Abstract
Background: Tumor metastasis is the major challenge for ovarian cancer treatment. Cancer-associated fibroblasts (CAFs), a major component existing in tumor microenvironment, can secrete several cytokines to interact with cancer epithelial cells, and promote cancer metastasis. Stanniocalcin 1 (STC1), a secretory glycoprotein hormone, has been proven to be an important factor in ovarian tumorigenesis.
Methods: In this study, we focused on the functional role of STC1 in ovarian cancer microenvironment, investigated STC1’s effects on the proliferation and metastasis of ovarian cancer cells, and explored the molecular mechanism underlying STC1-mediated cancer metastasis.
Results: By analyzing the GEO dataset and examined STC1 expression in CAFs isolated from ovarian cancer patients, we found that expression of STC1 was higher in ovarian cancer stroma and CAFs than in the normal ovarian stroma and normal fibroblasts (NFs). Addition of recombinant human STC1 (rhSTC1) promoted cell proliferation and metastasis in ovarian cancer, while adoption of STC1 neutralizing antibody (STC1 Ab) abolished the effects. Furthermore, our results revealed that STC1 promoted the phosphorylation of Akt (Ser473), and upregulated several epithelial–mesenchymal transition (EMT) markers including fibronectin,vimentin and slug. In addition, we demonstrated that STC1 in tumor microenvironment could mediate the conversion of NFs to CAFs.
Conclusion: Taken together, the study results suggested the crucial role of STC1 in tumor environment on the metastasis of ovarian cancer.
Keywords: STC1, tumor microenvironment, CAFs, EMT, metastasis
Introduction
Ovarian cancer has a high mortality rate among women, with an estimated 295,000 new cases and 185,000 deaths in 2018 worldwide.1 Approximately 70% of ovarian cancer patients present with an advanced stage (International Federation of Gynecology and Obstetrics [FIGO] IIIC/IV stage) with widespread cancer cells beyond ovaries at the time of diagnosis, and less than one-half of women survive beyond 5 years.2 For the treatment of ovarian cancer, understanding the molecular mechanism of tumor metastasis is still the biggest challenge for gynecological oncologists.
Tumor cell dissemination is the most important pattern for ovarian cancer metastasis. Increasing evidence indicates that the seeded tumor cells can spontaneously generate reactive oxygen species (ROS), chemokines, or cytokines that reprogram the surrounding cells to form the tumor microenvironment and potentiate tumor progression including angiogenesis, inflammation, or epithelial–mesenchymal transition (EMT).3–5 Cancer-associated fibroblasts (CAFs), a major constituent of the tumor stroma, are transformed quiescent resident fibroblasts upon interaction with cancer cells.6 In turn, CAFs stimulate carcinogenesis via multiple mechanisms including the secretion of pro-tumorigenic factors.7 In ovarian cancer, CAFs have been proved to be associated with tumor growth, angiogenesis, cell invasion,8 chemoresistance,9 and cell metabolism.10
Stanniocalcin 1 (STC1) is a paracrine protein that is widely expressed in various human tissues. Previous studies have shown that STC1 was involved in the development of numerous cancers, including colorectal,11 hepatocellular,12 renal,13 and ovarian carcinomas.14 Functional studies also revealed that STC1 was associated with inflammation, tumor growth, and cancer metastasis.15,16 A previous study from Liu showed that expression of STC1 protein was higher in ovarian cancer tissues than in normal ovarian tissues. STC1 overexpression promoted cellular proliferation by regulating the cell cycle through the increased expression of cyclin A, cyclin B, CDK2, and a short cyclin E isoform. Moreover, STC1 overexpression also promoted migration in ovarian cancer cells.14 However, the underlying molecular mechanism of STC1-regulated metastasis in ovarian cancer cells is not clear.
