Abstract
Objective: To assess long-term outcomes for effectiveness, safety, and treatment burden after injection of 0.2 µg/day fluocinolone acetonide [FAc] intravitreal implant (ILUVIEN®) in patients with persistent or recurrent diabetic macular edema (DME) and 6–18 months of follow-up.
Methods: Retrospective case series in 18 eyes (13 patients) treated with the FAc implant. Prior to the implant, eyes were treated with an anti-VEGF therapy, dexamethasone implant, or focal or panretinal photocoagulation. Effectiveness outcomes included changes in visual acuity and macular edema. Safety outcomes included intraocular pressure (IOP) changes, IOP drugs, and IOP-related surgeries/interventions. Treatment burden was assessed by comparing the number of DME treatments before and after FAc implantation.
Results: The FAc implant reduced macular volume in 16/18 (89%) eyes, with a statistically significant mean change of –1.33 mm3 (p=0.001). The average central retinal thickness reduction for all 18 eyes was statistically significant, decreasing from 444 µm at baseline to 359 µm after the FAc implant (p<0.001). In 90% of eyes, visual acuity was stable throughout the follow-up period, with increases or no worsening in Early Treatment Diabetic Retinopathy Study letter score. Although mean IOP was statistically higher after treatment, it was within the normal range at all timepoints, and most (83.3%) eyes remained in the IOP category 0–22 mmHg, and the number of IOP treatments required did not increase and no patients required IOP-lowering surgery. Treatment burden for DME was reduced after the implant was administered, with 56% of eyes not requiring any additional treatment. The average number of treatments was 1.3 in the 6 months after the FAc implant versus 4.6 in the 12 months preceding the implant.
Conclusion: The FAc implant is an appropriate option to incorporate earlier in the DME treatment process, leading to positive long-term outcomes with an acceptable safety profile, and a reduced treatment burden for patients, and reduced clinical staff time.
Keywords: persistent diabetic macular edema, recurrent diabetic macular edema, DME, visual acuity, macular volume, fluocinolone acetonide implant
Introduction
Diabetic retinopathy is a microvascular complication of type 1 and 2 diabetes mellitus that is caused by damage to the blood vessels of the retina.1 It affects one in three people with either form of diabetes and is the most common cause of blindness in adults aged 20–74 years in developed countries.1,2 Retinopathy advances from mild nonproliferative abnormalities, characterized by increased numbers of microaneurysms that may wax and wane, to moderate and severe nonproliferative diabetic retinopathy, evidenced by increased vascular permeability and occlusion, to proliferative diabetic retinopathy, represented by growth of new blood vessels on the retina and posterior surface of the vitreous.1 Diabetic retinopathy may lead to vision loss from impairment of central vision by diabetic macular edema (DME) due to increased vascular permeability and nonperfusion of capillaries, from distortion of the retina by new blood vessels and contraction of associated fibrous tissue, or from preretinal or vitreous hemorrhage due to bleeding of new blood vessels.1,3
DME results in retinal thickening in the macula and affects about 20 million people worldwide.2 It is deemed to be clinically significant if there is thickening of the retina at or within 500 µm of the center of the macula, or if hard exudates at or within 500 µm of the center of the macula are associated with thickening of the adjacent retina (excluding residual hard exudates remaining after disappearance of retinal thickening).The exact pathogenesis of DME is not fully understood,4 but it is defined by an abnormal collection of extracapillary fluid due to blood–retinal barrier breakdown because of increased production of inflammatory mediators and vascular permeability factors and loss of endothelial tight junctions.5 Vascular endothelial growth factor (VEGF) is believed to be a key mediator, promoting angiogenesis and breakdown of the blood–retinal barrier, resulting in accumulation of plasma proteins such as albumin, which exert high oncotic pressure in the neural interstitium, leading to interstitial edema.4,6 In DME, upregulation of multiple inflammatory cytokines occurs, and the resultant inflammatory cascade produces diverse anatomical and biochemical changes in the eye.6
Chronic hyperglycemia, hypertension, and hyperlipidemia are implicated in the development of diabetic retinopathy and DME,4 so management of blood sugar/diabetes, blood pressure, and lipids should be optimized to reduce the risk and slow progression of diabetic retinopathy.1,2 Although this can substantially decrease the risk of vision loss, a significant proportion of patients with diabetes develop DME or proliferative changes that require DME-specific treatments, such as laser therapy, anti-VEGF agents, and corticosteroids.1,4
The current first-line standard of care for clinically significant DME is anti-VEGF therapy, unless there is a clearly defined circinate exudate emanating from a defined microaneurysm outside the fovea.6 Intravitreous injections of anti-VEGF drugs are indicated for center-involved DME, which occurs beneath the foveal center and may threaten reading vision.1 Intravitreal anti-VEGF monotherapy is also considered for DME associated with proliferative diabetic retinopathy.2 Although many patients gain significant benefit from anti-VEGF therapies licensed for DME, which include ranibizumab, bevacizumab (off-label), aflibercept, and pegaptanib, a considerable number do not,6 and macular swelling after intravitreal anti-VEGF may require repeat treatments.4
