Abstract
Purpose
African Americans who shed JC polyomavirus (JCV) in their urine have reduced rates of nondiabetic chronic kidney disease (CKD). We assessed the associations between urinary JCV and urine BK polyomavirus (BKV) with CKD in African Americans with diabetes mellitus.
Methods
African Americans with diabetic kidney disease (DKD) and controls lacking nephropathy from the Family Investigation of Nephropathy and Diabetes Consortium (FIND) and African American-Diabetes Heart Study (AA-DHS) had urine tested for JCV and BKV using quantitative PCR. Of the 335 individuals tested, 148 had DKD and 187 were controls.
Results
JCV viruria was detected more often in the controls than in the patients with DKD (FIND: 46.6% vs 32.2%; OR, 0.52; 95% CI, 0.29 to 0.93; P = 0.03; AA-DHS: 30.4% vs 26.2%; OR, 0.63; 95% CI, 0.27 to 1.48; P = 0.29). A joint analysis adjusted for age, sex, and study revealed that JC viruria was inversely associated with DKD (OR, 0.56; 95% CI, 0.35 to 0.91; P = 0.02). Statistically significant relationships between BKV and DKD were not observed.
Main Conclusions
The results from the present study extend the inverse association between urine JCV and nondiabetic nephropathy in African Americans to DKD. These results imply that common pathways likely involving the innate immune system mediate coincident chronic kidney injury and restriction of JCV replication. Future studies are needed to explore causative pathways and characterize whether the absence of JC viruria can serve as a biomarker for DKD in the African American population.
Relationships between JC viruria and DKD were assessed. Just as in nondiabetic nephropathy, JC viruria showed a protective association with DKD.
Among individuals with two apolipoprotein L1 (APOL1) gene renal-risk variants (RRVs), only a subset will develop chronic kidney disease (CKD). Thus, disease modifiers are required to cause a renal phenotype (1–5). The most potent risk factor for nephropathy in individuals with APOL1-mediated genetic risk is untreated HIV infection leading to HIV-associated nephropathy (1–3). Thus, the NHAANS (Natural History of APOL1-Associated Nephropathy Study) evaluated first-degree relatives of African Americans with nondiabetic end-stage renal disease (ESRD) to detect the active replication of viruses with properties similar to those of HIV, because they might contribute to APOL1-associated CKD (6). Plasma cytomegalovirus and human herpes virus 6 were analyzed because they are lymphotropic, and urine JC polyomavirus (JCV) and BK polyomavirus (BKV) were analyzed because they maintain renal reservoirs of infection. NHAANS detected an unexpected protective association between urine JCV and CKD (6–8).
The US–Israel Binational Science Foundation (BSF) study recruited additional African Americans and replicated the substantial protective association between JCV viruria and nondiabetic CKD (9). A meta-analysis that included the NHAANS and BSF revealed the protective association with JC viruria was present in African Americans with and without APOL1 renal-risk genotypes (9). These results supported those from a Brazilian report in which greater rates of urinary JCV were seen in the general population compared with the rates in those with advanced kidney disease (10).
It is unknown whether a protective association exists between nephropathy and urine JCV in patients with diabetes mellitus. To address this, data from African Americans in the FIND (Family Investigation of Nephropathy and Diabetes) and AA-DHS (African American–Diabetes Heart Study) were analyzed. FIND enrolled self-reported African Americans with and without diabetes mellitus to identify the genes underlying diabetic kidney disease (DKD) (11). The AA-DHS was initiated to identify environmental and inherited factors contributing to the development and progression of cardiovascular disease (CVD) in African Americans with type 2 diabetes (T2D) (12). We report the associations between JC viruria and DKD in the FIND and AA-DHS participants.
Materials and Methods
Study populations
AA-DHS recruited self-reported African Americans with T2D at the Wake Forest School of Medicine (WFSM) from May 2007 through August 2010 (12). T2D was clinically diagnosed according to an age at onset >30 years, receipt of insulin and/or oral glucose-lowering agents, fasting glucose ≥7.0 mmol/L, nonfasting glucose ≥11.1 mmol/L, and/or glycosylated hemoglobin (GHb) >6.5% (48 mmol/mol) in the absence of diabetic ketoacidosis. Individuals with known previous serum creatinine concentrations of ≥176.8 μmol/L were not recruited. The baseline assessments included a medical history, anthropometric measures, blood pressure, fasting serum glucose, serum creatinine, GHb, and spot urine albumin/creatinine ratio (UACR).
The FIND design and genetic results have been previously reported (11, 13). Families of probands with DKD with a diabetic sibling with or without nephropathy were recruited. Where available, one nondiabetic sibling, parent, or other relative (i.e., avuncular, cousin, half-sibling, and grandparent affected pairs) was recruited. The present analysis was limited to African American FIND participants recruited at WFSM.
The WFSM institutional review board approved the AA-DHS and FIND, and all participants had provided written informed consent. The estimated glomerular filtration rate (eGFR) was computed using the creatinine-based Chronic Kidney Disease Epidemiology Collaboration equation (14). Individuals were considered patients with DKD if they had a Chronic Kidney Disease Epidemiology Collaboration eGFR of <60 mL/min/1.73m2 and/or a UACR of >300 mg/g. Those without nephropathy (controls) had an eGFR >60 mL/min/1.73m2 and a UACR <30 mg/g.
Nucleic acid extraction and quantitative PCR for JCV and BKV
Frozen urine (200 μL) samples from the participants were thawed, and total nucleic acid was extracted. The extraction procedure used the Magna Pure LC, version 2.0, apparatus and Magna Pure LC total Nucleic Acid Isolation Kit Reagents (Roche Diagnostics, Germany) according to the manufacturer’s instructions (elution volume, 100 µL).
