Abstract
Background: Breast cancer has become the most common malignant disease threatening women’s health. The cancer stem cell (CSC) has been recognized as a small subpopulation of cancer cells possesses stem cell properties, which is crucial in tumorigenicity, tumor invasion, drug resistance, and metastasis. The BCL11A plays a crucial role in breast cancer progression. To investigate the effect of BCL11A, a functional oncogene, we focused on its maintenance ability of stemness in breast cancer stem cells.
Methods: We assessed the BCL11A expression level in tumor and non-tumor tissues using RT-qPCR and IHC. We subsequently established BCL11A-modulating breast cancer cell lines MDA-MB-231 and MCF-7. CCK8, colony formation assays, and xenograft model were used to determine the effect of BCL11A on tumorigenicity. Transwell assay and lung metastasis model in vivo were conducted to validate its function in metastasis. Its effect on stemness was assessed by flow cytometry and mammosphere formation. Western blot further characterized the importance of Wnt/β-catenin signaling in BCL11A-regulated cancer cell stemness.
Results: A higher level of BCL11A was detected in clinical breast cancer samples. BCL11A promoted tumor formation, cancer cell mobility, spheroid forming, and epithelial-mesenchymal transition by activating the Wnt/β-catenin signaling. In addition, BCL11A was associated with lung metastasis and increased the breast cancer cells stemness. BCL11A high expression (BCL11Ahigh) cancer cells exhibited stem cell-like properties compared with BCL11Alow cells, including a higher percentage of CD24low/CD44high subpopulation, self-renewal spheroids formation, and higher tumorigenicity. Our studies demonstrated that the Wnt/β-catenin signaling activated by BCL11A plays a potential role in the initiation of the renewal of breast cancer stem cells.
Conclusions: BCL11A not only functions in breast cancer carcinogenesis but also enhanced the stemness of breast cancer through activating Wnt/β-catenin signaling, and may become a potential target for breast cancer treatment.
Keywords: BCL11A, CSC, breast cancer, Wnt/β-catenin pathway
Introduction
In breast, lung, prostate, colon, head and neck carcinomas, cancer stem cells (CSCs) are a small population of cells with their tumor-initiating properties.1–3 This subpopulation of tumor cells has been reported to exhibit self-renewal and multi-differentiation characteristics. CD44 is a well-known marker for CSCs. CSCs participate in initiating tumor growth, metastasis, drug resistance, and metastasis.4,5 In many experimental models of cancer treatment researches using chemotherapy or radiotherapy for bulk cancer cell population, a therapy-resistant subpopulation of cancer cells has emerged and presented with a more oncogenic, stem-like, and invasive property.6 The outgrowth of breast cancer CSCs appears to be one of the most reasonable explanations for the fact that chemotherapy or radiotherapy is not sufficient to prevent all the cancer progression, invasion, recurrence, and metastasis effectively.7,8 Therefore, it is of great importance to eliminate CSCs in breast cancer treatment. Moreover, It is important to investigate the important mechanisms of the stemness of cancer cell and develop more effective therapies targeting cancer genes.
BCL11A (B-Cell CLL/Lymphoma 11A) is a transcription factor that was initially recognized as a retroviral insertion site (Evi9) in myeloid leukemia of BXH-2 mouse. Lazarus et al showed that BCL11A interact with SOX2 to regulate epigenetic regulators in lung cancer, indicating the oncogenic capacity of BCL11A.9 Jiang et al also reported that BCL11A protein was significantly increased in non-small cell lung cancer.10 Khaled et al demonstrated that BCL11A is an oncogene in TNBC (triple-negative breast cancer) and its overexpression plays an essential role in tumor formation and invasion.11 Moody et al reported that BCL11A employs RBBP4/7 to recruit epigenetic complex to promote tumorigenesis in TNBC, further elucidating its mechanism.12 In the study of Smith et al, they discovered BCL11A is required for hematopoietic stem cell and immune functions, indicating a novel role in stem cell development.13 Furthermore, Khaled et al demonstrated that BCL11A deletion decreases the number of breast epithelial stem and progenitor cells, but the mechanism still remains unclear.11 These findings suggest that BCL11A could effectively function in breast cancer. However, the mechanism of the effect of BCL11A on breast cancer cell stemness, tumorigenesis and metastasis is still unknown.
Wnt/β-catenin pathway has been proved to be crucial in breast cancer stem cells. Lv et al demonstrated that the number of breast cancer stem cells, tumor-initiating ability, and metastasis to lung were significantly changed when Wnt/β-catenin signaling was interfered.14 Furthermore, Huang et al reported that inhibition of Wnt/β-catenin signaling pathway by Salinomycin exerted selective reduction of the number of cancer stem cells, leading to tumor suppression.15
Herein, we conducted this study to explore the function of BCL11A and the potential key mechanisms of its effect on breast cancer. Our results indicated that BCL11A promoted cancer cell stemness, invasion, metastasis via the Wnt/β-catenin signaling. Therefore, BCL11A may be a promising target in developing a new breast cancer treatment.
Method and materials
Cell lines and clinical samples
20 paired clinical surgical samples (breast cancer and adjacent normal tissues) were obtained from the breast cancer patients who underwent surgery at the first affiliated Hospital of Guangzhou Medical University (Guangzhou, People’s Republic of China) and immediately disposed using RNAlater (Thermo Fisher, USA) according to the manufacturer’s instructions. The Guangzhou Medical University Medical Ethics Committee has approved this study, and all the participants have signed the written informed consent. The study was conducted in accordance with the Declaration of Helsinki. All breast cancer cell lines including normal immortalized mammary cells and breast cancer cells (MCF-10A, 184A1, MCF-7, 4T1, T47D, SkBr3, MDA-MB-361, MDA-MB-468, BT-483, BT-474, MDA-MB-231) used in our study have been described in previous studies.16,17 The cell lines were purchased from the Cell Bank of Type Culture Collection of the Chinese Academy of Sciences, Shanghai Institute of Biochemistry and Cell Biology. MCF7 and MDA-MB-231 cells were cultured in DMEM medium supplemented with 10% fetal bovine serum (Invitrogen) and were grown without antibiotics in an atmosphere of 5% CO2 and 99% relative humidity at 37°C. The STR profile analysis of these cell lines used in this study was perform every six months after resuscitation by Beijing Microread Genetics, Co., Ltd. No mycoplasma infection was detected in all cell lines.