Laser treatment may be needed in patients with central DME and associated vision loss who have persistent retinal thickening despite anti-VEGF therapy.1,2 Laser treatment was once the gold standard of care, but its role has diminished with the emergence of pharmacological therapies. Focal/grid laser therapy is aimed directly at the affected area or applied in a contained, grid-like pattern to destroy damaged eye tissue and clear away scars that contribute to blind spots and vision loss.7 Panretinal photocoagulation aims to destroy areas where there are capillary nonperfusion and retinal ischemia.3 Although laser photocoagulation reduces the risk of further vision loss, improvement in vision only occurs in about 12% of patients after 3 years.1,4,6 Furthermore, side effects can include scotoma, altered color perception, night blindness, hemorrhage, transient visual loss, macular edema, and visual field defects.6,7
Corticosteroids act on inflammatory cytokines and pathogenic mechanisms in addition to those associated with VEGF.5,8 They inhibit edema, fibrin deposition, capillary dilation, leukocyte migration, capillary proliferation, fibroblast proliferation, deposition of collagen, and scar formation associated with inflammation, leading to visual gain and reduction of DME.5 The effect of DME on retinal vasculature9 has been investigated and optical coherence tomography-angiography (OCTA) has been used in an attempt to understand the pathogenesis of the disease. Toto et al9 concluded that retinal vessel density is reduced in DME eyes versus normal healthy controls and that DME mainly involved the deep capillary plexus. Interestingly, the authors concluded that vessel density did not recover after the administration of the dexamethasone intravitreal implant, which may be due to its relatively short duration of action. To date, no studies have investigated the retinal vascular effect of the 0.19 mg fluocinolone acetonide (FAc; ILUVIEN®; Alimera Sciences) intravitreal implant.
The FAc and dexamethasone implants4 are licensed for use in DME and provide sustained release of drug over a period of 3–4 months for the dexamethasone implant and up to 36 months for the FAc implant, thus reducing the need for repeat injections. In the past, concerns about increases in intraocular pressure (IOP) and worsening of cataracts4 meant that corticosteroids were used cautiously in this population and tended to be used later in the treatment pathway. Steroid effects can be managed, whereas edema causing retinal damage is a bigger issue that must be addressed early to preserve vision in the long term.
The FAc implant is a sterile nonbioerodable intravitreal implant containing 0.19 mg (190 µg) FAc in a 36-month sustained-release drug-delivery system.10 The implant is injected directly into the vitreous via a single-use applicator and releases FAc at an initial rate of 0.2 μg/day.10 In the USA, the implant is indicated for the treatment of DME in patients previously treated with a course of corticosteroids who did not have a clinically significant rise in IOP. Two 3-year, randomized clinical trials in patients with DME previously treated with laser photocoagulation showed that the FAc implant significantly improved best-corrected visual acuity from as early as 3 weeks after implantation, which was sustained for up to 3 years, and was associated with significantly lower foveal thickness than control.10,11
In the absence of head-to-head studies of the FAc implant and anti-VEGF drugs for the treatment of DME, case reports and case series from clinical practice offer additional evidence for the implant’s role in the real-life setting.8,12–17 Such evidence has shown improvements in visual acuity following injection of the FAc implant, compared with worsening in fellow eyes,14,15 as well as larger improvements in central foveal thickness,14,15,18 improvements in visual acuity in patients with pseudophakia and bilateral persistent or recurrent DME,12 and improvements in visual acuity and foveal thickness in patients with vitrectomized and nonvitrectomized eyes.17,18 Increases with IOP in real-world cases have not been notable or have been significant but small and managed successfully,8,12–18 although tests and risk factors for IOP such as ocular hypertension and glaucoma should be taken into account when deciding whether to use the FAc implant.8 A retrospective cost–analysis study in the UK found that switching to the FAc implant in patients with refractory DME was cost- and time-saving without any reduction in efficacy.19
In the current clinical practice, FAc is generally reserved for patients with persistent DME despite anti-VEGF therapy; however, the fact that the FAc implant has beneficial effects as early as 3 weeks after the start of treatment, which are sustained for up to 3 years, suggests that the FAc implant may be useful earlier in the treatment pathway for some patients.8,10,11 The case series reported here, therefore, investigated the effectiveness and safety of the FAc intravitreal implant for patients with persistent or recurrent DME, who had not received a full year of monthly anti-VEGF injections.