STC1 is also expressed in the tumor stroma, such as in the CAFs. A previous study showed that STC1 expression by CAFs mediated tumor metastasis in colorectal cancer. They showed that a tumor that formed in the presence of STC1-deficient fibroblasts presented decreased lymph vessel density and EMT marker expression in an orthotopic mouse model.11 In prostate cancer, immunohistochemical assays showed that STC1 was expressed in restricted regions of stroma next to tumor epithelia, suggesting that STC1 may be a marker for reactive stroma.17
In this study, we compared the expression of STC1 in ovarian CAFs and NFs, and investigated the effect of STC1 on metastasis of ovarian cancer cells. By detaching the fibroblasts from ovarian tissues, we found that STC1 expression was higher in CAFs than in NFs. Then, we found that addition of rhSTC1 promoted proliferation and metastasis in ovarian cancer. Western blot detection showed that STC1 treatment increased the expression of EMT markers including fibronectin, vimentin, and slug, while treatment with STC1-neutralizing antibody (STC1 Ab) reduced the expression of these EMT markers and further reversed STC1-mediated metastasis. Conditioned medium with STC1 overexpression also promoted cellular proliferation and metastasis in ovarian cancer cells, and STC1 was implicated in the conversion of NFs into CAFs. Moreover, we found that STC1 promoted the phosphorylation of Akt, STC1 Ab inhibited the activation of Akt, and inhibition of Akt activation by LY294002 reduced STC1-mediated metastasis. Our findings revealed the essential role of STC1 in tumor environment and its effect on metastasis of ovarian cancer, which may provide new insights into the treatment of ovarian cancer.
Materials and methods
Reagents and antibodies
Roswell Park Memorial Institute (RPMI) 1640 medium, MTT powder and LY294002 were purchased from Sigma-Aldrich. Fetal bovine serum (FBS) was purchased from Thermo Fisher Scientific. Corning® transwell inserts (8.0 μm) were obtained from Sigma-Aldrich. Antibody against STC1 (MAB2958) was purchased from R&D. Antibodies against α-SMA (#19245), slug (#9585), Akt (4685), and phospho-Akt (Ser473) (4060) were purchased from Cell Signaling Technology. Antibodies against fibronectin (sc-9068) and vimentin (sc-6260) were purchased from Santa Cruz Biotechnology. Horseradish peroxidase (HRP)-conjugated goat anti-rabbit (ab6721) and anti-mouse (ab6789) secondary antibodies were purchased from Abcam. Alexa Fluor® 594 AffiniPure Donkey Anti-Rabbit IgG (H+L) (111-585-003) was purchased from Jackson ImmunoResearch. Mounting Medium with DAPI (F6057) was purchased from Sigma-Aldrich. rhSTC1 (HOR-259) was obtained from ProSpec.
Cell lines and cell culture
Human epithelial ovarian cancer cell lines HEY and SKOV3 were acquired from the American Type Culture Collection. All cells were cultured in RPMI 1640 medium with 10% FBS in a cell culture incubator with 5% CO2 and 100% humidity at 37 °C. STR DNA profiling analysis was used for cell line authentication, and mycoplasma detection kit (Solarbio, CA1080) was used for routine testing of mycoplasma contamination.
GEO data sets analysis
Gene expression data (GSE40595) was downloaded as raw signals from GEO datasets (www.ncbi.nlm.nih.gov/geo), and analyzed and log2-scaled using the GEO2R online analysis tool (www.ncbi.nlm.nih.gov/geo/geo2r).
Isolation of NFs and CAFs
The study was approved by the Independent Ethics Committee of the Fifth Peoples’ Hospital of Shanghai Fudan University, and the study was conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all patients. Fibroblasts were isolated from ovarian tissue samples from patients with uterine fibroids (normal fibroblasts, NFs) and ovarian cancer (cancer-associated fibroblasts, CAFs) during operation at the Fifth Peoples’ Hospital of Shanghai, Fudan University. In brief, the tissues were dissected and immediately rinsed in sterilized phosphate buffered saline (PBS). Then, the tissues were cutted into small pieces in RPMI 1640 medium with 20% FBS. When cells were propagated to 70–80% confluence, fibroblasts were purified by trypsin digestion to exclude the epithelial cells. The fibroblasts were used at low passage number.
