Abstract
Background: Deceased muscle mass combined with increased visceral fat mass is reportedly linked to a higher risk of worsening the hepatic conditions of non-alcoholic fatty liver disease (NAFLD).
Objective: The aim of this study was conducted in a retrospective manner to investigate whether longitudinal changes in skeletal muscle mass to visceral fat area ratio (SV ratio), an index of sarcopenic obesity, are influential on the hepatic conditions and pathophysiology of NAFLD during the clinical course.
Design: The association of SV ratio with hepatic conditions and pathophysiology was evaluated longitudinally for 2–5.5 years (median 4.1 years) in 92 patients with NAFLD (36 men and 56 women; 17–78 years). The subjects were divided into three groups according to the change in their SV ratio: improved, stable, or worsened, and the changes in parameters associated with NAFLD were compared among the groups.
Results: In the group with a worsened SV ratio, visceral fat area increased (122±30–138±30 cm2; mean ± SD), whereas total muscle mass decreased (26.5±6.1–25.9±5.9 kg), which was especially noticeable in the lower extremities (14.8±3.3–14.3±3.1 kg). In accordance with the change of body composition, transient elastography showed higher levels of liver stiffness (7.7±5.4–9.0±6.0 kPa) and fat accumulation (265±43–293±48 dB/m). There were also higher levels of fasting plasma glucose (115±29–126±40 mg/dL) and HbA1c (6.0±1.1–6.3±1.0%). In contrast, deterioration in these parameters did not occur in the groups with improved or stable SV ratios.
Conclusion: Collectively, a progressive reduction in skeletal muscle mass accompanied by an increase in visceral fat mass during the clinical course of NAFLD is associated with a worsening of the hepatic conditions, fat accumulation and progression of fibrosis.
Keywords: non-alcoholic fatty liver disease, skeletal muscle mass to visceral fat area ratio, sarcopenic obesity, hepatic steatosis, hepatic fibrosis, clinical course
Introduction
Abnormal body composition, such as obesity coupled with low skeletal muscle mass, reportedly affects the pathophysiology of chronic liver disease.1 The relation between low skeletal muscle mass (sarcopenia) and poor survival outcome of patients with chronic liver disease has also been published previously.2 This may be because a reduction in exercise tolerance secondary to loss of skeletal muscle mass accelerates the progression of chronic liver disease to cirrhosis.2 Low skeletal muscle mass is also an independent prognostic factor for hepatocellular carcinoma.3,4 However, it also heightens the probability of developing NAFLD5–7 and exacerbating liver fibrosis.7
Based on the clinical findings above, alterations in body composition caused by an obesity-related increase in abdominal fat and a decrease in skeletal muscle mass are believed to be elements related to the onset and exacerbation of chronic liver diseases, in addition to the survival outcome of chronic liver disease patients. Therefore, both increases in abdominal fat and decreases in skeletal muscle mass are being increasingly shown to be important factors affecting the pathophysiology and survival outcome of those presented with chronic liver disease.
Recently, we reported on the cross-sectional study that a low skeletal muscle mass to visceral fat area ratio (SV ratio),8 an index of sarcopenic obesity, is related to exacerbation of the hepatic conditions in NAFLD; eg, moderate to severe steatosis and advanced fibrosis.9 When SV ratio was stratified using quartiles, the group with the lowest SV ratio were more likely to worsen NAFLD pathophysiological conditions such as insulin resistance, adipokine imbalance, and inflammatory or oxidative stress, leading to the exacerbation of hepatic function, hepatocyte apoptosis, and liver fibrosis. Thus, it is likely that a low SV ratio aggravates the risk of NAFLD onset and is an important factor for the progression of hepatic fibrosis. However, no studies have been published that assess the influence of longitudinal changes in SV ratio on the features of NAFLD.
Therefore, this study was undertaken in a retrospective manner to investigate the influence of longitudinal changes in SV ratio on the hepatic conditions and pathophysiology of NAFLD during the clinical course.
Patients and methods
Patients
From April 2011 to October 2015, 300 subjects (179 men and 121 women), who were referred to the Tsukuba University Hospital outpatient department for the first time from April 2011 to October 2015 after hepatic dysfunction or fatty liver was detected as a result of a complete medical checkup or medical examination, were diagnosed with NAFLD. The diagnosis of NAFLD was made on the basis of overeating or physical inactivity, high serum alanine aminotransferase (ALT), and the existence of at least two of three atypical findings using abdominal ultrasonography, as per the diagnostic guidelines for NAFLD in the Asia-Pacific region.10 The patients were removed from this study as follows: (a) the presence of other causes of liver disease, (b) psychiatric disease, and (c) those who were taking anti-diabetic or weight loss medication. Patients with an alcohol consumption of >20 g/day were also excluded.
Of the 300 recruited, 92 patients (36 men and 56 women of 17–78 years) whose clinical course (2–5.5 years; median, 4.1 years) were subsequently followed as outpatients who continuously visited the Hospital for treatment. The remaining 208 patients were referred from our university hospital to the related hospitals located near the patient’s residence according to their will. In the treatment of these 92 patients, guidance for lifestyle improvement was provided through consultation on diet and exercise at the first visit. Subsequently, patients with poorly controlled diabetes mellitus were treated with oral antidiabetic drugs, while patients with hepatic dysfunction were treated with ursodeoxycholic acid under the primary care physician. The patients visited the Hospital about once a year, and a consultation to improve lifestyle was conducted on each visit.
