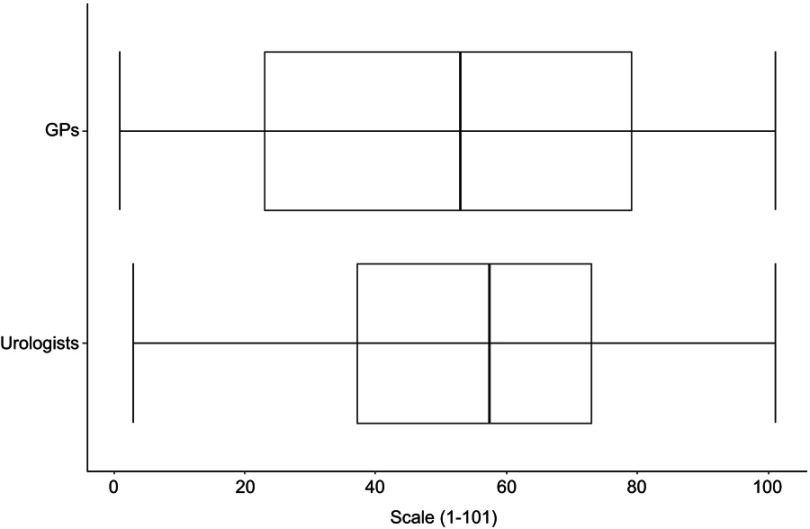
Figure 5 shows the boxplots of the change in handling PSA testing during the last 10 years for GPs ( n=41) and urologists ( n=14). For the urologists, the median of 57.5 is slightly higher than the corresponding one of the GPs (53.0).
Figure 5.
Change in handling PSA testing during the last 10 years for GPs (n=41) and urologists (n=14).
Abbreviations: GPs, general practitioners; PSA, prostate-specific antigen.
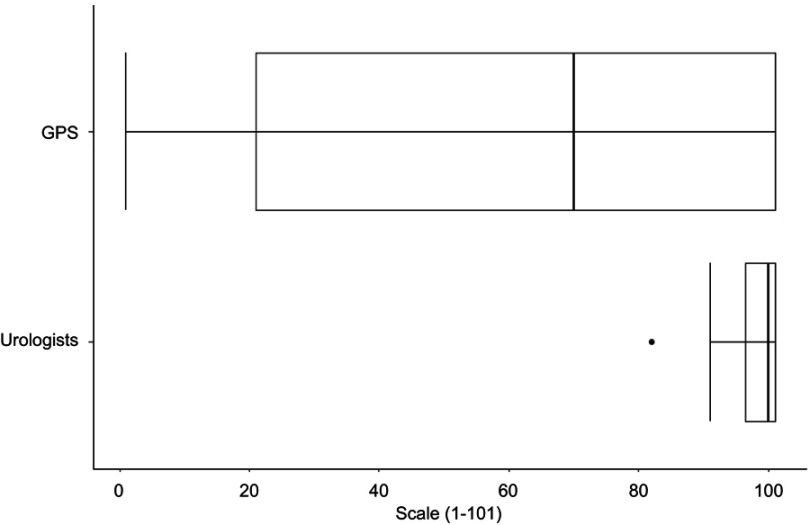
Figure 6 shows the boxplots of the score in agreement for undergoing a PSA test in the future for male GPs ( n=36) and male urologists ( n=14). The propensity to undergo a PSA test in the future is higher for urologists than for GPs (median 100.0 and 70.0, respectively).
Figure 6.
Score in agreement for undergoing a PSA test in the future for male general practitioners (GPs) (n=36) and male urologists (n=14).
Abbreviations:GPs, general practitioners; PSA, prostate-specific antigen.