Material and methods
Study design
This was a retrospective chart review. Eyes were treated with the FAc implant if they did not respond to anti-VEGF therapy, responded to anti-VEGF therapy but needed multiple monthly injections, or previously had a dexamethasone impant and achieved a response with a reduction in macular thickness without a clinically significant increase in IOP. Eyes were included in the chart review if they had received the FAc implant and had at least one assessment after implantation. Consent for the procedure was obtained. DME was defined as macular thickness of >250 µm, measured by TD-OCT. Mean duration of follow-up prior to FAc implant injection was calculated as the number of days between the first available record in the database and the date the implant was administered. Mean duration of follow-up after FAc implant injection was calculated as the number of days between the date of the initial implant administration and the last available record in the database.
Effectiveness outcome measures were visual acuity (visual acuity was assessed using a Snellen chart and converted to an Early Treatment Diabetic Retinopathy Study (ETDRS) letter score), macular edema, and retinal thickness. Safety outcomes included IOP changes, IOP drugs used, and any IOP-related surgeries or interventions. Treatment burden was assessed through the number of DME treatments before and after administration of the implant.
Statistical analysis
As data were not always available at all time points for all eyes, the last observation calculated (LOC; i.e. the last observation post-injecting the FAc implant) was used to maximize the number of eyes included in analyses. Statistical significance was taken as a p-value <0.05. P-values were based on statistical tests that included: Student one-sample t-tests (to compare LOC values after administration of the FAc implant with baseline values prior to administration of the FAc implant); Fisher's exact tests (to compare the proportion of eyes prior to and following the administration of the FAc implant); and, analysis of variance (to compare the number of treatments prior to and following the administration of the FAc implant).
Results
Patient population
In total, 18 eyes from 13 patients were included in this study (Table 1). The mean age was 67.8 years (range 56–85 years); nine (69.2%) patients were Caucasian and four (30.8%) were African American. Eleven (84.6%) patients had type 2 diabetes, while two (15.4%) had type 1 diabetes, with a mean duration of diabetes (n=8) of 26.5 years (range 5–72 years) and mean glycated hemoglobin (n=11) of 6.85% (range 5.9–8.1%).
Table 1.
Patient demographics at baseline
| Patients (n) | 13 |
|---|---|
| Eyes (n) | 18 |
| Mean (range) age (years) | 67.8 (56–85) |
| Sex (n, %) | |
| Male | 6 (46.2) |
| Female | 7 (53.8) |
| Ethnicity (n, %) | |
| Caucasian | 9 (69.2) |
| African American | 4 (30.8) |
| Type of diabetes (n, %) | |
| Type 1 | 2 (15.4) |
| Type 2 | 11 (84.6) |
| Mean (range) duration of diabetes (years) (n=8) | 26.5 (5–72) |
Fifteen (83.3%) eyes were pseudophakic and three (16.7%) phakic (Table 2). The mean duration of DME was 2.27 years (range 0.1–4.9 years). Before the FAc implant, the mean total number of treatments for DME was 4.6 for all 18 eyes that had any duration of follow-up before and after implantation. For the nine eyes that had ≥12 months follow-up before implantation and ≥6 months follow-up after implantation, the monthly mean number of treatments before the implant was 0.36, equivalent to one treatment every 2.77 months. For all 18 eyes that had any duration of follow-up before and after implantation, the monthly mean number of treatments before the implant was 0.34, equivalent to one treatment every 2.94 months. The mean number of previous treatments was three for anti-VEGF agents, 1.4 for corticosteroid, 0.1 for focal/grid laser therapy, and 0.1 for panretinal photocoagulation. Twelve (66.7%) eyes were known to have previously received dexamethasone. The most recent intervention was an anti-VEGF agent in seven (38.9%) eyes, dexamethasone in eight (44.4%), focal/grid laser therapy in two (11.1%), and panretinal photocoagulation in one (5.6%) eye. Five (27.8%) eyes received IOP-lowering medications before the implant was administered and these five (27.8%) eyes were receiving IOP-lowering medication on the date of the implantation. Mean duration of follow-up was 389 days (range 150–637 days) prior to administration of the implant and 335 days (range 70–748 days) after administration.
Table 2.
Ocular history at baseline
| Characteristic | Number (%) or mean (range) |
|---|---|
| Lens status (n, %) | |
| Pseudophakic | 15 (83.3) |
| Phakic | 3 (16.7) |
| Duration of DME (years) | 2.27 (0.1–4.9) |
| Prior treatments (n, %) | |
| Intravitreal dexamethasone | 12 (66.7) |
| Missing | 6 (33.3) |
| Most recent intervention (n, %) | |
| Anti-VEGF | 7 (38.9) |
| Dexamethasone | 8 (44.4) |
| Focal/grid laser | 2 (11.1) |
| Panretinal photocoagulation | 1 (5.6) |
| Number of previous treatments (n) | |
| Anti-VEGF | 3.0 (0–9) |
| Dexamethasone | 1.4 (0–4) |
| Focal/grid laser | 0.1 (0–1) |
| Panretinal photocoagulation | 0.1 (0—1) |
| Total | 4.6 (2–10) |
Abbreviations: DME, diabetic macular edema; VEGF, vascular endothelial growth factor.
