Abstract
Purpose: We sought to examine the role of pretreatment systemic immune-inflammation index (SII) in hormone receptor-negative, human epidermal growth factor receptor 2+ (HER2+) breast cancer patients.
Patients and methods: 155 HER2+ patients treated in our hospital from September 3, 2002, to September 21, 2012, were retrospectively enrolled. SII was established as neutrophil x platelet/lymphocyte counts. The median value of SII was used as cut-off value. We used the Kaplan-Meier method to evaluate the overall survival (OS), disease-free survival (DFS), distant metastasis-free survival (DMFS). To comparatively evaluate the survival rates between patients from two groups, we used the log-rank test. For identifying independent factors of prognosis, we used the Cox regression model, applying multivariate statistics.
Results: Analyses show that HER2+ patients with high and low SII had median DFS of 15.1 and 31.5 months, respectively (P<0.001), while the median DMFS in HER2+ patients with high SII was 18.4 and in patients with low SII was 33.0 months (P=0.001), and the median OS were 54.5 and 71.1 months respectively in high and low SII patients, respectively (P=0.002). Multivariate analysis had revealed increased SII independently linked to poor DFS (HR =1.46, 95% CI: 1.01–2.11, P=0.045). The difference between SII and DMFS bore no statistical significance. (HR =1.40, 95% CI: 0.96–2.03, P=0.078), while high SII independently predicted short OS (HR =1.51, 95% CI: 1.02–2.25, P=0.038).
Conclusion: Our findings suggest that increased SII independently predicts poor survival for hormone receptor-negative, HER2+ breast cancer patients. Prospective studies are, however, required to confirm the role of SII in the prognosis of patients with HER2+ before clinical use.
Keywords: breast cancer, SII, HER2, inflammation
Introduction
Breast cancer is still one of the primary reasons for cancer death among female patients, although treatment strategies have improved in recent years.1 That the human epidermal growth factor receptor-2 (HER2) is overexpressed is reportedly a concern in about 20–25% of breast cancers and is generally linked to poor outcomes. Trastuzumab is a humanized monoclonal antibody that acts against HER2’s extracellular domain. The antibody is integral in the treatment of patients with HER2+ tumors, given its beneficial features in both early and metastatic situations. In addition to Trastuzumab, newer drugs, such as Ado-Trastuzumab Emtansine, Pertuzumab, and Lapatinib that also target HER2 have boosted the treatment of HER2+ breast cancer, subsequently improving survival rates and the quality of life of patients. However, death tolls from HER2+ breast cancer remain high.2 HER2+ breast cancer frequently recurs after first-line treatment, resulting in metastasis and ultimately death.3 It is, therefore, particularly essential to evaluate HER2+ breast cancer patients’ prognosis, which is affected by several clinicopathological parameters, such as tumor size, lymph node status, patient age, and molecular biology characteristics.4,5
In the past, several investigations have paid more attention to inflammation and have established cancer-related inflammation to have a significant part in the development and prognosis of cancer.6–11 Systemic inflammation can be monitored using biochemical or hematologic markers, including elevated C-reactive protein, leukocyte, hypoalbuminemia, neutrophil, and platelet cell numbers.12–14 If these factors are combined, they can be used to derive predictive inflammatory scores. Examples of such scores are lymphocyte ratios for platelets (PLR) and neutrophil (NLR), and the Glasgow Prognostic Score. The disapproving role of elevated NLR and PLR has been vigorously scrutinized in various cancer types before, including breast cancer, lung cancer, ovarian cancer, colorectal cancer, gallbladder cancer, cervical cancer, gastric cancer.14–24
Liquid biopsy, while being a noninvasive and dynamic detector tool in oncology, also offers several clinical benefits, including diagnosis, prognosis, treatment selection, and monitoring disease burdens.25,26
Recently, an integrated indicator that is known as a systemic immune-inflammation index (SII), centered on peripheral platelet, neutrophil, and lymphocyte counts and defined as neutrophil × platelet/lymphocyte, has been assessed in various cancers.27 This indicator is noninvasive and offers a dynamic detection procedure during therapy. This indicator could be more suited in reflecting the balance between host inflammatory and immune status.27 Although SII has been inspected in various cancers, including colorectal cancer, nasopharyngeal carcinoma, hepatocellular carcinoma, pancreatic cancer, gastric cancer, and prostate cancer,10,27–34 researchers are still to comprehend its part in patients with breast cancer fully. Our investigation aimed to examine the predictive significance of SII in patients with HER2+ breast cancer.
Materials and methods
Subjects
155 hormone receptor-negative, HER2+ patients treated in our hospital from September 3, 2002, to September 21, 2012, were retrospectively enrolled. All patients were pathologically diagnosed. Progesterone receptor and estrogen receptor were negative when the immunohistochemistry test to be less than 1% of the positive invasive tumor nuclei. HER2 status was considered negative when the immunohistochemical result was equal to 0 or with the confirmation of negativity with fluorescence in situ hybridization. We excluded patients who had inflammatory breast cancer, liver, hematological, infectious, immune, inflammatory diseases and other tumors. The Ethical Committee of Shandong Cancer Hospital approved our investigation. Because of the characteristic of a retrospective study, the habitual written informed consent was not required, and all data was anonymous.
We set August 6, 2018, as the deadline for follow-up. Patients were regularly followed up every three and six months after surgery until the end of follow up or death. We frequently used magnetic resonance imaging, ultrasound imaging, computed tomography, and tumor biomarkers to evaluate patients’ tumor status. The criteria for determining relapse and distant metastasis is elucidated in the RECIST guideline (version 1.1). Relapse denotes both local and regional relapses, defined by the occurrence of detectable relapsing masses in the ipsilateral breast, ipsilateral chest wall, surgical scars, or local lymphatic drainage area. The classic clinical and radiographic and/or histological determination of distant detectable metastases larger than 0.2 mm confirm the presence of distant metastases. The overall survival (OS) begins with surgery up to the point of death or lost follow-up, disease-free survival time (DFS) represents the time beginning from surgery up to the first disease recurrence, death or lost follow-up, and distant metastasis-free survival (DMFS) covers surgery and spans the first distant metastasis phase, death or lost follow-up.
