Abstract
Objective: To evaluate the effect of butorphanol on the prevention of myoclonus induced by etomidate.
Materials and methods: We searched the PubMed, Embase, Cochrane Library, and China National Knowledge Infrastructure databases to collect relevant randomized controlled trials (RCTs) evaluating the effect of butorphanol on etomidate-induced myoclonus in January 2019 without any language restrictions. The primary outcome was the incidence of etomidate-induced myoclonus. Secondary outcomes included the incidence of myoclonus at various degrees and the incidence of adverse effects. Risk ratios (RRs) were calculated for binary outcomes. All statistical analysis were performed by using RevMan 5.3 software.
Results: We identified 6 RCTs involving a total of 608 patients who reported the incidence of etomidate-induced myoclonus. In pooled analyses, the incidence of etomidate-induced myoclonus in the butorphanol group was significantly lower than that in the control group (RR =0.15, 95% CI [0.10, 0.22], P<0.00001). Subgroup analyses showed that butorphanol significantly decreased the numbers of patients with mild myoclonus (RR =0.41, 95% CI [0.25, 0.68], P=0.0005), moderate myoclonus (RR =0.18, 95% CI [0.09, 0.34], P<0.00001), and severe myoclonus (RR =0.04, 95% CI [0.01, 0.10], P<0.00001). Additionally, butorphanol did not increase the incidence of postoperative nausea/vomiting (RR =3.0, 95% CI [0.32, 28.42], P=0.34) or dizziness (RR =6.79, 95% CI [0.84, 54.84], P=0.07) associated with etomidate.
Conclusion: Our findings suggest that butorphanol can effectively prevent the incidence of etomidate-induced myoclonus and alleviate the intensity of etomidate-induced myoclonus, without inducing postoperative nausea/vomiting and dizziness.
Keywords: butorphanol, etomidate, myoclonus
Introduction
Etomidate was widely introduced into clinical practice as a procedural sedation drug and a rapid sequence intubation (RSI) agent. Several properties, such as stable cardiovascular profile and minimal respiratory depression, make etomidate an attractive substitute to propofol in patients with compromised hemodynamic or cardiac reserves.1,2 However, intravenous bolus administration of etomidate is often associated with myoclonus, with a reported incidence up to 80% in unpremedicated patients.3,4 Etomidate-induced myoclonus may lead to the accidental dislodgement of intravenous access and monitoring devices and increase oxygen consumption and the rate of reflux aspiration, posing vital threats to patients with coronary artery disease and intracranial aneurysm. In addition, myoclonus complicates the procedure for which the patient is being sedated and most critically leads to difficulty in airway management in RSI.
Even though the study by Voss et al suggested that myoclonus induced by etomidate may represent a seizure-like activity,5 the mechanism of etomidate-induced myoclonus is still unclear. Several studies have shown that opioids, such as fentanyl and dezocine, could decrease the incidence of etomidate-induced myoclonus, while the risk of side effects, such as apnoea and chest wall rigidity, was significantly higher than that in the control group.6,7 Butorphanol, a new opioid receptor agonist–antagonist, plays an antiseizure role through primarily binding to and modulating κ-receptors,8 and several clinical trials have pushed for more study of the prevention of etomidate-induced myoclonus by use of butorphanol. However, no individual meta-analyses have focused on this topic to provide comprehensive evidence. Therefore, we conducted this systematic review and meta-analysis of randomized controlled trials (RCTs) to evaluate the hypothesis that butorphanol prevents etomidate-induced myoclonus.
Materials and methods
Search strategy
Two authors independently searched the Embase, PubMed, Cochrane Library, and China National Knowledge Infrastructure databases for relevant RCTs in January 2019 without language restriction. The references of the identified studies were also searched for any further relevant studies. The search terms included butorphanol, etomidate, and myoclonus, and the following search was fulfilled in PubMed: ([myoclonus] OR [myoclonic movements]) AND [butorphanol] AND [etomidate].
Eligibility criteria
We included RCTs studying butorphanol for the prevention of etomidate-induced myoclonus, while the control group received an equal volume of saline. Studies were excluded for the following criteria: 1) non-RCTs; 2) retrospective studies; 3) review and case reports; 4) without target outcomes of interest; and 5) etomidate was applied for procedural sedation. The primary outcome was the incidence of etomidate-induced myoclonus, and the secondary outcomes included the incidence of myoclonus at various degrees and the incidence of adverse effects. The intensity of myoclonus was similarly graded in each study and defined as follows: 0, no myoclonus; 1, mild myoclonus (short contraction of some muscle fibers, eg, a finger or shoulder); 2, moderate myoclonus (contraction of different groups of muscles, eg, face and leg); and 3, severe myoclonus (intense clonic movement in two or more muscle groups, eg, fast adduction of a limb or whole body movements).9 All the included studies used the grading system above.
