Abstract
Background: Non-small-cell lung cancer (NSCLC) was known as the most malignant tumor. Paclitaxel (PTX) is the effective drug used for the treatment of NSCLC; however, it also exhibits severe side effects. Emodin could induce apoptosis of NSCLC cells and serve as a potential cancer therapeutic agent. However, the effects of combination of emodin with PTX on NSCLC remain unclear. Thus, this study aimed to investigate the effects of emodin in combination with PTX on A549 cells.
Materials and methods: The effects of combination treatment on the proliferation, apoptosis and invasion of NSCLC cells were evaluated by CCK-8, flow cytometric and TUNEL assays, respectively. In addition, Western blotting was used to detect the expressions of Bax, Bcl-2, active caspase 3, p-Akt and ERK in cells.
Results: Combination of emodin with PTX synergistically inhibited the proliferation of A549 cells in vitro. In addition, we found that emodin significantly enhanced PTX-induced apoptosis in A549 cells via increasing the expressions of Bax and active caspase 3 and decreasing the levels of Bcl-2, p-Akt and p-ERK. Moreover, emodin markedly enhanced antitumor effect of PTX on A549 xenograft without significant side effects in vivo.
Conclusion: Our findings indicated that emodin could significantly enhance antitumor effect of PTX in vitro and in vivo. Therefore, the combination of emodin with PTX may serve as a potential strategy for the treatment of patients with NSCLC.
Keywords: non-small-cell lung cancer, emodin, paclitaxel, apoptosis
Introduction
Lung cancer is the main cause of cancer-related death in worldwide,1 which is characterized by high morbidity rates, high metastasis rates and high death rates.2 Non-small-cell lung cancer (NSCLC) is the most common malignant tumor in the world and accounting for 80 percent of all lung tumors.3 A 60% of patients with NSCLC were diagnosed with advanced stage of disease.4 Patients with advanced NSCLC have less response to chemotherapy, because of the side effects and drug tolerance.5,6 Although surgery is the most commonly method to treat patients with NSCLC, the 5-year survival rate is poor.7 Therefore, novel effective therapies for the treatment of NSCLC are imminently needed.
Paclitaxel (PTX) is an effective drug used for the treatment of cancer, which is extensively applied to clinical therapeutic treatment of NSCLC.8,9 However, PTX also exhibits severe side effects including vomiting, diarrhea and myelosuppression.8,10 In recent years, drug combination is an effective method to reduce cell toxicity and improve the efficacy of therapy.11 Recently, several traditional Chinese medicine (TCM) monomers, such as andrographolide, tomatine and piperlongumine, have been found to exhibit synergistically antitumor effects against various cancers when combined with PTX.8,11,12 Therefore, combination of TCM with PTX may be a potential therapeutic method for the treatment of NSCLC.
Emodin (1,3,8-trihydroxy-6-methyl-anthraquinone), a natural active anthraquinone compound, was extracted from the rhizome of Rheum palmatum (a Chinese medicinal herb). Emodin has been indicated to have a multiple of pharmacological and biological functions, including anti-inflammation, anti-neuro and anti-renal protection effects.13–15 In addition, emodin was reported to inhibited A549 cell invasion and migration via inducing apoptosis.16,17 Therefore, the present study aimed to explore the antitumor effects of combination of PTX with emodin on human NSCLC cells A549 in vitro and in vivo.
Materials and methods
Cell culture
A549 human NSCLC cell line was purchased from American Type Culture Collection (Rockville, MD, USA). A549 cells were cultured in Dulbecco’s Modified Eagle’s Medium (Thermo Fisher Scientific, Waltham, MA, USA) with 10% fetal bovine serum (Hyclone, Logan, UT, USA), 100 U/ml penicillin and 100 μg/ml streptomycin (Thermo Fisher Scientific) in a 5% CO2 humidified incubator at 37 °C.
CCK-8 assay
The cell viability value was measured by cell counting kit-8 (CCK8, Beyotime, Shanghai, China) according to the manufacturer’s protocols. Briefly, A549 cells (5 × 103 cells/well) were plated into each well of 96-well plate at 37°C overnight. Then, the cells were treated with emodin (0, 10, 20, 40, 80, 120 μM) and/or PTX (0, 2, 4, 8, 16, 32 μM) for 72 h. After that, 10 μL of CKK-8 reagent was added to each well for another 1 hr. Next, the absorbance at 450 nm was measured with a microplate reader (Bio-Rad Laboratories, Benicia, CA, USA). Emodin and PTX standard product were purchased from Sigma (St. Louis, MO, USA, #30,269; #580,555).