Outcome measures
Effectiveness
Macular volume was available at baseline and at least one assessment after the implant was administered for all 18 eyes. The change from baseline in the LOC population was statistically significant (–1.33±0.34 mm3, p=0.001). Mean changes in the LOC population were also statistically significant for men (–1.96±0.56 mm3, p=0.010); eyes with DME for <3 years (–1.52±0.43 mm3; p=0.003); pseudophakic eyes (–1.55±0.37 mm3; p<0.001); eyes for which the most recent intervention was steroid therapy (–1.93±0.40 mm3; p=0.002); or dexamethasone within 180 days prior to administration of the implant (–1.94±0.46 mm3; p=0.006); eyes with one to three prior DME treatments (–1.73±0.67 mm3; p=0.042); eyes with four to six prior DME treatments (–1.73±0.39 mm3; p=0.004); eyes with no DME treatment after administration of the implant (–1.30±0.42 mm3; p=0.013); and eyes with four to six DME treatments after administration of the implant (–3.15±0.15 mm3; p=0.030). Overall, 16 (89%) eyes in the LOC population had a decrease in macular volume, with a decrease of >2.0 mm3 in four (22.2%) eyes, >1.5–2.0 mm3 in one (5.6%), >1.0–1.5 mm3 in three (16.7%), >0.5–1.0 mm3 in five (27.8%), and >0–0.5 mm3 in three (16.7%) eyes. Two (11%) eyes had an increase in macular volume of >0–0.5 mm3 and >0.5–1.0 mm3, respectively.
Central retinal thickness was available at baseline and at least one assessment after the implant was administered for all 18 eyes (Table 3). Retinal thickness was markedly lower for the LOC population, with a reduction from 444 µm at baseline to 359 µm (p<0.001) and mean change in retinal thickness from baseline of –84.5±34.9 µm (range –417–170 µm; p=0.027). Mean changes in the LOC population were also statistically significant for women (–96.9±41.0 µm [range –334–67 µm]; p=0.042); eyes with DME for <3 years (–107.4±42.3 µm [range –417–170 µm]; p=0.025); pseudophakic eyes (–99.3±40.8 µm [range –417–170 µm]; p=0.031); eyes with baseline vision between 20/100 and 20/40 (–104.0±38.8 µm [range –257–97 µm]; p=0.040); eyes with baseline center subfield retinal thickness of 600–700 µm (–336.0±46.2 µm [range –417–257 µm]; p=0.018); and eyes with no previous treatment for DME (–113.4±50.2 µm [range –417–170 µm]; p=0.05). All 18 patients had a >200 µm decrease in retinal thickness: >200–300 µm for six (33.3%) eyes, >300–400 µm for six (33.3%) eyes, >400–500 µm for four (22.3%) eyes, and >600–700 µm for two (11.1%) eyes.
Table 3.
Visual acuity and retinal thickness before and after FAc implant
| Parameter | –12 months | Baseline | Last observation |
|---|---|---|---|
| Absolute VA, ETDRS letters | 51.0 (SD 27.58) (n=5) |
50.7 (SD 19.50) (n=10) |
56.8 (SD 61.0) (n=10) (p=0.108) |
| Absolute CFT, µm | 404.5 (SD 35.64) (n=10) |
443.7 (SD 34.29) (n=18) |
359.2 (SD 30.38) (n=18) (p=0.027) |
Abbreviations: CFT, central foveal thickness; ETDRS, Early Treatment Diabetic Retinopathy Study; FAc, fluocinolone acetonide; VA, visual acuity.
Visual acuity was available at baseline and at least one assessment after the implant was administered for 10 eyes and was stable throughout the follow-up periods (see Table 3). The mean change from baseline in the LOC population was 6.1±3.4 letters (range –9–30 letters; p=0.108). Overall, five (50%) eyes had an increase in approximate ETDRS letter score from baseline: three (30%) with a 10–14 letter increase and one (10%) each with a 5–9 letter increase or an increase of ≥15 letters. Four (40%) eyes showed no change and one (10%) eye had a 5–9 letter decrease.