Immunofluorescence
Fibroblasts were seeded on the cover slides in a 24-well plate and cultured in a humidified incubator with 5% CO2 at 37 °C overnight. On the next day, cells were fixed in pre-chilled methanol for 5 min, rinsed in PBS thrice, and blocked with 5% BSA for 1 h. Then cells were incubated with primary antibodies against α-SMA, vimentin, or STC1 at 4 °C overnight. Cells were rinsed in PBST for 15 min thrice and incubated with the secondary antibodies at 37 °C for 45 min in the dark. Cells were again washed in PBST for 15 min thrice. The slides were sealed with Mounting Medium with DAPI. Images were captured under a fluorescence microscope (Leica) and the quantification of fluorescence intensity was analyzed using Image J software.
Establishment of STC1-overexpression cell line and collection of conditioned medium
Plasmid construction and lentivirus production were performed according to the Lenti-XTM Tet-On® Advanced Inducible Expression System User Manual (Clontech). Ovarian cancer cells (SKOV3) were infected with pLVX-STC1 virus and screened using puromycin. Once treated with doxycycline, the established cells can express and secrete STC1-HA into the culture supernatant. In this study, cells were plated in 10-cm dishes in RPMI 1640 medium with 10% FBS and were maintained overnight. Then the supernatant was removed and replaced with RPMI 1640 without FBS, and 2 µg/mL doxycycline was added into the medium. After incubation for 48 h, the culture supernatant was collected and centrifuged to remove cell debris. The collected conditioned medium (CM) was stored at 4 °C for future use and the cell culture medium without doxycycline treatment (NM) was used as the control.
Western blot analysis
Whole cell extracts were generated using RIPA lysis buffer. Protein concentrations of the lysates were determined using Thermo Scientific™ Pierce™ BCA Protein Assay Kit. Samples were separated in sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto a polyvinylidene fluoride (PVDF) membrane. Then, the membrane was blocked with 5% nonfat milk for 1 h and incubated with a primary antibody at 4 °C overnight with shaking. After washing in TBST for 15 min thrice, the membrane was incubated with HRP-conjugated secondary antibody for 1 h. Protein bands were detected using an enhanced chemiluminescence substrate kit (Millipore) and visualized using FluorChem E system.
Cell proliferation assay
Cells were washed with PBS and detached by trypsinization. Cells (5×103 per well) were seeded in Corning® 96-well culture plates in 100 μL medium with 10% FBS and maintained overnight. On the next day, the medium was replaced with the desired medium without FBS and incubated for another 24 h and 48 h. Cell proliferation was detected using 5 mg/mL MTT solution according to the manufacturer’s instruction. The optical density (OD) at 490 nm was measured using a multi-well plate reader (Infinite 200 PRO). The assay was repeated three times independently.
Cell migration assay
Cells were seeded in the upper chamber of Corning® transwell inserts in 100 μL serum-free medium. Conditioned medium or medium with rhSTC1 and STC1 Ab was added into the lower chamber. After incubation for 48 h, the migrated cells were fixed with methanol and stained by crystal violet. The stained cells were counted and photographed in five randomly selected microscopic fields.
Cell invasion assay
60 μl diluted Matrigel matrix were added to the upper chamber of Corning® transwell inserts, and allow them fully condense in the cell incubator. Then cells were seeded in the upper chamber in 100 μL serum-free medium. Serum-free medium with rhSTC1 and STC1 Ab was added into the lower chamber. After incubation for 48 h, the invaded cells were fixed with methanol and stained by crystal violet. The stained cells were counted and photographed under a microscope
Statistics
All the data were presented as mean ± SEM. Statistical analyses were carried out using GraphPad PRISM. Differences between two groups were analyzed using Student’s t-test. Comparisons between multiple groups were performed by analysis of variance (ANOVA) followed by Tukey’s test. p<0.05 was considered statistically significant. *p<0.05, **p<0.01, ***p<0.0001.