Combination studies
The combination index (CI) by Chou was used to analyze the drug combination studies.18 Combinations of PTX with emodin were utilized in the cell treatments. A549 cells were exposed to solutions containing 0, 5, 10, 20, 40 μM emodin combined with PTX (range 0 from 32 μM). The CI for the combination of PTX and emodin in NSCLC can be described as CI = DA / ICx,A + DB/ICx,B. (DA and DB represent the concentrations of samples A and B in combination to reach x% inhibition; ICX,A and ICX,B represent concentrations of samples A and B to reach x% inhibition when used alone, respectively.) The above parameters can be automatically determined from the median-effect equation using CalcuSyn software (Biososoft, Ferguson, MO, USA). A 0.9≤ CI ≤1.1 indicates additive; 0.8≤ CI ≤0.9 indicates slight synergism; 0.6≤ CI ≤0.8 indicates moderate synergism; 0.4≤ CI ≤0.6 indicates synergism, 0.2≤ CI ≤0.4 indicates strong synergism. The dose reduction index (DRI) was used to evaluate the extent of dose reduction in the combination treatment compared with the dose of single treatment. DRI can be described as DRI = ICx,A/DA.
Immunofluorescence
A549 cells (4 × 104 cells/well) were plated into each well of 24-well plate at 37°C overnight, and then cells were treated with emodin (10 μM) and/or PTX (4 μM) for 72 hr. After that, cells were washed in PBS three times and fixed in pre-cold methanol for 10 min at −20°C. Next, cells were incubated with primary antibodies for anti-Ki67 (Abcam; ab15580) (1:1,000), DAPI (ab104139), at 4°C overnight. Subsequently, cells were incubated with goat anti-rabbit IgG second antibody (Abcam; ab150077) (1:5,000) at 37°C for 1 hr. The samples were observed by fluorescence microscope (Olympus CX23 Tokyo, Japan).
Flow cytometric analysis of cell apoptosis
A549 cells (5 × 104 cells/well) were plated into each well of 6-well plate at 37°C overnight, and then cells were treated with emodin (10 μM) and/or PTX (4 μM) for 72 hr. Later on, cells were washed in cold PBS three times and re-suspended with Annexin V binding buffer. Then, cell suspension was cultured with 5 μL Annexin V-FITC and 5 μL propidium for 15 min at room temperature in the dark, and apoptotic cells were measured by flow cytometer (BD, Franklin Lake, NJ, USA).
Western blot analysis
The cells were collected and protein concentrations were measured using BCA protein assay kit (Beyotime). Equal amounts of protein extracts (30 μg) were loaded in the sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) with 10% SDS polyacrylamide gel. Then, the proteins were transferred to polyvinylidene fluoride membranes (PVDF, Thermo Fisher Scientific, Waltham, MA, USA) for 2 hr and blocked with 5% defatted milk in TBST for another 1 hr. After that, the membranes were incubated with primary antibodies overnight at 4°C: Bax (Abcam; cat. no. ab32503) (1:1,000), Bcl-2 (Abcam; cat. no. ab32124) (1:1,000), active caspase 3 (Abcam; cat. no. ab2302) (1:1,000), Akt (Abcam; cat. no. ab8805) (1:1,000), p-Akt (Abcam; cat. no. ab38449) (1:1,000), ERK (Abcam; cat. no. ab54230) (1:1,000), p-ERK (Abcam; cat. no. ab50011) (1:1,000), and anti-GAPDH (Abcam; cat. no. ab8245) (1:1,000). After washing with PBS, the PVDF membrane was incubated with goat anti-rabbit IgG second antibody (Abcam; ab150077) (1:5,000) at room temperature for 1 hr. Finally, the immunoreactivity was detected using the ECL reagent (Santa Cruz Biotechnology).
Animal study
To investigate the anti-tumor effects of combination of PTX with emodin on NSCLC tumor-burdened animals, 16 male BALB/nude mice (aged 4–6 weeks) were purchased from Shanghai Slac Animal Center (Shanghai, China) and housed within a constant temperature of 18–23°C and 55–65% humidity. Sixteen nude mice were randomly divided into four groups (4 mice/group): vehicle, PTX (10 mg/kg), emodin (50 mg/kg), or PTX + emodin. Aliquots of A549 cells (5 × 106 cells, in 100 μL of PBS) were injected subcutaneously into the right armpit area of the mice. Tumor volume was measured by caliper according to the formula: 1/2× (length × width2) weekly for three weeks until mice were sacrificed under anesthesia. The body weight and tumor weight were recorded when nude mice were sacrificed on day 21. Parts of each tumor tissue were stored at −20°C for immunohistochemical staining. All animal experiments were performed in accordance with institutional guidelines, following a protocol approved by the Ethics Committees of Zhejiang University. National Institutes of Health guide for the care and use of laboratory animals was strictly followed by us.
TUNEL staining
Tumor tissues were stained using an APO-BrdU™ TUNEL Assay Kit (Thermo Fisher Scientific, #A23210), according to the manufacturer’s instructions.
