Abstract
Background
25-hydroxyvitamin D [25(OH)D] is lower in black compared with white Americans but is not consistently associated with outcomes in this group, possibly due to genetic and other biological differences. We examined the association of plasma 25(OH)D and renal outcomes in black Americans with a focus on effect modifiers.
Methods
We studied associations between baseline 25(OH)D with (i) annual rate of estimated glomerular filtration rate (eGFR) decline and (ii) incident chronic kidney disease (CKD) in the Jackson Heart Study, a prospective cohort of black Americans. Plasma 25(OH)D levels were corrected for monthly variation in sunlight exposure using the residual method. We used adjusted generalized linear models to evaluate outcomes and assessed potential effect modification by diabetes mellitus, vitamin D binding protein (DBP) genotype, obesity, dietary sodium intake, and use of renin-angiotensin-aldosterone system inhibitors.
Results
Among 5164 participants with 25(OH)D available, plasma 25(OH)D was 14.5 ± 6.5 ng/mL (mean ± SD), and eGFR was 94.1 ± 22.0 mL/min/1.73 m2. Over a median of 8 years, eGFR decline was 1.3 ± 2.0 mL/min/1.73 m2 per year in 3228 participants with complete data, and 220 out of 1803 eligible participants developed incident CKD. Overall, 25(OH)D was not associated with eGFR decline in fully adjusted models. However, higher 25(OH)D was associated with slower eGFR decline among those with diabetes: each 5 ng/mL higher 25(OH)D was associated with a 0.27 mL/min/1.73 m2/y slower eGFR decline (95% CI, 0.13 to 0.41; P < 0.001). Higher 25(OH)D was not associated with incident CKD overall, but it was associated with lower odds of incident CKD among participants with the GG or GT genotype at rs7041 in the gene encoding DBP [OR, 0.69 per 5 ng/mL higher 25(OH)D; 95% CI, 0.51 to 0.93; P-interaction = 0.005]. Other interactions were not significant.
Conclusion
These findings support a potential benefit of higher 25(OH)D for kidney health in black Americans with diabetes or specific variants in DBP.
We elucidate a potential benefit of higher plasma 25-hydroxyvitamin D for CKD in a subset of blacks with diabetes and genetic variants that confer lower levels of the biologically active hormone.
Low levels of 25-hydroxyvitamin D [25(OH)D] may be a risk factor for adverse outcomes in the general population, including chronic kidney disease (CKD) outcomes (1–4). Blacks have generally lower levels of 25(OH)D compared with whites (5) and experience disproportionately high rates of CKD (6, 7). However, although low 25(OH)D is often associated with adverse CKD outcomes in diverse or primarily white populations (1–4), low total 25(OH)D is often not associated with adverse outcomes in blacks (8–11). This may be attributable, in part, to ancestry-related genetic differences that affect bioavailability of 25(OH)D, such as well-described differences in vitamin D binding protein (DBP) genotypes. Specifically, allele frequencies at rs7041 and rs4588 in the GC gene encoding DBP vary by race (12, 13), with a higher frequency of the T allele at rs7041 and the C allele at rs4588 in blacks. The T and C alleles at rs7041 and rs4588, respectively, affect levels of circulating DBP (12, 13); thus, individuals with different alleles may have optimal bioavailable 25(OH)D despite low total 25(OH)D (14–16). Analyses of kidney outcomes have not evaluated the impact of these DBP alleles on associations with 25(OH)D in blacks.
Like DBP variants, obesity may alter the relationship between circulating 25(OH)D and outcomes. Adipose tissue is a storage depot for fat-soluble 25(OH)D (17, 18). Therefore, excess adipose tissue may alter the relationship between circulating levels and total 25(OH)D stores, rendering circulating levels a less effective biomarker. Here, we study a population with a high prevalence of obesity that allows us to elucidate potential interactions between 25(OH)D levels and adipose stores.
Comorbid factors that act downstream of 25(OH)D actions might also account for inconsistencies in its association with adverse outcomes in prior studies. For instance, one of the major hypothesized mechanisms linking low levels of 25(OH)D with CKD outcomes is stimulation of the renin-angiotensin-aldosterone system (RAAS) (19–21). Systemic and intrarenal RAAS activity is also augmented by diabetes mellitus or sodium loading but is lessened with RAAS inhibitors. Therefore, diabetes status, dietary sodium intake, and use of RAAS inhibitors may also modify the associations between circulating 25(OH)D and clinical outcomes.