Based on the retrospective analysis, the 92 subjects followed up in the Hospital were classified as having an “improved” SV ratio when the ratio had increased by >5% of its baseline value, while those classified as having a “stable” SV ratio had a ratio that had increased or decreased by <5% of its baseline value, and those classified as having a “worsened” SV ratio had a ratio that had decreased by >5% of its baseline value.
The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki, as reflected in prior approval by the Ethics Committee of the Tsukuba University Hospital. Written informed consent was collected from each patient.
Anthropometric measurements
Body weight and composition (fat mass, skeletal muscle mass, and abdominal visceral fat area) were analyzed by using an InBody 720 (Biospace Japan Inc., Tokyo, Japan), and then SV ratio (g/cm2)8 was calculated. Height was measured with a wall-mounted stadiometer (Muratec-KDS Co., Kyoto, Japan). Using these data, BMI was calculated (kg/m2). The maximum strength of the isometric contraction connected with knee extension to an angle of 90° in a seated position was measured using a hand-held dynamometer (μ-TasF-1; Anima Co., Tokyo, Japan). Isometric movement was conducted by exerting maximum effort to extend the knee joint for up to 5 seconds. The greater of two measurements was recorded for each subject and the data were normalized according to body mass (kg) and expressed as percentages.
Blood biochemical measurements
Blood was drawn from a median cubital vein. Measurements of blood biochemistry were conducted as previously reported.11 Surrogate markers were calculated from the laboratory measurements: for homeostasis model assessment of insulin resistance (HOMA-IR),12 and for NAFLD the NAFLD fibrosis score (NFS)13 and the fibrosis (FIB)-4 index.14
Ultrasonography
An ultrasound scanner (Aplio-400; Toshiba Co. Ltd., Tokyo) was utilized to gauge liver steatosis. The grade of steatosis (G0, normal echogenicity; G1, minimal, diffuse increase in fine echoes in liver parenchyma, but with normal visualization of the diaphragm and intrahepatic vessel borders; G2, modest, diffuse increase in fine echoes with slightly impaired visualization of intrahepatic vessels and the diaphragm; G3, significant increase in fine echoes with poor or non-visualization of the intrahepatic vessel borders, diaphragm, and posterior right lobe of the liver) was determined according to criteria reported previously,15 by a clinical radiologist, who was blinded to the treatment assignment of the patients. The scans were scored in an arbitrary order without the provision of any clinical data.
Liver stiffness and steatosis
Hepatic stiffness was determined by using a Fibroscan® (Echosens, Paris, France) with a 3.5-MHz standard probe. The principle and examination methodology have been reported previously.16 According to the validity criteria,17 the liver stiffness measurement (LSM) reliability includes the conditions as follows: 10 valid acquisitions are available; the success rate is ≥60%; and the interquartile range to median ratio (IQR/M) is <30%. We decided to use the LSM data when all the above conditions were met. Measurements of CAP and LSM were performed by a single laboratory technician. In addition, hepatic fat quantity was determined with a controlled attenuation parameter (CAP) designed to evaluate hepatic ultrasonic attenuation with a 3.5 MHz standard probe by using signals acquired by the Fibroscan. A detailed description of CAP has also been reported previously.18
Statistical analysis
The statistical analysis was conducted by using Statistical Package for the Social Sciences (SPSS) for Windows, version 23.0 (SPSS Inc., Chicago, USA). The measurement values are expressed as mean ± standard deviation. Comparisons on the time course of changes in each pathophysiological factor in the improved, stable, or worsened SV-ratio groups were analyzed by using ANCOVA with and without adjustment for age and gender as covariates. A P-value of <0.05 was defined as representing statistical significance.
Results
Longitudinal changes in the SV ratio
The 92 subjects followed up in the Hospital were divided into three groups according to the change in their SV ratio: 14 improved, 46 stable, and 32 worsened. Table 1 provides summarized information on age, gender, type 2 diabetes mellitus, and prevalence of other lifestyle-related diseases at baseline (when the observation was started) in the groups. While the gender composition was different, there were no significant differences in the age and prevalence of lifestyle-related diseases among the groups.
Table 1.
The baseline data on age, gender, and prevalence rates of life style-related diseases for 92 NAFLD patients with SV ratios that worsened, were stable, and improved
| Total n=92 |
Worsened SV ratio n=32 |
Stable SV ratio n=46 |
Improved SV ratio n=14 |
P-value | |||
|---|---|---|---|---|---|---|---|
| ANOVA | |||||||
| Worsened vs Stable | Worsened vs Improved | Stablevs. Improved | |||||
| Age | 55.5±14.3 | 52.8±14.0 | 57.3±15.1 | 55.9±11.9 | 0.369 | 0.789 | 0.941 |
| Chi-square test | |||||||
| Gender, male/female | 36/56 | 14/18 | 13/33 | 9/5 | 0.043 | ||
| Life style-related diseases | Chi-square test | ||||||
| Diabetes mellitus (%) | 42.4 | 34.4 | 47.8 | 42.9 | 0.497 | ||
| Dyslipidemia (%) | 57.6 | 56.3 | 56.5 | 64.3 | 0.860 | ||
| Hypertension (%) | 23.9 | 18.8 | 28.3 | 21.4 | 0.608 | ||
Note: Values are given as mean±SD.