Data extraction
One author extracted the following data from each trial by using standard data tables, and a second author checked the following data: 1) first author and year of publishing; 2) country; 3) sample size; 4) outcome measures (overall incidence of myoclonus, and degree of myoclonus); and 5) details of the intervention. Disagreements were discussed between the two authors, as well as a third author if necessary. We emailed the trial authors for further clarification if the data were missing or unclear.
Quality assessment
Two authors independently assessed the methodological quality of the included studies according to the Jadad scale (studies with 1–3 points were classified as low-quality publications and studies with 4–7 points were classified as high-quality publications).10 The following items were evaluated for each study: 1) whether randomization was performed and whether the method was correct; 2) whether allocation concealment was used and whether the method was correct; 3) whether blinding was performed and in whom the method was used; and 4) whether there were withdrawals or dropouts.
Statistical analysis
RevMan 5.3 software (Cochrane Collaboration, London, UK) was used to conduct all statistical analyses. The incidence of etomidate-induced myoclonus and its degrees were reported by risk ratio (RR) and 95% CIs. Heterogeneity of the included studies was assessed with the I2 statistic, and I2>50% was regarded as significant.11 Considering the heterogeneity between trials with respect to different doses of butorphanol administered to prevent etomidate-induced myoclonus, we used the random-effects model to calculate pooled effects.
To test the robustness of the pooled results, sensitivity analysis was performed by using both the random-effects and fixed-effects analysis models.
Results
Characteristics of the included studies
We identified 65 potentially relevant studies in the initial search, and 6 of these studies4,12–16 were eventually included in the meta-analysis based on the inclusion and exclusion criteria. Figure 1 shows the screening process and results. The basic characteristics of all of the included studies are shown in Table 1.
Figure 1.
Eligibility of studies for inclusion in meta-analysis.
Table 1.
Characteristics of the studies included in the meta-analysis
| First author | Published year | Country | Sample | Grouping | Dose of etomidate | Incidence of myoclonus | Jadad score | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Total | Mild | Moderate | Severe | |||||||
| Zhao15 | 2008 | China | 50 | Butorphanol 2 mg | 0.3 mg/kg | 2 | 2 | 0 | 0 | 2 |
| 50 | Normal saline | 41 | 7 | 18 | 16 | |||||
| Ren16 | 2013 | China | 50 | Butorphanol 15 µg/kg | 0.3 mg/kg | 8 | 4 | 3 | 1 | 3 |
| 50 | Normal saline | 37 | 7 | 11 | 19 | |||||
| He4 | 2014 | China | 54 | Butorphanol 15 µg/kg | 0.3 mg/kg | 7 | 3 | 3 | 1 | 7 |
| 54 | Normal saline | 43 | 7 | 11 | 25 | |||||
| Zhang 14 | 2015 | China | 40 | Butorphanol 15 µg/kg | 0.3 mg/kg | 5 | 4 | 1 | 0 | 3 |
| 40 | Normal saline | 32 | 5 | 12 | 15 | |||||
| Zhang 12 | 2015 | China | 50 | Butorphanol 15 µg/kg | 0.3 mg/kg | 7 | 5 | 2 | 0 | 7 |
| 50 | Normal saline | 37 | 15 | 12 | 10 | |||||
| Yan13 | 2015 | China | 60 | Butorphanol 20 µg/kg | 0.3 mg/kg | 3 | 2 | 1 | 0 | 2 |
| 60 | Normal saline | 43 | 9 | 8 | 26 | |||||
Incidence of etomidate-induced myoclonus
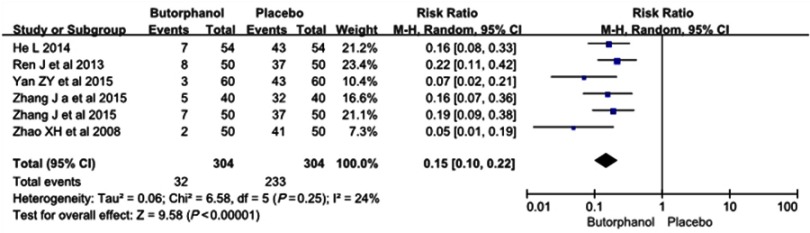
All 6 RCTs, involving a total of 608 patients, reported the incidence of etomidate-induced myoclonus. The incidence of etomidate-induced myoclonus in the butorphanol group and in the control group was 10.5% and 76.7%, respectively. Heterogeneity was not found among the studies (I 2=24%). The results showed that butorphanol could significantly decrease the etomidate-induced myoclonus compared with the control group (RR=0.15, 95% CI [0.10, 0.22], P<0.00001) (Figure 2).