Statistical analysis
Each group were performed at least three independent experiments and all values were expressed as the mean ± standard deviation. The comparison between two groups was analyzed by Student’s t-test. The comparisons among multiple groups were made with one-way analysis of variance followed by Dunnett’s test. For all tests, values of P<0.05 or P<0.01 were considered to indicate a statistically significant difference.
Results
Combination of emodin with PTX synergistically inhibited the proliferation of A549 cells
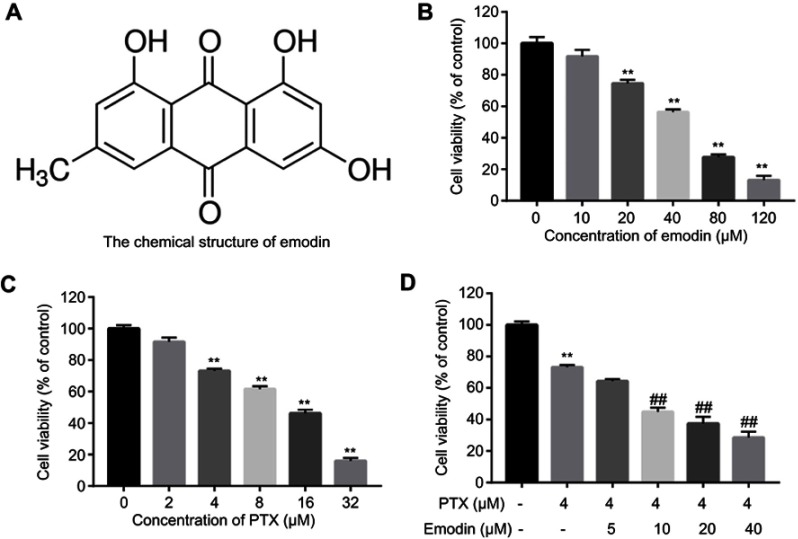
The chemical structure of emodin was indicated in Figure 1A. CCK-8 assay was used to evaluate the effects of emodin or/and PTX on the viability of A549 cells. The result indicated both emodin (range from 0 to120 μM) and PTX (range from 0 to 32 μM) inhibited the proliferation of A549 cells in a dose-dependent manner (Figure 1A and B). In addition, 4 μM PTX significantly inhibited the proliferation of A549 cells and exhibited about 60% growth inhibition (Figure 1C). Therefore, 4 μM PTX were utilized in the following experiments. Moreover, emodin (10, 20 or 40 μM) markedly enhanced the antiproliferative effect of PTX on A549 cells (Figure 1D).
Figure 1.
Combination of emodin with PTX synergistically inhibited the proliferation of A549 cells. (A) The chemical structure of emodin. (B) Cell viability was determined using CCK-8 assay in A549 cells treated with emodin (0, 10, 20, 40, 80, 120 μM) for 72 hr. (C) Cell viability was determined using CCK-8 assay in A549 cells treated with PTX (0, 2, 4, 6, 8, 16, 32 μM) for 72 hr. (D) Cell viability was determined using CCK-8 assay in A549 cells treated with PTX (4 μM) and emodin (0, 5, 10, 20, 40 μM) for 72 h. **P< 0.01 compared with 0 μM group; ##P< 0.01 compared with 4 μM PTX alone group. Each group performed at least three independent experiments.
Abbreviation: PTX, paclitaxel.
As shown in Table 1, IC50 value of PTX (alone treatment) was 10.08 μM. However, when PTX was combined with emodin (5, 10, 20 or 40 μM), the IC50 value of PTX was decreased in the range of 1.33–8.65 μM (Table 1). In addition, it was noticed that the CI values of emodin (10 or 20 μM) combined with PTX were less than 0.8, which indicated the moderate synergism (Table 1). Meanwhile, the CI value of 10 μM emodin combined with PTX treatment group was lower than other groups. Therefore, 10 μM emodin combined with 4 μM PTX was utilized in the subsequent experiments. Meanwhile, the value of DRI was dose-dependent (Table 1). These results suggested that combination of emodin with PTX synergistically inhibited the proliferation of A549 cells.
Table 1.
Evaluation of combination of PTX with emodin in NSCLC (72 hr treatment)
| Combination strategy | IC50 value of PTX | 95% CI values | DRI |
|---|---|---|---|
| PTX (range 0 from 32 µM) | IC50 = 10.08 ± 1.04 µM | – | – |
| PTX +5 µM emodin | IC50 = 8.65 ± 1.01 µM | 0.980 | 1.165 |
| PTX +10 µM emodin | IC50 = 4.68 ± 0.71 µM | 0.709 | 2.154 |
| PTX +20 µM emodin | IC50 = 2.33 ±0.52 µM | 0.720 | 4.326 |
| PTX +40 µM emodin | IC50 = 1.33 ± 0.33 µM | 1.109 | 7.579 |
Abbreviations: DRI, dose reduction index; NSCLC, non-small-cell; PTX, paclitaxel.