IHC (immunohistochemistry)
Paraffin tissue sections were heated twice in a microwave oven for 5 min each after being dewaxed, rehydrated and placed in 10 mmol/L citrate buffer (pH 6.0) as routine. The tissue slides were then incubated with purified anti-BCL11A and anti-CD44 primary antibody at 4 °C overnight after incubation with 3% H2O2 for 8 mins, PBS washing, 10% normal goat serum blocking for 30 minutes. The tissue sections were further stained with the commercial amplification system kit (DAKO, k5007) after washing and visualized under the microscope (NIKON ECLIPSE 80i) with photos taken.
In vitro tumor growth assays
The processed cancer cells were harvested, resuspended, and cultured at a density of 1×103 per well in 96-well plates for cell growth assays, and a MTT kit (ab211091, Abcam, UK) was used to detect the BCL11A-modulating cancer cell growth rate following the manufacturer’s instructions. 50 µL of serum-free media and 50 µL of MTT solution was added into each well. Incubate the plate at 37 °C for 3 hrs. After incubation, add 150 µL of MTT solvent into each well and read absorbance at OD=590 nm. In addition, 1×103 cells were cultured in six-well plates as above for the foci formation assay. The supernatant was removed, and the cell colonies were washed, stained, and counted using a crystal violet staining method. In our study, the data of three independent experiments are displayed as the mean ± SD.
Xenograft tumor growth assays
4 week-old nude mice were used for establishing the animal model for xenograft tumor growth assays. 17β-estradiol pellet was implanted subcutaneously to promote MCF-7 tumors in nude mice. We cut the skin around the third pair of breast and found the epithelia-cleared fat pads where the BCL11A-modulated cells and controls were injected. A total of 2×106 of MCF7 or MDA-MB-231 cells with BCL11A overexpression or knockdown were injected. Tumor weight was measured every 7 days to assess the tumor formation ability of these cells in nude mice. Mice were sacrificed at the end of experiment and the xenografts were stripped and photographed.Our animal model experiments were performed following Guangzhou Medical University standard ethics guidelines. The institutional animal ethics committee has approved our experiments. All experimental procedures were performed in accordance with relevant institutional and national guidelines and regulations.
In vitro cell metastasis assay
Subsequently, cells were seeded onto the basement membrane matrix present in the insert of a 24-well culture plate (EC matrix, Chemicon, Temecula, CA). Fetal bovine serum was added to the lower chamber as a chemoattractant. After an additional 48 hrs, the non-invading cells and EC matrix were gently removed with a cotton swab. Invasive cells located on the lower side of the chamber were stained with crystal violet, counted and imaged.
In vivo metastasis assay
Female nude mice (6–8 weeks old) were used for metastasis assays in vivo. Each independent experimental group consisted of three mice. For tail vein injection, 2×105 cells were harvested, washed twice with PBS, and injected for each female Balb/c nude mouse. 8 weeks later, we sacrificed all the mice, and then examined and counted the pulmonary metastatic nodules carefully. The lungs of each group were fixed by 4% paraformaldehyde for further study.
Mammosphere formation and cell sorting
A density of 1,000 cells MDA-MB-231 and MCF-7 cells were cultured in ultra-low attachment dishes (Corning, USA) to form spheres. The culture medium was formulated with DMEM/F12 medium, EGF (20 ng/ml, Invitrogen), insulin (10 μg/ml, Sigma), 1% B27 (Invitrogen, Carlsbad, CA), and bFGF (10 ng/ml, Invitrogen). Cells were incubated with primary antibody for 30 min, followed by secondary antibody staining for 20 min, prior to FACS analysis or sorting. The anti-CD44 and anti-CD24 antibodies used for FACS analysis were obtained from BD Bioscience. The organoids were collected by brief centrifugation (800 rpm for 2 min) and then digested with 0.25% trypsin-EDTA for 2 min at 37 °C and filtered through a 40 µm cell strainer to dissociate into single cells.
RNA isolation and RT-qPCR (real-time quantitative PCR)
The methods for isolating RNA was described in previous study.18 We used a PrimeScriptRT Reagent Kit for reverse transcription following the manufacturer’s instructions (Promega, Madison, WI). We performed the Real-time PCR on a Bio-Rad CFX100 using a SYBR Green SuperMix kit (Invitrogen, Carlsbad, CA). We used β-actin as a control for each group. The BCL11A and β-actin primer sequences: BCL11A-F 5-CAGCACTTAAGCAAACGGGAAT-3 and BCL11A-R 5-TTGTTTCCGTTTGTGCTCGATA-3; β-actin-F 5-GGACTTCGAGCAAGAGATGG-3 β-actin-R 5-ATCTGCTGGAAGGTGGACAG-3. The primers were purchased from Invitrogen (Shanghai, People’s Republic of China).
