Abstract
Aims: Extrahepatic cholangiocarcinoma (EHCC) is a highly malignant tumor with poor prognosis and intrinsic resistance to cytotoxic agents. The molecular mechanisms associated with high malignancy and resistance to chemotherapy and radiotherapy have not been fully elucidated. This study investigated the clinicopathological significances of FOXP1 and FOXO3a expression in EHCC.
Methods: We assayed FOXP1 and FOXO3a expressions in 100 EHCC, 30 peritumoral tissues, 10 adenoma and 15 normal biliary tract tissues using EnVision immunohistochemistry.
Results: The positive rates of FOXP1 and FOXO3a proteins were significantly lower in EHCC tumors than in peritumoral tissues, adenoma, and normal bile tract tissues (P<0.05 or P<0.01). Adenoma and pericancerous tissues with negative FOXP1 and/or FOXO3a protein expressions exhibited atypical hyperplasia. The positive correlation was established between the expression of FOXP1 and FOXO3a in EHCC (P<0.01). The positive rates of FOXP1 and FOXO3a expression were significantly higher in cases with well differentiation, no metastasis in lymph node, no invasion to surrounding tissues and organs, TNM I + II stage and radical resection (p<0.05 or p<0.01). Kaplan-Meier survival analysis showed that EHCC patients with positive FOXP1 and FOXO3a expression survived significantly higher than patients with negative FOXP1 and FOXO3a expression, respectively (P<0.001). Cox multivariate analysis revealed that negative FOXP1 or FOXO3a expressions were independent poor prognostic factors in EHCC patients. The AUCs for FOXP1 and FOXO3a were 0.676 (95% CI: 0.589–0.763, P<0.001) and 0.652 (95% CI: 0.563–741, P=0.002), respectively.
Conclusion: The present study indicates that negative FOXP1 and FOXO3a expressions are closely associated with the pathogenesis, clinical, pathological and biological behaviors, and poor prognosis in EHCC.
Keywords: extrahepatic cholangiocarcinoma, biliary tract adenoma, FOXP1, FOXO3a, immunohistochemistry
Introduction
Cholangiocarcinoma(CCA)occurring at every point of the biliary tree, is the second most frequent type of primary liver cancer and represents about 3% of all gastrointestinal tumor.1 CCAs are classified as intrahepatic CCA (iCCA) and extrahepatic CCA (eCCA or EHCC) which further divided into perihilar CCA (pCCA), also named Klatskin tumor, and distal CCA (dCCA).2 Although CCAs encompass a heterogenous group of tumors based on anatomic location, they commonly exhibit a dismal prognosis with an overall 5-year survival of <5%.3 The incidence rate of EHCC increased from 2001 to 2007 derived from a report of the International Classification of Diseases for Oncology.4 Until currently, the only curative treatment of EHCC was surgical resection or liver transplantation; overall 5-year survival rates after surgery were 20–30% and 27% for patients with pCCA and dCCA, respectively.5,6 For more than second-third of patients who were not candidates for surgery, systemic chemotherapy was routinely considered. However, the curative effect of chemotherapy was far from satisfaction, as the median progression-free survival was less than 11.7 months.7 Once progressing, recurring or relapsing bile duct cancer occur, the prognosis is very poor. Thus, it is therefore of remarkable importance to assess patients with EHCC and grade the risk for a poor outcome. Several patient-related and clinical factors, such as age, presence of biliary stones, chronic infection (liver flukes, hepatitis B virus and hepatitis C virus), inflammatory bowel disease, cirrhosis, and primary sclerosing cholangitis, are associated with the initiation, development and progression of CCA.8,9 However, these factors are lack of precision to tailor surveillance and anticipate prognosis accurately.
Pathological analysis is essential for confirming a diagnosis for CCA. Histologically, EHCC was divided into grades of well, moderately, poorly, and undifferentiated carcinoma. Accompanied by other pathological characteristics, such as lymph node metastasis, periductal tumor involvement and organ invasion, those are considered as independent prognostic factors. In patients who are not proceeding to surgical resection, it is particularly important to obtain positive pathological findings, contributing to treatment selection, clinical trials and predict prognosis. However, except in cases where surgery can be conducted, it is not easy to sample enough tissue for pathological documentation of EHCC. Positive cytology from brushings at ERCP/PTC or combined with biopsy is less than 70% for CCA.9 Thus, new exploration to reveal valuable protein biomarkers intimately associated with the pathological characteristics of EHCC is urgently needed.
FOXP1 (forkhead box protein P1) is a member of the FOXP subfamily. Previous studies have shown that FOXP1 may not only be a potential factor for predicting prognosis, but also used to develop FOXP1-directed therapeutic strategies.10 Current evidence indicated a heterogenous expression and divergent function of FOXP1 in various cancers. In non-small cell lung cancer (NSCLC), the expression of FOXP1 in tumoral tissues was significantly higher than that of corresponding peritumoral tissues, which was confirmed by RT-PCR and immunohistochemical analysis.11 Similarly, FOXP1 mRNA and protein was also more enriched in hepatocellular carcinoma (HCC) cells compared to normal hepatic cells.12 Reversely, a relatively low expression was observed in ovarian tumor tissues compared to normal ovarian tissue.13 As for the function of FOXP1, it was considered as a tumor suppressor, as it could inhibit cell proliferation and migration in colorectal and prostate cancer cells.14,15 In addition, it could also inhibit inflammatory reaction in colorectal cancer.14 When examine how valuable of FOXP1 as a prognosis factor, multiple lines of evidence showed attractive results. The level of FOXP1 expression is an independent prognosis factor for breast cancer, NSCLC, HCC, prostate cancer and ovarian cancer.11,13,15,16 Nevertheless, whether FOXP1 could function as a reliable prognosis factor in EHCC remains elusive.