Data collection
Data for differential blood counts were collected within 3 days before surgery or neoadjuvant chemotherapy. We collected neutrophil counts alone, platelet counts alone, and lymphocyte counts alone and then calculated the corresponding NLR (neutrophil/lymphocyte ratio), PLR (platelet/lymphocyte ratio), and SII (neutrophil x platelet/lymphocyte).27 Patients were partitioned into two groups of high or low SII following the median value (cut-off value), as well as in two groups each of high or low NLR, PLR, lymphocytes, platelets, and neutrophils in the Cox regression. The treatment approaches involved carrying out surgery, chemotherapy, and radiotherapy. All the patients were subjected to breast-conserving surgery or radical mastectomy. Patients received chemotherapy with Anthracyclines, Cyclophosphamide, and Paclitaxel during a treatment period spanning between 4 and 8 cycles (a median value of 5.7 cycles). Targeted therapy was used with Trastuzumab during a 1 year treatment period. The postoperative radiotherapy was performed with 50–60 Gy/25–30 fractions. Platelet count, lymphocyte count, neutrophil count, lymph node involvement, tumor differentiation, lymphovascular invasion, age, tumor stage, and surgical types for enrolled patients were evaluated. Tumor stage was evaluated by the 7th American Joint Committee on Cancer (AJCC) staging system.35
Statistical analysis
We used SPSS 21 software (SPSS, Chicago, IL, USA) to analyze data. We used the Kaplan-Meier method to evaluate the DFS, DMFS, and OS, and the Log-rank test to evaluate survival differences between patients partitioned into two groups according to the SII obtained. Chi-square or Fisher exact tests were employed, when necessary, to assess the association between SII and clinicopathological parameters, while the Cox regression model helped in the multivariate analysis to identify independent factors of prognosis. Differences at P<0.05 were considered statistically significant.
Results
Patient characteristics
Characteristics of the 155 HER2+ patients in our study are presented in Table 1. There are 31 young patients (age ≤35) and 124 older patients (age >35). 70, 64, and 21 HER2+ patients were at stage III, II, and I, respectively, according to the 7th AJCC stage. 77 patients had high SII (SII >578), and 78 patients had low SII (SII ≤578). There were 17, 76, 52, and 10 HER2+ patients with well, middle, poor, and unknown differentiation, respectively. Follow-up duration lasted 57.6 months (10.4–158.2 months). By the end of our study, 119 patients had died, 137 of them had experienced recurrence, all 155 had developed distant metastasis, and 9 had eventually gone missing during follow-up.
Table 1.
Patient characteristics of 155 hormone receptor-negative, HER2+ breast cancer patients
| Parameters | Number | % |
|---|---|---|
| Age (years) | ||
| ≤35 | 31 | 20.0 |
| >35 | 124 | 80.0 |
| Tumor stage | ||
| T1 | 29 | 18.7 |
| T2 | 84 | 54.2 |
| T3 | 35 | 22.6 |
| T4 | 7 | 4.5 |
| Tumor differentiation | ||
| I | 17 | 11.0 |
| II | 76 | 49.0 |
| III | 52 | 33.5 |
| Unknown | 10 | 6.5 |
| Lymph node involvement | ||
| N0 | 56 | 36.1 |
| N1 | 34 | 21.9 |
| N2 | 25 | 16.1 |
| N3 | 40 | 25.8 |
| AJCC stage | ||
| I | 21 | 13.5 |
| II | 64 | 41.3 |
| III | 70 | 45.2 |
| Pathological type | ||
| IDC | 126 | 81.3 |
| ILC | 29 | 18.7 |
| Ki-67 | ||
| ≤30% | 98 | 63.2 |
| >30% | 57 | 36.8 |
| Surgery | ||
| Breast-conserving surgery | 15 | 9.7 |
| Radical mastectomy | 140 | 90.3 |
| Radiotherapy | ||
| Yes | 92 | 59.4 |
| No | 63 | 40.6 |
| Chemotherapy | ||
| Adjuvant chemotherapy | 129 | 83.2 |
| Neoadjuvant chemotherapy | 26 | 16.8 |
| Targeted therapy | ||
| Yes | 81 | 52.3 |
| No | 74 | 47.7 |
| SII | ||
| ≤578 | 78 | 50.3 |
| >578 | 77 | 49.7 |
Abbreviations: AJCC, American Joint Committee on Cancer; SII, systemic immune-inflammation index; IDC, Intraductal Carcinoma; ILC, Intralobular Carcinoma.