TaqMan real time PCR
The primers and probes were derived from nucleotide sequences of the N terminus of the viral T antigen of JCV and BKV: JCV, 5′-GAGTGTTGGGATCCTGTGTTTT-3′ (forward); 5′-AGAAGTGGGATGAAGACCTGTTT-3′ (reverse); and Probe 5′FAM-TCATCACTGGCAAACATTTCTTCATGGC-BHQ-1-3′; BKV: 5′-TTGCTTCTTCATCACTGGCAA-3′ (forward); 5′-AGTCCTGGTGGAGTTCCTTTAATG-3′ (reverse); and probe 5′FAM-CATATCTTCATGGCAAAATAAATCTTCATCTCATCCCATTT-BHQ-1-3′.
The PCR reaction was performed in a total volume of 25 μL containing Absolute Blue quantitative PCR mix (Thermo Scientific, UK) in the presence of 10 μL target DNA, 300 nM of each primer and 200 nM of the probe. PCR was performed using the rotor gene 6000/Q instrument (Corbett Research/Qiagen, Hilden, Germany) under the following conditions: 15 minutes at 95°C and 45 cycles of 15 seconds at 95°C and 60 seconds at 60°C. For quantitative results analysis, an average standard curve was constructed using quantified JCV and BKV DNA (Advanced Biotechnology Industry, MD). The results are reported as the number of JCV/BKV genome copies per 1 mL of urine. The lowest detection level was 100 genomic copies per 1 mL.
Statistical analysis
Wilcoxon two-sample tests were computed to compare the distribution of continuous outcomes between JCV-positive and JCV-negative participants. An association between the categorical outcomes and JCV status was tested using the Fisher exact test when the cell counts were less than five and χ2 tests, as appropriate. Logistic regressions were fitted to test for association between JCV status and presence of DKD, adjusting for age and sex. The analyses were run separately for each study and in a combined data set that included participants from FIND and AA-DHS with adjustment for the study to account for differences between populations.
An inverse variance-weighted meta-analysis was performed of both FIND and AA-DHS participants and the results from 300 African American NHAANS participants and 200 unrelated BSF study participants (6, 7, 9). The percentage of variation due to the heterogeneity among the studies was computed (15, 16).
Results
African Americans with DKD (cases) and those without nephropathy (controls) in the FIND and AA-DHS were evaluated for the presence and titer of JCV and BKV viruria. The demographic and clinical characteristics for 205 FIND and 130 AA-DHS participants stratified by case-control status are presented in Table 1. Both cohorts included more women than men. Because the AA-DHS analyzed subclinical CVD and attempted to exclude individuals with advanced nephropathy, the FIND cases had a significantly lower median eGFR. In contrast, the AA-DHS cases had higher UACR and GHb. The diabetes duration was longer in the cases than in the controls in both studies. The FIND cases had a significantly longer diabetes duration than the AA-DHS cases. Although the frequency of JC viruria was greater in the controls from both studies compared with their respective cases, BK viruria did not differ significantly between the cases and controls. In FIND, 46.6% of the controls had JC viruria compared with 32.2% of DKD cases (P = 0.04). In AA-DHS, 30.4% of the controls had JC viruria compared with 26.2% of the controls (P = 0.47).
Table 1.
Baseline Demographic Characteristics and Clinical Parameters of the FIND and AA-DHS Cohorts
| Variable | Cases With DKD |
Controls Without Nephropathy |
||||
|---|---|---|---|---|---|---|
| AA-DHS (n = 61) | FIND (n = 87) | P Value | AA-DHS (n = 69) | FIND (n = 118) | P Value | |
| Without diabetes, % | 0 | 0 | 0 | 15 (n = 18) | 0.0002 | |
| Female sex, n (%) | 37 (61) | 55 (63) | 0.86 | 49 (71) | 78 (66) | 0.52 |
| Age at enrollment, y | 58 (51–66) | 62 (55–70) | 0.049 | 52 (48–58) | 58 (51–66) | 0.0003 |
| Diabetes duration, y | 13 (9–20) | 20 (14–25) | 0.0006 | 8 (6–12) | 11 (2–18) | 0.89 |
| CKD-EPI eGFR, mL/min/1.73m2 | 54 (45–86) | 48 (32–57) | 0.0014 | 117 (113–122) | 93 (79–113) | < 0.0001 |
| UACR, mg/g | 362 (47–917) | 49 (14–126) | <0.0001 | 5.9 (4.0–11.0) | 1.0 (0.3–2.7) | < 0.0001 |
| Hemoglobin A1c, % | 8.7 (7.6–10.2) | 7.4 (6.4–9.5) | 0.0069 | 7.7 (6.6–8.8) | 6.9 (5.9–8.5) | 0.0091 |
| APOL1 2 RRVs, n/N (%) | 10/59 (17) | 9/55 (16) | >0.99 | 6/68 (9) | 7/80 (9) | > 0.99 |
| Log (JC titers)a | 10.4 (9.3–16.2) | 12.1 (9.2–15.3) | 0.98 | 13.1 (11.1–13.7) | 14.3 (11.0–16.4) | 0.073 |
| JC virus, % | 26.2 | 32.2 | 0.47 | 30.4 | 46.6 | 0.032 |
| Log (BK titers)a | 5.1 (4.6–7.1) | 5.3 (4.3–6.4) | 0.98 | 6.7 (4.8–8.8) | 6.2 (5.0–8.2) | 0.93 |
| BK virus, % | 18.0 | 16.1 | 0.83 | 23.2 | 21.2 | 0.85 |
Data presented as median (25th percentile to 75th percentile) for continuous variables or % for categorical variables (continuous data were analyzed using Wilcoxon two-sample test and categorical data using Fisher exact test).
Abbreviation: CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration.
Data from subjects with virus present.
The results of the association analysis between JC viruria and DKD in African Americans from the FIND and AA-DHS, adjusted for age and sex, and a joint analysis adjusted for study are presented in Table 2. A significantly greater prevalence of JC viruria was detected in the controls compared with those with DKD in the joint analysis (OR, 0.56; 95% CI, 0.35 to 0.91; P = 0.019). Among the African Americans with JC viruria in the combined studies, the log(JCV) viral titers showed a trend toward greater levels in the non-nephropathy controls compared with the DKD cases (P = 0.07). No statistically significant effect was observed for presence of BK viruria in either study or in the combined studies (OR, 0.68; 95% CI, 0.38 to 1.20; P = 0.18). In those with BK viruria in the combined studies, the viral titers were not different between the non-nephropathy controls and DKD cases [median log(BKV), 6.4 and 5.1, respectively; P = 0.15].