Results
STC1 expression was upregulated in ovarian cancer stroma
To examine the expression pattern of STC1, we analyzed the expression of STC1 mRNA in a GEO dataset (GSE40595) including the microdissected normal ovarian stroma (n=8) and the microdissected ovarian cancer stroma (n=31). The results showed that STC1 mRNA level was significantly higher in the tumor stroma than in the normal stroma (Figure 1A). The tumor stroma also showed higher expression of vimentin and α-SMA (Figure 1B and C), which were usually considered as CAFs markers. As CAFs are the most prominent cell type existing in the tumor stroma, we isolated NFs and CAFs from the patients with uterine fibroids and ovarian cancer, respectively, and propagated them in RPMI 1640 medium with 20% FBS. Then, we investigated the protein expression of STC1 in NFs and CAFs using immunofluorescence. We found that STC1 expression was higher in CAFs than in NFs (Figure 1D and E); vimentin expression was positive in both NFs and CAFs, but was relatively higher in CAFs; and a-SMA was hardly detected in NFs, but showed higher expression in CAFs (Figure 1D and E). Taken together, these results showed that STC1 was more highly expressed in a cancer-associated microenvironment.
Figure 1.
STC1 is upregulated in the ovarian tumor stroma. (A–C) Boxplots showing the expression levels of STC1 (A), vimentin (B), α-SMA (C) in microdissected normal stroma and tumor stroma included in the ovarian dataset GSE40595 (Normal stroma, n=8; Tumor Stroma, n=31). (D) Immunofluorescence staining showing the relative expression of STC1, vimentin, and α-SMA in the NFs and CAFs from patients. (E) Quantification of fluorescence intensity using Image J software.
Abbreviations: NFs, normal fibroblasts; CAFs, cancer-associated fibroblasts.
STC1 in the tumor microenvironment promoted metastasis of ovarian cancer
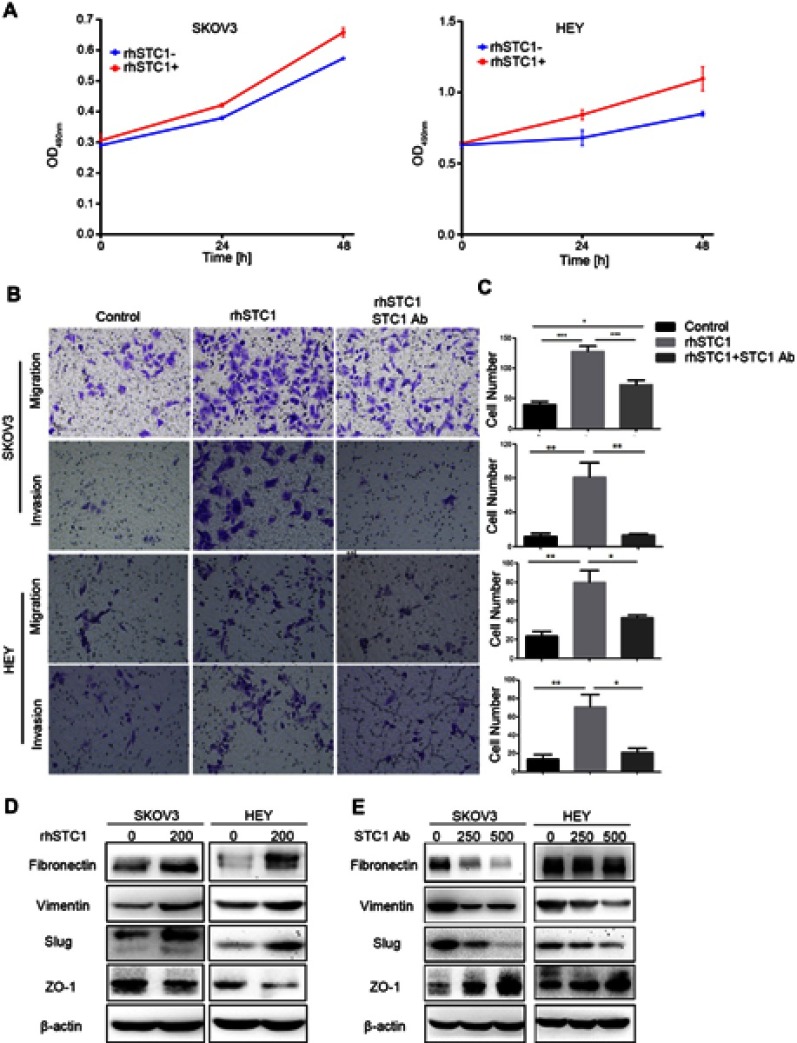
To investigate the role of STC1 in the tumor microenvironment, ovarian cancer cell lines (SKOV3 and HEY) were treated with rhSTC1 and STC1 Ab. As shown in Figure 2A, addition of rhSTC1 in the culture medium of ovarian cancer cell lines promoted cell proliferation. These findings coincide with previous reports on STC1-stimulated proliferation.14 Furthermore, we found that treatment with rhSTC1 significantly promoted metastasis of ovarian cancer cells, while the pro-metastatic effect was abolished by treatment with STC1 Ab (Figure 2B and C). To explore the molecular mechanism of STC1-regulated metastasis, we collected SKOV3 and