Univariate analysis
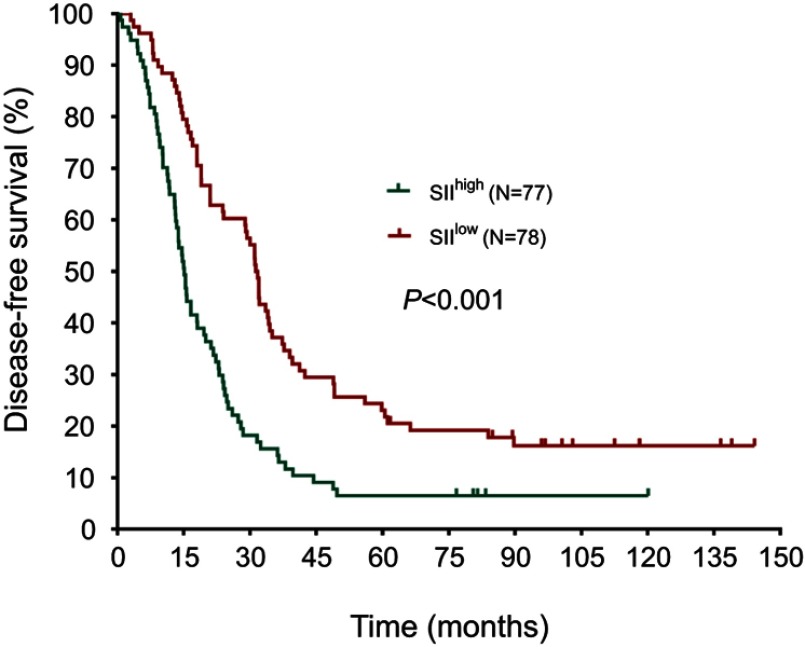
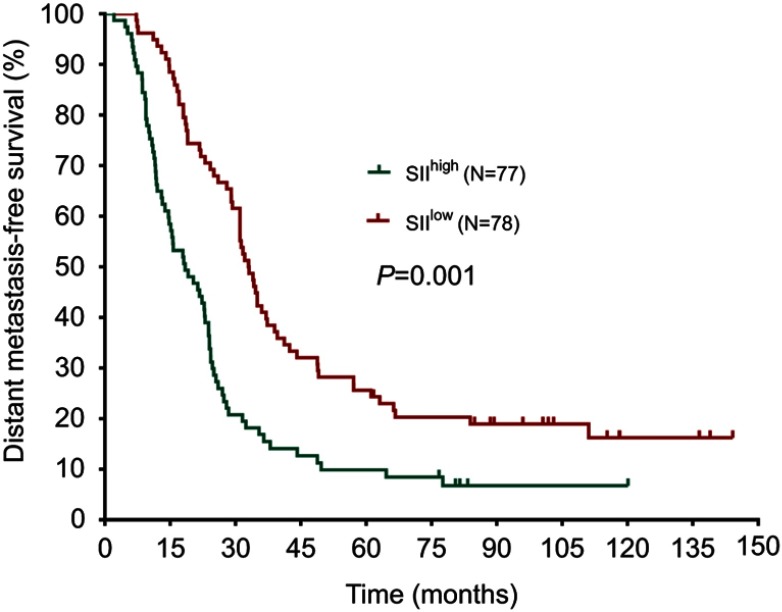
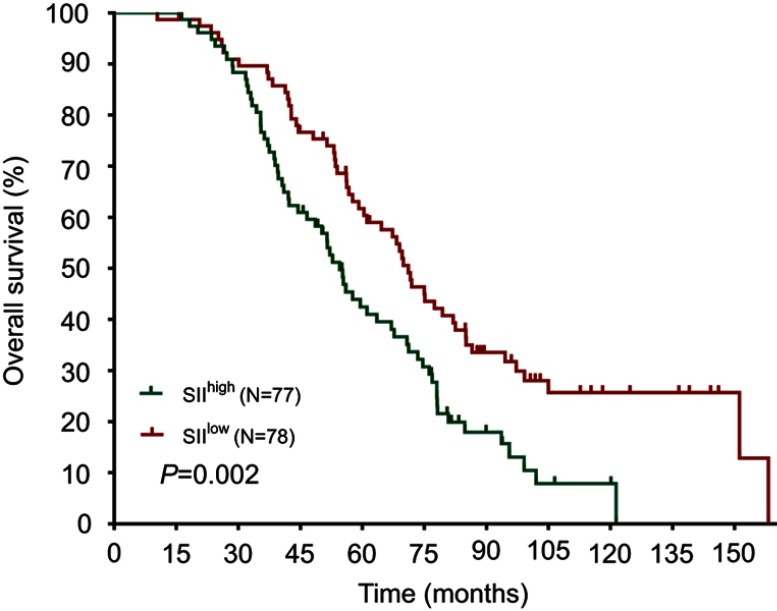
The median DFS in high and low SII HER2+ patients were 15.1 and 31.5 months, respectively (HR=1.88, 95% CI: 1.34–2.65, P<0.001, Figure 1, Table 2), the median DMFS in high SII HER2+ patients was 18.4, while that in patients with low SII was 33.0 months (HR =1.81, 95% CI: 1.28–2.55, P=0.001, Figure 2, Table 2), and the median OS were 54.5 and 71.1 months in high and low SII patients, respectively (HR=1.79, 95% CI: 1.24–2.60, P=0.002, Figure 3, Table 2). Besides, tumor stage, Ki-67 differentiation, lymph nodes involvement, lymphocytes, NLR and PLR also correlated with DFS, DMFS, OS (P<0.05).
Figure 1.
Disease-free survival rates of 155 patients with hormone receptor-negative, HER2+ breast cancer according to low and high SII (systemic immune-inflammation index).
Table 2.