Table 2.
Kidney Disease and Association with JC and BK Viruria in Diabetes-Affected African-American Cohorts
| Cohort | Patients, n | β (SE) | OR | 95% CI | P Value |
|---|---|---|---|---|---|
| JCV in AA-DHSa | 130 | −0.4552 (0.4337) | 0.63 | (0.27–1.48) | 0.29 |
| JCV in FINDa | 205 | −0.6616 (0.3018) | 0.52 | (0.29–0.93) | 0.028 |
| JCV in AA-DHS and FIND combinedb | 335 | −0.5768 (0.2464) | 0.56 | (0.35–0.91) | 0.019 |
| BKV in AA-DHSa | 130 | −0.3742 (0.4661) | 0.69 | (0.28–1.72) | 0.42 |
| BKV in FINDa | 205 | −0.3942 (0.3758) | 0.67 | (0.32–1.41) | 0.29 |
| BKV in AA-DHS and FIND combinedb | 335 | −0.3915 (0.2908) | 0.68 | (0.38–1.20) | 0.18 |
Models included adjustment for age and sex.
Model included additional adjustment for study.
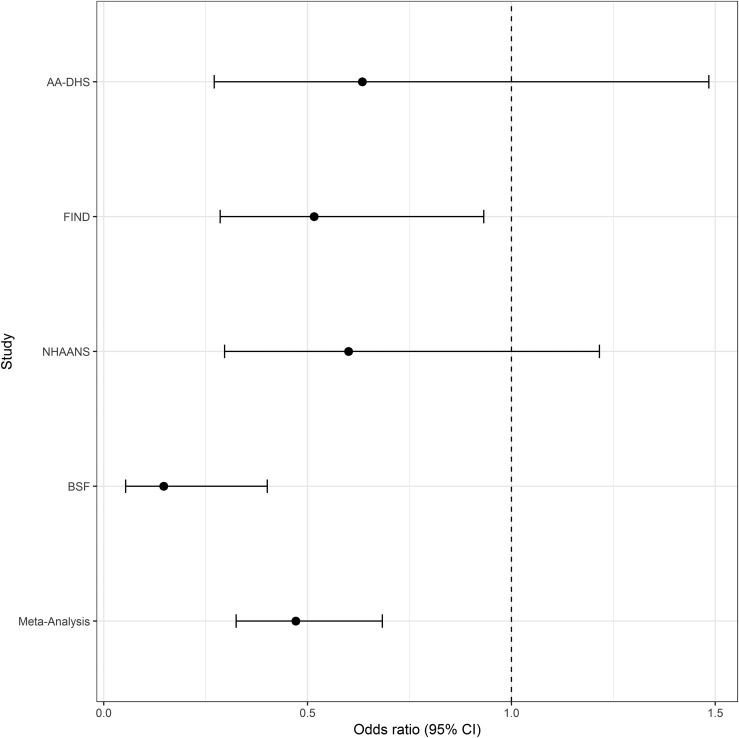
The results of a meta-analysis of 885 individuals that compared the presence vs absence of JC viruria in African American non-nephropathy controls and CKD cases from the FIND, AA-DHS, NHAANS, and BSF are shown in Fig. 1. In contrast to FIND and AA-DHS, the NHAANS and BSF cases had nondiabetic etiologies of CKD. This meta-analysis revealed a statistically significant protective association between JCV and all-cause CKD (OR, 0.47; 95% CI, 0.33 to 0.68; P = 0.0001). The heterogeneity between studies (I2) was 0.26. Single-study results from the FIND revealed a protective OR of 0.52 (95% CI, 0.29 to 0.93; P = 0.03). In AA-DHS, the OR was 0.63 (95% CI, 0.27 to 1.48; P = 0.29). In NHAANS, the OR was 0.60 (95% CI, 0.30 to 1.22; P = 0.16), and in BSF, the OR was 0.15 (95% CI, 0.05 to 0.40; P = 0.0002).
Figure 1.
Inverse-weighted meta-analysis for the relationship between JC viruria and CKD in African Americans. Effect presented as OR (95% CI); values <1.0 indicate a negative association between CKD in individuals with JC viruria.
We assessed whether the frequencies of JCV viruria differed by APOL1 genotype in FIND and AA-DHS. Of the 262 individuals with genotype data available, 16.7% (19 of 114) of DKD cases and 8.8% (13 of 148) of controls had two APOL1 RRVs (P = 0.059). In the participants with two APOL1 RRVs, 26% (5 of 19) of DKD cases and 38% (5 of 13) of controls had JC viruria (P = 0.70). Among those with zero or one APOL1 RRV, 34% (32 of 95) of DKD cases and 41% (55 of 135) of controls had JC viruria (P = 0.33).
The frequency of urinary tract coinfection with JCV and BKV was analyzed. Among the FIND and AA-DHS cases, 13.5% had BK viruria alone, 26.3% had JC viruria alone, 56.8% had neither virus, and 3.4% had both. Among the FIND and AA-DHS controls, 14.4% had BK viruria alone, 33.2% had JC viruria alone, 44.9% had neither virus, and 7.5% had both. Overall, JCV was detected in 37.6% of non-BK viruric individuals compared with 28.8% of BK viruric individuals (P = 0.20). No association, either multiplicative or inhibitory, was found between JCV and BKV. Thus, the presence of two simultaneous polyomavirus infections in the urinary tract was relatively uncommon and does not support an inhibitory interaction. In contrast, decreased JCV urine shedding has been related to increased excretion of the more nephropathic BKV (17).