Emodin could enhance PTX-induced apoptosis in A549 cells
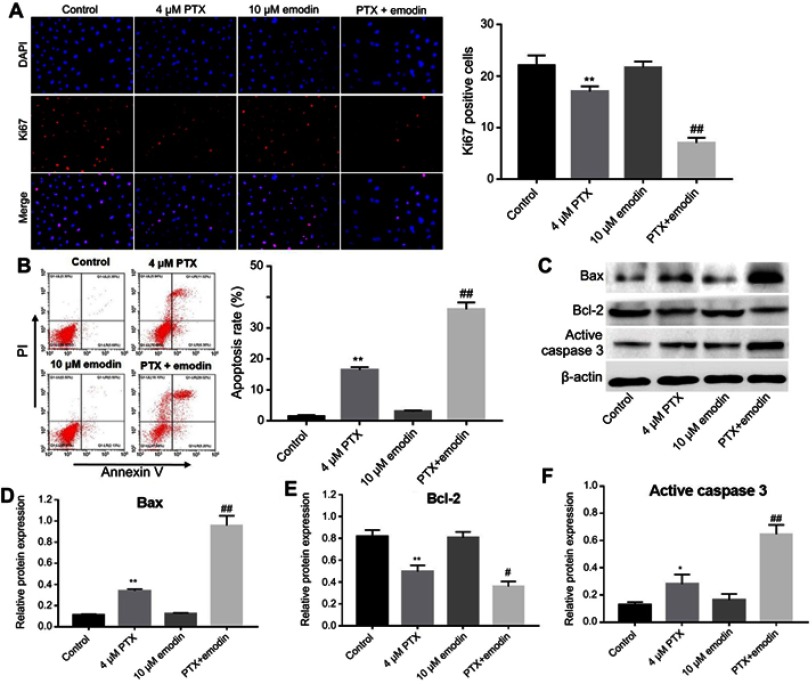
To further demonstrate the effects of combination of emodin with PTX on A549 cells, immunofluorescence and flow cytometry were applied. Ki67 is normally used as a detector for cells in a proliferative state. In immunofluorescence assay, 4 μM PTX significantly inhibited the proliferation of A549 cells, while 10 μM emodin had very limited effect on cell growth (Figure 2A). However, the antiproliferation effect of PTX was notably increased in the presence of emodin, compared with PTX alone (Figure 2A). In addition, PTX-induced cell apoptosis was markedly enhanced by treatment of emodin (Figure 2B).
Figure 2.
Emodin could enhance PTX-induced apoptosis in A549 cells. A549 cells were treated with 4 μM PTX or/and 10 μM emodin for 72 h.r (A) Relative fluorescence expression levels were quantified by Ki67 and DAPI staining. The number of Ki67 positive cells were counted. (B) Apoptotic cells were detected with Annexin V and PI double staining. The apoptosis cell rates were calculated. (C) The expressions of Bax, Bcl-2 and active caspase 3 were analyzed by Western blotting in A549 cells. (D) The relative expression of Bax was quantified by normalizing to β-actin. (E) The relative expression of Bcl-2 was quantified by normalizing to β-actin. (F) The relative expression of active caspase 3 was quantified by normalizing to β-actin. *P< 0.05, **P<0.01 compared with control group; #P< 0.05, ##P< 0.01 compared with 4 μM PTX alone group. Each group performed at least three independent experiments.
Abbreviation: PTX, paclitaxel.
Next, Western blotting was used to detect the apoptosis-related proteins Bax, Bcl-2 and active caspase-3. As shown in Figure 2C–F, PTX markedly increased the expressions of Bax and active caspase 3 and decreased the expression of Bcl-2, compared with the control group. As expected, the levels of Bax and active caspase 3 in cells were further increased by emodin, while the expression of Bcl-2 was further decreased, compared with the PTX alone (Figure 2C–D). All these data suggested that emodin enhanced PTX-induced apoptosis in A549 cells.