Univariate analysis for DFS, DMFS and OS
| Parameters | DFS | DMFS | OS | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Age (years) | ||||||
| ≤35 | 1 | 1 | 1 | |||
| >35 | 0.65 (0.43–0.97) | 0.038 | 0.61 (0.41–0.92) | 0.020 | 0.86 (0.56–1.33) | 0.514 |
| Tumor stage | ||||||
| T1 | 1 | 1 | 1 | |||
| T2–4 | 2.99 (1.84–4.86) | <0.001 | 2.94 (1.81–4.78) | <0.001 | 3.51 (2.01–6.12) | <0.001 |
| Tumor differentiation | ||||||
| I–II | 1 | 1 | 1 | |||
| III | 3.33 (2.26–4.88) | <0.001 | 3.14 (2.13–4.62) | <0.001 | 2.71 (1.83–4.02) | <0.001 |
| Lymph node involvement | ||||||
| N0 | 1 | 1 | 1 | |||
| N1–3 | 2.20 (1.54–3.17) | <0.001 | 2.14 (1.49–3.07) | <0.001 | 2.34 (1.58–3.48) | <0.001 |
| Pathological type | ||||||
| ILC | 1 | 1 | 1 | |||
| IDC | 0.86 (0.62–1.28) | 0.621 | 0.92 (0.75–1.41) | 0.381 | 1.13 (0.83–1.39) | 0.614 |
| Surgery | ||||||
| Breast-conserving surgery | 1 | 1 | 1 | |||
| Radical mastectomy | 1.57 (0.86–2.84) | 0.135 | 1.53 (0.84–2.78) | 0.157 | 2.07 (1.04–4.10) | 0.037 |
| Ki-67 | ||||||
| ≤30% | 1 | 1 | 1 | |||
| >30% | 2.65 (2.11–4.15) | <0.001 | 2.89 (1.82–5.36) | <0.001 | 2.93 (1.94–4.62) | <0.001 |
| Radiotherapy | ||||||
| No | 1 | 1 | 1 | |||
| Yes | 1.04 (0.74–1.46) | 0.836 | 0.99 (0.70–1.39) | 0.952 | 0.84 (0.59–1.22) | 0.364 |
| Chemotherapy | ||||||
| Adjuvant chemotherapy | 1 | 1 | 1 | |||
| Neoadjuvant chemotherapy | 0.85 (0.55–1.34) | 0.493 | 0.92 (0.59–1.44) | 0.714 | 1.18 (0.71–1.96) | 0.517 |
| Targeted therapy | ||||||
| Yes | 1 | 1 | 1 | |||
| No | 1.07 (0.76–1.49) | 0.692 | 0.96 (0.68–1.35) | 0.819 | 1.04 (0.72–1.50) | 0.839 |
| Neutrophils | ||||||
| ≤3.61 | 1 | 1 | 1 | |||
| >3.61 | 1.11 (0.79–1.56) | 0.518 | 1.06 (0.75–1.48) | 0.729 | 1.22 (0.85–1.75) | 0.276 |
| Lymphocytes | ||||||
| ≤1.65 | 1 | 1 | 1 | |||
| >1.65 | 0.63 (0.43–0.91) | 0.015 | 0.61 (0.42–0.89) | 0.010 | 0.57 (0.37–0.86) | 0.008 |
| Platelets | ||||||
| ≤224 | 1 | 1 | 1 | |||
| >224 | 1.05 (0.75–1.48) | 0.742 | 1.18 (0.84–1.65) | 0.332 | 1.28 (0.89–1.84) | 0.181 |
| NLR | ||||||
| ≤2.3 | 1 | 1 | 1 | |||
| >2.3 | 1.44 (1.02–2.03) | 0.034 | 1.41 (1.00–1.99) | 0.045 | 1.54 (1.06–2.23) | 0.022 |
| PLR | ||||||
| ≤160 | 1 | 1 | 1 | |||
| >160 | 1.68 (1.20–2.36) | 0.003 | 1.78 (1.26–2.51) | 0.001 | 1.69 (1.17–2.44) | 0.005 |
| SII | ||||||
| ≤578 | 1 | 1 | 1 | |||
| >578 | 1.88 (1.34–2.65) | <0.001 | 1.81 (1.28–2.55) | 0.001 | 1.79 (1.24–2.60) | 0.002 |
Abbreviations: DFS, disease-free survival; DMFS, distant metastasis-free survival; OS, overall survival; NLR, neutrophil to lymphocyte ratio; PLR, platelet to lymphocyte ratio; SII, systemic immune-inflammation index; IDC, Intraductal Carcinoma; ILC, Intralobular Carcinoma.
Figure 2.
Distant metastasis-free survival rates of 155 patients with hormone receptor-negative, HER2+ breast cancer according to low and high SII (systemic immune-inflammation index).
Figure 3.
Overall survival rates of 155 patients with hormone receptor-negative, HER2+ breast cancer according to low and high SII (systemic immune-inflammation index).
Multivariate analysis
Lymphocytes, NLR, PLR, and SII, alongside other clinicopathological parameters that were statistically significant after the univariate analysis were further evaluated using the multivariate Cox regression for DFS, DMFS, and OS. According to results from multivariate analysis, increased amounts of lymphocytes were independently linked to better OS (HR=0.62, 95% CI: 0.40–0.97, P=0.039, Table 3), but no statistical significances were noted between lymphocytes and DMFS (HR=0.72, 95% CI: 0.48–1.08, P=0.116, Table 3) and between lymphocytes and DFS (HR=0.76, 95% CI: 0.51–1.12, P=0.174, Table 3). NLR did not predict any of DFS (HR=1.38, 95% CI: 0.96–1.99, P=0.081, Table 3), DMFS (HR=1.32, 95% CI: 0.92–1.91, P=0.130, Table 3), or OS (HR=1.32, 95% CI: 0.90–1.95, P=0.153, Table 3) independently. PLR was also not an independent prognosticator for DFS (HR=1.23, 95% CI: 0.83–1.82, P=0.296, Table 3), DMFS (HR=1.36, 95% CI: 0.92–2.02, P=0.114, Table 3), or OS (HR=1.34, 95% CI: 0.88–2.04, P=0.167, Table 3).increased SII was independently linked to poor DFS (HR=1.46, 95% CI: 1.01–2.11, P=0.045, Table 3), but there was no statistical significance between SII and DMFS (HR=1.40, 95% CI: 0.96–2.03, P=0.078, Table 3), and high SII independently predicted short OS (HR=1.51, 95% CI: 1.02–2.25, P=0.038, Table 3). Furthermore, tumor stage, Ki-67 and tumor differentiation were independent predictors for DFS, DMFS, OS in patients with HER2+ breast cancer (P<0.05). We, therefore, note that SII has the best prognostic value among the 4 evaluated indices.