Figure 2.
Butorphanol reduced the incidence of etomidate-induced myoclonus.
Severity of etomidate-induced myoclonus
Mild myoclonus
All 6 RCTs, including a total of 608 patients, reported a degree of mild myoclonus.
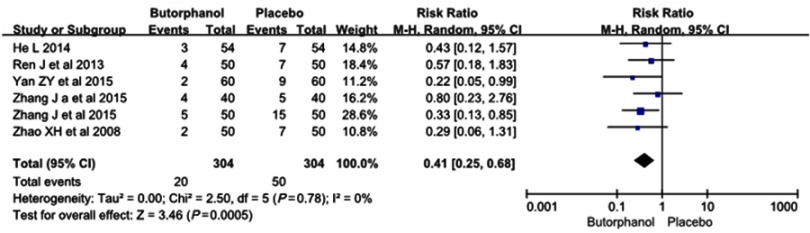
The incidence of mild myoclonus was 20 of 304 (6.6%) in the butorphanol group and 50 of 304 (16.4%) in the control group, respectively. No statistical heterogeneity was found among the study results (I 2=0%). The results showed that butorphanol could significantly decrease the incidence of etomidate-induced mild myoclonus compared with the control group (RR=0.41, 95% CI [0.25, 0.68], P=0.0005) (Figure 3).
Figure 3.
Butorphanol reduced the intensity of etomidate-induced myoclonus: mild myoclonus.
Moderate myoclonus
All 6 RCTs, including a total of 608 patients, reported a moderate degree of myoclonus.
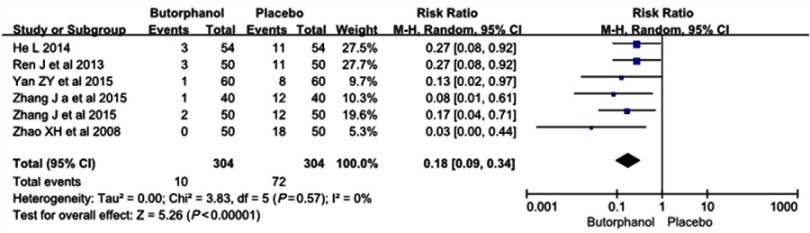
The incidence of moderate myoclonus was 10 of 304 (3.3%) in the butorphanol group and 72 of 304 (23.7%) in the control group, respectively. No statistical heterogeneity was found among the study results (I 2=0%). The results showed that butorphanol could significantly decrease the incidence of etomidate-induced moderate myoclonus compared with the control group (RR=0.18, 95% CI [0.09, 0.34], P<0.00001) (Figure 4).
Figure 4.
Butorphanol reduced the intensity of etomidate-induced myoclonus: moderate myoclonus.
Severe myoclonus
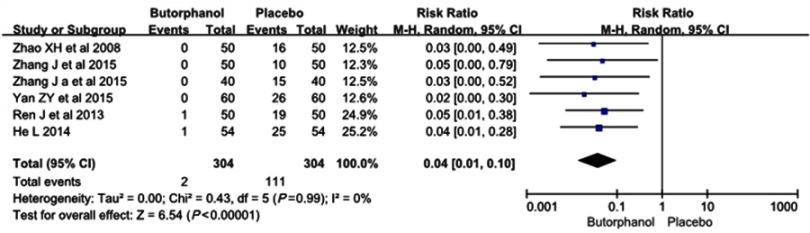
All 6 RCTs, including a total of 608 patients, reported a degree of etomidate-induced severe myoclonus. The incidence of severe myoclonus was 2 of 304 (0.6%) in the butorphanol group and 111 of 304 (36.5%) in the control group, respectively. No statistical heterogeneity (I 2=0%) was found. The results showed that butorphanol could significantly decrease the incidence of etomidate-induced severe myoclonus compared with the control group (RR=0.04, 95% CI [0.01, 0.10], P<0.00001) (Figure 5).
Figure 5.
Butorphanol reduced the intensity of etomidate-induced myoclonus: severe myoclonus.