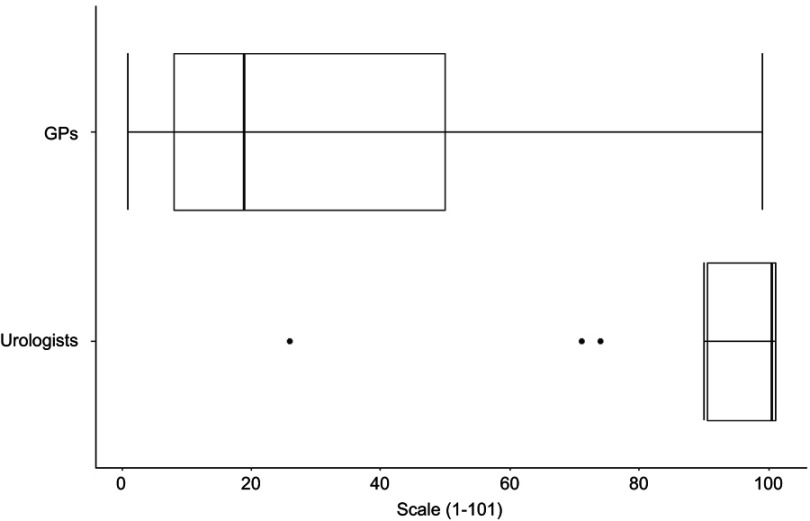
The boxplots of the scores of GPs ( n=41) and urologists ( n=14) on the question if they think that the reduction of PCa-related mortality by early detection based on PSA testing is proven are shown in Figure 7. The median score for the urologists is more than five times higher than the corresponding one for the GPs (19.0 and 100.5, respectively).
Figure 7.
Scores of GPs (n=41) and urologists (n=14) on the question if they think that the reduction of PCa-related mortality by early detection based on PSA testing is proven.
Abbreviations: GPs, general practitioners; PSA, prostate-specific antigen..
Discussion
This study aimed to give a first insight into the daily practice of PSA testing and potential differences between GPs and urologists regarding the early detection of PCa based on PSA testing in the northwest of Germany. The results of this study show that GPs and urologists differ on various aspects regarding PSA testing. In total, PSA testing was less accepted among GPs than among urologists. Further, differences in opinion on usefulness of the PSA test, in handling PSA results, consideration of guidelines in daily practice and knowledge about guidelines or actual study results on PSA testing were observed.
Our findings support earlier studies showing that variation in handling of PSA testing is due to, for example, insufficient knowledge or individual conviction of the doctor.19,27 Also the fact that a doctors’ intention to screen himself for PCa using PSA testing predicted their tendency to screen their patients is supported by our results.28 Because it is known that the way the patient is informed about the PSA test has a high impact on the patient’s satisfaction with early detection,16 this indirectly influences their satisfaction with the urological care. One study conducted in the United States found no difference between urologists and primary care physicians in the amount of PSA tests conducted.29 This is in contrast to our findings. Differences in health care systems and/or opinions on PSA testing might be an explanation for this.
The results also show that doctors have a different self-assessment than the study results show. All urologists indicated to know the S3 guideline in detail and 94.9% stated to consider the guideline in their daily practice, while the answers of some questions were not in consistence with the guideline. This is in accordance with other studies stating that although doctors have favorable attitudes toward guidelines on PCa, guideline knowledge is limited.30,31 One study stated that physicians handling after a normal or raised PSA seems to a large extent not in accordance with guidelines on PCa screening which agrees with our results.32
The “dissenting opinion” of the 2014 version of the German S3 guideline says that men, not broaching early PCa screening by PSA testing to the doctor, should not be actively approached by the GP, while urologists are advised to proactively inform these men about PSA testing.15 This might explain some differences between GPs and urologists, but not all. Apart from this “dissenting opinion” regarding a more reserved approach for GPs, the recommendations in the guideline (eg, retesting after a raised PSA, etc.) are identical for doctors with different specialties. Further, the results show that urologists know the urological guidelines better than GPs. However, this might not fully explain the observed differences between them.
Accordingly, there seems to be an urgent need to educate and support doctors more (GPs, as well as urologists and doctors with other specialties dealing with PSA testing) concerning PSA testing.19,27,30 This is important for further improving the quality of the urological health care.