Apart from FOXP1, another forkhead box (FOX) family member also attracts attention, that is FOXO3. FOX family which includes 19 sub-families of transcription factors, shares a highly conserved DNA-binding domain, the forkhead box domain (also known as the winged-helix domain). Within this family, the O subgroup contains four members: FOXO1 (FKHR), FOXO3 (FKHRL1), FOXO4 (AFX) and FOXO6.17 FOXO3 serves as a checkpoint that promotes cell cycle arrest and apoptosis, thus recognized as a tumor suppressor. Notably, FOXO contributes to the intrinsic feedback regulation of PI3K-AKT signaling and maintains activity of survival pathways in cancers.18,19 Along the same line, FOXO3a implements a process to detoxify and repair therapy-induced genotoxic stress which benefits developing drug resistance in cancers.20,21 In addition, evidence also suggested that FOXO3a has a pro-metastatic role in colorectal cancer through regulation of metastasis relevant genes.22 Regarding the differential expression throughout different tissues and multiple functions of FOXO3a in tumorigenesis and progression, it is noteworthy in understanding the expression of FOXO3a in EHCC tissues. As FOXO3a is aberrantly upregulated in drug-resistant and metastatic cells, it is suspected that malignant tissues with high expression of FOXO3a might indicate high malignancy and dismal prognosis.
Since the role of FOXP1 and FOXO3a in EHCC remains to be clarified and to gain further insight into the clinical significance of them, we evaluated FOXP1 and FOXO3a expression using immunohistochemistry in surgically resected specimens, which include EHCC, peritumoral tissues, adenoma and normal biliary tract. The clinicopathological significance and prognostic values of FOXP1 and FOXO3a expressions were analyzed.
Material and methods
Case selection
The present retrospective study was approved by the Ethics Committee for Human Research, Central South University, and was conducted according to the approved guidelines. The patients whose tissues were used provided written informed consent, in accordance with the Declaration of Helsinki. One hundred EHCC, thirty peritumoral tissues, ten bile tract adenoma, and fifteen normal biliary tissues were obtained at the Second Xiangya Hospital and the Third Xiangya Hospital, Central South University from January 2001 to December 2014. We confirm that all the donors of normal biliary tract tissues were voluntary organ donors, strictly according to civilian laws. All specimens obtained from the patients were histologically confirmed by two pathologists. Tumours were restaged according to the 7th TNM Classification of Malignant Tumours and classified following the World Health Organization (WHO) tumour classification system. Tumor differentiation degrees were defined according to the World Health Organization criteria (well differentiated, moderately differentiated and poorly differentiated).
Clinicopathological data for EHCC is summarized in Table 2. Among the 100 EHCC samples, 61 were from male patients and 39 were female (M/F =1.56) and patient ages ranged from 35 to 80 (58.8±10.2) years. Of the 100 EHCCs, 31 were well-differentiated (31.0%), 34 were moderately differentiated (34.0%) and 35 were poorly differentiated (35.0%). Among the 100 patients with EHCC, invasion of region tissues and/or organs was found in 67 (67.0%); 38 (38.0%) had regional lymph node metastasis; and 31 (31.0%) had gallstones. According to TNM staging, 35 of the 100 EHCCs were stage I+Ⅱ, 38 were stage Ⅲ and 27 were stage IV. Surgery included radical resection for 54 (54.0%), palliative resection for 36 (36.0%) and 10 only for biopsy (10.0%). Survival data for the 100 patients with EHCC was obtained through letters and/or telephone calls. The follow-up time was 30 months, and patients who survived longer than 30 months were included in the analysis as censored cases. Of the 100 EHCC patients, fifty-nine patients died within twelve months, twenty-four patients died within twenty-four months, ten patients died within thirty months, and patients (twelve cases) who survived longer than thirty months were included in the analysis as censored cases.
Table 2.
Correlations of FOXP1 and FOXO3a protein expression with the clinicopathological characteristics of EHCC
| CPC | Case No. | FOXP1 | FOXO3a | ||||
|---|---|---|---|---|---|---|---|
| Pos No. (%) | χ 2 | P-value | Pos No. (%) | χ 2 | P-value | ||
| Age (year) | |||||||
| ≤45 years | 17 | 10 (58.8) | 2.092 | 0.148 | 10 (58.8) | 1.356 | 0.244 |
| >45 years | 83 | 33 (39.8) | 36 (43.4) | ||||
| Sex | |||||||
| Male | 61 | 26 (42.6) | 0.009 | 0.924 | 30 (49.2) | 0.637 | 0.425 |
| Female | 39 | 17 (43.6) | 16 (41.0) | ||||
| Differentiation | |||||||
| Well | 31 | 21 (67.7) | 15.314 | 0.000 | 20 (64.5) | 8.574 | 0.014 |
| Moderately | 34 | 15 (44.1) | 16 (47.1) | ||||
| Poorly | 35 | 7 (20.0) | 10 (28.6) | ||||
| Tumor size | |||||||
| ≤3cm | 62 | 30 (58.4) | 1.932 | 0.165 | 32 (51.6) | 2.069 | 0.150 |
| >3cm | 38 | 13 (34.2) | 14 (36.8) | ||||
| Tumor position | |||||||
| Hilar site | 27 | 11 (40.7) | 0.690 | 0.708 | 8 (29.6) | 3.998 | 0.136 |
| Hepatic duct | 4 | 1 (25.0) | 2 (50.0) | ||||
| Distal duct | 69 | 31 (44.9) | 36 (52.2) | ||||
| Biliary stone | |||||||
| No | 69 | 35 (50.7) | 5.419 | 0.020 | 33 (47.8) | 0.299 | 0.585 |
| Yes | 31 | 8 (25.8) | 13 (41.9) | ||||
| Lymph node metastasis | |||||||
| No | 62 | 38 (61.3) | 22.269 | 0.000 | 37 (59.7) | 12.288 | 0.000 |
| Yes | 38 | 5 (13.2) | 9 (23.7) | ||||
| Invasion | |||||||
| No | 33 | 23 (69.7) | 14.323 | 0.000 | 22 (66.7) | 8.469 | 0.004 |
| Yes | 67 | 20 (29.9) | 24 (35.8) | ||||
| TNM stage | |||||||
| I+II | 35 | 27 (77.1) | 29.046 | 0.000 | 24 (68.6) | 12.210 | 0.002 |
| III | 38 | 13 (34.2) | 15 (39.5) | ||||
| IV | 27 | 3 (11.1) | 7 (25.9) | ||||
| Surgery | |||||||
| Radical | 54 | 34 (63.0) | 19.655 | 0.000 | 35 (64.8) | 16.808 | 0.000 |
| Palliative | 36 | 6 (16.9) | 9 (25.0) | ||||
| Biopsy | 10 | 3 (30.0) | 2 (20.0) | ||||
Abbreviations: CPC, clinicopathological characteristics; EHCC, extrahepatic cholangiocarcinoma; Pos, positive.