Safety
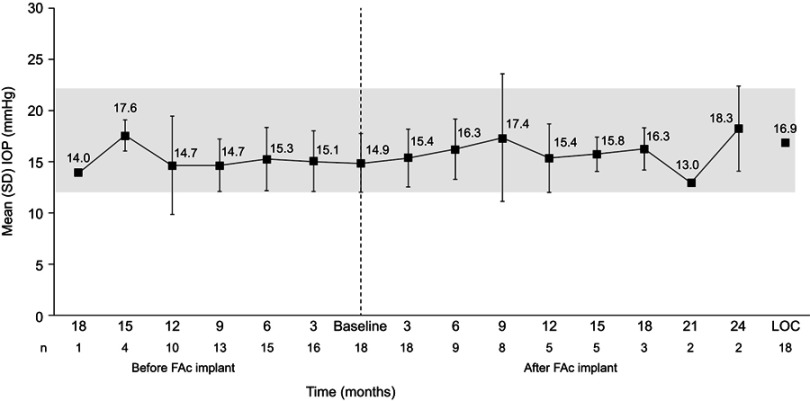
Figure 1 shows mean IOP before and after administration of the implant. Although the mean change in IOP from baseline in the LOC population showed a statistically significant increase (2.0±0.8 mmHg; p=0.020), mean IOP was within the normal range at all timepoints (16.9±0.72 mmHg). Statistically significant changes in mean IOP were seen for eyes with a duration of DME of <3 years (2.4±0.9 mmHg; p=0.018), with a maximum IOP of 23 mmHg; eyes considered pseudophakic at baseline (2.3±0.9 mmHg; p=0.021), with a maximum IOP of 23 mmHg; and eyes with a baseline IOP ≤15 mmHg (3.1±0.8 mmHg; p=0.005), with a maximum IOP of 19 mmHg.
Figure 1.
Mean IOP before and after FAc implant Note: Gray box indicates normal range of 12–22 mmHg.
Abbreviations: FAc, fluocinolone acetonide; IOP, intraocular pressure; LOCF, last observation calculated.
At last observation, seven (38.9%) eyes had an IOP of >10–15 mmHg, eight (44.4%) had an IOP of >15–20 mmHg, and three (16.7%) had an IOP of >20–25 mmHg. Five (27.8%) eyes had a decrease in IOP of 1–4 mmHg from baseline, two (11.1%) had no change, seven (38.9%) had a 1–4 mmHg increase, and four had a 5–7 mmHg increase.
The IOP category at baseline was 0–20 mmHg in all 18 eyes; after the implant was administered, and at last observation, 15 eyes remained in this category, while three eyes moved to the IOP category 21–25 mmHg.
A comparison of IOP events (any IOP-related events, and IOP elevation to >21 and >25 mmHg) before and after administration of the FAc implant was performed. In all three cases no statistical difference was observed (p>0.05): IOP-related events, n=2 vs 4 (before vs after, respectively); IOP elevation to >21 mmHg, 2 vs 4; and, IOP elevation to >25 mmHg, 1 vs 1. No cases of IOP elevation >30 mmHg were observed.
No change in the number of IOP-lowering medications was seen after administration of the implant, with the same five eyes (27.8%) receiving these drugs before, on the date of, and after implantation. No differences in IOP were observed based on patients’ sex, duration of diabetes, phakic status, number or type of previous treatments, baseline vision, or retinal thickness was observed. Furthermore, the comparison of IOP changes at last observation showed little difference in the magnitude of changes at last observation (+1.9±2.9 mmHg vs 2.2±4.6 mmHg [without vs with IOP-lowering medications]) and that mean IOP was well controlled with IOP-lowering medications at last observation (15.5±2.5 mmHg vs 13.4±3.4 mmHg [without vs with IOP-lowering medications]).
Treatment burden
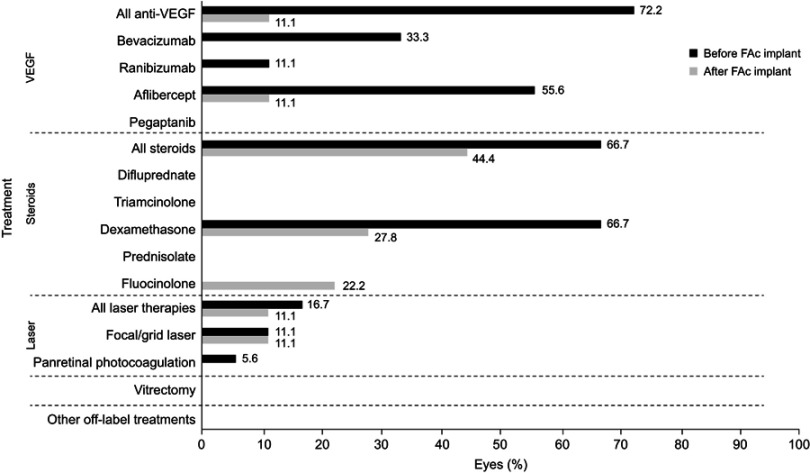
The average number of treatments was more than three times lower in the 6 months after the FAc implant was administered than in the 12 months preceding administration of the implant, with an average of 1.3 versus 4.6 treatments (Figure 2). To normalize any differences between follow-up before and after administration of the implant, treatments were also calculated as the mean number of treatments per month to compare treatment burden before and after the administration: for the nine eyes that had ≥12 months follow-up before implantation and ≥6 months follow-up after implantation, the average monthly number of treatments was 0.36 before, equivalent to one treatment every 2.78 months, versus 0.15, equivalent to one treatment every 6.67 months, after the FAc implant.