Western blotting and antibodies
We used a Quantified Protein BCA Assay Kit (Bio-Rad) to measure the concentration of protein lysates. The SDS-PAGE gels were used to load the total proteins of cell lysates. After electrophoresis, the proteins were transferred onto polyvinylidene fluoride (PVDF) membranes following the manufacturer’s instructions (Millipore, Billerica, MA), and then the membranes were incubated with anti-human primary antibodies to c-myc (Cell Signaling Technology), β-actin (Cell Signaling Technology), CD44 (Cell Signaling Technology), β-catenin (Cell Signaling Technology), cyclinD1 (Cell Signaling Technology), fibronectin (Cell Signaling Technology), slug (Cell Signaling Technology), snail (Cell Signaling Technology), and BCL11A (Abcam, Cambridge, U.K.). The blots were visualized with ECL (Enhanced Chemiluminescence) (Amersham Biosciences) using a chemiluminescent method. We quantified the results by normalized the blotting according to β-actin.
Statistics
Comparisons between groups were analyzed using two-tailed Student's t-tests and chi-square tests. Differences between groups were considered significant when P<0.05. Statistical analyses were performed using the SPSS 22.0 statistical software (SPSS Inc., Chicago, IL, USA).
Results
BCL11A expression in breast cancer
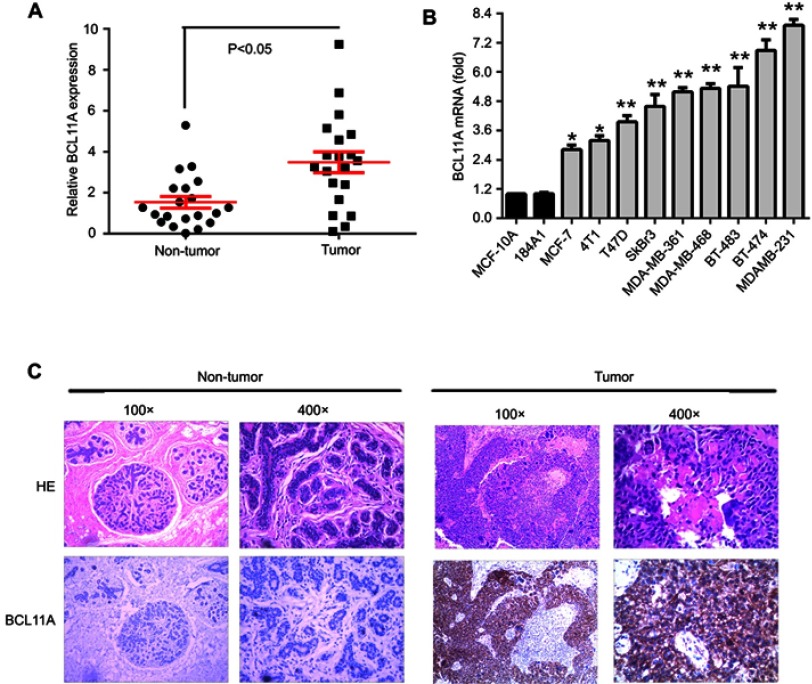
To investigate the functions of BCL11A in breast cancer, we detected the BCL11A expression level in clinical breast cancer specimens. Relative BCL11A expression level of 20 pairs of adjacent nontumor tissues and primary breast cancer was measured using qRT-PCR. BCL11A overexpression was detected in tumor tissues. BCL11A expression was significantly higher in breast cancer tumor tissues than that in corresponding adjacent normal tissues (Figure 1A, paired Student’s t-test, P<0.05). To further determine the role of BCL11A in breast cancer tumorigenesis and metastasis, we examined the expression level in a panel of breast cancer cell lines by qRT-PCR. BCL11A was overexpressed in all the breast cancer cell lines compared with normal human mammary epithelial cell lines (MCF-10A and 184A1) examined in our study, with the highest level in MDA-MB-231 and the lowest level in MCF7 (Figure 1B). We further tried to extend our results to the intracellular distribution of BCL11A and their expression in clinical samples using IHC. BCL11A overexpression was detected in breast cancer samples compared with paired normal mammary tissues. Furthermore, BCL11A was mainly distributed in the cytoplasm of breast cancer cell (Figure 1C).
Figure 1.
BCL11A mRNA overexpression level in breast cancer. (A) The relative BCL11A expression level was detected in 20 pairs of breast cancer samples. (B) qRT-PCR analysis of BCL11A expression in two normal immortalized breast cell lines (184A1 and MCF-10A) and breast cancer cell lines. **p<0.01; *p<0.05. (C) HE-staining and immunohistochemical-staining of BCL11A protein in a clinical sample and its adjacent normal tissue. Original magnification, 100× and 400×.