In the future, non-PSA-based effective screening tests for early detection of PCa based on urine analysis may be more acceptable to the GPs because of probably improved specificity. Current studies aim the development of such innovative diagnostic tools which may improve the characterization of the disease biology, allowing to determine whether the PCa will be aggressive or indolent, in order to avoid overtreatment.33 For example, urinary polyamines (eg, spermine) show potential to serve as novel PCa diagnostic biomarkers, which may be helpful to address the limited sensitivity and specificity problem of the serum PSA test.34
Certain limitations must be considered. One limitation is the fact that the GPs who were invited belonged to the teaching network of the University of Oldenburg. It is conceivable that the doctors belonging to this network are more interested in science. This selection bias could lead to an underestimation of the results. Although none of the urologists surveyed is currently working at a hospital, the urologists, in general, could have a different attitude toward PSA testing than GPs and other physicians due to their earlier training in clinical oncologic sites. The low response proportions of 27.9% for the GPs and 13.2% for the urologists are another limitation. However, other studies among doctors like GPs and psychiatrists show comparable response proportions.35,36 A possible cause for this could be that physicians in Germany receive a lot of surveys, which partially are commercial and also well-paid. This survey was relatively extensive or time-consuming and without reimbursement. Thus, the participating physicians possibly had surpassing scientific interest and knowledge about clinical guidelines. As mentioned above, this circumstance may lead to selection bias.
Our pilot study provides an insight into the PSA tests conducted in the northwest of Germany. Till now, in Germany, only data from the German Health Interview and Examination Survey for Adults (2008–2011) are available, reporting that 30.6% of men aged 45 years or older had received a PSA test.37 In other countries, these data often are determined based on secondary data.38 As PSA testing is not an early detection service of the German statutory health insurance, these data are not gathered by the national health insurance.
If a patient wants a PSA test to be conducted, he has to pay the test by himself. The medical doctor “earns” a certain amount of money for every PSA test he or she conducts. Because financial incentives could affect doctors’ treatment choices, this could be a motivation for a doctor to conduct a PSA test.39,40 Our results also showed that 50.0% of the urologists analyzed the blood samples in their own practice. This could be an extra motivational factor for doctors to stimulate men to conduct a PSA test. To determine the influence of financial factors on conducting a PSA test further research is needed.
Conclusion
This pilot study argues for differences in various aspects regarding PSA testing between GPs and urologists, wherein a low response rate represents a limitation in some respects. Physicians with surpassing scientific interest are probably over-represented, which may lead to selection bias. There is an urgent need to educate and support doctors more on topics related to PSA testing. This is important for further improving the quality of urological health care. Therefore, the interdisciplinary exchange needs to be continued and extended to achieve a consistent level of knowledge among doctors with different specialties.
To validate the results of this study and to constitute the consequences of the different information levels on the urological care, a follow-up project is planned. This project will be conducted in different regions in Germany and will include GPs as well as practicing urologists and those working at hospitals. Further, a survey among men aged >45 years is planned to assess the satisfaction with the urological health care in Germany.
Acknowledgments
We would like to thank the GPs and the urologists who took the time to participate in this survey. Further, we would like to express our thanks to the teaching network of the Carl von Ossietzky University Oldenburg and the Professional Association of German Urologists (Berufsverband der Deutschen Urologen [BvDU]) for forwarding the study invitation letters and the questionnaire to the GPs and the urologists. This study was funded by the research pool of the Carl von Ossietzky University Oldenburg.
Ethics approval and consent to participate
The study was approved by the Medical Ethics Committee of the Carl von Ossietzky University Oldenburg (No. 041/2016).
Availability of data and materials
Data and materials supporting the conclusion were included in the main paper. Further data were available from the corresponding author on reasonable request.
Abbreviation list
ACS, American Cancer Society; ASR, Age-standardized rate; AUA, American Urological Association; BvDU, Professional Association of German Urologists (Berufsverband der Deutschen Urologen); BPH, benign prostatic hyperplasia; DEGAM, German College of General Practice and Family Medicine (Deutsche Gesellschaft für Allgemeinmedizin und Familienmedizin); EAU, European Association of Urology; ERSPC, European Randomized Study of Screening for Prostate Cancer; GP, General practitioner; NCCN, National Comprehensive Cancer Network; ng/mL, nanograms per milliliter; PCa, prostate cancer; PLCO, Prostate, Lung, Colorectal and Ovarian study; PSA, prostate-specific antigen; USPSTF, US Preventive Services Task Force.