Abbreviation: SV ratio, skeletal muscle mass to visceral fat area ratio.
Anthropometric characteristics in the SV ratio groups
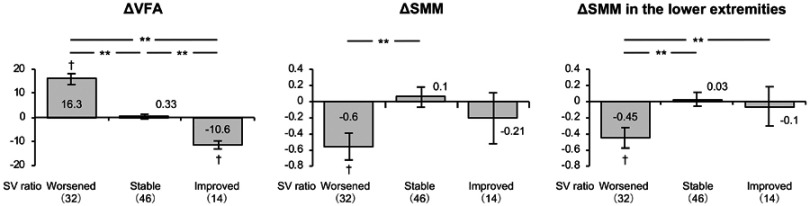
The mean SV ratio across all of the subjects with NAFLD decreased, and the visceral fat area and waist to hip ratio increased, during the observation period, as shown in Table 2. When the subjects were divided into groups according to their change of SV ratio (improved, stable, or worsened), the group with a worsened SV ratio demonstrated an increase in visceral fat area and a reduction in total muscle mass, especially in the lower extremities (Figure 1). Both the waist-hip ratio and fat mass increased. However, in the group with an improved SV ratio, visceral fat area, BMI, waist to hip ratio, and fat mass decreased, and in the group with a stable SV ratio, fat mass also decreased. Regarding the height of the subjects, the difference among the SV-ratio worsened group, unchanged group, and improved group was examined, which showed no statistically significant difference.
Table 2.
Changes in anthropomatic characteristics, insulin resistance, hepatic abnormalities, lipid profile, and fibrosis markers in NAFLD patients with SV-ratio that worsened, were stable or improved during the study
| Observation period, years | Total | Worsened SV ratio | Stable SV ratio | Improved SV ratio | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n=92 | n=32 | n=46 | n=14 | |||||||||
| 4.1±1.1 | 4.5±0.9 | 3.9±1.2 | 4.0±1.2 | |||||||||
| Baseline | After | Change | Baseline | After | Change | Baseline | After | Change | Baseline | After | Change | |
| Height, cm | 161±10 | – | — | 162±8 | — | — | 158±11 | — | — | 165±10 | — | — |
| SV ratio | 209±72 | 199±60 | −10.0** | 232±90 | 195±57 | −37.5** | 189±55 | 189±55 | −0.3 | 220±60 | 241±67 | 21** |
| VFA, cm2 | 128±31 | 132±32 | 4.1* | 122±30 | 138±30 | 16.3** | 134±34 | 134±35 | 0.33 | 121±15 | 110±15 | −10.6** |
| Total SMM, kg | 25.4±6.5 | 25.2±6.4 | −0.2 | 26.5±6.1 | 25.9±5.9 | −0.6** | 24.5±7.0 | 24.6±7.2 | 0.1 | 26.0±5.3 | 25.8±4.9 | −0.21 |
| Upper extremities, kg | 5.02±1.51 | 4.99±1.53 | −0.04 | 5.25±1.50 | 5.21±1.43 | −0.05 | 4.84±1.60 | 4.83±1.69 | −0.01 | 5.12±1.23 | 5.00±1.17 | −0.12 |
| Truncus, kg | 21.1±4.8 | 21.6±7.8 | 0.51 | 21.8±4.6 | 21.8±4.4 | −0.08 | 20.5±5.1 | 20.4±5.3 | −0.1 | 21.5±4.1 | 25.3±16.1 | 3.8 |
| Lower extremities, kg | 14.3±3.7 | 14.1±3.6 | −0.15* | 14.8±3.3 | 14.3±3.1 | −0.45** | 13.7±4.1 | 13.8±4.1 | 0.03 | 15.0±3.1 | 14.9±2.8 | −0.1 |
| Body mass index, kg/m2 | 27.9±5.1 | 27.7±5.3 | −0.2 | 28.0±4.7 | 28.5±5.1 | 0.5 | 28.5±5.7 | 28.1±5.7 | −0.3 | 26.0±4.0 | 24.5±2.8 | −2** |