Thirty peritumoral tissues were collected from twenty male (66.6%) and patient ages ranged from 35 to 72 (48.5±9.2) years. The pathological examination showed 12 normal tissues, 8 mild dysplasia, 6 moderately dysplasia and four severe dysplasia. Ten bile tract adenoma tissues collected from six male (66.6%) and patient ages ranged from 33 to 70 (46.7±10.2) years. The pathological examination showed 6 simple adenoma tissues, 2 mild dysplasia and 2 moderate to severe dysplasia. Fifteen normal biliary tract tissues were collected from contributors of liver transplantion and pathological examination being normal billary tract tissues.
All tissues were treated with 4% formaldehyde for 24 to 48 hrs, and then were routinely embedded in paraffin.
Immunohistochemistry
Rabbit anti-human FOXP1 and FOXO3a polyclonal antibody were purchased from Dako Corporation (Carpentaria, CA, USA). EnVisionTM Detection Kit was purchased from Dako Laboratories (CA, USA). Positive controls were provided with the EnVisionTM Detection Kit. EnVision immunohistochemistry of FOXP1 and FOXO3a was performed by following the user manual. Briefly, 4 μM-thick sections were cut from paraffin-embedded tissues. The sections were deparaffinized and then incubated with 3% H2O2 in the dark for 15 min. The heat-induced epitope retrieval was conducted with sodium citrate buffer (10 mM Sodium citrate, 0.05% Tween 20, pH 6.0) at 96 ℃ for 30 min. The sections were incubated with rabbit anti-human FOXP1 and FOXO3a primary antibody (1:100 dilution) for 2 hrs after they were soaked in PBS for 3×5 min. The sections were incubated with several drops of Solution A (ChemMateTMEnVison + /HRP) for 30 min followed by DAB staining and haematoxylin counter-staining. The sections were dehydrated, soaked in xylene, and mounted with neutral balsam. Five hundred cells from ten random fields were examined per section by 2 observers independently. An average of the percentages from these two observers was used for final evaluation. Cases with positive cells ≥25% were considered positive whereas other cases were considered negative.
Statistical analysis
Data was analyzed using the SPSS 17.0 (statistical package for the Social Sciences, Version 17.0). The inter-relationship of FOXP1 and FOXO3a with histological or clinical factors was analyzed using χ2 test or Fisher’s exact test, as appropriate. The overall survival of patients with EHCC was analyzed using Kaplan-Meier univariate survival analysis and log-rank tests. Multivariate analysis was performed with Cox proportional hazards model and the 95% confidence interval was calculated. p<0.05 was considered statistically significant.
Results
FOXP1 and FOXO3a protein expression in EHCC, peritumoral tissues, adenoma, and normal tissues
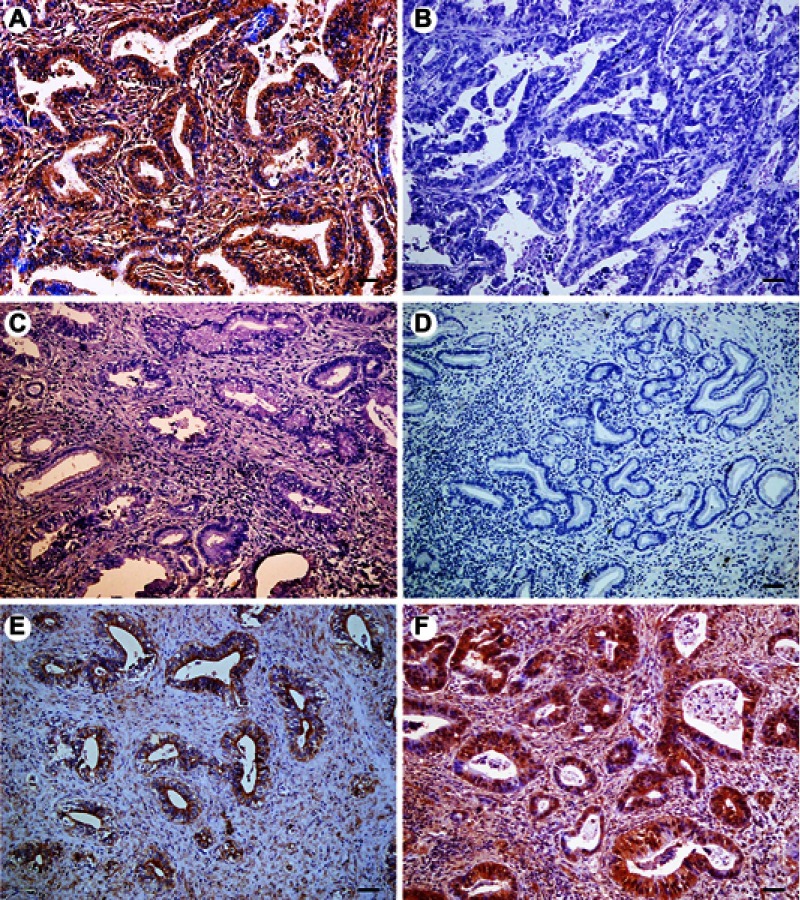
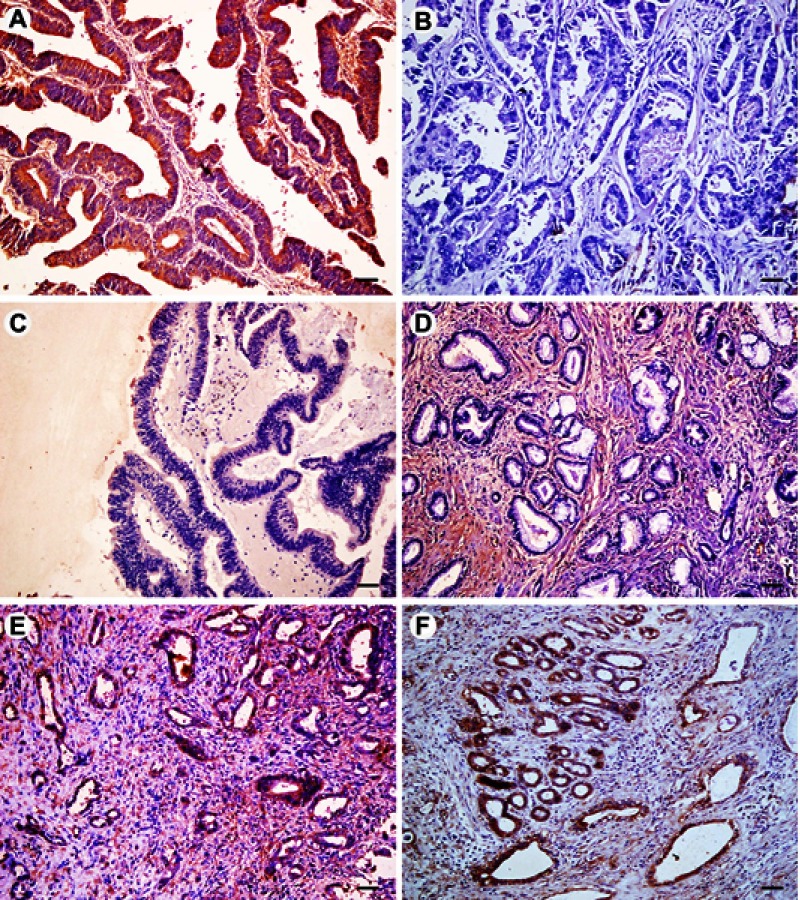