Figure 2.
Anti-DME treatments before and after FAc implant.
Abbreviations: DME, diabetic macular edema; FAc, fluocinolone acetonide; VEGF, vascular endothelial growth factor.
No further DME treatment was required after the FAc implant was administered in 10 (55.6%) of all 18 eyes. Most eyes that did need treatment after implantation required fewer than four treatments – nine (50.0%) of all eyes and four (56.1%) of the eyes with ≥360 days of follow-up before and after implantation – although the one eye with any follow-up and baseline vision worse than 20/200 required four to six treatments postimplantation. After administration of the implant, steroid therapy was the most commonly used treatment, used in eight (44.4%) eyes, followed by anti-VEGF agents and laser treatment, used in two (11.1%) eyes each.
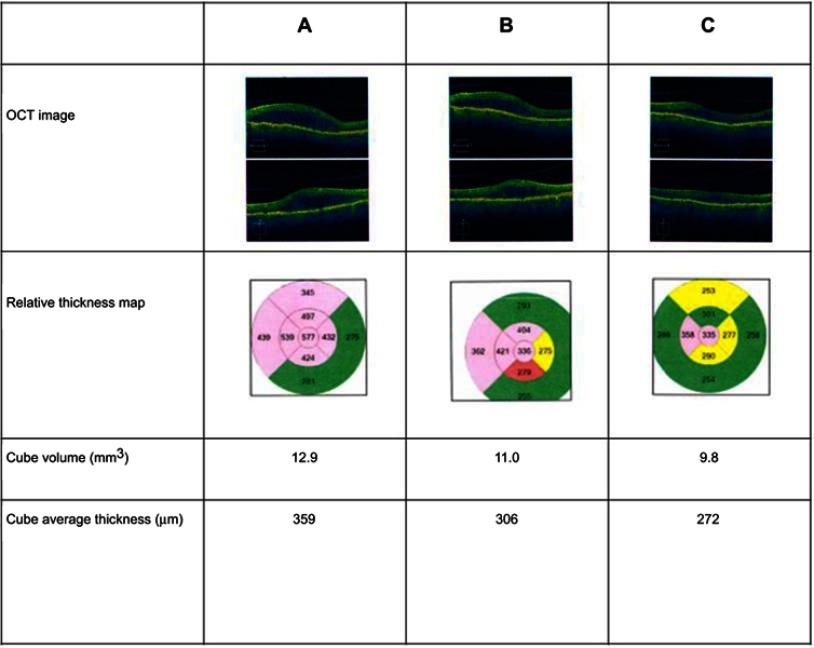
Figure 3 is a case study for one eye and shows the macular thickness and volume in the left eye on the day the FAc implant was administered and 1 and 6 months after implantation. IOP in the left eye was 11 mmHg on the day of the injection, with 15 mmHg being the highest pressure measured since the implant was administered (Box 1).
Box 1.
Case study illustrating early use of the fluocinolone acetonide (FAc) implant
| A 75-year-old man with type 1 diabetes mellitus developed diabetic macular edema. He was treated in 2009 with focal laser coagulation in each eye. Since 2016, his right eye has received intravitreal injections of bevacizumab (three courses), aflibercept (two courses), and dexamethasone intravitreal implant but with a poor response to all. After one course of bevacizumab and three courses of aflibercept to his left eye, a fluocinolone acetonide (FAc) implant was administered on February 12, 2018. His visual acuity on the day of implantation was 20/30, improving to 20/25 on March 19, 2018 and 20/20 by August 30, 2018. |
Figure 3.
Case study: Macular thickness and volume in the left eye on the day a fluocinolone acetonide (FAc) implant was administered (A) and 1 (B) and 6 (C) months after implantation.
Discussion
In this retrospective case series of patients with persistent or recurrent DME, the FAc implant led to positive long-term outcomes with acceptable tolerability and a reduced treatment burden. The implant led to significant improvements in macular volume, which was reduced in 89% of eyes. Visual acuity was stable throughout the follow-up periods, with increases or no worsening in ETDRS letter score in around 90% of cases (ie, 9 of 10 eyes) and all eyes had a >200 µm decrease in retinal thickness. Treatment burden was reduced after administration of the FAc implant, with no further DME treatment required in just over half of all eyes, and most eyes that did need postimplant intervention required fewer than four treatments during the follow-up period. Overall, mean IOP was statistically higher after treatment and was within the normal range at all timepoints. A comparison, comparing patients receiving and not receiving IOP-lowering drops, revealed that IOP increases were similar between groups (~2 mmHg) at the last observation point. The group receiving IOP-lowering drops (n=5) had done so prior to and following administration of the FAc implant. In this group, mean IOP was well controlled with IOP-lowering drops with mean values similar and even slightly lower than the group that did not receive IOP-lowering drops.