| Waist to hip ratio | 0.967±0.054 | 0.974±0.055 | 0.01** | 0.955±0.055 | 0.976±0.056 | 0.02** | 0.982 ±0.055 |
0.985±0.053 | 0.003 | 0.943±0.033 | 0.934±0.036 | −0.01** |
| Fat mass, kg | 26.1±10.6 | 25.8±10.9 | −0.3 | 25.2±10.0 | 27.7±11.1 | 2.4** | 27.5±11.6 | 26.4±11.4 | −1.0** | 23.4±8.1 | 19.3±6.3 | −4** |
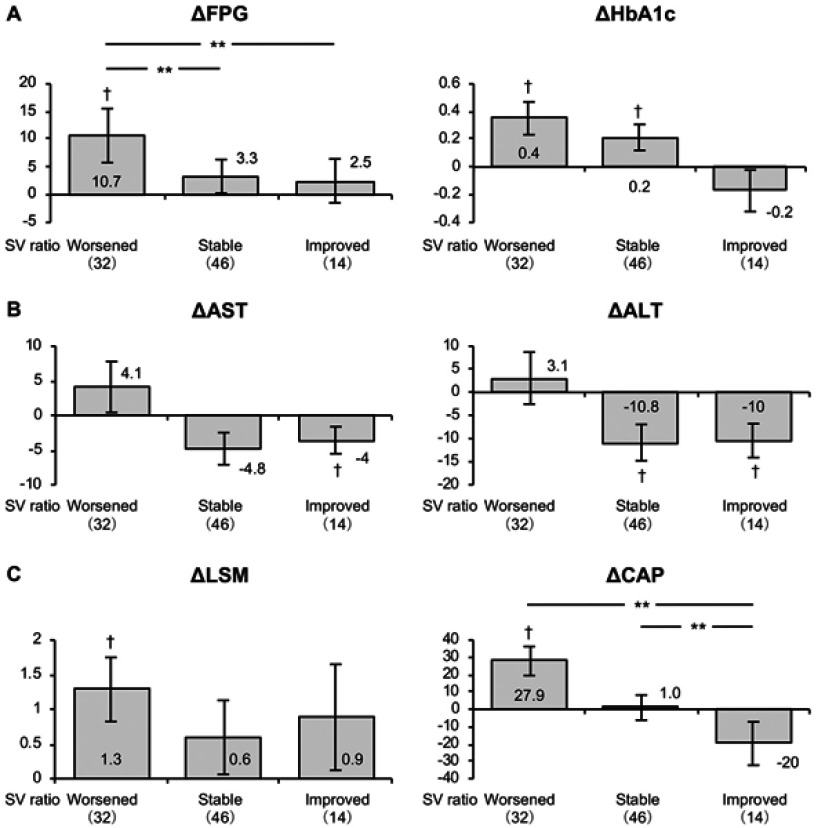
| FPG, mg/dl | 118±30 | 124±33 | 5.7* | 115±29 | 126±40 | 10.7* | 119±29 | 122±29 | 3.3 | 122±34 | 124±30 | 2.5 |
| Insulin, μU/ml | 14±20 | 13±11 | −1.0 | 15±14 | 16±14 | 0.9 | 15±25 | 12±10 | −2.2 | 11±6 | 10±5 | −1.4 |
| HOMA-IR | 4.8±10.9 | 4.1±3.9 | −0.7 | 4.3±4.5 | 5.0±5.1 | 0.7 | 5.5±14.9 | 3.9±3.4 | −1.6 | 3.5±2.2 | 3.0±1.6 | −0.4 |
| HbA1c, % | 6.2±1.0 | 6.4±1.0 | 0.2** | 6.0±1.1 | 6.3±1.0 | 0.4** | 6.3±0.8 | 6.5±0.9 | 0.2** | 6.5±1.3 | 6.3±1.2 | −0.2 |
| Platelet, x 109/l | 214±63 | 219±66 | 4.6 | 205±55 | 214±61 | 8.4 | 220±66 | 225±69 | 5.4 | 217±69 | 210±71 | −7 |
| Ferritin, ng/ml | 118±117 | 99±71 | −19.5 | 133±171 | 112±74 | −20.1 | 106±69 | 89±66 | −17.1* | 125±94 | 99±82 | −26 |
| Alb, g/dl | 4.4±0.3 | 4.3±0.3 | −0.1** | 4.4±0.3 | 4.3±0.3 | −0.1 | 4.4±0.2 | 4.3±0.2 | −0.1 | 4.5±0.4 | 4.4±0.2 | −0.1 |
| AST, U/l | 35±22 | 34±22 | −1.5 | 35±20 | 39±27 | 4.1 | 37±24 | 32±19 | −4.8 | 31±20 | 27±18 | −4* |
| ALT, U/l | 44±39 | 38±32 | −6.1* | 44±36 | 47±37 | 3.1 | 45±46 | 34±30 | −10.8* | 39±22 | 28±15 | −10** |
| γ-GT, U/l | 50±53 | 51±57 | 1.1 | 45±36 | 54±53 | 8.8 | 53±63 | 54±64 | 0.5 | 50±54 | 36±34 | −14 |
| CHO, mg/dl | 198±40 | 201±38 | 3.5 | 197±29 | 202±32 | 4.3 | 199±47 | 202±38 | 2.8 | 195±41 | 200±50 | 4 |
| TG, mg/dl | 141±91 | 141±105 | 0.2 | 135±57 | 131±68 | −3.7 | 130±71 | 140±103 | 9.9 | 191±175 | 167±172 | −24 |
| CAP, dB/m | 272±49 | 279±48 | 7.2 | 265±43 | 293±48 | 27.9** | 275±52 | 276±45 | 1.0 | 279±51 | 259±50 | −20 |
| LSM, kPa | 7.6±4.6 | 8.5±5.4 | 0.9** | 7.7±5.4 | 9.0±6.0 | 1.3** | 7.7±4.1 | 8.3±5.2 | 0.6 | 7.2±4.4 | 8.0±5.0 | 0.9 |
| Hyaluronic acid, ng/ml | 49±45 | 64±82 | 14.6 | 51±49 | 72±89 | 20.3 | 47±45 | 53±49 | 5.9 | 51±41 | 80±134 | 30 |
| Type IV collagen, ng/ml | 135±44 | 140±47 | 5.0 | 143±60 | 146±64 | 3.6 | 127±33 | 135±38 | 8.4 | 142±28 | 139±28 | −2 |
| NAFLD fibrosis score | −1.3±1.4 | −1.1±1.5 | 0.2** | −1.3±1.1 | −1.2±1.2 | 0.1 | −1.1±1.6 | −0.9±1.6 | 0.2* | −1.6±1.4 | −1.3±1.7 | 0.2 |
| FIB-4 index | 1.63±1.04 | 1.83±1.35 | 0.2** | 1.52±0.83 | 1.70±1.04 | 0.2* | 1.75±1.20 | 1.94±1.59 | 0.2 | 1.51±0.88 | 1.78±1.18 | 0.3 |
Notes: Values are given as mean±SD. *P< 0.05, **P< 0.01; significantly different from the baselines.