Table 3.
Multivariate analysis for DFS, DMFS and OS
| Parameters | DFS | DMFS | OS | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Age (years) | ||||||
| ≤35 | 1 | 1 | / | / | ||
| >35 | 0.79 (0.51–1.21) | 0.285 | 0.72 (0.47–1.11) | 0.142 | / | |
| Tumor stage | ||||||
| T1 | 1 | 1 | 1 | |||
| T2–4 | 2.32 (1.39–3.89) | 0.001 | 2.31 (1.38–3.89) | 0.001 | 2.73 (1.50–4.97) | <0.001 |
| Tumor differentiation | ||||||
| I–II | 1 | 1 | 1 | |||
| III | 2.42 (1.54–3.81) | <0.001 | 2.34 (1.49–3.66) | <0.001 | 1.85 (1.20–2.87) | 0.005 |
| Lymph node involvement | ||||||
| N0 | 1 | 1 | 1 | |||
| N1–3 | 1.21 (0.77–1.87) | 0.396 | 1.16 (0.75–1.81) | 0.485 | 1.22 (0.75–2.00) | 0.409 |
| Surgery | ||||||
| Breast-conserving surgery | / | / | 1 | |||
| Radical mastectomy | / | / | / | / | 1.58 (0.73–3.39) | 0.238 |
| Ki-67 | ||||||
| ≤30% | 1 | 1 | 1 | |||
| >30% | 1.98 (1.36–2.68) | 0.001 | 2.06 (1.74–3.92) | <0.001 | 2.31 (1.81–3.46) | <0.001 |
| Lymphocytes | ||||||
| ≤1.65 | 1 | 1 | 1 | |||
| >1.65 | 0.76 (0.51–1.12) | 0.174 | 0.72 (0.48–1.08) | 0.116 | 0.62 (0.40–0.97) | 0.039 |
| NLR | ||||||
| ≤2.3 | 1 | 1 | 1 | |||
| >2.3 | 1.38 (0.96–1.99) | 0.081 | 1.32 (0.92–1.91) | 0.130 | 1.32 (0.90–1.95) | 0.153 |
| PLR | ||||||
| ≤160 | 1 | 1 | 1 | |||
| >160 | 1.23 (0.83–1.82) | 0.296 | 1.36 (0.92–2.02) | 0.114 | 1.34 (0.88–2.04) | 0.167 |
| SII | ||||||
| ≤578 | 1 | 1 | 1 | |||
| >578 | 1.46 (1.01–2.11) | 0.045 | 1.40 (0.96–2.03) | 0.078 | 1.51 (1.02–2.25) | 0.038 |
Notes: Lymphocytes, NLR, and PLR are evaluated using the multivariate Cox regression for DFS, DMFS, and OS alongside other clinicopathological parameters that were found to be statistically significant after the univariate analysis. Only each of lymphocytes, NLR, and PLR alone are listed in this table.
Abbreviations: DFS, disease-free survival; DMFS, distant metastasis-free survival; OS, overall survival; NLR, neutrophil to lymphocyte ratio; PLR, platelet to lymphocyte ratio; SII, systemic immune-inflammation index.
Correlation findings between SII and baseline characteristics
Table 4 displays the correlation between SII and baseline characteristics. Patients with a higher tumor stage have increased SII (P=0.038) and the positivity of lymph nodes metastasis correlated with high SII (P=0.012). We did not find significant correlations with SII for other baseline characteristics.
Table 4.
Correlation between SII and baseline characteristics
| Parameters | SII ≤578 | SII >578 | P-value |
|---|---|---|---|
| Age (years) | |||
| ≤35 | 13 | 18 | 0.322 |
| >35 | 65 | 59 | |
| Tumor stage | |||
| T1 | 20 | 9 | 0.038 |
| T2–4 | 58 | 68 | |
| Tumor differentiation | |||
| I–II | 53 | 40 | 0.119 |
| III | 22 | 30 | |
| Lymph node involvement | |||
| N0 | 36 | 20 | 0.012 |
| N1–3 | 42 | 57 | |
| AJCC stage | |||
| I | 14 | 7 | 0.158 |
| II–III | 64 | 70 | |
| Pathological type | |||
| ILC | 12 | 17 | 0.310 |
| IDC | 66 | 60 | |
| Surgery | |||
| Breast-conserving surgery | 11 | 4 | 0.101 |
| Radical mastectomy | 67 | 73 |
Abbreviations: AJCC, American Joint Committee on Cancer; SII, systemic immune-inflammation index; IDC, Intraductal Carcinoma; ILC, Intralobular Carcinoma
Discussion
SII prognoses have already been reported in prostate cancer, pancreatic cancer, nasopharyngeal carcinoma, colorectal cancer, hepatocellular carcinoma, and gastric cancer.10,27–29,31–34,36 Hu et al27 initially identified SII as a novel and independent prognostic pointer in hepatocellular carcinoma, with higher preoperative SII patients presenting increased recurrence rates and surviving lesser than their lower SII counterparts. Tong et al32 in examining the association between SII and each of therapy response and overall patient survival at stage III of non-small cell lung cancer, proved SII’s prognostic value was independent. Studies on the prognostic significance of preoperative SII in patients with gastric cancer revealed that high SII correlated with poor clinicopathological characteristics and could act as a predictor of inferior prognosis.