HEY cells that were treated with rhSTC1 (200 ng/mL) for western blot. We found that treatment with rhSTC1 enhanced the expression of mesenchymal markers including fibronectin, vimentin and slug, and reduced the expression of epithelial marker ZO-1 (Figure 2D), while treatment with STC1Ab (250 ng/mL and 500 ng/mL) downregulated these mesenchymal markers and upregulated epithelial marker in both SKOV3 and HEY cells (Figure 2E). These results indicated that STC1 promoted metastasis of ovarian cancer cells through EMT.
Figure 2.
STC1 in the tumor microenvironment promotes proliferation and metastasis in ovarian cancer. (A) Addition of rhSTC1 promoted cell proliferation in both SKOV3 and HEY cell lines. Data were presented as mean ± SEM from at least three independent experiments. (B) Treatment with rhSTC1 promoted metastasis in ovarian cancer, while treatment with STC1Ab abrogated the effect of rhSTC1 on metastasis. (C) Quantification of metastasis by counting the number of migrated or invaded cells. Data were presented as mean ± SEM from at least three independent experiments. (D) Treatment with rhSTC1 promoted EMT in ovarian cancer. (E) Treatment with STC1Ab inhibited EMT in ovarian cancer.
Abbreviations: rhSTC1, recombinant human STC1; STC1 Ab, STC1 neutralizing antibody.
Secretory STC1 promoted metastasis and was implicated in the conversion of NFs into CAFs
To further investigate the effect of STC1 in the tumor microenvironment on the metastasis of ovarian cancer, we utilized a Tet-inducible system to establish STC1-overexpressing cells, in which addition of doxycycline can induce endogenous STC1-HA expression. Then, we collected the cell culture medium and examined the STC1 protein level in the induced (CM) and non-induced medium (NM). The results showed that overexpression of STC1 increased the secretion of STC1 into the cultured medium; CM contained higher STC1 protein than NM (Figure 3A). To investigate the function of the secreted STC1, we co-cultured the ovarian cancer cell lines with either CM or NM. The results showed that the secreted STC1 promoted cellular proliferation (Figure 3B) and metastasis (Figure 3C and D) in both SKOV3 and HEY cells. Moreover, the EMT markers fibronectin, vimentin, and slug were more highly expressed in SKOV3 and HEY cells co-cultured with CM (Figure 3E). These results were consistent with the results we obtained from the exogenous addition of rhSTC1, confirming that the STC1 in the tumor microenvironment promoted metastasis in ovarian cancer.
Figure 3.