Adverse effects
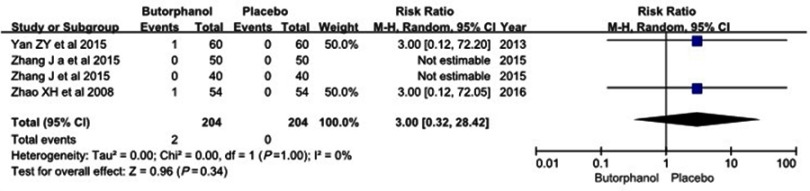
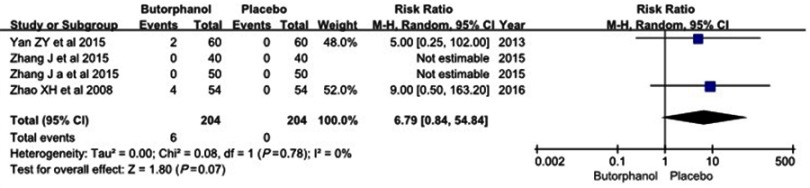
Four studies12–15 reported incidences of nausea/vomiting and dizziness, and no statistical heterogeneity was found among the study results (I 2=0%). No differences were observed in the incidence of nausea/vomiting between the two groups (RR=3.0, 95% CI [0.32, 28.42], P=0.34) (Figure 6). Additionally, there was no significant difference in the incidence of dizziness between the two groups (RR=6.79, 95% CI [0.84, 54.84], P=0.07) (Figure 7).
Figure 6.
Forest plot for the incidence of nausea/vomiting with and without butorphanol.
Figure 7.
Forest plot for the incidence of dizziness with and without butorphanol.
Sensitivity analysis
A fixed-effects model was used to perform the meta-analysis, and sensitivity analysis did not alter the pooled results, indicating the robustness of the pooled results (Table 2).
Table 2.
Sensitivity analysis of included studies
| Outcomes | FEM | ||
|---|---|---|---|
| RR (95% CI) | P-value | I2 | |
| Incidence of myoclonus | 0.14 (0.10, 0.19) | <0.00001 | 24% |
| Mild myoclonus | 0.40 (0.24, 0.65) | 0.0003 | 0% |
| Moderate myoclonus | 0.14 (0.08, 0.27) | <0.00001 | 0% |
| Severe myoclonus | 0.04 (0.01, 0.09) | <0.00001 | 0% |
| Nausea/vomiting | 3.00 (0.32, 28.42) | 0.34 | 0% |
| Dizziness | 7.00 (0.88, 55.98) | 0.07 | 0% |
Abbreviations: FEM, fixed-effects model; RR, risk ratio.
Discussion
This systematic review described the effects associated with the administration of butorphanol in a total of 608 patients in 6 RCTs. The meta-analysis showed that the incidence of etomidate-induced myoclonus associated with the administration of butorphanol was significantly decreased (RR =0.15, 95% [CI] [0.10, 0.22]). We also found a lower incidence of etomidate-induced mild, moderate, and severe myoclonus associated with the administration of butorphanol, without nausea, vomiting, and dizziness.
Etomidate-induced myoclonus is an undesirable complication during anesthesia induction. It is not only a challenging situation to anesthesiologists but also threatens unpremedicated patients.17,18 The precise mechanism of etomidate-induced myoclonus remains unclear, although numerous studies have been conducted on this case.5,19–22 One possible mechanism is that myoclonus may be a form of convulsive seizure, similar to the mechanism of epilepsy.5,19 In addition, some studies have suggested that large doses of etomidate may inhibit the cerebral cortex before depressing subcortical neurons, resulting in myoclonus. Etomidate may also inhibit the central nervous reticular activating system, allowing the occurrence of autonomic nervous conduction by acting on the γ-aminobutyric acid receptor.20 Furthermore, several clinical factors, such as gender, age, and preoperative anxiety, have been implicated in the causes of myoclonic movements.21,22
Butorphanol, a mixed opioid receptor agonist–antagonist, mainly binds to and modulates κ opiate receptors, and the administration of butorphanol for preventing etomidate-induced myoclonus has been used for many years in daily clinical practice. Compared with μ-opioid receptor agonists, such as fentanyl, sufentanil, and remifentanil, butorphanol seems to be more appropriate in relieving etomidate-induced myoclonus for lower incidence of induced respiratory depression and chest rigidity.23,24 The results in our meta-analysis also acknowledged butorphanol-associated reductions in myoclonic movement, despite the site of inhibitory action being obscure. The mechanism of butorphanol to prevent etomidate-induced myoclonus during anesthesia induction may be that the κ-receptor agonist produces a strong anticonvulsant effect8 and interacts with a variety of neurotransmitter systems involved in antiseizure activity,25 such as n-methyl-d-aspartate receptors, γ-aminobutyric acid a–benzodiazepine coupled receptor system, and γ-aminobutyric acid receptors. The fact that pretreatment with butorphanol for decreasing myoclonus may affect intraoperative opioid consumption and recovery quality must be considered. However, all outcome measures indicated in the published trials were limited merely to the anesthetic induction, and few studies examined adverse effects associated with the administration of butorphanol during the recovery period. The absence of data regarding the effects of butorphanol provides an opportunity for future research.