Combination of emodin with PTX inhibited Akt and ERK pathways in A549 cells
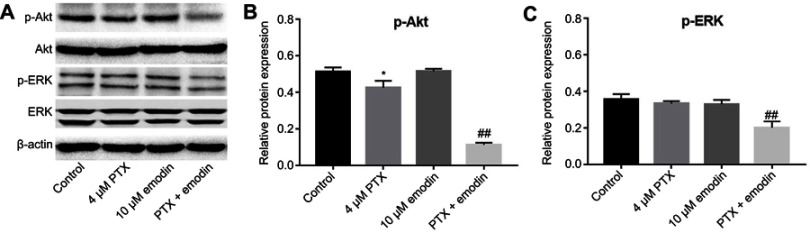
To explore the mechanisms by which the combination inhibited the proliferation of A549 cells, Akt and ERK pathways were investigated. As indicated in Figure 3A–C, combination of emodin with PTX significantly downregulated the expressions of phosphorylated Akt (p-Akt) and phosphorylated ERK (p-ERK), compared with PTX alone or control group. This data suggested that combination of emodin with PTX inhibited Akt and ERK pathways in A549 cells.
Figure 3.
Combination of emodin with PTX inhibited Akt and ERK pathways in A549 cells. A549 cells were treated with 4 μM PTX or/and 10 μM emodin for 72 hr. (A) The expressions of p-Akt and p-ERK in A549 cells were analyzed by Western blotting. (B) The relative expression of p-Akt was quantified by normalizing to Akt. (C) The relative expression of p-ERK was quantified by normalizing to ERK. *P< 0.05 compared with control group. ##P< 0.01 compared with 4 μM PTX group. Each group performed at least three independent experiments.
Abbreviation: PTX, paclitaxel.
Emodin could enhance the antitumor effects of the PTX on NSCLC in vivo
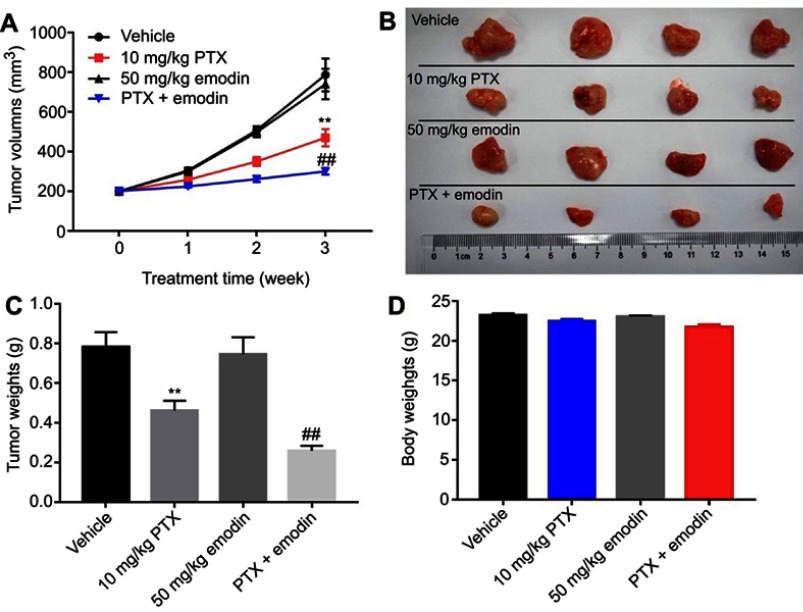
To further confirm the effects of the combination on tumor growth in vivo, a xenograft mouse model was used. As shown in Figure 4A and B, 10 mg/kg PTX decreased the tumor volume, while 50 mg/kg emodin had very limited effect on the tumor volume on day 21. Similar to in vitro data, the volumes of the tumors were further decreased by the combination treatment compared with the PTX group. In addition, the tumor weights in combination treatment group were notably decreased, compared with 10 mg/kg PTX or control group (Figure 4C). Meanwhile, the combined treatment had no effect on the body weight changes of mice, which indicated this treatment strategy was tolerable (Figure 4D). These results illustrated that emodin could enhance the antitumor effects of PTX on NSCLC in vivo.
Figure 4.
Emodin could enhance the antitumor effects of the PTX on NSCLC in vivo. (A) Tumor volumes of xenograft were measured weekly post-inoculation of A549 cells. (B) Tumors were isolated from xenografts and pictured after 3 weeks of treatment. (C) Tumor weights in each group were calculated. (D) Body weights of mice were calculated. N = 4, **P< 0.01 compared with vehicle group; ##P< 0.01 compared with 10 mg/kg PTX group.
Abbreviations: NSCLC, non-small-cell; PTX, paclitaxel.