Abbreviation: SV ratio, skeletal muscle mass to visceral fat area ratio.
Figure 1.
Changes in total muscle mass, muscle mass of the lower extremities, and visceral fat area in NAFLD patients with SV ratios that worsened, were stable, or improved during the study. To compare between groups, all dependent variables were analyzed by using ANCOVA with adjustment for age and gender as covariates. †P<0.05 versus baseline; **P<0.01 between the groups.
Changes in insulin resistance in the SV ratio groups
The mean fasting plasma glucose (FPG) and HbA1c increased across all the subjects during the observation period, as shown in Table 2. In the group with a worsened SV-ratio, FPG and HbA1c increased, and in the group with a stable SV ratio, HbA1c increased, but the magnitude of the increase in HbA1c was greater in the worsened than in the stable group (Figure 2). HOMA-IR tended to increase in the worsened group but decreased in the stable and improved groups.
Figure 2.
Changes in FPG and HbA1c (A), AST and ALT (B), and CAP and LSM (C) in NAFLD patients with SV ratios that worsened, were stable, or improved during the study. To compare between groups, all dependent variables were analyzed by using ANCOVA with adjustment for age and gender as covariates. †P<0.05 versus baseline; **P<0.01 between the groups.
Biochemical findings in the SV ratio groups
The mean albumin and ALT levels across all the subjects decreased during the observation period, as shown in Table 2. Although no significant changes were observed in the group with a worsened SV ratio, the ALT in the stable group, and the AST and ALT in the improved group, decreased during the study (Figure 2). In addition, ferritin concentration decreased in the stable group.
The NAFLD-related hepatic conditions in the SV ratio groups
Although the mean CAP-value, indicating the level of hepatic fat accumulation, remained unchanged, the mean LSM, which indicates the level of hepatic fibrosis and/or inflammation, increased across all of the subjects during the observation period, as shown in Table 2. However, CAP increased in the group with a worsened SV ratio, and the magnitude of the increase in LSM was greater in the worsened group (Figure 2). In addition, the mean NFS and the mean FIB-4 index increased across all the subjects, reflecting a progression of fibrosis during the observation period. Specifically, these changes were because of increases in NFS in the stable group and FIB-4 index in the worsened group.
Discussion
The results of this retrospective study can be summarized as follows. (i) In the NAFLD patients with an SV ratio that worsened during the clinical course, visceral fat area increased and total muscle mass decreased, especially in the lower extremities. (ii) Transient elastography showed that liver stiffness and fat accumulation had worsened. (iii) Blood biochemistry showed a worsening of hepatic dysfunction, insulin resistance, and liver fibrosis scores (eg, FIB-4 index). Thus, a progressive reduction in skeletal muscle mass coupled with an increase in visceral fat mass is associated with a worsening of hepatic conditions and pathophysiology of NAFLD.
In this study, abnormal body composition and disease pathological conditions in patients with NAFLD are discussed using skeletal muscle mass and visceral fat area measured by BIA. The measurement of body composition by BIA is not the gold standard. However, it is less expensive, easy, and reproducible and does not cause radiation exposure. It is one of the measurement methods that are easily applied in the clinical setting. While abdominal CT scan is one of the gold standards in measuring visceral fat mass, it has been reported that visceral fat mass measured by BIA is highly correlated with the visceral fat mass measured by abdominal CT scan.19
The disadvantage of this study is the absence of information on liver histopathology. Instead, liver pathological conditions were evaluated in detail using blood biochemistry, diagnostic imaging of ultrasonography and transient elastography in addition to prediction models that have been used conventionally for evaluation of liver fibrosis. Devised combinations of blood biochemistry, ultrasonography and transient elastography are supportive for the better understanding of the stage of liver fibrosis.