BCL11A correlates with breast cancer tumorigenicity in vivo and in vitro
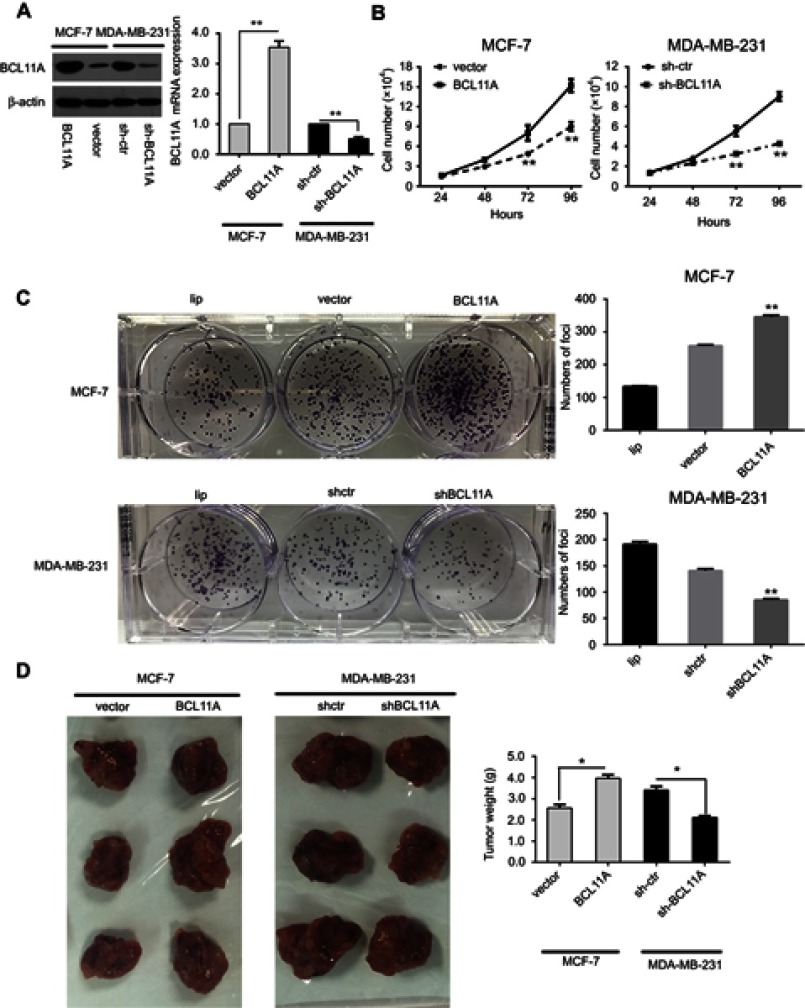
Because a significant correlation between BCL11A expression and tumorigenicity was examined in clinical samples, stable overexpressing and knockdown cells were necessary for further study. We used shRNA and plasmid transfection to establish stable BCL11A-knockdown cells (MDA-MB-231-shBCL11A) and BCL11A-overexpressing cells (MCF7-BCL11A) to assess the role of BCL11A in tumor progression. Western blot analysis and qPT-PCR was used to confirm the BCL11A expression in the modified cells. Using scramble shRNA and empty vector-transfected cells as controls, BCL11A was effectively downregulated or regulated (Figure 2A).The role of BCL11A in tumorigenicity was further confirmed using several assays. The cell growth assays were applied to BCL11A-transfectant MCF7 cells and indicated higher growth rates compared with control cells, and sh-BCL11A MDA-MB-231 group shows lower proliferation rates (Figure 2B, P<0.01). In the focus formation assay, it showed that BCL11A could significantly increase the number of foci. BCL11A-expressing cells yield larger and more colonies (P<0.01 for MDAMB231-shBCL11A cells, P<0.01 for MCF7-BCL11A cells), indicating an effect on tumorigenicity (Figure 2C). We perform animal experiment via orthotopic injection of the BCL11A-modulated cell into the fat pad of nude mice. The mice were sacrificed after four weeks, the tumors were harvested, and the wet weights of each tumor were recorded. Compared with control groups, the BCL11A-expression group yielded significantly higher and heavier tumors (P<0.01 for MDAMB231-shBCL11A cells; P<0.01 for MCF7-BCL11A cells) (Figure 2D). The tumor formation and focus formation assay experiment in animal model showed that BCL11A played a role in tumorigenicity. Our findings indicate that BCL11A may play a crucial role in tumor proliferation of breast cancer both in vivo and in vitro.
Figure 2.
BCL11A has strong oncogenic functions. (A) A Western blot assay was used to characterize the expression of BCL11A in BCL11A-overexpressing and control vector-transfected cells. shRNA against BCL11A effectively decreased BCL11A expression detected by Western blotting (left). BCL11A expression was confirmed by qRT-PCR (right), and β-actin was used as a loading control. (B) MTT assays were performed to compare the cell growth rates between BCL11A-overexpressing and control cells and between BCL11A-silenced and control cells. (C) Representative images of the increased foci formation ability induced by BCL11A in MCF-7 and MDAMB231 cell lines. Quantitative analyses of foci numbers are shown in the right panel. (D) Representative images of xenografts and a summary of tumor weight in nude mice. The weights of xenograft tumors are summarized in the right panel. All results are expressed as the mean ± SD of three independent experiments, *p<0.05; **p<0.01.
Active BCL11A confers increased cell migration and tumor metastasis in breast cancer
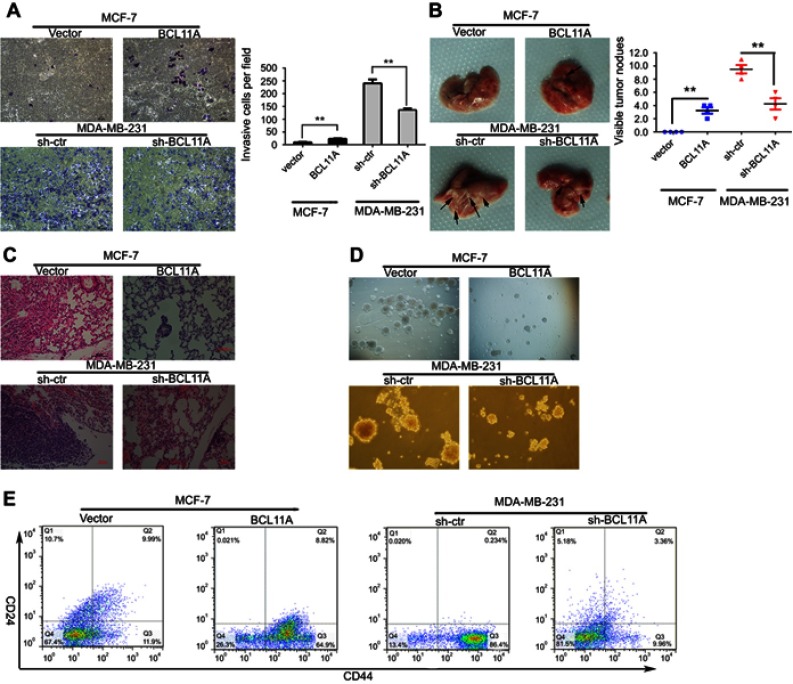
Because the role of BCL11A in breast cancer metastasis still remains unknown, we further assessed whether the presence of BCL11A confers greater breast cancer migratory and invasive capacity. Compared with controls, the BCL11A-expressing cells significantly yield a more invasive property (Figure 3A, P<0.01 for MDAMB231-shBCL11A cells; P<0.01 for MCF7-BCL11A cells). To further investigate the role of BCL11A in promoting tumor metastasis in vivo, BCL11A-modulated breast cancer cells were injected intravenously into the tail vein of the nude mice. After eight weeks, the mice were sacrificed, and we counted the number of the metastatic nodules on the lungs surface. There was no visible metastatic nodule on the surface of the lung in the MCF-7 control group, whereas BCL11A overexpression significantly increased the amount of pulmonary metastatic nodules in the BCL11A-expression group (Figure 3B upper). Moreover, BCL11A knockdown reduced the visible metastatic nodules in mice injected with MDAMB231 cells compared with controls (P<0.01, independent Students’ t-test; Figure 3B bottom). The metastatic modules on the surface of the lung were further confirmed as metastatic tumors using HE staining (Figure 3C).