Secretory STC1 promotes metastasis and is implicated in the conversion of NFs to CAFs. (A) Examination of the expression of STC1 in condensed culture medium. (B) Cells incubated with CM showed higher proliferation rates than those with NM. Data were presented as mean ± SEM from at least three independent experiments. (C) Treatment with CM enhanced metastasis in ovarian cancer cell lines. (D) Quantification of metastasis by counting the number of migrated cells. Data were presented as mean ± SEM from at least three independent experiments. (E) Treatment with CM upregulated the expression of fibronectin, vimentin, and slug. (F) Co-culture of NFs with CM promoted the expression of CAFs markers, including a-SMA, vimentin, and STC1.
Moreover, we found that NFs co-cultured with CM showed higher expression of α-SMA, vimentin, and STC1 than those cultured with NM (Figure 3F). The results indicated that STC1 in the tumor microenvironment could activate fibroblasts and promote the conversion of NFs to CAF-like cells; Moreover, the upregulated STC1 expression may facilitate the maintenance of the CAF state. Taken together, STC1 was implicated in the conversion of NFs into CAFs.
STC1 activated the phosphorylation of Akt at Ser473
Growing evidence suggests that Akt signaling pathway is vital to the induction of EMT, so we investigated whether the activation of Akt was involved in STC1-mediated metastasis. The result showed that rhSTC1 enhanced the phosphorylation of Akt at Ser473 (Figure 4A), while the phosphorylation decreased when STC1 Ab was added (Figure 4B). Previous studies have reported that the activation of Akt via phosphorylation at Ser473 promoted metastasis by up-regulating the expression of several EMT proteins, including fibronectin, vimentin and slug, we utilized LY294002 to inhibit the phosphorylation of Akt, and found that STC1-stimulated metastasis was suppressed (Figure 4C and D). Taken together, our results suggest that STC1 promotes metastasis through Akt-mediated EMT.
Figure 4.
STC1 in the microenvironment activates Akt by enhancing the phosphorylation at Ser473. (A) Treatment with rhSTC1 upregulated the phosphorylation of Akt at Ser473. (B) Treatment with STC1Ab inhibited the phosphorylation of Akt at Ser473. (C) Treatment with LY294002 inhibited STC1-stimulated metastasis. (D) Quantification of metastasis by counting the number of migrated cells. Data were presented as mean ± SEM from at least three independent experiments. (E) Schematic model of microenvironmental STC1-mediated metastasis in ovarian cancer.
Discussion
Recent studies have shown that targeting the tumor microenvironment could be an effective strategy for cancer treatment.3–5 Fibroblasts that existed in the tumor stroma are called CAFs, they are the major component of the tumor microenvironment. These cells promote tumorigenesis by secreting pro-inflammatory cytokines, such as IL-6 and CXCL1,18 as well as exosomes.19 Identification of novel secretory cytokines, especially those that could be specific markers for CAFs, will facilitate the development of new strategies for anti-cancer treatment.
In our study, we analyzed the different expression profiles of normal ovarian stroma and ovarian cancer stroma in a GEO dataset and found that STC1 mRNA was more highly expressed in cancer stroma than in normal stroma. We also confirmed that STC1 protein expression was higher in CAFs than in NFs, which we isolated from ovarian tissues of patients with either ovarian cancer or uterine fibroids, respectively, and cultured in vitro. The results demonstrated that STC1, a known secretory protein, was upregulated in the tumor microenvironment of ovarian cancer. Then, we investigated the effect of STC1 on tumor progression. The results showed that addition of rhSTC1 in the culture medium promoted proliferation and metastasis in ovarian cancer, while STC1 Ab in the culture medium suppressed metastasis. Further investigation demonstrated that rhSTC1 enhanced the expression of several mesenchymal markers, including fibronectin, vimentin and slug, and inhibited the expression of epithelial marker ZO-1, treatment with STC1Ab attenuated the expression of these mesenchymal markers and upregulated epithelial marker. These results suggested that STC1 promoted metastasis through EMT. We also found that STC1 secreted by endogenous overexpression model promoted metastasis and EMT. Furthermore, NFs treated with CM, the medium collected from STC1 overexpression cells, showed CAF-like characteristics; CAF markers including a-SMA and vimentin were significantly upregulated, suggesting that STC1 may be involved in the conversion of NFs to CAFs. Finally, we showed that STC1 promoted metastasis through the Akt signaling pathway. Phosphorylation of Akt at Ser473 was enhanced with rhSTC1 treatment, but was suppressed upon treatment with STC1Ab. Previous studies have demonstrated that the Akt signaling pathway could upregulate the expression of fibronectin,20vimentin,21 and slug.22,23 Thus, we proposed that the Akt pathway may be involved in STC1-mediated metastasis. Further study showed that LY294002, a potent inhibitor of Akt activation, inhibited STC1-induced metastasis. Taken together, our results demonstrated that STC1 in the tumor microenvironment promoted metastasis in ovarian cancer by regulating Akt-mediated EMT (Figure 4E).