Discussion
The results from the present study extend the protective association between JC viruria and nondiabetic nephropathy in African Americans to that associated with diabetes. An effort is ongoing to identify non–HIV viral triggers as second hits to explain the variable risk of developing APOL1-associated nephropathy in individuals with two APOL1 RRVs. These analyses were motivated by the powerful APOL1 association with HIV-associated nephropathy (1–5). However, instead of identifying an injurious infection, an initial study had identified a protective association between urine shedding of JCV and CKD (6). A subsequent study revealed that the protective relationship with JCV in nondiabetic CKD was not confined to those with APOL1 high-risk genotypes (9).
Given the lack of an association between APOL1 RRVs and DKD (18–20), the present analyses explored whether urine JCV excretion was greater in patients with diabetes who lacked kidney disease compared with those with DKD. A protective association between JC viruria in African Americans with DKD was again observed, expanding the spectrum of kidney diseases associated with reduced rates of JC viruria. However, cases with DKD in the diabetic FIND and AA-DHS cohorts had greater rates of urine JCV shedding compared with cases with nondiabetic CKD in the BSF study (29.7% vs 8.75%) (6, 9). This might imply that although diabetic and nondiabetic CKD share common pathways that interact with JCV replication (most likely mediators of the innate immune system), the interaction might be more robust in nondiabetic CKD.
Our findings are in accordance with a report by Pires et al. (10) of increased detection of JC viruria in the general Brazilian population compared with those with ESRD. They hypothesized that a reduced renal mass or a high concentration of inhibitory molecules might perturb the urine PCR reaction in uremia (10). However, in contemporary cohorts (6, 9), FIND and AA-DHS, the median eGFR in cases with nephropathy was far greater than that in ESRD. This suggests that a reduced renal mass fails to explain this observation (6, 9). As Cheng et al. (17) reported, inhibitory interactions might be present between urinary tract infection and JCV and BKV. In the present report, 3.4% and 7.5% of the FIND and AA-DHS participants (cases and controls, respectively) had coinfections. These results are inconsistent with an inhibitory interaction in which decreased JCV urine shedding is related to increased excretion of the more nephropathic BKV (17). However, we could not exclude potential inhibitory interactions with other pathogens that exploit similar endocytic trafficking to JCV, namely clathrin-dependent endocytosis and uptake via caveolin-1–positive endosomal compartments (21) nor other uncharacterized shared pathways.
Just as in all cross-sectional studies, it was difficult to discriminate between causality vs consequence of CKD. The trend toward greater viral titers in the controls (vs the cases) with JCV infection was reassuring and suggests a potential dose–effect interaction. However, this could not determine whether JCV viruria confers a causal protective cellular mechanism antagonizing upregulated injurious pathways in CKD or whether reduced viruria is an epiphenomenon that reflects upregulated pathways mediating kidney injury and, in parallel, restrict JCV replication. The latter could include enhanced inflammatory signaling (22–24).
Biologic differences mediating the protective association of JCV (but not BKV) with DKD are not known. The increased urinary excretion of JCV in the controls contrasted with the similar excretion of BKV in DKD cases and controls, as we reported previously in nondiabetic kidney disease (6, 9). Although BKV and JCV belong to the polyomavirus family and share many similarities, they differ with respect to their interaction with the innate immune system. Their target organs for injury in immunosuppressed individuals differ, with BKV targeting the kidney and causing nephropathy, and JCV targeting the central nervous system and causing progressive multifocal leukoencephalopathy (25, 26). JCV- and BKV-specific antibodies are detected in ~44% to 92% and ~55% to 85% of adults, respectively. The seroprevalence of JCV increases with aging, but the opposite occurs with BKV. Thus, JCV immunologic stimulation might continue in immunocompetent hosts, either by virus reactivation or reinfection (26–29). Primary, usually subclinical, infection with transient viremia occurs in childhood. Subsequently, the virus produces a persistent latent infection in the kidney epithelial cells and lymphocytes and is shed into the urine at variable titers throughout life (25–31). Low levels of JCV replication and shedding are common in immunocompetent individuals (25–27, 29, 31); however, surprisingly, the incidence of symptomatic viruria with impaired renal allograft function is uncommon, compared with the nephropathic BKV (17). Similarly, only BK viruria (but not JC viruria) will be increased in immunocompromised patients with HIV infection and correlates inversely with the CD4+ cell count (31–33). Thus, the state of immune suppression (mainly in kidney and other transplant recipients) enhances BKV shedding in the urine, which entails an increased risk of BK nephropathy (17). In contrast, JC shedding reflects an immunocompetent state that only partially suppresses viral replication and might translate into improved graft survival with fewer acute rejection episodes after kidney transplantation (17). These findings suit the concept of a “virus–immune system equilibrium,” in which the JC viral load does not have a pathogenic role, similar to the nonpathogenic course of infection by simian immunodeficiency viruses in their natural hosts, such as sooty mangabey monkeys (34).
We postulated that the reduced JC viruria in kidney disease might reflect enhanced inflammatory signaling (e.g., via the type I interferon signaling cascade that abrogates JC replication; Fig. 2). In accordance with this hypothesis, Assetta et al. (35) deciphered the different interactions between polyomaviruses with regard to interferon signaling. They demonstrated that JCV infection of primary human renal epithelial cells remained constant and low for 3 weeks, and BKV spread efficiently and killed the cells by day 15. Although JCV- and BKV-infected cells released IFN-β, BKV-infected cells did not activate the antiviral response mediated by interferon, which was confirmed by the lack of phosphorylated STAT1 in the nucleus of BKV-infected cells. Instead, two critical suppressors of cytokine signaling were induced, SOCS3 and SOCS1. In direct contrast, JCV significantly induced interferon-stimulated genes. Phosphorylated STAT1 and interferon regulatory factor 9 were found in the nucleus of JCV-infected cells, and the block of interferon α/β receptor signaling partially increased JCV infectivity. These observations point to divergent innate immune responses that control JCV replication but fail to control BKV (35). We postulated that decreased shedding of JCV might reflect activation of the innate immune system, limiting viral replication in diabetic and nondiabetic kidney disease.