In the multiple parallel hit hypothesis for NAFLD,20 various disorder factors engage in complex crosstalk under the combination of pathophysiologies between the liver and various organs as components of the disease. Not only insulin resistance but also exacerbation of inflammatory/oxidative stress conditions related to sarcopenia and sarcopenic obesity and an increase in hepatic apoptosis related to these conditions are affected in the pathological background.
In the SV-ratio worsened group, an increase in FIB-4 index, which is a prediction model, along with an increase in LSM, as well as an increase in fasting blood glucose and HbA1c levels, and a tendency toward an increase in HOMA-IR were observed (Table 2), suggesting that a decrease in SV ratio is involved in exacerbation of hepatic conditions of NAFLD and glucose metabolic diseases. In addition, the LSM reflects not only hepatic fibrosis but also inflammatory/oxidative stress conditions or congestion, etc., that promote hepatic fibrogenesis.21 Exacerbation of inflammatory/oxidative stress conditions related to sarcopenia and sarcopenic obesity and an increase in hepatic apoptosis related to these conditions are affected in the pathological background. Based on these results, the decrease in SV ratio is considered to be related to exacerbation of hepatic pathological conditions. It is unlikely that many of the patients with NAFLD with worsened SV ratio have genetically increased NAFLD disease susceptibility.22
In this study, the period of follow-up for the patients was largely variable ranging from 2 to 5.5 years. Therefore, the effect of the length of the observation period on the changes in LSM and CAP by transient elastography was examined in the SV-ratio worsened group. The SV-ratio worsened group was divided into two groups: less than 4.5 years and not less than 4.5 years of the median observation period; the changes in LSM and CAP in these two groups were compared. No significant difference was observed in the change in LSM and CAP based on the length of the observation period in the SV-ratio worsened group (data not shown). Therefore, the length of the observation period is considered to have no substantial effect on the exacerbation of hepatic conditions of NAFLD in the SV-ratio worsened group.
Consistent with these findings, we showed using multiple regression analysis that SV ratio is an independent factor affecting CAP, which reflects hepatic fat accumulation, and LSM, which reflects hepatic inflammation and/or fibrosis.9 Here, a low SV ratio was apparently associated with the worsened hepatic conditions. The hepatic conditions of NAFLD are thus likely to be impacted by not only the main defects in the liver but also defects in other organs or tissues, such as skeletal muscle and abdominal fat.
It has been suggested that insulin resistance, leptin resistance, inflammation, and oxidative stress are highly influential on the qualitative deterioration of muscle in sarcopenic obesity. Moreover, in our recent paper,9 worsening of these pathogenic factors was found in subjects in the lowest SV ratio quartile. Insulin resistance is associated with glycogenesis and an acceleration of muscular protein degradation, which leads to a loss of lower limb muscle and grip strength.23 Sarcopenic obesity also involves inflammatory conditions; the pro-inflammatory cytokines produced by adipose tissues act on skeletal muscle, which might also tend to reduce muscle mass and strength. Indeed, an adverse correlation has been indicated between blood pro-inflammatory cytokine concentrations and skeletal muscle mass.24 In particular, interleukin (IL)-6 and the soluble IL-6 receptor are present at high circulating concentrations in patients with sarcopenic obesity.25 An increase in IL-6 is known to reduce muscle strength and movement capability in aged persons, together with an inhibition of the anabolic effects of insulin-like growth factor-1 in skeletal muscle, resulting in net muscle protein degeneration.
Moreover, myostatin (MSTN), a member of the transforming growth factor β family, is a potent contributor to muscle wasting. Serum myostatin levels are high in subjects in the lowest SV-ratio quartile;9 therefore, it is also likely to play an important role in the observed loss of muscle mass. However, MSTN also promotes the development of fatty liver through the induction of insulin resistance.26,27 The MSTN receptor, activin IIbr, has been found in hepatic stellate cells,28 implying that MSTN may activate these fibrogenic cells. In support of this suggestion, MSTN levels are high in patients with advanced liver disease.29
NAFLD can be subdivided into two categories: NAFL, thought to be the benign phenotype, linked to a lower risk of progression to cirrhosis, and NASH, thought to be the aggressive phenotype, linked to a significant risk of progression to cirrhosis.30,31 Recent studies have indicated that some subjects with NAFLD may quickly progress to cirrhosis.32 Thus, a study was held to determine the extended prognostic relevance of distinct histologic features of NAFLD in those biopsy-diagnosed with NAFLD.33 In multivariable analyses, independent predictors of mortality were the fibrosis stage, age in years, presence of diabetes, and current smoking, but only fibrosis stages 3 and 4 were independent predictors of liver-related events in this cohort study.
The presence of associated defects, for example, insulin resistance, leptin resistance, inflammation, or oxidative stress, deleteriously affects patients in the lowest SV ratio quartile or in those with a worsened SV ratio, by exacerbating the hepatic conditions of NAFLD, for example being associated with the progression of fibrosis. This also underlies the greater mortality of such patents from liver-related events, including variceal bleeding, ascites, hepatocellular carcinoma, cirrhosis, or liver transplantation. Therefore, medical support, including appropriate nutrition and exercise, are required to help prevent decreases in SV ratio and/or as an intervention if it decreases.