Since STC1 is a secretory protein, the mechanism of how STC1 transduced signaling into the cells, caused the phosphorylation of Akt and induced the downstream effects needs further research; we speculate that certain receptors located on the cell membrane may be involved in the process, but these receptors remain to be identified.
According to other research, STC1 is also highly expressed in ovarian cancer cells. As a paracrine protein, STC1 may be a crucial factor in the interaction between tumor epithelial and stromal cells. STC1 in the tumor microenvironment can promote the metastasis of tumor epithelial cells, as well as facilitate the conversion of NFs into CAFs, which in turn secrete more STC1 to further enhance metastasis. Thus, tumor epithelial cells and stromal cells co-evolve and communicate with each other to synergistically create a tumor microenvironment that promotes tumor progression.
In conclusion, we demonstrated that STC1 in tumor microenvironment stimulated tumor metastasis via the Akt-mediated EMT and may be implicated in the conversion of NFs to CAFs. Our results indicated that STC1, as a potential biomarker and a therapeutic target, should be taken into account in the treatment of ovarian cancer.
Acknowledgments
This work was supported by the Shanghai Municipal Commission of Health and Family Planning (grant number: 201440484).
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2.Torre LA, Trabert B, DeSantis CE, et al. Ovarian cancer statistics, 2018. CA Cancer J Clin. 2018;68(4):284–296. doi: 10.3322/caac.21456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nwani NG, Sima LE, Nieves-Neira W, Matei D. Targeting the microenvironment in high grade serous ovarian cancer. Cancers (Basel). 2018;10(8). doi: 10.3390/cancers10110400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang L, Lin PC. Mechanisms that drive inflammatory tumor microenvironment, tumor heterogeneity, and metastatic progression. Semin Cancer Biol. 2017;47:185–195. doi: 10.1016/j.semcancer.2017.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Casey SC, Amedei A, Aquilano K, et al. Cancer prevention and therapy through the modulation of the tumor microenvironment. Semin Cancer Biol. 2015;35(Suppl):S199–S223. doi: 10.1016/j.semcancer.2015.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mitra AK, Zillhardt M, Hua Y, et al. MicroRNAs reprogram normal fibroblasts into cancer-associated fibroblasts in ovarian cancer. Cancer Discov. 2012;2(12):1100–1108. doi: 10.1158/2159-8290.CD-12-0206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21(3):309–322. doi: 10.1016/j.ccr.2012.02.022 [DOI] [PubMed] [Google Scholar]
- 8.Zhang Y, Tang H, Cai J, et al. Ovarian cancer-associated fibroblasts contribute to epithelial ovarian carcinoma metastasis by promoting angiogenesis, lymphangiogenesis and tumor cell invasion. Cancer Lett. 2011;303(1):47–55. doi: 10.1016/j.canlet.2011.01.011 [DOI] [PubMed] [Google Scholar]
- 9.Leung CS, Yeung TL, Yip KP, et al. Cancer-associated fibroblasts regulate endothelial adhesion protein LPP to promote ovarian cancer chemoresistance. J Clin Invest. 2018;128(2):589–606. doi: 10.1172/JCI95200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Curtis M, Kenny HA, Ashcroft B, et al. Fibroblasts mobilize tumor cell glycogen to promote proliferation and metastasis. Cell Metab. 2019;29(1):141–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pena C, Cespedes MV, Lindh MB, et al. STC1 expression by cancer-associated fibroblasts drives metastasis of colorectal cancer. Cancer Res. 2013;73(4):1287–1297. doi: 10.1158/0008-5472.CAN-12-1875 [DOI] [PubMed] [Google Scholar]