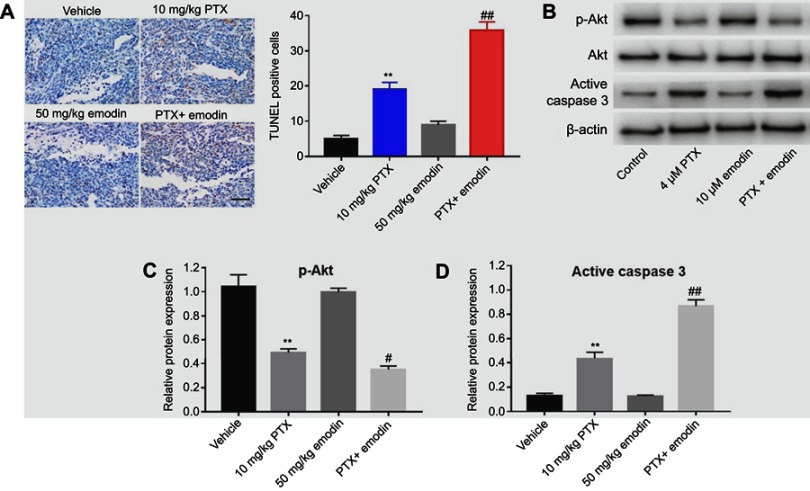
Combination of emodin with PTX-induced apoptosis in tumor tissues via inhibition of Akt signaling pathway in vivo
We next examined the effects of the combination on apoptosis and Akt signaling in A549 tumor tissues in vivo. The data of TUNEL indicated that emodin markedly enhanced PTX-induced cell apoptosis in tumor tissues compared with the 10 mg/kg PTX group (Figure 5A). In addition, 10 mg/kg PTX significantly downregulated the expression of p-Akt and upregulated the expression of active caspase 3 in tumor tissues compared with vehicle group. Similar to in vitro data, the expressions of these proteins were further decreased (p-Akt) or increased (active caspase 3) in the presence of emodin (Figure 5B–D). All these results suggested that combination of emodin with PTX induced apoptosis in tumor tissues via inhibition of Akt signaling pathway in vivo.
Figure 5.
Combination of emodin with PTX-induced apoptosis in tumor tissues via inhibition of Akt signaling pathway in vivo. (A) TUNEL staining of tumor tissues in each group. Quantification of TUNEL positive cells in tissues. (B) Expressions of p-Akt and active caspase 3 were analyzed by Western blotting in A549 cells. (C) Relative expression of p-Akt was quantified by normalizing to Akt. (D) Relative expression of active caspase 3 was quantified by normalizing to β-actin. **P< 0.01 compared with vehicle group; #P< 0.05, ##P< 0.01 compared with 10 mg/kg PTX group. Each group performed at least three independent experiments.
Abbreviation: PTX, paclitaxel.
Discussion
PTX is a common and an effective drug used for the treatment of cancer, while PTX also exhibits severe side effects.8,9 The use of TCM monomer and PTX in combination is a prospective method to reduce cytotoxicity induced by PTX and to increase the curative effect. In this study, we aimed to investigate the antitumor effect of emodin and PTX on A549 cells in vitro and in vivo. Our results indicated that emodin significantly enhanced the antiproliferative and proapoptotic effects of PTX in vitro and in vivo.
The results suggested that low-dose emodin markedly enhanced the PTX-induced antitumor activities on A549 cells in vitro and in vivo. This finding similar to previous reports that combination of carboplatin with PTX or combined PTX with triptolide-coloaded could synergistically inhibited tumor growth in lung cancer.19,20 In addition, our data suggested that 50 mg/kg emodin had very limited effect on the tumor weight or body weight of mice. Moreover, the combination treatment significantly reduced the tumor weight and had no effect on the body weight changes of mice, which indicated that this treatment strategy had no obvious systemic toxicity. Consistently, li et al found that emodin increased cisplatin-induced cell apoptosis in xenograft tumors without obvious systemic toxicity.21 All these results demonstrated that combination treatments could inhibit A549 cell proliferation without systemic toxicity in vivo.
On the other hand, emodin enhanced the PTX-induced apoptosis in A549 cells via increasing the level of Bax and active caspase 3 and decreasing the level of Bcl-2. Lai et al found that emodin induced apoptosis in A549 cells via upregulating the expression of Bax in lung cancer.22 PTX in combination with 5-demethylnobiletin induced cell apoptosis through regulating caspase 3 pathway in lung cancer.23 Consistent with these results, the combination of emodin with PTX could induce apoptosis via upregulation of Bax and active caspase 3 and downregulation of Bcl-2 in A549 cells.
Next, the mechanisms underlying the antitumor effects of the combination on NSCLC were further investigated. Akt and ERK signaling pathways are key modulators of cell proliferation and apoptosis in NSCLC, which can promote tumor cell growth and inhibition of apoptosis.24,25 Previous evidence has indicated that activation of Akt/ERK could increase PTX-induced gastric cancer cell resistance.26 Previous studies also suggested that inhibition of Akt could increase PTX sensitivity in human ovarian cancer cells.27 In addition, Bartling et al also found that PTX induced lung cancer cell apoptosis via inhibition of ERK and Akt kinases.28 Therefore, we detected the level of the Akt and ERK signaling pathway proteins in A549 cells treated with emodin or/and PTX in the present study. Our results demonstrated that the combination of emodin with PTX synergistically induced apoptosis via inhibition of Akt and ERK pathways in A549 cells. Consistent with these results, PTX combined with alpha-tomatine synergistically induced apoptosis via inhibition of Akt signaling pathway in human prostate cancer PC-3.11 Meanwhile, emodin induced apoptosis via inhibition of ERK and Akt pathways in human lung adenocarcinoma cells.29 These data clearly confirmed that the combination treatment induced apoptosis via inhibition of Akt and ERK pathways in A549 cells. However, the effect of combination of emodin with PTX on PTX-resistant A549 cells remained unclear and further investigations are needed.