Mounting research findings have established an existing prognostic relationship between different cancer types and the inflammatory system, typified by the pretreatment tallies of inflammatory cells, such as lymphocytes, neutrophils, and monocytes.37–43 Lymphocytes that infiltrate tumors could serve to prevent tumor development and spread through their antitumor immune response; lymphocytes are associated with better survival chances in cancer patients.44,45 Platelets, on the other hand, may not only be able to promote the angiogenesis and metastases of tumors but could as well protect tumor cells from the impact of antitumor immune response by shielding them.46 Neutrophils are an inflammatory and immune parameter renowned for their role in tumor proliferation and metastasis owing to their release of inflammatory mediators (matrix metalloproteinase-9, neutrophil elastase, and interleukin-8).47
Our research has, for the first time, presented the clinical role of SII in Chinese patients with HER2+ breast cancer. We report that increased SII independently predicts short DFS and OS in HER2+ breast cancer patients. These findings are consistent with previous investigations.28,36,48 Prominent among those is Jiang et al’s33 substantiation of the independent predictive role of SII for survival in nasopharyngeal carcinoma, where tumor stage, differentiation, and lymphovascular invasion independently associated with bad survival for nasopharyngeal cancer patients.
A 1-year trastuzumab therapy is the standard treatment for high-risk, early Her2+ breast cancer patients. However, the Her2-targeted therapy played no significant role on prognosis as shown in Table 2. In this retrospective study, early stage patients (AJCC I and II) accounted for over 50% of the cases. Those who underwent the Her2-targeted therapy were mainly AJCC stage III individuals. And tumor stage is an independent predictor of DFS, DMFS and OS. A simple separation into two groups, designated “targeted therapy” or “non-targeted therapy” could weaken the role of targeted therapy on prognosis. This study was conducted on a small sample size; only 81 of the 155 patients received targeted therapy, with it possible that the effect of targeted therapy could be influenced by simple grouping easily.
The poor prognosis in this study, when compared to previously reported investigations, may be due partly to poor compliance to normative adjuvant therapy by some Chinese patients.49 The lesser standard form of adjuvant therapy contributed to the poor survival. Furthermore, only 81 of 155 patients received targeted therapy in this study, with the outcome of worse survival. Findings from a study investigating the prognostic significance of derived NLR in 310 patients who had HER2+ breast cancer and underwent neoadjuvant chemotherapy, amassing a median follow-up time of 62.5 months (9.6–138.5 months) established 270 cases of recurrent or metastatic disease, 246 dead cases, and 16 lost follow-up cases.50 These cases had poor prognoses comparative to those in our study.
Despite the novelty and potential of our studies, there are some limitations to it. Firstly, our investigation consisted of a single-center retrospective study without a validation group, and it consisted of a relatively small sample size. Furthermore, factors such as hypercholesterolemia, metabolic syndrome, abnormal thyroid function, smoking, and alcohol consumption may potentially affect SII. That notwithstanding, if proper validation is made in the future, the study could serve as a potential boost in the fight against HER2 breast cancer.
Conclusion
Our results suggest that increased SII independently predicts poor survival for patients with HER2+ breast cancer. However, further in-depth studies are required to confirm the potential role of SII in HER2+ breast cancer patients before contemplations of any potential clinical use.
Acknowledgment
This study was funded by The National Key Research and Development Program of China (No.2018YFC1313200).
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. doi: 10.3322/caac.21442 [DOI] [PubMed] [Google Scholar]
- 2.Fan L, Strasser-Weippl K, Li JJ, et al. Breast cancer in China. Lancet Oncol. 2014;15(7):e279–e289. doi: 10.1016/S1470-2045(13)70567-9 [DOI] [PubMed] [Google Scholar]
- 3.Zhu X, Ying J, Wang F, Wang J, Yang H. Estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2 status in invasive breast cancer: a 3,198 cases study at National Cancer Center, China. Breast Cancer Res Treat. 2014;147(3):551–555. doi: 10.1007/s10549-014-3136-y [DOI] [PubMed] [Google Scholar]
- 4.Bianchini G, Balko JM, Mayer IA, Sanders ME, Gianni L. Triple-negative breast cancer: challenges and opportunities of a heterogeneous disease. Nat Rev Clin Oncol. 2016;13(11):674–690. doi: 10.1038/nrclinonc.2016.66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brewster AM, Chavez-MacGregor M, Brown P. Epidemiology, biology, and treatment of triple-negative breast cancer in women of African ancestry. Lancet Oncol. 2014;15(13):e625–e634. doi: 10.1016/S1470-2045(14)70364-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Troppan K, Deutsch A, Gerger A, et al. The derived neutrophil to lymphocyte ratio is an independent prognostic factor in patients with diffuse large B-cell lymphoma. Br J Cancer. 2014;110(2):369–374. doi: 10.1038/bjc.2013.763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Szkandera J, Gerger A, Liegl-Atzwanger B, et al. The derived neutrophil/lymphocyte ratio predicts poor clinical outcome in soft tissue sarcoma patients. Am J Surg. 2015;210(1):111–116. doi: 10.1016/j.amjsurg.2014.10.021 [DOI] [PubMed] [Google Scholar]