Conclusion
The results of this study suggest that a progressive reduction in SV ratio during the clinical course is of pathophysiological importance for NAFLD and worsens the features of the hepatic conditions, in particular liver fibrosis, which is a critical factor determining the prognosis of NAFLD. Thus, in the clinical management of NAFLD patients, especially in those with low SV ratios, it may be necessary to perform an intervention that hinders a reduction in skeletal muscle mass, in addition to preventing an exacerbation of obesity. The promotion of hepatic rehabilitation should, therefore, be diligently conducted by medical teams incorporating diet and exercise therapies.
Acknowledgments
This work was supported in part by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology, Japan (No. 17H02174, 17K19887, 18H02884, 18H03172, 18H03197, 18K07930, 18K11094). We thank Mark Cleasby, PhD, from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Berzigotti A, Garcia-Tsao G, Bosch J, et al; The Portal Hypertension Collaborative Group. Obesity is an independent risk factor for clinical decompensation in patients with cirrhosis. Hepatology. 2011;54:555–561. doi: 10.1002/hep.24418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alameri HF, Sanai FM, Dukhayll MA, et al. Six-minute walk test to assess functional capacity in chronic liver disease patients. World J Gastoenterol. 2007;13:3996–4001. doi: 10.3748/wjg.v13.i29.3996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iritani S, Imai K, Takai K, et al. Skeletal muscle depletion is an independent prognostic factor for hepatocellular carcinoma. J Gastoenterol. 2015;50:323–332. doi: 10.1007/s00535-014-0964-9 [DOI] [PubMed] [Google Scholar]
- 4.Fujiwara N, Nakagawa H, Kudo Y, et al. Sarcopenia, intramuscular fat deposition, and visceral adiposity independently predict the outcomes of hepatocellular carcinoma. J Hepatol. 2015;63:131–140. doi: 10.1016/j.jhep.2015.02.031 [DOI] [PubMed] [Google Scholar]
- 5.Hong HC, Hwang SY, Choi HY, et al. Relationship between sarcopenia and nonalcoholic fatty liver disease: the Korean Sarcopenic Obesity Study. Hepatology. 2014;59:1772–1778. doi: 10.1002/hep.26716 [DOI] [PubMed] [Google Scholar]
- 6.Lee Y-H, Jung KS, Kim SU, et al. Sarcopaenia is associated with NAFLD independently of obesity and insulin resistance: nationwide surveys (KNHANES 2008-2011). J Hepatol. 2015;63:486–493. doi: 10.1016/j.jhep.2015.02.051 [DOI] [PubMed] [Google Scholar]
- 7.Lee Y-H, Kim SU, Song K, et al. Sarcopenia is associated with significant liver fibrosis independently of obesity and insulin resistance in nonalcoholic fatty liver disease: nationwide surveys (KNHANES 2008-2011). Hepatology. 2016;63:776–786. doi: 10.1002/hep.28376 [DOI] [PubMed] [Google Scholar]
- 8.Kim TN, Park MS, KIl L, et al. Skeletal muscle mass to visceral fat area ratio is associated with metabolic syndrome and arterial stiffness: the Korean Sarcopenic Obesity Study (KSOS). Diabetes Res Clin Pract. 2011;93:285–295. doi: 10.1016/j.diabres.2011.06.013 [DOI] [PubMed] [Google Scholar]
- 9.Shida T, Akiyama K, Oh S, et al. Skeletal muscle mass to visceral fat area ratio is an important determinant affecting hepatic conditions of nonalcoholic fatty liver disease. J Gastroenterol. 2018;53:535–547. doi: 10.1007/s00535-017-1377-3 [DOI] [PubMed] [Google Scholar]
- 10.Farrell GC, Chitturi S, Lau GK, et al. Guidelines for the assessment and management of non-alcoholic fatty liver disease in the Asia-Pacific region: executive summary. J Gastroenterol Hepatol. 2007;22:775–777. doi: 10.1111/j.1440-1746.2007.05002.x [DOI] [PubMed] [Google Scholar]
- 11.Oh S, Shida T, Yamagishi K, et al. Moderate to vigorous physical activity volume is an important factor for managing nonalcoholic fatty liver disease: a retrospective study. Hepatology. 2015;61:1205–1215. doi: 10.1002/hep.27544 [DOI] [PubMed] [Google Scholar]
- 12.Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. [DOI] [PubMed] [Google Scholar]
- 13.Angulo P, Hui JM, Marchesini G, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology. 2007;45:846–854. doi: 10.1002/hep.21496 [DOI] [PubMed] [Google Scholar]
- 14.Vallet-Pichard A, Mallet V, Nalpas B, et al. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. Comparison with liver biopsy and fibrotest. Hepatology. 2007;46:32–36. doi: 10.1002/hep.21669 [DOI] [PubMed] [Google Scholar]