Author contributions
VJ and AW were responsible for the study design. SK, VJ and AW did the literature search. SK, VJ, AW and MHF developed the questionnaire. SK, VJ, AW and MHF were responsible for conducting the questionnaire. SK and VJ performed the descriptive analysis and the data management. SK, VJ and AW interpreted the data. SK, VJ and AW drafted the manuscript, while MHF revised it. All authors have read and approved the final manuscript and agreed to be accountable for all aspect of the work.
Disclosure
MHF reports personal fees from DAK Gesundheit, outside the submitted work. The authors report no other conflicts of interest in this work.
Supplementary materials
References
- 1.Zhou CK, Check DP, Lortet-Tieulent J, et al. Prostate cancer incidence in 43 populations worldwide: an analysis of time trends overall and by age group. Int J Cancer. 2016;138(6):1388–1400. doi: 10.1002/ijc.29894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robert Koch-Institut. Gesellschaft der epidemiologischen Krebsregister in Deutschland e.V., eds. Krebs in Deutschland Berlin; 2015. [Google Scholar]
- 3.Dörr M, Hölzel D, Schubert-Fritschle G, Engel J, Schlesinger-Raab A. Changes in prognostic and therapeutic parameters in prostate cancer from an epidemiological view over 20 years. Oncol Res Treat. 2015;38(1–2):8–14. doi: 10.1159/000371717 [DOI] [PubMed] [Google Scholar]
- 4.Winter A, Sirri E, Jansen L, et al. Comparison of prostate cancer survival in Germany and the United States: can differences be attributed to differences in stage distributions? BJU Int. 2017;119(4):550–559. doi: 10.1111/bju.135372016. [DOI] [PubMed] [Google Scholar]
- 5.Larrañaga N, Galceran J, Ardanaz E, et al. Prostate cancer incidence trends in Spain before and during the prostate-specific antigen era: impact on mortality. Ann Oncol. 2010;21(suppl3):iii83–iii89. doi: 10.1093/annonc/mdq087 [DOI] [PubMed] [Google Scholar]
- 6.Schroder FH, Hugosson J, Roobol MJ, et al. Screening and prostate cancer mortality: results of the European Randomised Study of Screening for Prostate Cancer (ERSPC) at 13 years of follow-up. Lancet. 2014;384(9959):2027–2035. doi: 10.1016/S0140-6736(14)60525-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robert Koch-Institut, Gesellschaft der epidemiologischen Krebsregister in Deutschland e.V., eds. Krebs in Deutschland 2007/2008. Berlin; 2012. [Google Scholar]
- 8.Eckersberger E, Finkelstein J, Sadri H, et al. Screening for prostate cancer: a review of the ERSPC and PLCO trials. Rev Urol. 2009;11(3):127. [PMC free article] [PubMed] [Google Scholar]
- 9.Andriole GL, Crawford ED, Grubb III RL, et al. Mortality results from a randomized prostate-cancer screening trial. N Engl J Med. 2009;360(13):1310–1319. doi: 10.1056/NEJMoa0810696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andriole GL, Crawford ED, Grubb RL, et al. Prostate cancer screening in the randomized prostate, lung, colorectal, and ovarian cancer screening trial: mortality results after 13 years of follow-up. J Natl Cancer Inst. 2012;104:125–132. doi: 10.1093/jnci/djr500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Koning HJ, Gulati R, Moss SM, et al. The efficacy of prostate-specific antigen screening: impact of key components in the ERSPC and PLCO trials. Cancer. 2018 Mar 15;124(6):1197–1206. doi: 10.1002/cncr.31178. Epub 2017 Dec 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McDavid K, Lee J, Fulton JP, Tonita J, Thompson TD. Prostate cancer incidence and mortality rates and trends in the United States and Canada. Public Health Reports. 2004;119(2):174–186. doi: 10.1177/003335490411900211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Winter A, Vohmann C, Wawroschek F, Kieschke J. Increase in uro-oncological health care needs due to demographic change: extrapolation of cancer incidence numbers through 2030 as a basis for directed regional planning. Urologe A. 2015;54(9):1261–1268. doi: 10.1007/s00120-014-3698-7 [DOI] [PubMed] [Google Scholar]