- 12.Chan KK, Leung CO, Wong CC, et al. Secretory Stanniocalcin 1 promotes metastasis of hepatocellular carcinoma through activation of JNK signaling pathway. Cancer Lett. 2017;403:330–338. doi: 10.1016/j.canlet.2017.06.034 [DOI] [PubMed] [Google Scholar]
- 13.Ma X, Gu L, Li H, et al. Hypoxia-induced overexpression of stanniocalcin-1 is associated with the metastasis of early stage clear cell renal cell carcinoma. J Transl Med. 2015;13:56. doi: 10.1186/s12967-015-0541-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu G, Yang G, Chang B, et al. Stanniocalcin 1 and ovarian tumorigenesis. J Natl Cancer Inst. 2010;102(11):812–827. doi: 10.1093/jnci/djq127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang AC, Doherty J, Huschtscha LI, et al. STC1 expression is associated with tumor growth and metastasis in breast cancer. Clin Exp Metastasis. 2015;32(1):15–27. doi: 10.1007/s10585-014-9687-9 [DOI] [PubMed] [Google Scholar]
- 16.Nguyen A, Chang AC, Reddel RR. Stanniocalcin-1 acts in a negative feedback loop in the prosurvival ERK1/2 signaling pathway during oxidative stress. Oncogene. 2009;28(18):1982–1992. doi: 10.1038/onc.2009.65 [DOI] [PubMed] [Google Scholar]
- 17.Orr B, Riddick AC, Stewart GD, et al. Identification of stromally expressed molecules in the prostate by tag-profiling of cancer-associated fibroblasts, normal fibroblasts and fetal prostate. Oncogene. 2012;31(9):1130–1142. doi: 10.1038/onc.2011.312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Erez N, Glanz S, Raz Y, Avivi C, Barshack I. Cancer associated fibroblasts express pro-inflammatory factors in human breast and ovarian tumors. Biochem Biophys Res Commun. 2013;437(3):397–402. doi: 10.1016/j.bbrc.2013.06.089 [DOI] [PubMed] [Google Scholar]
- 19.Li YY, Tao YW, Gao S, et al. Cancer-associated fibroblasts contribute to oral cancer cells proliferation and metastasis via exosome-mediated paracrine miR-34a-5p. EBioMedicine. 2018;36:209–220. doi: 10.1016/j.ebiom.2018.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.You D, Jung SP, Jeong Y, Bae SY, Lee JE, Kim S. Fibronectin expression is upregulated by PI-3K/Akt activation in tamoxifen-resistant breast cancer cells. BMB Rep. 2017;50(12):615–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu QS, Rosenblatt K, Huang KL, et al. Vimentin is a novel AKT1 target mediating motility and invasion. Oncogene. 2011;30(4):457–470. doi: 10.1038/onc.2010.421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fenouille N, Tichet M, Dufies M, et al. The epithelial-mesenchymal transition (EMT) regulatory factor SLUG (SNAI2) is a downstream target of SPARC and AKT in promoting melanoma cell invasion. PLoS One. 2012;7(7):e40378. doi: 10.1371/journal.pone.0040378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carpenter RL, Paw I, Dewhirst MW, Lo HW. Akt phosphorylates and activates HSF-1 independent of heat shock, leading to slug overexpression and epithelial-mesenchymal transition (EMT) of HER2-overexpressing breast cancer cells. Oncogene. 2015;34(5):546–557. doi: 10.1038/onc.2013.582 [DOI] [PMC free article] [PubMed] [Google Scholar]