Figure 3.
BCL11A promotes tumor metastasis, cancer cell migration, and the stemness of breast cancer cells. BCL11A promotes cell metastasis ability, confirmed by Transwell invasion assay in vitro. (A) Representative invaded cells image (Left), and the statistical analysis of the invaded cell number (Right) are shown. All the data was shown as the mean ± SD, and three independent experiments were performed (**p<0.01; *p<0.05). (B) Representative image of lung metastasis nodules on the lung of xenograft model (arrows). 8 weeks after tail vein injection of BCL11A-modulating MDA-MB-231 and MCF-7 cells, the number of lung nodules on lungs surface of nude mice was counted and analysed (N=3) (**p<0.01; *p<0.05). (C) Hematoxylin and eosin stained metastatic nodules on the surface of the lung. Representative image of sections was shown. Original magnification, 100×. (D) BCL11A increases the sphere-forming ability of MDA-MB-231 and MCF-7 cells. Original magnification: ×100. (E) Representative dot plots of CD44+/CD24− cell surface markers from mammospheres in BCL11A-osverexpressing MCF-7 and sh-BCL11A MDA-MB-231 and their controls.
BCL11A is essential for the maintenance of breast cancer stemness
The MCF-7 and MDA-MB-231 cancer cells were cultured with conditioned DMEM/F12 medium on special plates with ultra-low attachment property. The cells grew in spheroids. To further investigate the effect of BCL11A on the breast cancer stemness, we conducted the sphere-forming assay. Cancer stem cells marked with CD133 or CD44 were further enriched through fluorescence-activated cell sorting. The sphere-forming capacities of MDA-MB-231-shBCL11A were significantly decreased compared with controls. BCL11A increase the sphere-forming capacities of MCF7-BCL11A cells. In corresponding cultures, spheroids formed by MDA-MB-231-shBCL11A cells were easy to be disrupted mechanically, but controls formed compact spheroids resistant to there disruptions. Spheroids formed by MCF7-BCL11A cells were larger and more than those in the control group (Figure 3D). To further assess the effect of BCL11A on the percentage of breast cancer stem cells in MCF7 and MDA-MB-231 cells, we use flow cytometry to evaluate the CD44high/CD24low subpopulation in BCL11A-modulating cells. In MCF7 cells, FACS analysis demonstrated that CD44high/CD24low cells were significantly increased in the BCL11A-expressing group compared with controls (64.9% vs 11.9%), and MDA-MB-231-shBCL11A group yield a lower percentage of CD44high/CD24low cells than control cells (9.96% vs 86.4%) (Figure 3E). These results indicate that BCL11A can enhance breast cancer tumor cell stemness.
BCL11A potentiates Wnt/β-catenin signaling in breast cancer cells
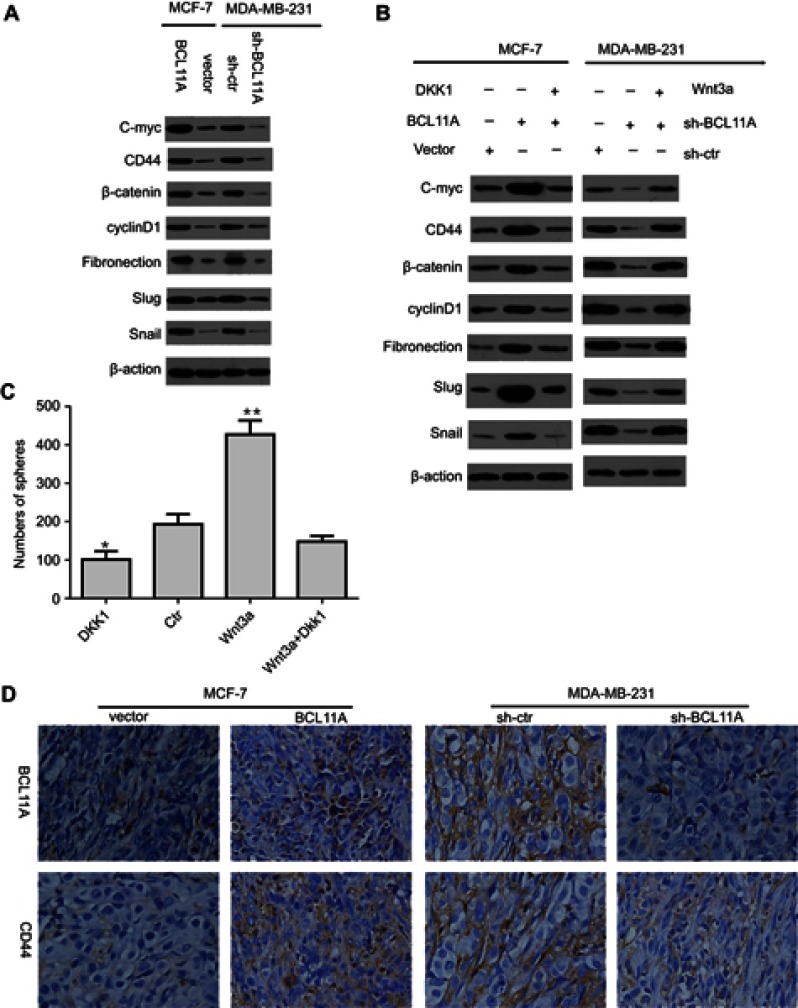
As epithelial-mesenchymal transition (EMT) was proved to be involved in cell invasion and metastasis, we measured the expression of EMT markers or EMT-related transcription factors in BCL11A-modulating cells to study the effect of BCL11A on EMT. Western blot analysis demonstrated that BCL11A could significantly upregulate the C-myc, CD44, β-catenin, and cyclinD1 as well as EMT markers (Fibronectin, Slug, and Snail) (Figure 4A). In MDA-MB-231 cells, consistent results were observed when BCL11A was knocked down.