- 14.Meer S, Kollen BJ, Hirdes WH, et al. Impact of the European Randomized Study of Screening for Prostate Cancer (ERSPC) on prostate‐specific antigen (PSA) testing by Dutch general practitioners. BJU Int. 2013;112(1):26–31. doi: 10.1111/bju.12029 [DOI] [PubMed] [Google Scholar]
- 15.Leitlinienprogramm Onkologie (Deutsche Krebsgesellschaft, Deutsche Krebshilfe, AWMF): Interdisziplinäre Leitlinie der Qualität S3 zur Früherkennung, Diagnose und Therapie der verschiedenen Stadien des Prostatakarzinoms, Langversion 3.1, 2014 AWMF Registernummer: 034/022OL. Available from: http://leitlinienprogrammonkologie.de/Leitlinien.7.0.html. Accessed October 15, 2014. [Google Scholar]
- 16.Orom H, Biddle C, Underwood W 3rd, Nelson CJ, Homish DL. What is a “good” treatment decision? Decisional control, knowledge, treatment decision making, and quality of life in men with clinically localized prostate cancer. Med Decis Making. 2016;36(6):714–725. doi: 10.1177/0272989X16635633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thomas R, Glasziou P, Rychetnik L, Mackenzie G, Gardiner R, Doust J. Deliberative democracy and cancer screening consent: a randomised control trial of the effect of a community jury on men’s knowledge about and intentions to participate in PSA screening. BMJ Open. 2014;4(12):e005691. doi: 10.1136/bmjopen-2014-005691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pucheril D, Dalela D, Sammon J, et al. The influence of physician recommendation on prostate-specific antigen screening. Urol Oncol. 2015;33(10):424e421–427. doi: 10.1016/j.urolonc.2015.06.013 [DOI] [PubMed] [Google Scholar]
- 19.Drummond FJ, Carsin AE, Sharp L, Comber H. Factors prompting PSA-testing of asymptomatic men in a country with no guidelines: a national survey of general practitioners. BMC Fam Pract. 2009;10:3. doi: 10.1186/1471-2296-10-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hjertholm P, Fenger-Gron M, Vestergaard M, et al. Variation in general practice prostate-specific antigen testing and prostate cancer outcomes: an ecological study. Int J Cancer. 2015;136(2):435–442. doi: 10.1002/ijc.29008 [DOI] [PubMed] [Google Scholar]
- 21.Kötter T. “Family Practioners‘ counseling regarding PSA screening”: Practice recommendation of the German College of General Practitioners and Family Physicians (DEGAM). Z Allg Med. 2016;92(12):495-499. [Google Scholar]
- 22.Mottet N, Bellmunt J, Bolla M, et al. EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur Urol. 2017 Apr;71(4):618-629. doi: 10.1016/j.eururo.2016.08.003. Epub 2016 Aug 25. [DOI] [PubMed] [Google Scholar]
- 23.Ballentine Carter H, Albertsen PC, Barry MJ, et al. Early detection of prostate Cancer: AUA Guideline. J Urol. 2013;190(2):419–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.(NCCN) NCCN. NCCN Clinical Practice Guidelines in Oncology – Prostate Cancer Early Detection, Version 2.2015. National Comprehensive Cancer Network (NCCN); 2015. [DOI] [PubMed] [Google Scholar]
- 25.Wolf AM, Wender RC, Etzioni RB, et al.American Cancer Society Prostate Cancer Advisory Committee. American Cancer Society guideline for the early detection of prostate cancer: update 2010. CA Cancer J Clin. 2010;60(2):70-98. doi: 10.3322/caac.20066. [DOI] [PubMed] [Google Scholar]
- 26.Moyer VA. U.S. Preventive services task force. Screening for prostate cancer: U.S. preventive services task force recommendation statement. Ann Intern Med. 2012;157(2):120-34. doi: 10.7326/0003-4819-157-2-201207170-00459. [DOI] [PubMed] [Google Scholar]