The expression of FOXP1 and FOXO3a proteins was evaluated by immunohistochemistry in 100 EHCCs, 30 peritumoral tissues, 10 adenomas, and 15 normal tissues. Immunohistochemical staining showed that positive FOXP1 and FOXO3a expression was located on the cytoplasm and nuclear (representative microphotographs were showed in Figure 1A,B and Figure 2A,B, respectively). In EHCCs, 43 and 46 was positive for FOXP1 (43.0%) and FOXO3a (46.0%), respectively. While many stromal cells were positively stained in EHCC tissues. In peritumoral tissues, positive expression of FOXP1 and FOXO3a was both found in 20 of 30 cases (66.7%), representative pictures showed in Figure 1C,E, and Figure 2C,E, respectively. In adenomas, both 8 cases of 10 were positive staining for FOXP1 (80.0%) and FOXO3a (80.0%), representative pictures showed in Figure 1D,F, and Figure 2D,F, respectively. Fifteen normal tissues were all positive for FOXP1 and FOXO3a expressions. The positive rates of FOXP1 or FOXO3a in EHCC were significantly lower than that in peritumoral, adenoma, and normal tissues (P<0.05 or P<0.01) (Table 1). Furthermore, peritumoral tissues and adenoma with negative expression of FOXP1 or negative FOXO3a expression exhibited moderate to severe dysplasia.
Figure 1.
Immunohistochemical expression of FOXP1 in extrahepatic cholangiocarcinoma (EHCC), peritumoral tissues, adenoma, and normal tissues. All results are displayed by ×200 magnification. (A) Positive expression of FOXP1 in well differentiated EHCC. (B) Negative expression of FOXP1 in poorly differentiated EHCC. (C) Negative expression of FOXP1 in peritumoral tissues. (D) Negative expression of FOXP1 in adenoma. (E) Positive expression of FOXP1 in peritumoral tissues. (F) Positive expression of FOXP1 in adenoma. Scale bars correspond to 50 μm.
Figure 2.
Immunohistochemical expression of FOXO3a in extrahepatic cholangiocarcinoma (EHCC), peritumoral tissues, adenoma, and normal tissues. All results are displayed by ×200 magnification. (A) Positive expression of FOXO3a in well differentiated EHCC. (B) Negative expression of FOXO3a in moderately differentiated EHCC. (C) Negative expression of FOXO3a in peritumoral tissues. (D) Negative expression of FOXO3a in adenoma. (E) Positive expression of FOXO3a in peritumoral tissues. (F) Positive expression of FOXO3a in adenoma. Scale bars correspond to 50 μm.
Table 1.
Expression of FOXP1 and FOXO3a in normal tissue, adenoma, peritumoral tissue and EHCC
| Tissue types | Case Number | FOXP1 positive (%) | FOXO3a positive (%) |
|---|---|---|---|
| EHCC | 100 | 43 (43.0) | 46 (47.0) |
| Peritumoral tissue | 30 | 20 (66.7) * | 20 (66.7) * |
| Adenoma | 10 | 8 (80.0) * | 8 (80.0) * |
| Normal tissue | 15 | 15 (100.0) ** | 15 (100.0) ** |
Notes: *Compared to EHCC, P<0.05;**compared to EHCC, P<0.01.
Abbreviation: EHCC, extrahepatic cholangiocarcinoma.