We hypothesized that 25(OH)D would be associated with CKD more strongly in persons with diabetes mellitus, consuming high dietary sodium, not using RAAS inhibitors, and with lower adipose stores and in those with alleles in the GC gene conferring less biologically available 25(OH)D. To evaluate this, we used detailed clinical and genetic data collected within the Jackson Heart Study (JHS), a community-based cohort of black Americans in Jackson, MS.
Materials and Methods
Study population
The JHS is a prospective cohort comprising 5306 African Americans, aged 21 to 94 years at baseline (2000 to 2004), who were recruited from the Jackson, MS, metropolitan area (Hinds, Madison, and Rankin counties). As previously detailed (22, 23), the JHS used probability-based sampling and random sampling with the Jackson driver’s license registry and a commercially available list as the sampling frame to enroll participants. A subset of participants also enrolled in the Atherosclerosis Risk in Communities (ARIC) study were eligible for enrollment in JHS along with their relatives.
After providing written informed consent, JHS participants completed baseline assessments from 2000 to 2004 (exam 1) and two follow-up examinations: from 2005 to 2008 (exam 2) and from 2009 to 2013 (exam 3). Institutional review boards at the University of Mississippi Medical Center, Jackson State University, and Tougaloo College approved the JHS protocol.
Plasma 25(OH)D assessment
The JHS measured plasma 25(OH)D2 and 25(OH)D3 using tandem mass spectrometry as previously described (24). In these analyses, we used measurements from JHS baseline (exam 1) and adjusted them for monthly variation in sunlight exposure using the residual method. Briefly, we regressed 25(OH)D3 on the month of blood draw and then added the residuals from this model to the overall mean to obtain adjusted values. Because 25(OH)D2 levels are not influenced by sunlight, we did not adjust 25(OH)D2. For participants with a 25(OH)D2 level below the limit of detection (i.e., <1.0 ng/mL), we imputed a value of 0.05 ng/mL. The sum of the adjusted plasma 25(OH)D3 and 25(OH)D2 comprised total 25(OH)D, which was used as the primary exposure in this study.
Ascertainment of VDP genotype
Genotype data for JHS participants, including two single-nucleotide polymorphisms (SNP) in the DBP genotype, rs4588 and rs7041, are derived from Affymetrix 6.0 genotyping platform. Briefly, the Birdseed genotype-calling algorithm was used to impute to 1000 Genomes project (1000G) based on the all ancestry panel (i.e., the cosmopolitan or worldwide panel) from 1000G Phase I integrated Release Version 3 Haplotypes (2010 to 2011 data freeze) using IMPUTE2 software. This reference panel contained haplotypes of 1092 individuals of all ethnic backgrounds and excludes monomorphic and singletons. The imputation qualities of rs4588 and rs7041 were ≥85%, which is an acceptable range for candidate gene analysis. Other quality control included tests of Hardy-Weinberg Equilibrium with a cutoff P value of 1 × 10−6 and call rate >0.99.
Because SNP genotypes are represented by dosages of imputed allele variants ranging continuously from 0 to 2, we used near-certain genotypes 0, 1, and 2 in the primary analysis, and uncertain genotype values were coded as missing. Concordance between these imputed genotypes and direct genotyping by the IBC Array where available was 99% for rs4588 and 98% for rs7041. We then dichotomized the SNP variables as AA or AC vs CC for rs4588 and GG or GT vs TT for rs7041 labeled in accordance with their most common nomenclature in the literature using the (+) strand. These labels correspond to TT or TG vs GG for rs4588 and CC or CA vs AA for rs7041 according to the (−) strand. In sensitivity analysis, we included all participants with imputed SNP genotypes, including those with uncertain genotypes, in an additive genetic model treating continuous SNP dosages as predictors.