- 27.Clements A, Watson E, Rai T, Bukach C, Shine B, Austoker J. The PSA testing dilemma: GPs’ reports of consultations with asymptomatic men: a qualitative study. BMC Fam Pract. 2007;8(1):35. doi: 10.1186/1471-2296-8-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tun Firzara AM, Ng CJ. Knowledge and practice of prostate cancer screening among general practitioners in Malaysia: a cross-sectional study. BMJ Open. 2016;6:9. doi: 10.1136/bmjopen-2016-011467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zavaski ME, Meyer CP, Sammon JD, et al. Differences in prostate-specific antigen testing among urologists and primary care physicians following the 2012 USPSTF recommendations. JAMA Intern Med. 2016;176(4):546–547. doi: 10.1001/jamainternmed.2015.7901 [DOI] [PubMed] [Google Scholar]
- 30.Sutton J, Melia J, Kirby M, Graffy J, Moss S. GPs views and understanding of PSA testing, screening and early detection; survey. Int J Clin Pract. 2016;70(5):389–395. doi: 10.1111/ijcp.12794 [DOI] [PubMed] [Google Scholar]
- 31.Birrenbach T, Kraehenmann S, Perrig M, Berendonk C, Huwendiek S. Physicians’ attitudes toward, use of, and perceived barriers to clinical guidelines: a survey among Swiss physicians. Adv Med Educ Pract. 2016;7:673–680. doi: 10.2147/AMEP.S115149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van der Meer S, Lowik SA, Hirdes WH, et al. Prostate specific antigen testing policy worldwide varies greatly and seems not to be in accordance with guidelines: a systematic review. BMC Fam Pract. 2012;13:100. doi: 10.1186/1471-2296-13-52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bax C, Taverna G, Eusebio L, et al. Innovative diagnostic methods for early prostate cancer detection through urine analysis: a review. Cancers (Basel). 2018;10(4):E123. doi: 10.3390/cancers10110400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tsoi T-H, Chan C-F, Chan W-L, et al. Urinary polyamines: a pilot study on their roles as prostate cancer detection biomarkers. PLoSONE. 2016;11:e0162217. doi: 10.1371/journal.pone.0162217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Przydacz M, Golabek T, Sobanski JA, et al. Perception of lower urinary tract symptoms by psychiatrists in mentally affected patients. Psychiatr Pol. 2017;51(5):963–978. doi: 10.12740/PP/74365 [DOI] [PubMed] [Google Scholar]
- 36.Martini F, Lazzati A, Fritsch S, Liagre A, Iannelli A, Paolino L. General practitioners and bariatric surgery in france: are they ready to face the challenge? Obes Surg. 2018;28:1754–1759. doi: 10.1007/s11695-017-3090-y [DOI] [PubMed] [Google Scholar]
- 37.Starker A, Saß A-C. Inanspruchnahme von Krebsfrüherkennungsunter-suchungen. Bundesgesundheitsblatt - Gesundheitsforschung - Gesund-heitsschutz. 2013;56(5):858–867. doi: 10.1007/s00103-012-1655-4 [DOI] [PubMed] [Google Scholar]
- 38.Haider MR, Qureshi Z, Horner R, Bennett C. Factors predicting receipt of Prostate Specific Antigen (PSA) testing: evidence from the national ambulatory Medical Care Survey (Namcs) data. Value Health. 2015;18(3):A274. doi: 10.1016/j.jval.2015.03.1598 [DOI] [Google Scholar]
- 39.Liu Y-M, Yang Y-HK, Hsieh C-R. Financial incentives and physicians’ prescription decisions on the choice between brand-name and generic drugs: evidence from Taiwan. J Health Econ. 2009;28(2):341–349. doi: 10.1016/j.jhealeco.2008.10.009 [DOI] [PubMed] [Google Scholar]
- 40.Croxson B, Propper C, Perkins A. Do doctors respond to financial incentives? UK family doctors and the GP fundholder scheme. J Public Econ. 2001;79(2):375–398. doi: 10.1016/S0047-2727(00)00074-8 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data and materials supporting the conclusion were included in the main paper. Further data were available from the corresponding author on reasonable request.