Conclusion
In conclusion, emodin enhanced the antitumor effects of PTX on A549 cells in vitro and in vivo. The results suggested that the combination of emodin with PTX may serve as a potent strategy in the treatment of patients with NSCLC.
Acknowledgment
This study was supported by Hospital Research Fund (B1519) and Clinical Research Fund of Zhejiang Medical Association (2016ZYC-A20).
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Wu J, He K, Zhang Y, et al. Inactivation of SMARCA2 by promoter hypermethylation drives lung cancer development. Gene. 2019;687:193–199. doi: 10.1016/j.gene.2018.11.032 [DOI] [PubMed] [Google Scholar]
- 2.Gong J, Xu L, Li Z, et al. A clinical prognostic score to predict survival of advanced or metastatic non-small cell lung cancer (NSCLC) patients receiving first-line chemotherapy: a retrospective analysis. Med Sci Monit. 2018;24:8264–8271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheng ZJ, Miao DL, Su QY, et al. THZ1 suppresses human non-small-cell lung cancer cells in vitro through interference with cancer metabolism. Acta Pharmacol Sin. 2018. doi: 10.1038/s41401-018-0187-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. doi: 10.3322/caac.20107 [DOI] [PubMed] [Google Scholar]
- 5.Scheff RJ, Schneider BJ. Non-small-cell lung cancer: treatment of late stage disease: chemotherapeutics and new frontiers. Semin Intervent Radiol. 2013;30(2):191–198. doi: 10.1055/s-0033-1342961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou GH, Lu YY, Xie JL, et al. Overexpression of miR-758 inhibited proliferation, migration, invasion, and promoted apoptosis of non-small cell lung cancer cells by negatively regulating HMGB. Biosci Rep. 2019;39(1). doi: 10.1042/BSR20180855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhong C, Ding S, Xu Y, Huang H. MicroRNA-222 promotes human non-small cell lung cancer H460 growth by targeting p27. Int J Clin Exp Med. 2015;8(4):5534–5540. [PMC free article] [PubMed] [Google Scholar]
- 8.Yuan H, Sun B, Gao F, Lan M. Synergistic anticancer effects of andrographolide and paclitaxel against A549 NSCLC cells. Pharm Biol. 2016;54(11):2629–2635. doi: 10.1080/13880209.2016.1176056 [DOI] [PubMed] [Google Scholar]
- 9.Chen X, Sun X, Guan J, et al. Rsf-1 influences the sensitivity of non-small cell lung cancer to paclitaxel by regulating NF-kappaB pathway and its downstream proteins. Cell Physiol Biochem. 2017;44(6):2322–2336. doi: 10.1159/000486116 [DOI] [PubMed] [Google Scholar]
- 10.Parayath NN, Nehoff H, Norton SE, et al. Styrene maleic acid-encapsulated paclitaxel micelles: antitumor activity and toxicity studies following oral administration in a murine orthotopic colon cancer model. Int J Nanomedicine. 2016;11:3979–3991. doi: 10.2147/IJN.S110251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee ST, Wong PF, Hooper JD, Mustafa MR. Alpha-tomatine synergises with paclitaxel to enhance apoptosis of androgen-independent human prostate cancer PC-3 cells in vitro and in vivo. Phytomedicine. 2013;20(14):1297–1305. doi: 10.1016/j.phymed.2013.07.002 [DOI] [PubMed] [Google Scholar]
- 12.Liu Q, Zhao D, Zhu X, et al. Coloaded nanoparticles of paclitaxel and piperlongumine for enhancing synergistic antitumor activities and reducing toxicity. J Pharm Sci. 2017;106(10):3066–3075. doi: 10.1016/j.xphs.2017.05.027 [DOI] [PubMed] [Google Scholar]
- 13.Kuo YC, Meng HC, Tsai WJ. Regulation of cell proliferation, inflammatory cytokine production and calcium mobilization in primary human T lymphocytes by emodin from Polygonum hypoleucum Ohwi. Inflamm Res. 2001;50(2):73–82. doi: 10.1007/s000110050727 [DOI] [PubMed] [Google Scholar]
- 14.Dou F, Liu Y, Liu L, et al. Aloe-emodin ameliorates renal fibrosis via inhibiting PI3K/Akt/mTOR signaling pathway in vivo and in vitro. Rejuvenation Res. 2018. doi: 10.1089/rej.2018.2104 [DOI] [PubMed] [Google Scholar]