Demographics, lifestyle factors, and comorbidities
Our models considered additional covariates, including demographic characteristics (age, sex, household income, occupation), lifestyle factors (dietary sodium intake, American Heart Association’s health categorizations for nutrition and physical activity, use of RAAS inhibitors), and comorbidities [waist circumference, body mass index (BMI), systolic blood pressure (BP), and diabetes mellitus]. JHS participants self-reported demographic characteristics, medical history, lifestyle factors, and medications. We assessed diet using the Delta Nutrition Intervention Research Initiative food frequency questionnaire, which was validated specifically for use in the JHS (25).
We categorized household income as ≤1.5 times the poverty level, >1.5 times the poverty level, or missing; occupation as outdoor (farming, construction, military) vs indoor (professional/management, service, sales, production, student, unemployed, and retired) to account for occupation-related sunlight exposure; and smoking status as never vs current or former smoker. Categories for physical activity and dietary quality included poor, intermediate, or ideal health according to the American Heart Association’s Life Simple 7 guidelines (26). For diet quality categorization, ideal and intermediate health were combined due to a limited number of participants with ideal nutrition metrics (27).
Blood pressure and body anthropometrics were measured directly at in-person study visits. Systolic BP, waist circumference (measured in cm), and BMI (kg/m2) were analyzed as continuous variables in all analyses. Diabetes mellitus was defined based on fasting glucose ≥126 mg/dL, HbA1c ≥6.5%, or use of diabetic medication ≤2 weeks prior to baseline exam.
CKD outcome measurements
The primary outcomes were estimated glomerular filtration rate (eGFR) decline and incident CKD based on standardized serum creatinine and the CKD-EPI equation (28). We defined eGFR decline as the annual rate of kidney function decline between exam 1 and exam 3 using the equation: 365.25 × (eGFR at exam 1 – eGFR at exam 3)/(number of days between exam 1 and exam 3). Incident CKD was defined as eGFR <60 mL/min/1.73 m2 at exam 3 along with a 25% decline in eGFR between exam 1 and exam 3, or new-onset albuminuria. New-onset albuminuria was defined as a spot urine albumin-to-creatinine ratio (ACR) ≥30 mg/g at exam 3 among subjects without CKD (eGFR ≥60 mL/min/1.73 m2 and no albuminuria) at exam 1. If spot urine was missing, a 24-hour urine collection was used for the ACR when available.
Statistical analysis
We described baseline continuous covariates using mean ± SD or median (interquartile range) and categorical covariates as frequency and proportion according to tertiles of plasma 25(OH)D at baseline. We used generalized linear regression models with the identity (eGFR decline) or logit (incident CKD) link to estimate the association between 25(OH)D and CKD outcomes. We adjusted models for age, sex, household income, occupation, smoking status, physical activity, nutritional status, BMI, diabetes mellitus, and systolic BP in the base model (model 1). We additionally adjusted for baseline ACR (model 2, eGFR decline outcome) and baseline eGFR (model 3, all renal outcomes). We assessed model quality for fit and model assumptions.
To assess effect modification of 25(OH)D and CKD outcomes by DBP genotype, diabetes status, dietary sodium intake, RAAS activation, and obesity measures, we added interaction terms between 25(OH)D and the potential modifier to the fully adjusted regression model (model 3) and used a likelihood ratio test to determine evidence of modification. If there was evidence of modification, we reported the association between 25(OH)D and CKD outcomes based on the cross-product of the main and interactive effects. In sensitivity analyses, we examined the potential influence of background genetic admixture and familial relatedness within the JHS cohort on the interaction between 25(OH)D and DBP genotype on CKD outcomes by adjusting for all 10 eigenvectors derived from the full SNP panel as markers of ancestry and familial correlation using pedigree data. We confirmed that the above models were robust to uncertainty of the imputed genotype data in an additive genetic model with the continuous SNP dosages using probABEL v.0.5.0 from the GenABEL suite of programs, which handles genotype uncertainty (29).
In other sensitivity analyses, we evaluated the influence of our incident CKD definition on our results by assessing purely eGFR-based definitions and a definition with a more rigorous threshold for albuminuria (i.e., ≥300 mg/g at exam 3). To evaluate the potential impact of missing follow-up data, we also performed sensitivity analyses with imputed event status for participants who were lost to follow-up, died, or otherwise had missing data at exam 3. In these models, we assumed extreme scenarios in which all individuals with missing event status either did, or did not, develop incident CKD.