FOXP1 and FOXO3a protein expressions were associated with clinicopathological characteristics of EHCC
We further evaluated potential association between positive expression of FOXP1 or FOXO3a proteins and clinicopathological data for the included EHCC cases (Table 2). Positive expressions of FOXP1 and FOXO3a were significantly higher in cases with well differentiation compared to cases with poor differentiation (P=P<0.001 and 0.014, respectively). The expressions of FOXP1 and FOXO3a were significantly correlated with lymph node metastasis (both P<0.001) and high expressions of them were found in cases with no lymph node metastasis. Similarly, in cases with no invasion to surrounding tissues and organs, positive expression of FOXP1 and FOXO3a were significantly higher than that of cases with invasion occurring (both P<0.001). The expressions of FOXP1 and FOXO3a were also associated with TNM stage (P<0.001) and surgical modality (P<0.001). Cases with TNM I+II and radical surgery showed high levels of both proteins compared to that in cases with TNM III or IV and biopsy. Furthermore, our data showed that the expressions of FOXP1 and FOXO3a exhibited no significant association with sex, age, biliary stone, tumor site and tumor diameter (p>0.05). To examine the correlation between FOXP1 and FOXO3a expression in EHCC, χ 2 test was also employed. Among the 43 cases with positive FOXP1 expression, 30 cases had positive FOXO3a expression. Among the 57 cases with negative FOXP1 expression, 41 cases had negative FOXO3a expression. The expression of FOXP1 was positively correlated with FOXO3a in EHCC (χ 2=15.702, P= P<0.001).
FOXP1 and FOXO3a protein expressions correlated with overall survival in patients with EHCC
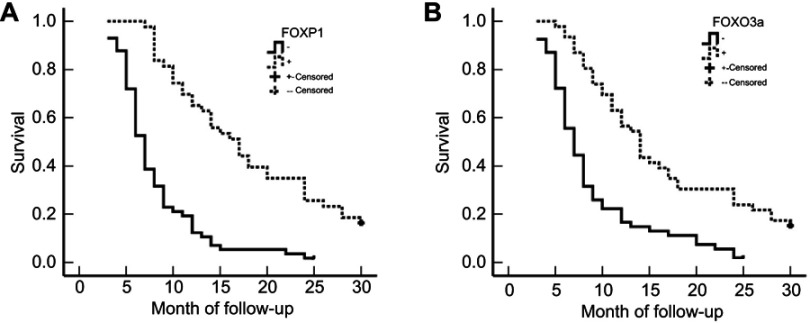
Survival information of included patients with EHCC was collected and listed in section of CASE SELCTION. Briefly, 59 patients died within 12 months; 24 patients died within 24 months; 10 patients died within 30 months, and patients (12 cases) who survived longer than 30 months in this analysis were marked as censored cases. We first examined which clinicopathological characteristics correlated to the survival of EHCC patients (Table 3). Kaplan-Meier survival analysis revealed that several factors were significantly associated with the average overall survival time of patients with EHCC, those including the differentiation (P=0.001), lymph node metastasis (P=0.011), invasion (P=0.025), TNM stage (P=0.001) and surgical modality (P=0.008). Average overall survival time for FOXP1 or FOXO3a positive patients was significantly longer than those with negative FOXP1 or FOXO3a expression (FOXP1: positive vs negative, 17.93 months vs 8.14 months, P= P<0.001; FOXO3a: positive vs negative, 16.44 months vs 8.87 months, P= P<0.001). The results were also presented in Table 3 and Figure 3.
Table 3.
Correlations of clinicopathological characteristics, FOXP1 and FOXO3a expression with the mean survival in patients with EHCC
| Group | Case No. (n) | Mean survival (month) | Chi-square | P-value |
|---|---|---|---|---|
| Sex | ||||
| Male | 61 | 12.67 (3–30) | 0.001 | 0.980 |
| Female | 39 | 12.59 (4–30) | ||
| Age (year) | ||||
| ≤45 | 17 | 13.82 (3–30) | 0.667 | 0.414 |
| >45 | 83 | 12.20 (3–30) | ||
| Differentiation | ||||
| Well | 31 | 18.46 (5–30) | 27.655 | 0.000 |
| Moderately | 34 | 11.41 (3–30) | ||
| Poorly | 35 | 7.97 (3–30) | ||
| Tumor size | ||||
| ≤3cm | 62 | 12.62 (3–30) | 0.235 | 0.628 |
| >3cm | 38 | 12.03 (3–30) | ||
| TNM stage | ||||
| I+II | 35 | 18.57 (7–30) | 57.569 | 0.000 |
| III | 38 | 11.05 (3–30) | ||
| IV | 27 | 6.26 (3–13) | ||
| Lymph node metastasis | ||||
| No | 62 | 15.52 (4–30) | 39.001 | 0.000 |
| Yes | 38 | 7.18 (3–25) | ||
| Invasion | ||||
| No | 33 | 17.52 (4–30) | 17.399 | 0.000 |
| Yes | 67 | 9.87 (3–30) | ||
| Surgery | ||||
| Radical | 54 | 16.62 (3–30) | 48.388 | 0.000 |
| Palliative | 36 | 7.58 (4–24) | ||
| Biopsy | 10 | 6.90 (3–14) | ||
| FOXP1 | ||||
| - | 57 | 8.14 (3–25) | 41.286 | 0.000 |
| + | 43 | 17.93 (7–30) | ||
| FOXO3a | ||||
| - | 54 | 8.87 (3–25) | 24.908 | 0.000 |
| + | 36 | 16.44 (5–30) |
Figure 3.
Association between survival and expression of FOXP1 and FOXO3a in patients with extrahepatic cholangiocarcinoma (EHCC). (A) Kaplan–Meier plots of overall survival in patients with FOXP1 positive and negative tumors. (B) Kaplan–Meier plots of overall survival in patients with FOXO3a positive and negative tumors.