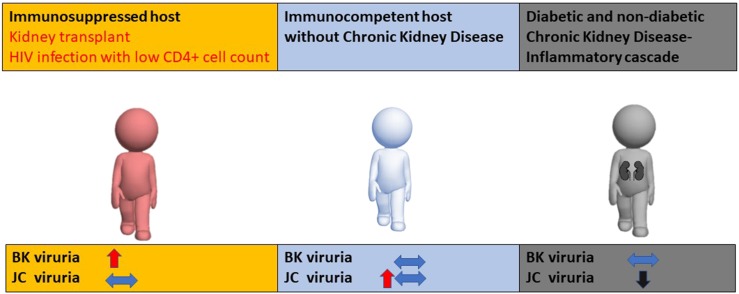
Figure 2.
Postulated association between immune system suppression and activation with polyomaviruria. (Left) Immunosuppressed host: BK viruria increases and can cause pathologic BK virus nephropathy, with no effect on JC viruria. (Middle) Immunocompetent host without CKD: JC or BK viruria could be present without clinical consequences. (Right) Host with CKD: upregulated inflammatory signaling restricts JC viruria with no effect on BK virus replication.
Increased interferon signaling by JCV would be expected to lead to increased translation of APOL1 protein, because APOL1 is an interferon-stimulated gene (4). This might predict an association of JC viruria with an increased risk of kidney disease. However, no correlations were found between APOL1 expression in kidney biopsies from patients with CKD or circulating APOL1 plasma levels and CKD (36–39), nor was the protective association of viruria with kidney disease dependent on APOL1 genotype, as described in the present study and in previous data. Therefore, interferon-stimulated increases in APOL1 do not appear to play a pathogenic role in individuals with JC viruria.
The lack of association with APOL1 high-risk genotypes is in accordance with the similar JCV protective association with DKD and nondiabetic CKD (6, 9). We hypothesized that upregulation of common upstream inflammatory pathways that restrict JCV replication might play a role in a wide spectrum of kidney diseases and that the downstream pathways would diverge into those specifically mediating DKD and nondiabetic kidney disease (including APOL1-associated nephropathy). Similar upstream inflammatory pathways for APOL1-associated nephropathy and DKD are supported by the shared predictive association of biomarkers, including tumor necrosis factor receptor-1 and -2 and kidney injury molecule-1 with the composite renal outcome of ESRD and sustained renal functional decline in patients with and without diabetes with two APOL1 RRVs (40, 41). Inflammation plays a major role in the progression of APOL1-mediated forms of nephropathy. APOL1 expression is induced by interferon. Collapsing focal segmental glomerulosclerosis has been described in patients with APOL1 high-risk genotypes treated with interferon; increased transcripts of CXCL9 and CXCL11 have been demonstrated in kidney biopsies of participants at high genotypic risk in the NEPTUNE (Nephrotic Syndrome Study Network) cohort; and pyroptotic cell death mediates APOL1-induced cell death (42–44). Similarly, accumulating data have emphasized the critical role of inflammation in the pathogenesis of DKD. The expression of cell adhesion molecules, growth factors, chemokines, and proinflammatory cytokines are increased in the renal tissues of patients with diabetes, and the serum and urinary levels of cytokines and cell adhesion molecules have correlated with albuminuria (23). Specific pathways potentially contributing to JCV restriction and reduced urine shedding remain to be characterized. Hence, we postulated that JCV restriction is an epiphenomenon that might imply ongoing inflammatory signaling in the kidney and, thereby, potentially serving as a biomarker that correlates with activation of the innate immune system (e.g., interferon signaling). Nevertheless, a causative protective role for JCV in CKD could not be excluded.
The present study had strengths and limitations. The strengths included the assessment of virus replication in well-characterized diabetes-affected cohorts. The limitations included the following. JCV was measured only once, and we could not exclude fluctuations in JC viruria over time (30). We did not measure JCV replication in the plasma among those with urinary tract replication, because JCV viremia is rare. We also lacked kidney biopsy specimens from the FIND and AA-DHS participants to directly examine the presence of JCV DNA. However, JCV DNA has been shown to be present in the human kidney (9). Also, the FIND African American sample included 18 controls without diabetes; therefore, we reanalyzed the FIND data, excluding the nondiabetic controls. Despite the reduced power, the results were in the same direction, with increased rates of JC viruria (P = 0.13) and higher viral titers in the diabetic controls compared with the diabetic cases with JC viruria (P = 0.026). Because most study subjects did not have kidney biopsy specimens, it is possible that some did not have DKD. Finally, the protective association between JCV and DKD was stronger in FIND than in AA-DHS, although a meta-analysis revealed a statistically significant relationship after adjustment for the study. FIND and AA-DHS had markedly different study designs. FIND was enriched for participants with DKD and attempted to identify the genetic basis of DKD. In contrast, AA-DHS focused on subclinical CVD. AA-DHS attempted to exclude participants with advanced DKD owing to the known relationship between CKD and subclinical CVD. Despite their differences, the association results in both studies were consistent in direction.
In conclusion, the results from the present study have confirmed an inverse association between urine JCV and nephropathy and extended the protective relationship to diabetes-related kidney disease. This clinical observation suggests that common upstream shared pathways, most likely in the innate immune system, jointly mediate the risk of nephropathy and contribute to the restriction of JCV replication and urinary shedding. Further studies are required to explore the involved pathways and characterize whether the absence of JC viruria can serve as a predictive biomarker antedating the risk of DKD in longitudinal cohorts. Such a biomarker could prove useful in identifying individuals with early DKD and informing practitioners to implement therapies to delay kidney disease progression.