Several potential limitations related to this meta-analysis must be acknowledged. First, the sample size was relatively small. Second, the included studies were only from China, and consequently data from foreign language publications and unpublished studies may be deficient. Third, the dose of butorphanol and observation period was different across studies; further studies are necessary to determine the optimal dose of butorphanol and length of observation. Fourth, there is too little data regarding the adverse effects, such as hypoxia and aspiration, which are the most common complications of using butorphanol. Therefore, the safety of butorphanol for preventing the etomidate-induced myoclonus needs to be further explored. All these limitations may affect the objectivity and reliability of the systemic evaluation; therefore, more high-quality, large-sample studies are required to confirm the present findings.
In summary, the currently available evidence showed that pretreatment with butorphanol can decrease the incidence of etomidate-induced myoclonus and ease the severity of myoclonus. In addition, it did not increase the incidence of dizziness and nausea/vomiting. Further studies focusing on the safety of butorphanol for preventing the etomidate-induced myoclonus are still needed.
Acknowledgments
This work was financially supported by grants from the National Natural Science Foundation of China (NSFC-81371242, 81671084); the Nature Science Foundation of Jiangsu Province (BK20161175); the “Six One” Project of Jiangsu Province (LGY2016039) and Jiangsu Provincial Medical Youth Talent (QNRC2016796).
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Erdoes G, Basciani RM, Eberle B. Etomidate – A review of robust evidence for its use in various clinical scenarios. Acta Anaesthesiol Scand. 2014;58:380–389. doi: 10.1111/aas.12289 [DOI] [PubMed] [Google Scholar]
- 2.Desai PM, Kane D, Sarkar MS. Cardioversion: what to choose? Etomidate or propofol. Ann Card Anaesth. 2015;18(3):306–311. doi: 10.4103/0971-9784.159798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Doenicke AW, Roizen MF, Kugler J, Kroll H, Foss J, Ostwald P. Reducing myoclonus after etomidate. Anesthesiology. 1999;90:113–119. doi: 10.1097/00000542-199901000-00017 [DOI] [PubMed] [Google Scholar]
- 4.He L, Ding Y, Chen H, et al. Butorphanol pre-treatment prevents myoclonus induced by etomidate: a randomised, double-blind, controlled clinical trial. Swiss Med Wkly. 2014;144:w14042. [DOI] [PubMed] [Google Scholar]
- 5.Voss LJ, Sleigh JW, Barnard JP, Kirsch HE. The howling cortex: seizures and general anesthetic drugs. Anesth Analg. 2008;107(5):1689–1703. doi: 10.1213/ane.0b013e3181852595 [DOI] [PubMed] [Google Scholar]
- 6.Zhu Y, Yang Y, Zhou C, Bao Z. Using dezocine to prevent etomidate-induced myoclonus: a meta-analysis of randomized trials. Drug Des Devel Ther. 2017;11:2163–2170. doi: 10.2147/DDDT.S137464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Isitemiz I, Uzman S, Toptaş M, et al. Prevention of etomidate-induced myoclonus: which is superior: fentanyl, midazolam, or a combination? A retrospective comparativestudy. Med Sci Monit. 2014;20:262–267. doi: 10.12659/MSM.889833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Loacker S, Sayyah M, Wittmann W, Herzog H, Schwarzer C. Endogenous dynorphin in epileptogenesis and epilepsy: anticonvulsant net effect via kappa etomidate receptors. Brain. 2007;130(Pt 4):1017–1028. doi: 10.1093/brain/awl384 [DOI] [PubMed] [Google Scholar]
- 9.Liou JT, Hsu JC, Liu FC, et al. Pretreatment with small-dose ketamine reduces withdrawal movements associated with injection of rocuronium in pediatric patients. Anesth Analg. 2003;97(5):1294–1297. [DOI] [PubMed] [Google Scholar]