- 15.Saadeh S, Younossi ZM, Remer EM, et al. The utility of radiological imaging in nonalcoholic fatty liver disease. Gastroenterology. 2002;123:745–750. [DOI] [PubMed] [Google Scholar]
- 16.Sandrin L, Fourquet B, Hasquenoph JM, et al. Transient elastography: a new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med Biol. 2003;29:1705–1713. [DOI] [PubMed] [Google Scholar]
- 17.Castera L, Forns X, Alberti A. Non-invasive evaluation of liver fibrosis using transient elastography. J Hepatol. 2008;48:835–847. doi: 10.1016/j.jhep.2008.02.008 [DOI] [PubMed] [Google Scholar]
- 18.Sasso M, Beaugrand M, de Ledinghen V, et al. Controlled attenuation parameter (CAP): a novel VCTE guided ultrasonic attenuation measurement for the evaluation of hepatic steatosis: preliminary study and validation in a cohort of patients with chronic liver disease from various causes. Ultrasound Med Biol. 2010;36:1825–1835. doi: 10.1016/j.ultrasmedbio.2010.07.005 [DOI] [PubMed] [Google Scholar]
- 19.Ogawa H, Fujitani K, Tsujinaka T, et al. InBody 720 as a new method of evaluating visceral obesity. Hepatogastroenterology. 2011;58:42–44. [PubMed] [Google Scholar]
- 20.Tilg H, Moschen AR. Evolution of inflammation in nonalcoholic fatty liver disease: the multiple parallel hits hypothesis. Hepatology. 2010;52:1836–1846. doi: 10.1002/hep.24001 [DOI] [PubMed] [Google Scholar]
- 21.Mueller S, Sandrin L. Liver stiffness: a novel parameter for the diagnosis of liver disease. Hepatic Med. 2010;2:19–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stefan N, Häring HU, Cusi K. Non-alcoholic fatty liver disease: causes, diagnosis, cardiometabolic consequences, and treatment strategies. Lancet Diabetes Endocrinol. 2018. doi: 10.1016/S2213-8587(18)30154-2 [DOI] [PubMed] [Google Scholar]
- 23.Abbatecola AM, Ferrucci L, Ceda G, et al. Insulin resistance and muscle strength in older persons. J Gerontol A Biol Sci-Med Sci. 2005;60A:1278–1282. doi: 10.1093/gerona/60.10.1278 [DOI] [PubMed] [Google Scholar]
- 24.Cesari M, Kritchevsky SB, Baumgartner RN, et al. Sarcopenia, obesity, and inflammation —results from the trial of angiotensin converting enzyme inhibition and novel cardiovascular risk factors study. Am J Clin Nutr. 2005;82:428–434. doi: 10.1093/ajcn.82.2.428 [DOI] [PubMed] [Google Scholar]
- 25.Schrager ME, Metter EJ, Simonsick E, et al. Sarcopenic obesity and inflammation in the In CHIANTI study. J Appl Physiol. 2007;102:919–925. doi: 10.1152/japplphysiol.00627.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bonala S, McFarlane C, Ang J, et al. Pid1 induces insulin resistance in both human and mouse skeletal muscle during obesity. Mol Endocrinol. 2013;27:1518–1535. doi: 10.1210/me.2013-1048 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 27.Zhang C, McFarlane C, Lokireddy S, et al. Myostatin-deficient mice exhibit reduced insulin resistance through activating the AMP-activated protein kinase signalling pathway. Diabetologia. 2011;54:1491–1501. doi: 10.1007/s00125-010-2040-1 [DOI] [PubMed] [Google Scholar]
- 28.Merli M, Dasarathy S. Sarcopenia in non-alcoholic fatty liver disease: targeting the real culprit? J Hepatol. 2015;63:309–311. doi: 10.1016/j.jhep.2015.05.014 [DOI] [PubMed] [Google Scholar]
- 29.Garcia PS, Cabbabe A, Kambadur R, Nicholas G, Csete M. Elevated myostatin levels in patients with liver disease: a potential contributor to skeletal muscle wasting. Anesthesia Analg. 2010;111:707–709. doi: 10.1213/ANE.0b013e3181eac1c9 [DOI] [PubMed] [Google Scholar]
- 30.Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology. 2012;142:1592–1609. doi: 10.1053/j.gastro.2012.04.001 [DOI] [PubMed] [Google Scholar]
- 31.Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Non- alcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116:1413–1419. doi: 10.1016/S0016-5085(99)70506-8 [DOI] [PubMed] [Google Scholar]
- 32.Singh S, Allen AM, Wang Z, Prokop LJ, Murad MH, Loomba R. Fibrosis progression in nonalcoholic fatty liver vs nonalcoholic steatohepatitis: a systematic review and meta-analysis of paired-biopsy studies. Clin Gastroenterol Hepatol. 2015;13:643–654. doi: 10.1016/j.cgh.2014.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Angulo P, Kleiner DE, Dam-Larsen S, et al. Liver fibrosis, but no other histologic features, is associated with long- term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology. 2015;149:389–397. doi: 10.1053/j.gastro.2015.04.043 [DOI] [PMC free article] [PubMed] [Google Scholar]