Acknowledgments
We acknowledge the support provided by the Israel Science Foundation, an academic research grant from GlaxoSmithKline, the Ernest and Bonnie Beutler Research Grant Program in Genomic Medicine, and the Kaylie Kidney Research Center of Excellence at Rambam. A list of the members of the FIND Research Group follows (key: *principal investigator; **co-investigator; #program coordinator; §University of California, Davis; †University of California, Irvine; ‡study chair): Genetic Analysis and Data Coordinating Center, Case Western Reserve University, *S. K. Iyengar,**R. C. Elston,**K. A. B. Goddard, **J. M. Olson, S. Ialacci, #J. Fondran, A. Horvath, R. Igo, Jr., G. Jun, K. Kramp, J. Molineros, S. R. E. Quade; Case Western Reserve University, *J. R. Sedor, **J. Schelling, #A. Pickens, L. Humbert, L. Getz-Fradley; Harbor-University of California, Los Angeles, Medical Center, *S. Adler, **E. Ipp, **†M. Pahl, **§M. F. Seldin, **S. Snyder, **J. Tayek, #E. Hernandez, #J. LaPage, C. Garcia, J. Gonzalez, M. Aguilar; Johns Hopkins University, *M. Klag, *R. Parekh, **L. Kao, **L. Meoni, T. Whitehead, #J. Chester; National Institute of Diabetes and Digestive and Kidney Diseases, Phoenix, AZ, *W. C. Knowler, **R. L. Hanson, **R. G. Nelson, **J. Wolford, #L. Jones, R. Juan, R. Lovelace, C. Luethe, L. M. Phillips, J. Sewemaenewa, I. Sili, B. Waseta; University of California, Los Angeles, *M. F. Saad, *S. B. Nicholas, **Y.-D. I. Chen, **X. Guo, **J. Rotter, **K. Taylor, M. Budgett, #F. Hariri; University of New Mexico, Albuquerque, *P. Zager, *V. Shah, **M. Scavini, #A. Bobelu; University of Texas Health Science Center at San Antonio, *H. Abboud, **N. Arar, **R. Duggirala, **B. S. Kasinath, **F. Thameem, **M. Stern; Wake-Forest University, *‡B. I. Freedman, **D. W. Bowden, **C. D. Langefeld, **S. C. Satko, **S. S. Rich, #S. Warren, S. Viverette, G. Brooks, R. Young, M. Spainhour; Laboratory of Genomic Diversity, National Cancer Institute, Frederick, MD, *C. Winkler, **M. W. Smith, M. Thompson, #R. Hanson, B. Kessing; Minority Recruitment Centers, Loyola University, *D. J. Leehey, #G. Barone; University of Alabama at Birmingham, *D. Thornley-Brown, #C. Jefferson; University of Chicago, *O. F. Kohn, #C. S. Brown; National Institute of Diabetes and Digestive and Kidney Diseases program office, J. P. Briggs, P. L. Kimmel, R. Rasooly; External Advisory Committee, D. Warnock (chair), L. Cardon, R. Chakraborty, G. M. Dunston, T. Hostetter, S. J. O’Brien (ad hoc), J. Rioux, R. Spielman. We acknowledge the contributions of the Wake Forest participants and coordinators Joyce Byers, Carrie Smith, Mitzie Spainhour, Cassandra Bethea, and Sharon Warren and the contributions of the FIND participants and physicians and CHOICE patients, staff, laboratory, and physicians at Dialysis Clinic Inc. and Johns Hopkins University.
Financial Support: The FIND study was supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases and in part by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases. The present study was supported by the Israel Science Foundation (grant 182/15), an academic research grant from GlaxoSmithKline, the Ernest and Bonnie Beutler Research Grant Program in Genomic Medicine, and the Kaylie Kidney Research Center of Excellence at Rambam. This research was also supported by the US–Israel Binational Science Foundation Proposal (grant 2013504 to B.I.F. and K.L.S.) and the National Institutes of Health (grants U01 DK57298 to B.I.F. and R01 DK084149 to B.I.F.).
Disclosure Summary: Wake Forest University Health Sciences and B.I.F. have rights to an issued US patent related to APOL1 genetic testing (available at: www.apol1genetest.com). B.I.F. is a consultant for AstraZeneca Pharmaceuticals. The remaining authors have nothing to disclose.
Glossary
Abbreviations:
- AA-DHS
African American-Diabetes Heart Study
- APOL1
apolipoprotein L1
- BKV
BK polyomavirus
- BSF
US–Israel Binational Science Foundation
- CKD
chronic kidney disease
- CVD
cardiovascular disease
- DKD
diabetic kidney disease
- ESRD
end-stage renal disease
- eGFR
estimated glomerular filtration rate
- FIND
Family Investigation of Nephropathy and Diabetes Consortium
- GHb
glycosylated hemoglobin
- JCV
JC polyomavirus
- NHAANS
Natural History of APOL1-Associated Nephropathy Study
- RRV
renal-risk variant
- T2D
type 2 diabetes
- UACR
urine albumin/creatinine ratio
- WFSM
Wake Forest School of Medicine
References
- 1. Kopp JB, Nelson GW, Sampath K, Johnson RC, Genovese G, An P, Friedman D, Briggs W, Dart R, Korbet S, Mokrzycki MH, Kimmel PL, Limou S, Ahuja TS, Berns JS, Fryc J, Simon EE, Smith MC, Trachtman H, Michel DM, Schelling JR, Vlahov D, Pollak M, Winkler CA. APOL1 genetic variants in focal segmental glomerulosclerosis and HIV-associated nephropathy. J Am Soc Nephrol. 2011;22(11):2129–2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kasembeli AN, Duarte R, Ramsay M, Mosiane P, Dickens C, Dix-Peek T, Limou S, Sezgin E, Nelson GW, Fogo AB, Goetsch S, Kopp JB, Winkler CA, Naicker S. APOL1 risk variants are strongly associated with HIV-associated nephropathy in black South Africans. J Am Soc Nephrol. 2015;26(11):2882–2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Freedman BI, Skorecki K. Gene-gene and gene-environment interactions in apolipoprotein L1 gene-associated nephropathy. Clin J Am Soc Nephrol. 2014;9(11):2006–2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kruzel-Davila E, Wasser WG, Skorecki K. APOL1 nephropathy: a population genetics and evolutionary medicine detective story. Semin Nephrol. 2017;37(6):490–507. [DOI] [PubMed] [Google Scholar]