Anatomical improvements in macular volume and retinal thickness were statistically significant for eyes with DME of <3 years, indicating that the implant may be useful in patients with short duration and early disease. Improvements in macular volume were also statistically significant, irrespective of whether eyes had received one to three or four to six previous treatments, and improvements in retinal thickness were significant for eyes with no previous treatment, again indicating that the implant may be as effective early in the treatment pathway as when eyes have received multiple treatments. Statistically significant improvements were also seen in patients for whom the most recent treatment was steroid, specifically dexamethasone, so the FAc implant may bring additional benefits even in eyes that have been previously treated with other steroids. The FAc implant also provided benefits in patients who had not already received a full year of monthly anti-VEGF injections.
The results of this case series are in line with the findings of the FAc in diabetic macular edema (FAME) clinical trials and previous case series with the FAc implant, showing improvements in macular volume and retinal thickness, and, typically, improvements or no worsening of visual acuity.8,10–17 Increases in IOP in clinical practice were generally small, typically within the normal range, and manageable with minimal intervention;8,10–17 however, corticosteroid provocation tests, baseline optic disc and visual field status, and risk factors for IOP, such as ocular hypertension and glaucoma, should be considered when deciding whether to use the FAc implant.8
The nature of retrospective observational studies means that there are some limitations to this case series, including a small population from a single center. Without a defined protocol for outcomes and follow-up prior to the retrospective chart review, the duration of follow-up before and after administration of the FAc implant is variable, which limits the number of patients with follow-up at all timepoints, and evaluated outcomes were not consistently available for all patients, again limiting the number of patients who could be included in analyses. The LOC population has, therefore, been used to maximize the number of patients included in analyses, but this can result in bias in terms of the treatment effect. Outcomes, effectiveness, and safety may have been misclassified during the chart review, although data were taken from patient notes and electronic medical records. There was no control group for comparison, and fellow eyes without DME were not included for comparison of outcomes. Furthermore, individual patient outcomes were not included in the analyses. Data relating to other potential safety concerns, such as formation of cataracts and migration of implants, were not collected on the case report form during this chart review. However, the results reflect the impact of the FAc implant in clinical practice, including in patients who might have been excluded from clinical trials – for example, patients with a history of raised IOP with ocular steroids – thus reflecting the effectiveness and safety profiles of the implant in the real-world population. The fact that the data all derive from a single center is a limitation but also a strength, as outcomes were measured using standardized measurements, equipment, and procedures – for example, visual acuity was measured by approximate ETDRS letter score for all patients, and OCT has been performed using the same make and model of equipment. When comparing treatment burden, treatments were calculated as the mean number per month to normalize any differences between follow-up before and after administration of the implant.
Conclusion
The FAc intravitreal implant is a unique drug delivery option that can be incorporated early in the DME treatment process, leading to positive long-term outcomes with an acceptable safety profile, and a reduced treatment burden for patients and the health care systems in which they are treated.
Acknowledgment
We would like to thank Alyson Evans, an employee of Alimera Sciences Incorporated, for reviewing the manuscript.
Statement of ethics
This article does not contain any new studies with human or animal subjects performed by any of the authors. Patient consent to review medical records was not required by the institutional review board as this was not a clinical trial but rather a retrospective audit of the usage of the implant in general clinical practice. All data are anonymized, confidential, and complies with the Declaration of Helsinki.
Disclosure
The publication of this article was supported by Alimera Sciences Ltd. Jessica D McCluskey has attended advisory boards and speaker engagements and remunerated for these by Alimera Sciences. The authors report no further conflicts of interest relating to this publication.
References
- 1.Solomon SD, Chew E, Duh EJ, et al. Diabetic retinopathy: a position statement by the American Diabetes Association. Diabetes Care. 2017;40(3):412–418. doi: 10.2337/dc16-2641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.International Council of Ophthalmology. ICO Guidelines for Diabetic Eye Care. San Francisco (CA): ICO; 2017. [Google Scholar]
- 3.Royal College of Ophthalmogists. Diabetic Retinopathy Guidelines. London: RCO; 2012. [Google Scholar]
- 4.Mathew C, Yunirakasiwi A, Sanjay S. Updates in the management of diabetic macular edema. J Diabetes Res. 2015;2015:8. doi: 10.1155/2015/815839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goncalves RS, Teixeira C, Coelho P. Recurrent diabetic macular edema: what to do. Case Rep Ophthalmol. 2017;8(3):465–474. doi: 10.1159/000480119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dugel PU, Parrish R. ILUVIEN – a new approach to the treatment of diabetic macular edema. US Ophthalmic Rev. 2015;8:110–115. doi: 10.17925/USOR.2015.08.02.110 [DOI] [Google Scholar]
- 7.Haddrill M. 2017. Treatment of Diabetic Retinopathy And Macular Edema. Available from: http://www.allaboutvision.com/conditions/diabetic-treatment.htm. Accessed April3, 2017.