- 8.Deng Q, He B, Liu X, et al. Prognostic value of pre-operative inflammatory response biomarkers in gastric cancer patients and the construction of a predictive model. J Transl Med. 2015;13:66. doi: 10.1186/s12967-015-0541-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang L, Wang C, Wang J, Huang X, Cheng Y. A novel systemic immune-inflammation index predicts survival and quality of life of patients after curative resection for esophageal squamous cell carcinoma. J Cancer Res Clin Oncol. 2017;143(10):2077–2086. doi: 10.1007/s00432-017-2451-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aziz MH, Sideras K, Aziz NA, et al. The systemic-immune-inflammation index independently predicts survival and recurrence in resectable pancreatic cancer and its prognostic value depends on bilirubin levels. Ann Surg. 2018; doi: 10.1097/sla.0000000000002660. [DOI] [PubMed] [Google Scholar]
- 11.Russo A, Franchina T, Ricciardi GRR, et al. Baseline neutrophilia, derived neutrophil-to-lymphocyte ratio (dNLR), platelet-to-lymphocyte ratio (PLR), and outcome in non small cell lung cancer (NSCLC) treated with Nivolumab or Docetaxel. J Cell Physiol. 2018. doi: 10.1002/jcp.26609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436–444. doi: 10.1038/nature07205 [DOI] [PubMed] [Google Scholar]
- 13.Shankaran V, Ikeda H, Bruce AT, et al. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 2001;410(6832):1107–1111. doi: 10.1038/35074122 [DOI] [PubMed] [Google Scholar]
- 14.Yu X, Wen Y, Lin Y, et al. The value of preoperative Glasgow Prognostic Score and the C-Reactive Protein to Albumin Ratio as prognostic factors for long-term survival in pathological T1N0 esophageal squamous cell carcinoma. J Cancer. 2018;9(5):807–815. doi: 10.7150/jca.22755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He X, Li JP, Liu XH, et al. Prognostic value of C-reactive protein/albumin ratio in predicting overall survival of Chinese cervical cancer patients overall survival: comparison among various inflammation based factors. J Cancer. 2018;9(10):1877–1884. doi: 10.7150/jca.23320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kwon BS, Jeong DH, Byun JM, et al. Prognostic value of preoperative lymphocyte-monocyte ratio in patients with ovarian clear cell carcinoma. J Cancer. 2018;9(7):1127–1134. doi: 10.7150/jca.24057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liang Y, Wang W, Li J, et al. Combined use of the neutrophil-lymphocyte and platelet-lymphocyte ratios as a prognostic predictor in patients with operable soft tissue sarcoma. J Cancer. 2018;9(12):2132–2139. doi: 10.7150/jca.24871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shao S, Risch E, Burner D, Lu L, Minev B, Ma W. IFNγ enhances cytotoxic efficiency of the cytotoxic T lymphocytes against human glioma cells. Int Immunopharmacol. 2017;47:159–165. doi: 10.1016/j.intimp.2017.04.003 [DOI] [PubMed] [Google Scholar]
- 19.Suzuki R, Wei X, Allen PK, Welsh JW, Komaki R, Lin SH. Hematologic variables associated with brain failure in patients with small-cell lung cancer. Radiother Oncol. 2018;128:505–512. doi: 10.1016/j.radonc.2018.05.026 [DOI] [PubMed] [Google Scholar]
- 20.Lu L, Bai Y, Wang Z. Elevated T cell activation score is associated with improved survival of breast cancer. Breast Cancer Res Treat. 2017;164(3):689–696. doi: 10.1007/s10549-017-4281-x [DOI] [PubMed] [Google Scholar]
- 21.Liu C, Wu S, Meng X, et al. Predictive value of peripheral regulatory T cells in non-small cell lung cancer patients undergoing radiotherapy. Oncotarget. 2017;8(26):43427–43438. doi: 10.18632/oncotarget.15238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Petrillo A, Laterza MM, Tirino G, et al. Systemic-inflammation-based score can predict prognosis in metastatic gastric cancer patients before first-line chemotherapy. Future Oncology 2018. Jul 3;14(24):2493–505. doi: 10.2217/fon-2018-0167 [DOI] [PubMed] [Google Scholar]
- 23.Huang W, Wang S, Zhang H, Zhang B, Wang C. Prognostic significance of combined fibrinogen concentration and neutrophil-to-lymphocyte ratio in patients with resectable non-small cell lung cancer. Cancer Biol Med. 2018;15(1):88–96. doi: 10.20892/j.issn.2095-3941.2017.0124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ethier JL, Desautels D, Templeton A, Shah PS, Amir E. Prognostic role of neutrophil-to-lymphocyte ratio in breast cancer: a systematic review and meta-analysis. Breast Cancer Res. 2017;19(1):2. doi: 10.1186/s13058-016-0794-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu L, Zeng H, Gu X, Ma W. Circulating tumor cell clusters-associated gene plakoglobin and breast cancer survival. Breast Cancer Res Treat. 2015;151(3):491–500. doi: 10.1007/s10549-015-3416-1 [DOI] [PubMed] [Google Scholar]
- 26.Lu L, Risch HA. Exosomes: potential for early detection in pancreatic cancer. Future Oncol. 2016;12(8):1081–1090. doi: 10.2217/fon-2015-0005 [DOI] [PubMed] [Google Scholar]
- 27.Hu B, Yang XR, Xu Y, et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res. 2014;20(23):6212–6222. doi: 10.1158/1078-0432.CCR-14-0442 [DOI] [PubMed] [Google Scholar]