- 10.Clark HD, Wells GA, Hut C, et al. Assessing the quality of randomized trials: reliability of the jadad scale. Control Clin Trials. 1999;20:448–452. doi: 10.1016/S0197-2456(99)00026-4 [DOI] [PubMed] [Google Scholar]
- 11.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557e560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang J, Liu L, Lyu G. Comparison of the effects of intravenous pre-treatment of butorphanol and dezocine on prevention of etomidate-induced myoclonus. Tianjin Med J. 2015;43(12):1450–1453. [Google Scholar]
- 13.Yan ZY, Guo GJ, Tia NJT. Influence of preinjection butorphanol on myoclonus caused by etomidate during induction of general anesthesia. Med Innovation China. 2015;12(2):32–35. [Google Scholar]
- 14.Zhang J, Liu L, Liu HP, Lv GY. Comparison of butorphanol or midazolam alone and combination of the two drugs in preventing etomidate-induced myoclonus during anesthesia induction. Chin J Anesthesiol. 2015;35(11):1325–1327. [Google Scholar]
- 15.Zhao XH, Li JB, Deng XM, Xiong YC, Zhao JH, Ning JS. Comparison of the effects of intravenous pretreatment with butorphanol and fentanyl on the etomidate-induced myoclonus. Chin J Anesthesiol. 2008;28(3):280–281. [Google Scholar]
- 16.Ren J, Lan P, Yuan RM. Effect of butorphanol on the etomidate-induced myoclonus during general anesthesia induction. Shandong Med J. 2013;48(53):58–60. [Google Scholar]
- 17.Berry JM, Merin RG. Etomidate myoclonus and the open globe. Anesth Analg. 1989;69:256–259. [PubMed] [Google Scholar]
- 18.Sedighinejad A, Naderi Nabi B, Haghighi M, et al. Comparison of the effects of low-dose midazolam, magnesium sulfate, remifentanil and low-dose etomidate on prevention of etomidate-induced myoclonus in orthopedic surgeries. Anesthesiology Pain Med. 2016;6(2):e35333. doi: 10.5812/aapm.35333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herrera-Peco I, Wix-Ramos R, Dominguez-Gadea L, et al. Cambios en la perfusión cerebral inducidos por etomidato en pacientes con epi-lepsia del lóbulo temporal. [Changes in cerebral perfusion induced by etomidate in patients with temporal lobe epilepsy]. Rev Neurol. 2009;49(11):561–565. [PubMed] [Google Scholar]
- 20.Gancher S, Laxer KD, Krieger W. Activation of epileptogenic activity by etomidate. Anesthesiology. 1984;61:616–618. [DOI] [PubMed] [Google Scholar]
- 21.Kelsaka E, Karakaya D, Sarihasan B, Baris S. Remifentanil pretreatment reduces myoclonus after etomidate. J Clin Anesth. 2006;18(2):83–86. doi: 10.1016/j.jclinane.2005.05.004 [DOI] [PubMed] [Google Scholar]
- 22.Li X, Liu J, Zhou M, Zhou C. Parecoxib sodium pretreatment reduces myoclonus after etomidate: A prospective, double-blind, randomized clinical trial. Int J Clin Pharmacol Ther. 2017;55(7):601–605. doi: 10.5414/CP202768 [DOI] [PubMed] [Google Scholar]
- 23.Wang J, Li QB, Wu YY, Wang B-N, Kang J-L, Xu X-W. Efficacy and safty of opioids for the prevention of etomidate-induced myoclonus: a meta-analysis. Am J Ther. 2018;25(5):e517–e523. doi: 10.1097/MJT.0000000000000404 [DOI] [PubMed] [Google Scholar]
- 24.Zou L, Yuan H, Wang HY, Geng Z-Y, Xu L, Sun L. Role of target controlled infusion of remifentanil for the prevention of etomidate induced myoclonus during general anesthesia. Zhongguo yi xue ke xue yuan xue bao. Acta Academiae Medicinae Sinicae. 2013;35(1):112–115. [PubMed] [Google Scholar]
- 25.Manocha A, Mediratta PK, Sharma KK. Studies on the anticonvulsant effect of U50488H on maximal electroshock seizure in mice. Pharmacol Biochem Behav. 2003;76(1):111–117. doi: 10.1016/S0091-3057(03)00218-1 [DOI] [PubMed] [Google Scholar]