- 8.Saedon H, Anand A, Yang YC. Clinical utility of intravitreal fluocinolone acetonide (ILUVIEN®) implant in the management of patients with chronic diabetic macular edema: a review of the current literature. Clin Ophthalmol. 2017;11:583–590. doi: 10.2147/OPTH.S131165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Toto L, D’Aloisio R, Di Nicola M, et al. Qualitative and quantitative assessment of vascular changes in diabetic macular edema after dexamethasone implant using optical coherence tomography angiography. Int J Mol Sci. 2017;2(6):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Campochiaro PA, Brown DM, Pearson A, et al. Long-term benefit of sustained-delivery fluocinolone acetonide vitreous inserts for diabetic macular edema. Ophthalmology. 2011;118(4):626.e622–635.e622. doi: 10.1016/j.ophtha.2010.12.028 [DOI] [PubMed] [Google Scholar]
- 11.Campochiaro PA, Brown DM, Pearson A, et al. Sustained delivery fluocinolone acetonide vitreous inserts provide benefit for at least 3 years in patients with diabetic macular edema. Ophthalmology. 2012;119(10):2125–2132. doi: 10.1016/j.ophtha.2012.04.030 [DOI] [PubMed] [Google Scholar]
- 12.Elaraoud I, Attawan A, Quhill F. Case series investigating the efficacy and safety of bilateral fluocinolone acetonide (ILUVIEN®) in patients with diabetic macular edema. Ophthalmol Ther. 2016;5(1):95–104. doi: 10.1007/s40123-016-0045-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meireles A, Goldsmith C, El-Ghrably I, et al. Efficacy of 0.2 mug/day fluocinolone acetonide implant (ILUVIEN) in eyes with diabetic macular edema and prior vitrectomy. Eye (Lond). 2017;31(5):684–690. doi: 10.1038/eye.2016.303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Currie CJ, Holden SE, Berni E, Owens DR. Evaluation of the clinical effectiveness of fluocinolone acetonide 190 microg intravitreal implant in diabetic macular edema: a comparison between study and fellow eyes. Curr Med Res Opin. 2017;33(sup2):19–31. doi: 10.1080/03007995.2017.1366659 [DOI] [PubMed] [Google Scholar]
- 15.Currie CJ, Holden SE, Owens DR. Patterns of retinal thickness prior to and following treatment with fluocinolone acetonide 190 microg intravitreal implant for diabetic macular edema. Curr Med Res Opin. 2017;33(sup2):33–43. doi: 10.1080/03007995.2017.1366662 [DOI] [PubMed] [Google Scholar]
- 16.Holden SE, Currie CJ, Owens DR. Evaluation of the clinical effectiveness in routine practice of fluocinolone acetonide 190 microg intravitreal implant in people with diabetic macular edema. Curr Med Res Opin. 2017;33(sup2):5–17. doi: 10.1080/03007995.2017.1366645 [DOI] [PubMed] [Google Scholar]
- 17.Schmit-Eilenberger VK. A novel intravitreal fluocinolone acetonide implant (ILUVIEN®) in the treatment of patients with chronic diabetic macular edema that is insufficiently responsive to other medical treatment options: a case series. Clin Ophthalmol. 2015;9:801–811. doi: 10.2147/OPTH.S79785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pessoa B, Coelho J, Correia N, Ferreira N, Beirao M, Meireles A. Fluocinolone acetonide intravitreal implant 190 mug (ILUVIEN®) in vitrectomized versus nonvitrectomized eyes for the treatment of chronic diabetic macular edema. Ophthalmic Res. 2018;59(2):68–75. doi: 10.1159/000484091 [DOI] [PubMed] [Google Scholar]
- 19.Ch’ng SW, Brent AJ, Empeslidis T, Konidaris V, Banerjee S. Real-world cost savings demonstrated by switching patients with refractory diabetic macular edema to intravitreal fluocinolone acetonide (ILUVIEN): a retrospective cost analysis study. Ophthalmol Ther. 2018;7(1):75–82. doi: 10.1007/s40123-017-0114-6. Epub 2017 Nov 10. [DOI] [PMC free article] [PubMed] [Google Scholar]