Figure 4.
BCL11A promotes the breast cancer cell stemness and epithelial-mesenchymal transition by activating the Wnt/b-catenin pathway. (A) Relative C-myc, fibronectin, β-catenin, CD44, cyclinD1, slug, and snail expression level between BCL11A-modulating cells and controls of MDA-MB-231 and MCF7 was characterized by Western blotting. (B) The C-myc, CD44, fibronectin, β-catenin, cyclinD1, slug, and snail expression level was effectively decreased by Dkk1, a Wnt inhibitor, in BCL11A-overexpressing MCF-7 cells, whereas effectively increased by Wnt3a, a Wnt agonist, in MDA-MB-231 cells transfected with shBCL11A. (C) Wnt3a significantly promoted the formation of more spheres, whereas the Wnt inhibitor DKK1 reversed this trend and reduced tumor spheres number. All the data was shown as the mean ± SD, and three independent experiments were performed (**p<0.01; *p<0.05). (D) BCL11A and CD44 expression were further confirmed in xenograft tumors of nude mice through immunohistochemistry, and representative images were shown. Original magnification, 200×.
Wnt signaling was commonly involved in cancer proliferation and metastasis. The previous study has reported that the activation of Wnt/β-catenin signaling was involved in the EMT process by interacting with snail.18 The latter is proved to be a target gene of β-catenin. The functional β-catenin is crucial in EMT, which leads to the nuclear localization and stabilization of snail, promoting the EMT and cell migrating of cancer cells. We further examined the Wnt/β-catenin signaling pathway in MCF-7 and MDA-MB-231 cells to further explore the potential molecular mechanisms of the effect of BCL11A on cancer proliferation and metastasis. To further investigate the role of Wnt/β-catenin signaling in the oncogenic effect of BCL11A, we used the Wnt signaling inhibitor Dkk1 to determine the association between Wnt target genes and the activation of β-catenin induced by BCL11A in BCL11A-modulated MCF-7 and MDA-MB-231 cells. In MCF-7 cells, C-myc, CD44, cyclinD1, β-catenin, Fibronectin, Snail, and Slug proteins were significantly upregulated compared with the controls. Moreover, this effect could be effectively inhibited by Dkk1. Compared with control cells, the expression of these EMT- related proteins was downregulated in BCL11A-knockdown MDA-MB-231 cells, whereas Wnt3a significantly reversed these results (Figure 4B). Our findings indicate that BCL11A functions in the EMT through the activation of Wnt/β-catenin signaling pathway in breast cancer cells, leading to tumor metastasis.
BCL11A and Wnt/β-catenin signaling pathway is necessary for CSCs derived from breast cancer spheroids
Previous studies have demonstrated that the Wnt/β-catenin pathway is crucial for the acquisition of uncontrolled cell proliferation and expansion.19 In the sphere-forming assay, Wnt3a addition significantly increased the number of spheres, while the Wnt inhibitor Dkk1 led to a different result, which indicated a weaker capability to enhance breast cancer cell stemness in vitro. Moreover, the effect of Wnt3a addition on the number of spheres could be reversed by Dkk1 (Figure 4C). To further elucidate the correlation between BCL11A and CD44, we examined the endogenous BCL11A, and CD44 in xenograft tumor derived from BCL11A-modulating MDA-MB-231 and MCF-7 cells (Figure 4D). The BCL11A and CD44 mainly distributed in cancer cell membrane and cytoplasm. The CD44 was significantly increased in BCL11A-expression tumors, while BCL11A-knockdown tumors yielded an opposing result. Therefore, our results indicate that BCL11A is correlated with breast cancer stemness and metastasis through the activation of Wnt/β-catenin signaling.
Discussion
BCL11A, which is a novel breast cancer gene associated with cell proliferation, signal transduction, cell cycle, and apoptosis, was upregulated in breast cancer. Previous researchers have investigated its ability to promote cancer cell stemness and metastasis.8,9,20 Experimentally, we have shown that disrupting BCL11A expression in breast cancer cell lines and mouse model significantly affected tumor development and stemness maintenance. We showed that BCL11A promotes breast cancer cell tumorigenesis, proliferation, invasion and metastasis by the activation of Wnt/β-catenin signaling. Moreover, we found that BCL11A significantly increased the percentage of breast CSCs and it is crucial for breast cancer the stemness maintenance. To our knowledge, we are the first to report the correlation between BCL11A and Wnt/β-catenin signaling and their regulatory function of stemness in breast cancer.