- 28.Chen L, Yan Y, Zhu L, et al. Systemic immune-inflammation index as a useful prognostic indicator predicts survival in patients with advanced gastric cancer treated with neoadjuvant chemotherapy. Cancer Manag Res. 2017;9:849–867. doi: 10.2147/CMAR.S151026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang J, Guo X, Wang M, Ma X, Ye X, Lin P. Pre-treatment inflammatory indexes as predictors of survival and cetuximab efficacy in metastatic colorectal cancer patients with wild-type RAS. Sci Rep. 2017;7(1):17166. doi: 10.1038/s41598-017-17130-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lolli C, Basso U, Derosa L, et al. Systemic immune-inflammation index predicts the clinical outcome in patients with metastatic renal cell cancer treated with sunitinib. Oncotarget. 2016;7(34):54564–54571. doi: 10.18632/oncotarget.10515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fan L, Wang R, Chi C, et al. Systemic immune-inflammation index predicts the combined clinical outcome after sequential therapy with abiraterone and docetaxel for metastatic castration-resistant prostate cancer patients. Prostate. 2018;78(4):250–256. doi: 10.1002/pros.23465 [DOI] [PubMed] [Google Scholar]
- 32.Tong Y-S, Tan J, Zhou X-L, Song Y-Q, Song Y-J. Systemic immune-inflammation index predicting chemoradiation resistance and poor outcome in patients with stage III non-small cell lung cancer. J Transl Med. 2017;15(1):221. doi: 10.1186/s12967-017-1326-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jiang W, Chen Y, Huang J, et al. Systemic immune-inflammation index predicts the clinical outcome in patients with nasopharyngeal carcinoma: a propensity score-matched analysis. Oncotarget. 2017;8(39):66075–66086. doi: 10.18632/oncotarget.19796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhong JH, Huang DH, Chen ZY. Prognostic role of systemic immune-inflammation index in solid tumors: a systematic review and meta-analysis. Oncotarget. 2017;8(43):75381–75388. doi: 10.18632/oncotarget.18856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17(6):1471–1474. doi: 10.1245/s10434-010-0985-4 [DOI] [PubMed] [Google Scholar]
- 36.Fukuda H, Takagi T, Kondo T, Shimizu S, Tanabe K. Predictive value of inflammation-based prognostic scores in patients with metastatic renal cell carcinoma treated with cytoreductive nephrectomy. Oncotarget. 2018;9(18):14296–14305. doi: 10.18632/oncotarget.24507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yao M, Liu Y, Jin H, et al. Prognostic value of preoperative inflammatory markers in Chinese patients with breast cancer. Onco Targets Ther. 2014;7:1743–1752. doi: 10.2147/OTT.S69657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen Y, Chen K, Xiao X, et al. Pretreatment neutrophil-to-lymphocyte ratio is correlated with response to neoadjuvant chemotherapy as an independent prognostic indicator in breast cancer patients: a retrospective study. BMC Cancer. 2016;16:320. doi: 10.1186/s12885-016-2352-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamagishi T, Fujimoto N, Nishi H, et al. Prognostic significance of the lymphocyte-to-monocyte ratio in patients with malignant pleural mesothelioma. Lung Cancer. 2015;90(1):111–117. doi: 10.1016/j.lungcan.2015.07.014 [DOI] [PubMed] [Google Scholar]
- 40.Templeton AJ, McNamara MG, Seruga B, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst. 2014;106(6):dju124. doi: 10.1093/jnci/dju061 [DOI] [PubMed] [Google Scholar]
- 41.Song S, Li C, Li S, et al. Derived neutrophil to lymphocyte ratio and monocyte to lymphocyte ratio may be better biomarkers for predicting overall survival of patients with advanced gastric cancer. Onco Targets Ther. 2017;10:3145–3154. doi: 10.2147/OTT.S138039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cannon NA, Meyer J, Iyengar P, et al. Neutrophil-lymphocyte and platelet-lymphocyte ratios as prognostic factors after stereotactic radiation therapy for early-stage non-small-cell lung cancer. J Thorac Oncol. 2015;10(2):280–285. doi: 10.1097/JTO.0000000000000399 [DOI] [PubMed] [Google Scholar]
- 43.Grenader T, Nash S, Plotkin Y, et al. Derived neutrophil lymphocyte ratio may predict benefit from cisplatin in the advanced biliary cancer: the ABC-02 and BT-22 studies. Ann Oncol. 2015;26(9):1910–1916. doi: 10.1093/annonc/mdv253 [DOI] [PubMed] [Google Scholar]
- 44.Ali HR, Provenzano E, Dawson SJ, et al. Association between CD8+ T-cell infiltration and breast cancer survival in 12,439 patients. Ann Oncol. 2014;25(8):1536–1543. doi: 10.1093/annonc/mdu191 [DOI] [PubMed] [Google Scholar]
- 45.Mohammed ZM, Going JJ, Edwards J, McMillan DC. The role of the tumour inflammatory cell infiltrate in predicting recurrence and survival in patients with primary operable breast cancer. Cancer Treat Rev. 2012;38(8):943–955. doi: 10.1016/j.ctrv.2012.04.011 [DOI] [PubMed] [Google Scholar]
- 46.Franco AT, Corken A, Ware J. Platelets at the interface of thrombosis, inflammation, and cancer. Blood. 2015;126(5):582–588. doi: 10.1182/blood-2014-08-531582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Houghton AM, Rzymkiewicz DM, Ji H, et al. Neutrophil elastase-mediated degradation of IRS-1 accelerates lung tumor growth. Nat Med. 2010;16(2):219–223. doi: 10.1038/nm.2084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yu J, Wu X, Yu H, et al. Systemic immune-inflammation index and circulating T-cell immune index predict outcomes in high-risk acral melanoma patients treated with high-dose interferon. Transl Oncol. 2017;10(5):719–725. doi: 10.1016/j.tranon.2017.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Iwamoto T, Fukui N, Kinoshita T, et al. Comprehensive prognostic report of the Japanese Breast Cancer Society registry in 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li Y, Shao Y, Bai L, Zhou X. Increased derived neutrophil-to-lymphocyte ratio and Breast Imaging-Reporting and Data System classification predict poor survival in patients with non-distant metastatic HER2+ breast cancer treated with neoadjuvant chemotherapy. Cancer Manag Res. 2018;10:3841–3847. doi: 10.2147/CMAR.S174537 [DOI] [PMC free article] [PubMed] [Google Scholar]