Multivariate analysis
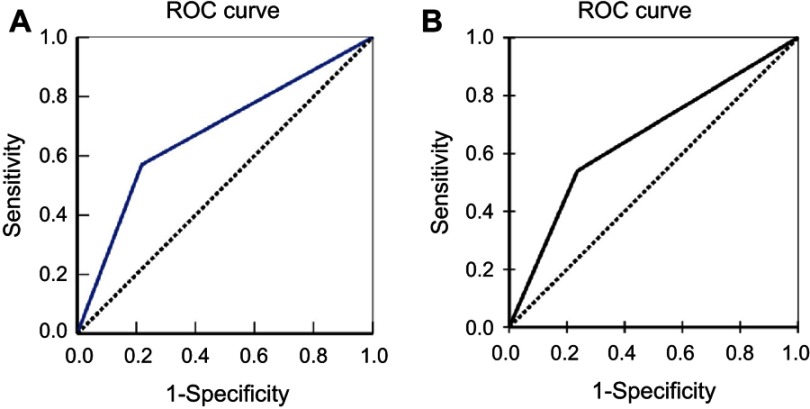
Cox multivariate analysis showed that poor differentiation, lymph node metastasis, invasion, and high TNM stage (III or IV) negatively correlated with overall survival and positively correlated with mortality (Table 4). Furthermore, high levels of FOXP1 and FOXO3a expression positively correlated with overall survival and negatively correlated with mortality (Table 4). Positive expressions of FOXP1 and FOXO3a were both independent prognostic factors. Finally, we calculated the AUC for FOXP1 expression (AUC=0.676, 95% CI: 0.589–0.763, P= P<0.001), and FOXO3a expression (AUC =0.652, 95% CI: 0.563–741, P=0.002), presented in Figure 4.
Table 4.
Multivariate Cox regression analysis of survival rate in patients with EHCC
| Groups | Factors | β | SE | Wald | P | RR | 95% CI | |
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | |||||||
| Differentiated degree | Well/moderately/poorly | 0.529 | 0.152 | 12.112 | 0.001 | 1.697 | 1.260 | 2.286 |
| Tumor size | ≤3cm/>3cm | 0.421 | 0.210 | 4.019 | 0.045 | 1.523 | 1.009 | 2.299 |
| Lymph node metastasis | No/yes | 0.720 | 0.283 | 6.473 | 0.011 | 2.054 | 1.180 | 3.578 |
| Invasion | No/yes | 0.804 | 0.359 | 5.016 | 0.025 | 2.234 | 1.106 | 4.516 |
| TNM stage | I/II/III/IV | 0.791 | 0.246 | 10.339 | 0.001 | 2.206 | 1.362 | 3.572 |
| Surgery | Radical/Palliative/Biopsy | 0.505 | 0.190 | 7.064 | 0.008 | 1.657 | 1.142 | 2.405 |
| FOXP1 | -/+ | -0.752 | 0.281 | 7.162 | 0.007 | 0.471 | 0.272 | 0.818 |
| FOXO3a | -/+ | -0.590 | 0.252 | 5.482 | 0.019 | 0.554 | 0.338 | 0.908 |
Abbreviations: β, regression coefficients; EHCC, extrahepatic cholangiocarcinoma; SE, standard error; RR, Relative risk.
Figure 4.
Multivariate analysis. ROC of Diagonal segments was produced by ties of FOXP1 (A) and FOXO3a (B) in extrahepatic cholangiocarcinoma.
Discussion and conclusion
The expressions of FOXP1 and FOXO3a in EHCC have not been previously reported, although their expressions have been associated with the progression and prognosis in a variety of cancers. Our study investigated the protein expression of FOXP1 and FOXO3a in EHCC, peritumoral tissues, adenoma, and normal biliary tract using immunohistochemistry. A significant decrease of FOXP1 and FOXO3a expression in EHCC was observed, and highly positive staining was observed in stromal cells. Negative FOXP1 and FOXO3a expressions were both associated with poor differentiation, high TNM stages, increasing invasion and lymph node metastasis, as well as poor prognosis in EHCC.
FOXP1 was initially investigated in the development of B cell, where pro-B cell stage was blocked during mature procedure in bone marrow if FOXP1 was artificially interfered.10 Studies revealing a common genetic event that loss of heterozygosity of chromosome 3p was found in multiple cancers have led to explore its role in tumorigenesis and development.10 Based on RNA and protein expression analysis, several reports show a significantly lower expression of FOXP1 in malignant tissues compared to normal ones, which include gastrointestinal cancers, lung cancer, head and neck cancers, breast cancer, endometrial cancer and ovarian cancer.23–25 Targeted deletion of FOXP1 leads to increased frequency of tumor formation in mice, indicating a role of tumor suppressor in cancer. However, several lines of evidence reversely suggest an oncogenic role of FOXP1 in various cancers. For instance, B-cell tumors especially ABC-diffuse large B-cell lymphoma express enhanced level of FOXP1, and furthermore, its strong nuclear expression is positively correlated with a worse prognosis.26 In hepatocellular carcinoma, high expression of FOXP1 indicates large tumor diameter, later TNM stage and poor prognosis.27 As the first analysis of FOXP1 expression in EHCC, we find a significantly decreased level of it compared to normal tissues. The negative correlation between FOXP1 expression and pathological features, and prognosis suggested that FOXP1 may function as a tumor suppressor in EHCC. It was reported that elevated expression of FOXP1 would lead to inhibit tumor growth. More studies are needed to further illuminate the potential molecular mechanism of FOXP1 in EHCC.10
FOXO transcription factors are involved in multiple physiological and pathological processes, including apoptosis, aging, proliferation, metabolism, immunity, and tumorigenesis.28,29 We focus our attention on FOXO3, a member of the FOXO family of transcription factors.30,31 Previous studies showed that FOXO3a was a suppressor of primary tumor growth and was negatively regulated by growth factors.28,32 During tumor development, inhibition of the transcriptional activity of FOXO3a promotes cell transformation, tumor progression, and angiogenesis.29,33,34 Reversely, FOXO3a overexpression leads to inhibition of tumor growth and decreasing of tumor size in breast cancer.34 Strikingly, FOXO3a was demonstrated playing an essential role in the control of differentiation and tumorigenicity of glioblastoma cancer stem cells.27 FOXO3a defect led to