All hypothesis tests were two-sided at a significance level of 0.05 for the overall analyses and 0.008 for the effect modification analyses to account for testing six interactions per outcome measure. SAS 9.4 (SAS institute, Cary, NC) and R 3.1.3 (R Core Team, Vienna, Austria) were used for all analyses.
Results
Participant characteristics
Among 5164 (97% of JHS) participants with the required data, mean ± SD plasma 25(OH)D was 14.5 ± 6.5 ng/mL, 63% were female, mean age was 55 ± 13 years, 7% had an outdoor occupation, and mean dietary sodium intake was 3360 ± 630 mg/d. Those in the highest (vs lowest) tertile of 25(OH)D were older (age 58 vs 52 years) with lower eGFR at baseline (90 ± 21 vs 99 ± 22 mL/min/1.73 m2) and were more likely to be male (43% vs 28%), to have an outdoor occupation (8% vs 4%), and to have intermediate or ideal dietary quality score (45% vs 34%). However, comorbidities, including BMI, waist circumference, systolic and diastolic BP, diabetes status, and use of RAAS inhibitors, were generally similar across tertiles of 25(OH)D (Table 1).
Table 1.
Baseline Characteristics of Participants Stratified by Tertiles of Plasma 25(OH)D
| Baseline Characteristics |
Plasma 25(OH)D Levels, by Tertiles
|
|||
|---|---|---|---|---|
| All | Lowest | Middle | Highest | |
| No. of participants | 5164 | 1722 | 1721 | 1721 |
| Total plasma 25(OH)D, ng/mL | 14.5 ± 6.5 | 8.1 ± 1.9 | 13.4 ± 1.6 | 22.0 ± 4.7 |
| Plasma 25(OH)D2, ng/mL | 1.3 ± 3.1 | 0.2 ± 0.6 | 0.8 ± 1.7 | 2.9 ± 4.7 |
| Plasma 25(OH)D3, ng/mLa | 13.2 ± 5.9 | 7.8 ± 1.9 | 12.7 ± 2.2 | 19.1 ± 5.7 |
| Demographics | ||||
| Age, y | 55.3 ± 12.8 | 52.1 ± 12.8 | 55.5 ± 12.7 | 58.3 ± 12.2 |
| Male, n (%) | 1900 (36.8) | 477 (27.7) | 686 (39.9) | 737 (42.8) |
| Lower-middle income, n (%)b | 1747 (33.8) | 610 (35.4) | 587 (34.1) | 550 (32.0) |
| Outdoor job, n (%)c | 337 (6.5) | 74 (4.3) | 134 (7.8) | 129 (7.5) |
| Lifestyle factors | ||||
| Cigarette smoking, n (%) | 1680 (32.5) | 541 (31.4) | 584 (33.9) | 555 (32.3) |
| AHA dietary quality score, n (%) | ||||
| Poor | 2521 (48.8) | 897 (52.1) | 856 (49.7) | 768 (44.6) |
| Intermediate/ideal | 1995 (38.6) | 580 (33.7) | 650 (37.8) | 765 (44.5) |
| Dietary sodium intake, mg/d | ||||
| Unadjusted | 3360 ± 1424 | 3365 ± 1463 | 3441 ± 1429 | 3275 ± 1375 |
| Adjusted for total caloric intaked | 3360 ± 630 | 3392 ± 635 | 3380 ± 647 | 3310 ± 604 |
| AHA physical activity score, n (%) | ||||
| Poor | 2537 (49.1) | 900 (52.3) | 881 (51.2) | 756 (43.9) |