Previous work from our study also showed that in clinical samples, BCL11A expression was significantly correlated with the DFS (Disease-free survival) and OS (Overall survival) of patients with TNBC.21 Previous researchers determined that the BCL11A level is upregulated in cancer samples compared with adjacent normal nontumor samples, promoting cancer invasion and metastasis.22–24 Cancer stem cells have proved to be involved in cancer recurrence, drug-resistance, invasion, and metastasis. Yun et al and Satterwhite et al reported that BCL11A promotes the lymphoid formation, suppressing the function of p53 and indicating a crucial role in tumor metastasis.25,26 We have shown that the BCL11A deletion leads to a reduction of breast epithelial stem cells and tumor metastasis. Taken together, the evidence indicates that BCL11A functions in breast cancer cell stemness maintenance, leading to tumor progression and metastasis.
We found a significantly higher level of BCL11A in clinical breast cancer samples compared with adjacent nontumor tissues. Subsequently, BCL11A promotes tumor growth through our functional studies. In the nude mice tumor xenograft experiment, BCL11A overexpression significantly promotes tumor growth and lung metastasis in vivo. Moreover, BCL11A knockdown could effectively reverse this effect. Experiment in vivo and in vitro assay also determined that BCL11A upregulation was significantly correlated with breast cancer stemness, indicating a key role in stemness maintenance.
Many studies determined that Epithelial-mesenchymal Transition (EMT) is of great importance in tumor invasion and metastasis, leading to a weaker cell-cell adhesion, stronger invasion ability and increased cancer cell mobility.27,28 We studied the influence of BCL11A knockdown or overexpression on epithelial-mesenchymal transition. Snail and slug, two of the important EMT markers, were upregulated as expected. Furthermore, silencing BCL11A expression could inhibit the EMT phenotype invasive ability in breast cancer cell lines. We further determined that BCL11A induce EMT through the Wnt/β-catenin pathway activity. The Wnt/β-catenin pathway is well known to be of great significance in modulating EMT process as tumor metastasis initiation.29,30 The snail is a well-known transcription factor targeted by β-catenin and an EMT regulatory factor. We have shown that when BCL11A was knockdown or overexpressed, the snail and β-catenin expression level changed subsequently. Moreover, the cancer stem cell features of breast cancer have been proved to be involved in EMT. Some researchers have directly demonstrated EMT may cause a CSCs state, while other researchers reported the EMT of cancer cells causes an increased percentage of stem cell-like cells which yield a self-renewal property and may affect the spheroid forming ability. In our research, BCL11A induced EMT in breast cancer, leading to a stem cell state.
We further explored whether BCL11A could serve as a cancer stem cell marker or enhance stemness in breast cancer. The characteristics of CSCs include the ability to differentiate into origin tumor cell types, higher tumorigenicity in the xenograft model, and self-renewal feature.31 We examined the BCL11Ahigh and BCL11Alow subpopulation to assess the differences in these characteristics. BCL11Ahigh cells yielded a stronger spheroid-forming ability compared with BCL11Alow cells. In the xenograft model, BCL11Ahigh cells presented higher tumorigenicity in vivo.
We have also reported that BCL11A regulates CSCs behavior through the Wnt/β-catenin pathway and is essential for breast cancer cell renewal. Although some studies have supported this notion,32 the role of BCL11A in breast cancer CSCs via Wnt signaling remains unexplored. Based on our results, BCL11A plays an important role in stemness maintenance of breast cancer CSCs through Wnt/β-catenin pathway activity. Besides, we provide proof that BCL11A also influences the cancer cell cycle. We intend to elucidate the function of Wnt and BCL11A, including the BCL11Ahigh subpopulation as breast cancer stem cells.
Conclusions
This study elucidates that BCL11A plays an important role in breast cancer tumorigenicity and stemness maintenance through the Wnt/β-catenin pathway activity. This may provide a novel target for developing novel anti-cancer drugs and breast cancer prevention, and even individualized target therapy for breast cancer patients. However, its specific mechanism still needs further investigation.
Acknowledgment
This work was supported by funds from the National Science Foundation of Guangdong Province (2017A030313554).
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer. 2008;8:755–768. doi: 10.1038/nrc2499 [DOI] [PubMed] [Google Scholar]
- 2.Grotenhuis BA, Wijnhoven BP, van Lanschot JJ. Cancer stem cells and their potential implications for the treatment of solid tumors. J Surg Oncol. 2012;106:209–215. doi: 10.1002/jso.23069 [DOI] [PubMed] [Google Scholar]
- 3.Ailles LE, Weissman IL. Cancer stem cells in solid tumors. Curr Opin Biotechnol. 2007;18:460–466. doi: 10.1016/j.copbio.2007.10.007 [DOI] [PubMed] [Google Scholar]
- 4.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167 [DOI] [PubMed] [Google Scholar]
- 5.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Creighton CJ, Li X, Landis M, et al. Residual breast cancers after conventional therapy display mesenchymal as well as tumor-initiating features. Proc Natl Acad Sci U S A. 2009;106:13820–13825. doi: 10.1073/pnas.0905718106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tanei T, Morimoto K, Shimazu K, et al. Association of breast cancer stem cells identified by aldehyde dehydrogenase 1 expression with resistance to sequential Paclitaxel and epirubicin-based chemotherapy for breast cancers. Clin Cancer Res. 2009;15:4234–4241. doi: 10.1158/1078-0432.CCR-08-1479 [DOI] [PubMed] [Google Scholar]