induce differentiation and reduce tumorigenicity of cancer stem cells. These results indicate a suppressor role of FOXO3a playing in various tumors. Additionally, the aberrant expression of FOXO3a is negatively correlated with the survival for patients with breast cancer, gastric cancer, ovarian cancer, and colon cancer.34–36 Although there is still no evidence concerning the functions of FOXO3a playing in CCA or EHCC, pathological expression of it based on clinical samples could implicate its potential role. Our result suggests a similar expression profile of FOXO3a in EHCC compared with the reported cancer tissues, and depressed expression of it associated with high malignancy based on correlation analysis with pathological features and survival analysis. From the insight of molecular mechanism, FOXO3a was found to be a negative regulator of cell cycle arrest, cell death and invasiveness.20
In the present study, the percentage of cases with negative FOXP1 and FOXO3a expression was significantly larger in EHCC patients with poor differentiation, lymph node metastasis, invasion, and TNM stage III/IV disease than in patients with well differentiation, no lymph node metastasis and invasion, and TNM stage I/II disease (P<0.05 or P<0.01). In biliary tract epithelia, pericancerous tissues and adenoma tissues with negative FOXP1 and/or FOXO3a protein expression exhibited moderate to severe dysplasia. Kaplan-Meier survival analysis showed that EHCC patients with negative FOXP1 and FOXO3a expression survived significantly shorter than patients with positive FOXP1 and FOXO3a expression. Cox multivariate analysis suggested that negative FOXP1 and FOXO3a expressions are independent prognostic factors for poor prognosis in patients with EHCC.The AUC for FOXP1 and FOXO3a showed might have role for carcinogenesis, progression and early finding or prevention of EHCC.
In conclusion, FOXP1 and FOXO3a are involved in the tumorigenesis and progression of EHCC, and negative FOXP1 and FOXO3a expressions were associated with poor prognosis in patients with EHCC.
Disclosure
The authors declared no conflicts of interest existing in this work.
References
- 1.Banales JM, Cardinale V, Carpino G, et al. Expert consensus document: cholangiocarcinoma: current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA). Nat Rev Gastroenterol Hepatol. 2016;13(5):261–280. doi:10.1038/nrgastro.2016 [DOI] [PubMed] [Google Scholar]
- 2.Liang Z, Liu X, Zhang Q, Wang C, Zhao Y. Diagnostic value of microRNAs as biomarkers for cholangiocarcinoma. Digestive Liver Dis. 2016;48(10):1227–1232. doi: 10.1016/j.dld.2016.07.006 [DOI] [PubMed] [Google Scholar]
- 3.Mosconi S, Beretta GD, Labianca R, Zampino MG, Gatta G, Heinemann V. Cholangiocarcinoma. Crit Rev Oncol Hematol. 2009;69(3):259–270. doi: 10.1016/j.critrevonc.2008.09.008 [DOI] [PubMed] [Google Scholar]
- 4.Razumilava N, Gores GJ. Classification, diagnosis, and management of cholangiocarcinoma. Clin Gastroenterol Hepatol. 2013;11(1):13–21.e1. doi: 10.1016/j.cgh.2012.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosen C, Heimbach J, Gores G. Surgery for cholangiocarcinoma: the role of liver transplantation. Hpb. 2008;10(3):186–189. doi: 10.1080/13651820801992542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeOliveira ML, Cunningham SC, Cameron JL, et al. Cholangiocarcinoma: thirty-one-year experience with 564 patients at a single institution. Ann Surg. 2007;245(5):755. doi: 10.1097/01.sla.0000251366.62632.d3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khan SA, Davidson BR, Goldin RD, et al. Guidelines for the diagnosis and treatment of cholangiocarcinoma: an update. Gut. 2012;61(12):1657–1669. doi: 10.1136/gutjnl-2011-301748 [DOI] [PubMed] [Google Scholar]
- 8.Razumilava N, Gores GJ. Cholangiocarcinoma. Lancet. 2014;383(9935):2168–2179. doi: 10.1016/S0140-6736(13)61903-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang CK, Aihara A, Iwagami Y, et al. Expression of transforming growth factor β1 promotes cholangiocarcinoma development and progression. Cancer Lett. 2016;380(1):153–162. doi: 10.1016/j.canlet.2016.05.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koon HB, Ippolito GC, Banham AH, Tucker PW. FOXP1: a potential therapeutic target in cancer. Expert Opin Ther Targets. 2007;11(7):955–965. doi: 10.1517/14728222.11.7.955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ijichi N, Shigekawa T, Ikeda K, et al. Association of double-positive FOXA1 and FOXP1 immunoreactivities with favorable prognosis of tamoxifen-treated breast cancer patients. Hormones Cancer. 2012;3(4):147–159. doi: 10.1007/s12672-012-0111-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Y, Zhang S, Wang X, et al. Prognostic significance of FOXP1 as an oncogene in hepatocellular carcinoma. J Clin Pathol. 2012;65(6):528–533. doi: 10.1136/jclinpath-2011-200547 [DOI] [PubMed] [Google Scholar]
- 13.Hu Z, Zhu L, Gao J, et al. Expression of FOXP1 in epithelial ovarian cancer (EOC) and its correlation with chemotherapy resistance and prognosis. Tumor Biol. 2015;36(9):7269–7275. doi: 10.1007/s13277-015-3383-5 [DOI] [PubMed] [Google Scholar]