| Intermediate | 1630 (31.6) | 523 (30.4) | 547 (31.8) | 560 (32.5) |
| Ideal | 989 (19.2) | 295 (17.1) | 292 (17.0) | 402 (23.4) |
| Comorbidity | ||||
| Body mass index, kg/m2 | 31.8 ± 7.2 | 33.3 ± 8.1 | 31.7 ± 6.9 | 30.3 ± 6.3 |
| Waist circumference, cm | 100.8 ± 16.1 | 103.1 ± 17.8 | 100.9 ± 15.7 | 98.3 ± 14.4 |
| Male | 101.2 ± 15.0 | 102.8 ± 17.7 | 102.3 ± 14.5 | 99.2 ± 13.3 |
| Female | 100.5 ± 16.7 | 103.2 ± 17.7 | 100 ± 16.4 | 97.6 ± 15.2 |
| Systolic BP, mm Hg | 127.5 ± 16.9 | 126.7 ± 17.2 | 127.7 ± 16.6 | 128.0 ± 16.9 |
| Diastolic BP, mm Hg | 75.8 ± 8.8 | 75.8 ± 8.9 | 75.9 ± 8.9 | 75.5 ± 8.5 |
| Diabetes mellitus, n (%) | 1120 (21.7) | 388 (22.5) | 390 (22.7) | 342 (19.9) |
| Use of RAAS inhibitors, n (%) | 862 (16.7) | 269 (15.6) | 302 (17.6) | 291 (16.9) |
| Baseline kidney function | ||||
| eGFR, mL/min per 1.73 m2 | 94.1 ± 22.0 | 98.8 ± 22.3 | 93.7 ± 22.0 | 89.9 ± 20.7 |
| Median ACR (IQR), mg/g | 6 (4–13) | 6 (4–13) | 6 (4–14)] | 6 (4–11) |
Values are mean ± SD unless otherwise stated.
Abbreviations: AHA, American Heart Association; IQR, interquartile range.
Adjusted for seasonal variation in plasma 25(OH)D3 levels by the residual method.
Family income ≤1.5 times the poverty level.
Selected to account for differential sunlight exposure: construction, farming, and military vs all others (management or professional, service, sales, production, sick, unemployed, retired, student).
Adjusted for total caloric intake by the residual method.
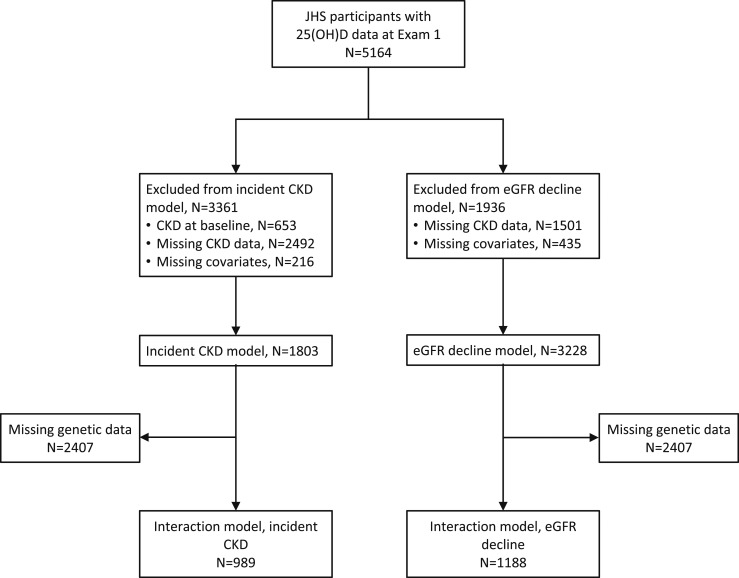
Out of these 5164 total participants, 3228 (63%) had measures of eGFR at exams 1 and 3 for calculation of annualized eGFR decline and were included in these models. We included 1803 (35%) participants in models of incident CKD after excluding those with prevalent CKD at exam 1 (n = 653) and those for whom we could not determine CKD status at exam 1 or 3 (n = 2492) (Fig. 1). Models that included genetic covariates were restricted to individuals who provided genetic consent. These models included 1188 participants for models of eGFR decline and 989 for models of incident CKD (Fig. 1).
Figure 1.
Flow diagram depicting cohort derivation. More patients are missing CKD data in incident CKD models compared with eGFR decline models due to inclusion of urine albumin to creatinine ratio in these definitions. Interaction models are between 25(OH)D and single nucleotide polymorphisms in the VDP genotype (rs4588 and rs7041) in the eGFR decline and incident CKD models.