- 8.Shafee N, Smith CR, Wei S, et al. Cancer stem cells contribute to cisplatin resistance in Brca1/p53-mediated mouse mammary tumors. Cancer Res. 2008;68:3243–3250. doi: 10.1158/0008-5472.CAN-07-5480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lazarus KA, Hadi F, Zambon E, et al. BCL11A interacts with SOX2 to control the expression of epigenetic regulators in lung squamous carcinoma. Nat Commun. 2018;9:3327. doi: 10.1038/s41467-018-05790-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiang BY, Zhang XC, Su J, et al. BCL11A overexpression predicts survival and relapse in non-small cell lung cancer and is modulated by microRNA-30a and gene amplification. Mol Cancer. 2013;12:61. doi: 10.1186/1476-4598-12-61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khaled WT, Choon Lee S, Stingl J, et al. BCL11A is a triple-negative breast cancer gene with critical functions in stem and progenitor cells. Nat Commun. 2015;6:5987. doi: 10.1038/ncomms6987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moody RR, Lo MC, Meagher JL, et al. Probing the interaction between the histone methyltransferase/deacetylase subunit RBBP4/7 and the transcription factor BCL11A in epigenetic complexes. J Biol Chem. 2018;293:2125–2136. doi: 10.1074/jbc.M117.811463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith EC, Luc S, Croney DM, et al. Strict in vivo specificity of the Bcl11a erythroid enhancer. Blood. 2016;128:2338–2342. doi: 10.1182/blood-2016-08-736249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lv C, Li F, Li X, et al. MiR-31 promotes mammary stem cell expansion and breast tumorigenesis by suppressing Wnt signaling antagonists. Nat Commun. 2017;8:1036. doi: 10.1038/s41467-017-01059-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang X, Borgstrom B, Stegmayr J, et al. The molecular basis for inhibition of stemlike cancer cells by Salinomycin. ACS Cent Sci. 2018;4:760–767. doi: 10.1021/acscentsci.8b00257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zeisberg M, Neilson EG. Biomarkers for epithelial-mesenchymal transitions. J Clin Invest. 2009;119:1429–1437. doi: 10.1172/JCI36183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lim J, Thiery JP. Epithelial-mesenchymal transitions: insights from development. Development. 2012;139:3471–3486. doi: 10.1242/dev.071209 [DOI] [PubMed] [Google Scholar]
- 18.Tang H, Deng M, Tang Y, et al. miR-200b and miR-200c as prognostic factors and mediators of gastric cancer cell progression. Clin Cancer Res. 2013;19:5602–5612. doi: 10.1158/1078-0432.CCR-13-1326 [DOI] [PubMed] [Google Scholar]
- 19.Yang L, Tang H, Kong Y, et al. LGR5 promotes breast cancer progression and maintains stem-like cells through activation of Wnt/beta-catenin signaling. Stem Cells. 2015;33:2913–2924. doi: 10.1002/stem.2083 [DOI] [PubMed] [Google Scholar]
- 20.Luc S, Huang J, McEldoon JL, et al. Bcl11a deficiency leads to hematopoietic stem cell defects with an aging-like phenotype. Cell Rep. 2016;16:3181–3194. doi: 10.1016/j.celrep.2016.08.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen B, Wei W, Huang X, et al. circEPSTI1 as a prognostic marker and mediator of triple-negative breast cancer progression. Theranostics. 2018;8:4003–4015. doi: 10.7150/thno.24106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen F, Luo N, Hu Y, Li X, Zhang K. MiR-137 suppresses triple-negative breast cancer stemness and tumorigenesis by perturbing BCL11A-DNMT1 interaction. Cell Physiol Biochem. 2018;47:2147–2158. doi: 10.1159/000491526 [DOI] [PubMed] [Google Scholar]
- 23.Yin J, Zhang F, Tao H, et al. BCL11A expression in acute phase chronic myeloid leukemia. Leuk Res. 2016;47:88–92. doi: 10.1016/j.leukres.2016.05.018 [DOI] [PubMed] [Google Scholar]
- 24.Zhou J, Yang Y, Zhang D, Zhou L, Tao L, Lu LM. Genetic polymorphisms and plasma levels of BCL11A contribute to the development of laryngeal squamous cell carcinoma. PLoS One. 2017;12:e0171116. doi: 10.1371/journal.pone.0171116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Satterwhite E, Sonoki T, Willis TG, et al. The BCL11 gene family: involvement of BCL11A in lymphoid malignancies. Blood. 2001;98:3413–3420. [DOI] [PubMed] [Google Scholar]
- 26.Yu Y, Wang J, Khaled W, et al. Bcl11a is essential for lymphoid development and negatively regulates p53. J Exp Med. 2012;209:2467–2483. doi: 10.1084/jem.20121846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trimboli AJ, Fukino K, de Bruin A, et al. Direct evidence for epithelial-mesenchymal transitions in breast cancer. Cancer Res. 2008;68:937–945. doi: 10.1158/0008-5472.CAN-07-2148 [DOI] [PubMed] [Google Scholar]
- 28.Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442–454. doi: 10.1038/nrc822 [DOI] [PubMed] [Google Scholar]
- 29.Wu Y, Zhou BP. New insights of epithelial-mesenchymal transition in cancer metastasis. Acta Biochim Biophys Sin (Shanghai). 2008;40:643–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huber MA, Kraut N, Beug H. Molecular requirements for epithelial-mesenchymal transition during tumor progression. Curr Opin Cell Biol. 2005;17:548–558. doi: 10.1016/j.ceb.2005.08.001 [DOI] [PubMed] [Google Scholar]
- 31.Visvader JE, Lindeman GJ. Cancer stem cells: current status and evolving complexities. Cell Stem Cell. 2012;10:717–728. doi: 10.1016/j.stem.2012.05.007 [DOI] [PubMed] [Google Scholar]
- 32.Clevers H, Loh KM, Nusse R; Stem cell signaling. An integral program for tissue renewal and regeneration: Wnt signaling and stem cell control. Science (New York, NY). 2014;346:1248012. doi: 10.1126/science.1248012 [DOI] [PubMed] [Google Scholar]