- 5. Dummer PD, Limou S, Rosenberg AZ, Heymann J, Nelson G, Winkler CA, Kopp JB. APOL1 kidney disease risk variants: an evolving landscape. Semin Nephrol. 2015;35(3):222–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Divers J, Núñez M, High KP, Murea M, Rocco MV, Ma L, Bowden DW, Hicks PJ, Spainhour M, Ornelles DA, Kleiboeker SB, Duncan K, Langefeld CD, Turner J, Freedman BI. JC polyoma virus interacts with APOL1 in African Americans with nondiabetic nephropathy. Kidney Int. 2013;84(6):1207–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kopp JB. JC viruria and kidney disease in APOL1 risk genotype individuals: is this a clue to a gene × environment interaction? Kidney Int. 2013;84(6):1069–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Divers J, Langefeld CD, Lyles DS, Ma L, Freedman BI. Protective association between JC polyoma viruria and kidney disease. Curr Opin Nephrol Hypertens. 2019;28(1):65–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Freedman BI, Kistler AL, Skewes-Cox P, Ganem D, Spainhour M, Turner J, Divers J, Langefeld CD, Murea M, Hicks PJ, Hemal AK, Snipes JA, Zhao L, Abend JR, Lyles DS, Ma L, Skorecki KL. JC polyoma viruria associates with protection from chronic kidney disease independently from apolipoprotein L1 genotype in African Americans. Nephrol Dial Transplant. 2018;33(11):1960–1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pires EP, Bernardino-Vallinoto CV, Alves DM, Migone SR, Machado LF, Ishak MO, Ishak R, Cayres-Vallinoto IM, Vallinoto AC. Prevalence of infection by JC and BK polyomaviruses in kidney transplant recipients and patients with chronic renal disease. Transpl Infect Dis. 2011;13(6):633–637. [DOI] [PubMed] [Google Scholar]
- 11. Iyengar SK, Sedor JR, Freedman BI, Kao WH, Kretzler M, Keller BJ, Abboud HE, Adler SG, Best LG, Bowden DW, Burlock A, Chen YD, Cole SA, Comeau ME, Curtis JM, Divers J, Drechsler C, Duggirala R, Elston RC, Guo X, Huang H, Hoffmann MM, Howard BV, Ipp E, Kimmel PL, Klag MJ, Knowler WC, Kohn OF, Leak TS, Leehey DJ, Li M, Malhotra A, März W, Nair V, Nelson RG, Nicholas SB, O’Brien SJ, Pahl MV, Parekh RS, Pezzolesi MG, Rasooly RS, Rotimi CN, Rotter JI, Schelling JR, Seldin MF, Shah VO, Smiles AM, Smith MW, Taylor KD, Thameem F, Thornley-Brown DP, Truitt BJ, Wanner C, Weil EJ, Winkler CA, Zager PG, Igo RP Jr, Hanson RL, Langefeld CD; Family Investigation of Nephropathy and Diabetes (FIND) . Genome-wide association and trans-ethnic meta-analysis for advanced diabetic kidney disease: Family Investigation of Nephropathy and Diabetes (FIND). PLoS Genet. 2015;11(8):e1005352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Freedman BI, Langefeld CD, Lu L, Palmer ND, Smith SC, Bagwell BM, Hicks PJ, Xu J, Wagenknecht LE, Raffield LM, Register TC, Carr JJ, Bowden DW, Divers J. APOL1 associations with nephropathy, atherosclerosis, and all-cause mortality in African Americans with type 2 diabetes. Kidney Int. 2015;87(1):176–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Knowler WC, Coresh J, Elston RC, Freedman BI, Iyengar SK, Kimmel PL, Olson JM, Plaetke R, Sedor JR, Seldin MF; Family Investigation of Nephropathy and Diabetes Research Group . The Family Investigation of Nephropathy and Diabetes (FIND): design and methods. J Diabetes Complications. 2005;19(1):1–9. [DOI] [PubMed] [Google Scholar]
- 14. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF III, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) . A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. [DOI] [PubMed] [Google Scholar]
- 17. Cheng XS, Bohl DL, Storch GA, Ryschkewitsch C, Gaudreault-Keener M, Major EO, Randhawa P, Hardinger KL, Brennan DC. Inhibitory interactions between BK and JC virus among kidney transplant recipients. J Am Soc Nephrol. 2011;22(5):825–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Genovese G, Friedman DJ, Ross MD, Lecordier L, Uzureau P, Freedman BI, Bowden DW, Langefeld CD, Oleksyk TK, Uscinski Knob AL, Bernhardy AJ, Hicks PJ, Nelson GW, Vanhollebeke B, Winkler CA, Kopp JB, Pays E, Pollak MR. Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science. 2010;329(5993):841–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tzur S, Rosset S, Shemer R, Yudkovsky G, Selig S, Tarekegn A, Bekele E, Bradman N, Wasser WG, Behar DM, Skorecki K. Missense mutations in the APOL1 gene are highly associated with end stage kidney disease risk previously attributed to the MYH9 gene. Hum Genet. 2010;128(3):345–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Friedman DJ, Kozlitina J, Genovese G, Jog P, Pollak MR. Population-based risk assessment of APOL1 on renal disease. J Am Soc Nephrol. 2011;22(11):2098–2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Querbes W, O’Hara BA, Williams G, Atwood WJ. Invasion of host cells by JC virus identifies a novel role for caveolae in endosomal sorting of noncaveolar ligands. J Virol. 2006;80(19):9402–9413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Anders HJ, Lichtnekert J, Allam R. Interferon-alpha and -beta in kidney inflammation. Kidney Int. 2010;77(10):848–854. [DOI] [PubMed] [Google Scholar]