- 15.Li Y, Xu QQ, Shan CS, et al. Combined use of emodin and ginsenoside Rb1 exerts synergistic neuroprotection in cerebral ischemia/reperfusion rats. Front Pharmacol. 2018;9:943. doi: 10.3389/fphar.2018.00943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wan L, Zhang L, Fan K, Wang J. Aloin promotes A549 cell apoptosis via the reactive oxygen speciesmitogen activated protein kinase signaling pathway and p53 phosphorylation. Mol Med Rep. 2017;16(5):5759–5768. doi: 10.3892/mmr.2017.7379 [DOI] [PubMed] [Google Scholar]
- 17.Ok S, Kim SM, Kim C, et al. Emodin inhibits invasion and migration of prostate and lung cancer cells by downregulating the expression of chemokine receptor CXCR4. Immunopharmacol Immunotoxicol. 2012;34(5):768–778. doi: 10.3109/08923973.2012.654494 [DOI] [PubMed] [Google Scholar]
- 18.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. [DOI] [PubMed] [Google Scholar]
- 19.Ramalingam SS, Blais N, Mazieres J, et al. Randomized, placebo-controlled, phase II study of veliparib in combination with carboplatin and paclitaxel for advanced/metastatic non-small cell lung cancer. Clin Cancer Res. 2017;23(8):1937–1944. doi: 10.1158/1078-0432.CCR-15-3069 [DOI] [PubMed] [Google Scholar]
- 20.Liu J, Cheng H, Han L, et al. Synergistic combination therapy of lung cancer using paclitaxel- and triptolide-coloaded lipid-polymer hybrid nanoparticles. Drug Des Devel Ther. 2018;12:3199–3209. doi: 10.2147/DDDT.S172199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li X, Wang H, Wang J, et al. Emodin enhances cisplatin-induced cytotoxicity in human bladder cancer cells through ROS elevation and MRP1 downregulation. BMC Cancer. 2016;16:578. doi: 10.1186/s12885-016-2640-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lai JM, Chang JT, Wen CL, Hsu SL. Emodin induces a reactive oxygen species-dependent and ATM-p53-Bax mediated cytotoxicity in lung cancer cells. Eur J Pharmacol. 2009;623(1–3):1–9. doi: 10.1016/j.ejphar.2009.08.031 [DOI] [PubMed] [Google Scholar]
- 23.Tan KT, Li S, Li YR, et al. Synergistic anticancer effect of a combination of paclitaxel and 5-demethylnobiletin against lung cancer cell line in vitro and in vivo. Appl Biochem Biotechnol. 2018. doi: 10.1007/s12010-018-2869-1 [DOI] [PubMed] [Google Scholar]
- 24.Crosbie PA, Crosbie EJ, Aspinall-O’Dea M, et al. ERK and AKT phosphorylation status in lung cancer and emphysema using nanocapillary isoelectric focusing. BMJ Open Respir Res. 2016;3(1):e000114. doi: 10.1136/bmjresp-2015-000114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wan J, Wu W. Hyperthermia induced HIF-1a expression of lung cancer through AKT and ERK signaling pathways. J Exp Clin Cancer Res. 2016;35(1):119. doi: 10.1186/s13046-016-0399-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu G, Qin XQ, Guo JJ, Li TY, Chen JH. AKT/ERK activation is associated with gastric cancer cell resistance to paclitaxel. Int J Clin Exp Pathol. 2014;7(4):1449–1458. [PMC free article] [PubMed] [Google Scholar]
- 27.Weng D, Song X, Xing H, et al. Implication of the Akt2/survivin pathway as a critical target in paclitaxel treatment in human ovarian cancer cells. Cancer Lett. 2009;273(2):257–265. doi: 10.1016/j.canlet.2008.08.027 [DOI] [PubMed] [Google Scholar]
- 28.Bartling B, Hofmann HS, Silber RE, Simm A. Differential impact of fibroblasts on the efficient cell death of lung cancer cells induced by paclitaxel and cisplatin. Cancer Biol Ther. 2008;7(8):1250–1261. doi: 10.4161/cbt.7.8.6264 [DOI] [PubMed] [Google Scholar]
- 29.Su YT, Chang HL, Shyue SK, Hsu SL. Emodin induces apoptosis in human lung adenocarcinoma cells through a reactive oxygen species-dependent mitochondrial signaling pathway. Biochem Pharmacol. 2005;70(2):229–241. doi: 10.1016/j.bcp.2005.04.026 [DOI] [PubMed] [Google Scholar]