- 14.De Smedt L, Palmans S, Govaere O, et al. Expression of FOXP1 and colorectal cancer prognosis. Lab Med. 2015;46(4):299–311. doi: 10.1309/LM7IHV2NJI1PHMXC [DOI] [PubMed] [Google Scholar]
- 15.Takayama KI, Suzuki T, Tsutsumi S, et al. Integrative analysis of FOXP1 function reveals a tumor-suppressive effect in prostate cancer. Mol Endocrinol. 2014;28(12):2012–2024. doi: 10.1210/me.2014-1171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feng J, Zhang X, Zhu H, Wang X, Ni S, Huang J. High expression of FoxP1 is associated with improved survival in patients with non–small cell lung cancer. Am J Clin Pathol. 2012;138(2):230–235. doi: 10.1309/AJCPDHQFNYJZ01YG [DOI] [PubMed] [Google Scholar]
- 17.Fu Z, Tindall D. FOXOs, cancer and regulation of apoptosis. Oncogene. 2008;27(16):2312. doi: 10.1038/onc.2008.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Y, Gan B, Liu D, Paik JH. FoxO family members in cancer. Cancer Biol Ther. 2011;12(4):253–259. doi: 10.4161/cbt.12.4.15954 [DOI] [PubMed] [Google Scholar]
- 19.Kelly-Spratt K, Philipp-Staheli J, Gurley KE, Hoon-Kim K, Knoblaugh S, Kemp CJ. Inhibition of PI-3K restores nuclear p27 Kip1 expression in a mouse model of Kras-driven lung cancer. Oncogene. 2009;28(41):3652. doi: 10.1038/onc.2009.226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Brachène AC, Demoulin JB. FOXO transcription factors in cancer development and therapy. Cell Mol Life Sci. 2016;73(6):1159–1172. doi: 10.1007/s00018-015-2112-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prasad SB, Yadav SS, Das M, et al. Down regulation of FOXO1 promotes cell proliferation in cervical cancer. J Cancer. 2014;5(8):655. doi: 10.7150/jca.6554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tenbaum SP, Ordóñez-Morán P, Puig I, et al. β-catenin confers resistance to PI3K and AKT inhibitors and subverts FOXO3a to promote metastasis in colon cancer. Nat Med. 2012;18(6):892. doi: 10.1038/nm.2772 [DOI] [PubMed] [Google Scholar]
- 23.Banham AH, Beasley N, Campo E, et al. The FOXP1 winged helix transcription factor is a novel candidate tumor suppressor gene on chromosome 3p. Cancer Res. 2001;61(24):8820–8829. [PubMed] [Google Scholar]
- 24.Zabarovsky ER, Lerman MI, Minna JD. Tumor suppressor genes on chromosome 3p involved in the pathogenesis of lung and other cancers. Oncogene. 2002;21(45):6915. doi: 10.1038/sj.onc.1205835 [DOI] [PubMed] [Google Scholar]
- 25.Fox SB, Brown P, Han C, et al. Expression of the forkhead transcription factor FOXP1 is associated with estrogen receptor α and improved survival in primary human breast carcinomas. Clin Cancer Res. 2004;10(10):3521–3527. doi: 10.1158/1078-0432.CCR-03-0461 [DOI] [PubMed] [Google Scholar]
- 26.Fox AH, Lam YW, Leung AKL, et al. Paraspeckles: a novel nuclear domain. Curr Biol. 2002;12(1):13–25. [DOI] [PubMed] [Google Scholar]
- 27.Sunayama J, Sato A, Matsuda KI, et al. FoxO3a functions as a key integrator of cellular signals that control glioblastoma stem‐like cell differentiation and tumorigenicity. Stem Cells. 2011;29(9):1327–1337. doi: 10.1002/stem.696 [DOI] [PubMed] [Google Scholar]
- 28.Yang XB, Zhao JJ, Huang CY, et al. Decreased expression of the FOXO3a gene is associated with poor prognosis in primary gastric adenocarcinoma patients. PLoS One. 2013;8(10):e78158. doi: 10.1371/journal.pone.0078158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arden KC. Multiple roles of FOXO transcription factors in mammalian cells point to multiple roles in cancer. Exp Gerontol. 2006;41(8):709–717. doi: 10.1016/j.exger.2006.05.015 [DOI] [PubMed] [Google Scholar]
- 30.Storz P. Forkhead homeobox type O transcription factors in the responses to oxidative stress. Antioxid Redox Signal. 2011;14(4):593–605. doi: 10.1089/ars.2010.3405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang L, Li L, Wei H, et al. Transcriptional factor FOXO3 negatively regulates the expression of nm23‐H 1 in non‐small cell lung cancer. Thoracic Cancer. 2016;7(1):9–16. doi: 10.1111/1759-7714.12260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Accili D, Arden KC. FoxOs at the crossroads of cellular metabolism, differentiation, and transformation. Cell. 2004;117(4):421–426. [DOI] [PubMed] [Google Scholar]
- 33.Greer EL, Brunet A. FOXO transcription factors at the interface between longevity and tumor suppression. Oncogene. 2005;24(50):7410. doi: 10.1038/sj.onc.1209086 [DOI] [PubMed] [Google Scholar]
- 34.Hu MCT, Lee DF, Xia W, et al. IκB kinase promotes tumorigenesis through inhibition of forkhead FOXO3a. Cell. 2004;117(2):225–237. [DOI] [PubMed] [Google Scholar]
- 35.Yu S, Yu H, Wu H, et al. Activation of FOXO3a suggests good prognosis of patients with radically resected gastric cancer. Inter J Clin Exp Pathol. 2015;8(3):2963. [PMC free article] [PubMed] [Google Scholar]
- 36.Lu M, Zhao Y, Xu F, Wang Y, Xiang J, Chen D. The expression and prognosis of FOXO3a and Skp2 in human ovarian cancer. Med Oncol. 2012;29(5):3409–3415. doi: 10.1007/s12032-012-0275-z [DOI] [PubMed] [Google Scholar]