- 23. Duran-Salgado MB, Rubio-Guerra AF. Diabetic nephropathy and inflammation. World J Diabetes. 2014;5(3):393–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Anders HJ, Ryu M. Renal microenvironments and macrophage phenotypes determine progression or resolution of renal inflammation and fibrosis. Kidney Int. 2011;80(9):915–925. [DOI] [PubMed] [Google Scholar]
- 25. Doerries K. Human polyomavirus JC and BK persistent infection. Adv Exp Med Biol. 2006;577:102–116. [DOI] [PubMed] [Google Scholar]
- 26. Knowles WA. Discovery and epidemiology of the human polyomaviruses BK virus (BKV) and JC virus (JCV). Adv Exp Med Biol. 2006;577:19–45. [DOI] [PubMed] [Google Scholar]
- 27. Egli A, Infanti L, Dumoulin A, Buser A, Samaridis J, Stebler C, Gosert R, Hirsch HH. Prevalence of polyomavirus BK and JC infection and replication in 400 healthy blood donors. J Infect Dis. 2009;199(6):837–846. [DOI] [PubMed] [Google Scholar]
- 28. Taguchi F, Kajioka J, Miyamura T. Prevalence rate and age of acquisition of antibodies against JC virus and BK virus in human sera. Microbiol Immunol. 1982;26(11):1057–1064. [DOI] [PubMed] [Google Scholar]
- 29. Polo C, Pérez JL, Mielnichuck A, Fedele CG, Niubò J, Tenorio A. Prevalence and patterns of polyomavirus urinary excretion in immunocompetent adults and children. Clin Microbiol Infect. 2004;10(7):640–644. [DOI] [PubMed] [Google Scholar]
- 30. Eash S, Manley K, Gasparovic M, Querbes W, Atwood WJ. The human polyomaviruses. Cell Mol Life Sci. 2006;63(7-8):865–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Knowles WA, Pillay D, Johnson MA, Hand JF, Brown DW. Prevalence of long-term BK and JC excretion in HIV-infected adults and lack of correlation with serological markers. J Med Virol. 1999;59(4):474–479. [PubMed] [Google Scholar]
- 32. Markowitz RB, Thompson HC, Mueller JF, Cohen JA, Dynan WS. Incidence of BK virus and JC virus viruria in human immunodeficiency virus-infected and -uninfected subjects. J Infect Dis. 1993;167(1):13–20. [DOI] [PubMed] [Google Scholar]
- 33. Behzad-Behbahani A, Klapper PE, Vallely PJ, Cleator GM, Khoo SH. Detection of BK virus and JC virus DNA in urine samples from immunocompromised (HIV-infected) and immunocompetent (HIV-non-infected) patients using polymerase chain reaction and microplate hybridisation. J Clin Virol. 2004;29(4):224–229. [DOI] [PubMed] [Google Scholar]
- 34. Silvestri G. Naturally SIV-infected sooty mangabeys: are we closer to understanding why they do not develop AIDS? J Med Primatol. 2005;34(5-6):243–252. [DOI] [PubMed] [Google Scholar]
- 35. Assetta B, De Cecco M, O’Hara B, Atwood WJ. JC polyomavirus infection of primary human renal epithelial cells is controlled by a type I IFN-induced response. MBio. 2016;7(4):e00903-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Madhavan SM, O’Toole JF, Konieczkowski M, Barisoni L, Thomas DB, Ganesan S, Bruggeman LA, Buck M, Sedor JR. APOL1 variants change C-terminal conformational dynamics and binding to SNARE protein VAMP8. JCI Insight. 2017;2(14):92581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Madhavan SM, O’Toole JF, Konieczkowski M, Ganesan S, Bruggeman LA, Sedor JR. APOL1 localization in normal kidney and nondiabetic kidney disease. J Am Soc Nephrol. 2011;22(11):2119–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bruggeman LA, O’Toole JF, Ross MD, Madhavan SM, Smurzynski M, Wu K, Bosch RJ, Gupta S, Pollak MR, Sedor JR, Kalayjian RC. Plasma apolipoprotein L1 levels do not correlate with CKD. J Am Soc Nephrol. 2014;25(3):634–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kozlitina J, Zhou H, Brown PN, Rohm RJ, Pan Y, Ayanoglu G, Du X, Rimmer E, Reilly DF, Roddy TP, Cully DF, Vogt TF, Blom D, Hoek M. Plasma levels of risk-variant APOL1 do not associate with renal disease in a population-based cohort. J Am Soc Nephrol. 2016;27(10):3204–3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nadkarni GN, Chauhan K, Verghese DA, Parikh CR, Do R, Horowitz CR, Bottinger EP, Coca SG. Plasma biomarkers are associated with renal outcomes in individuals with APOL1 risk variants. Kidney Int. 2018;93(6):1409–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kruzel-Davila E, Skorecki K. “Biomarking” the transition from genetic risk to kidney disease. Kidney Int. 2018;93(6):1270–1272. [DOI] [PubMed] [Google Scholar]
- 42. Nichols B, Jog P, Lee JH, Blackler D, Wilmot M, D’Agati V, Markowitz G, Kopp JB, Alper SL, Pollak MR, Friedman DJ. Innate immunity pathways regulate the nephropathy gene apolipoprotein L1. Kidney Int. 2015;87(2):332–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Beckerman P, Bi-Karchin J, Park AS, Qiu C, Dummer PD, Soomro I, Boustany-Kari CM, Pullen SS, Miner JH, Hu CA, Rohacs T, Inoue K, Ishibe S, Saleem MA, Palmer MB, Cuervo AM, Kopp JB, Susztak K. Transgenic expression of human APOL1 risk variants in podocytes induces kidney disease in mice. Nat Med. 2017;23(4):429–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sampson MG, Robertson CC, Martini S, Mariani LH, Lemley KV, Gillies CE, Otto EA, Kopp JB, Randolph A, Vega-Warner V, Eichinger F, Nair V, Gipson DS, Cattran DC, Johnstone DB, O’Toole JF, Bagnasco SM, Song PX, Barisoni L, Troost JP, Kretzler M, Sedor JR; Nephrotic Syndrome Study Network . Integrative genomics identifies novel associations with APOL1 risk genotypes in black NEPTUNE subjects. J Am Soc Nephrol. 2016;27(3):814–823. [DOI] [PMC free article] [PubMed] [Google Scholar]