Baseline characteristics of participants were nearly identical across subgroups of DBP genotype. However, participants with vs those without diabetes at baseline were more likely to have poor physical activity (58% vs 47%), intermediate/ideal diet quality (51% vs 35%), and on-going use of RAAS inhibitors (37% vs 11%) (30).
25(OH)D levels and CKD outcomes overall
Over a median follow-up of 8 years (interquartile range, 7 to 8), annual eGFR decline among the 3228 participants was 1.27 ± 1.96 mL/min/1.73 m2 per year, and 220 of 1803 (12%) participants developed incident CKD. Every 5-ng/mL higher plasma 25(OH)D level was associated modestly with 0.09 mL/min/1.73 m2 per year (95% CI, 0.02 to 0.15) slower annual eGFR decline after adjustment for demographics, lifestyle factors, comorbidity, and baseline ACR (model 2). This association was no longer evident after additional adjustment for baseline eGFR (model 3) (Table 2). Plasma 25(OH)D level was not associated with odds of incident CKD after adjustment for demographics, lifestyle factors, and comorbidity (OR, 0.97 per 5 ng/mL higher 25(OH)D; 95% CI, 0.86 to 1.09). Additional adjustment for baseline eGFR yielded similar findings (OR, 0.94; 95% CI, 0.83 to 1.06) (Table 2).
Table 2.
Differences in Annual eGFR Decline and OR of Incident CKD per 5 ng/mL Higher Plasma 25(OH)D
| Models a |
eGFR Decline
|
Incident CKD
|
||
|---|---|---|---|---|
| No. Included | Difference in Annual eGFR Decline in mL/min/1.73 m2/y (95% Cl) b | No. of Events/No. Included | OR (95% CI) | |
| 1 | 3228 | −0.07 (−0.13 to −0.02) | 220/1803 | 0.97 (0.86 to 1.09) |
| 2 | 2138 | −0.09 (−0.15 to −0.02) | NA | NA |
| 3 | 2138 | −0.04 (−0.11 to 0.02) | 220/1803 | 0.94 (0.83 to 1.06) |
Model 1: adjusted for age, sex, income, occupation, smoking, physical activity score, diet quality score, BMI, diabetes mellitus, and systolic blood pressure. Model 2: additionally adjusted for baseline albumin-to-creatinine ratio (for the eGFR decline model). Model 3: additionally adjusted for baseline eGFR.
Negative difference represents slower eGFR decline.
25(OH)D and CKD outcomes by potential modifiers
The association between 25(OH)D levels and CKD outcomes differed by DBP genotype and diabetes status (Table 3) but not by other effect modifiers.
Table 3.
Differences in Annual eGFR Decline and OR of Incident CKD per 5 ng/mL Higher Plasma 25(OH)D and According to DBP Genotype and Diabetes Status
| Interactions With 25(OH)D |
eGFR Decline
|
Incident CKD
|
||||
|---|---|---|---|---|---|---|
| No. Included (% of JHS) | Difference in Annual eGFR Decline in mL/min/1.73 m2/y (95% Cl) a | P-Interaction Value | No. of Events/No. Included | OR of Incident CKD (95% CI) | P-Interaction Value | |
| DBP genotypeb | ||||||
| rs4588 (overall) | 1181 | −0.05 (−0.14 to 0.03) | 0.467 | 118/983 | 0.97 (0.82 to 1.14) | 0.310 |
| AA/AC | 201 | 0.01 (−0.19 to 0.22) | 16/173 | 1.18 (0.78 to 1.79) | ||
| CC | 980 | −0.07 (−0.16 to 0.03) | 102/810 | 0.94 (0.78 to 1.12) | ||
| rs7041 (overall) | 1171 | −0.06 (−0.14 to 0.03) | 0.303 | 117/979 | NA | 0.005 |
| GG/GT | 349 | −0.12 (−0.28 to 0.03) | 46/293 | 0.69 (0.51 to 0.93) | ||
| TT | 828 | −0.03 (−0.13 to 0.07) | 71/686 | 1.13 (0.93 to 1.37) | ||
| Diabetes mellitus | ||||||
| Overall | 2138 | NAc | <0.001 | 220/1803 | 0.94 (0.83 to 1.06) | 0.60 |
| No diabetes | 1736 | 0.01 (−0.06 to 0.07) | 145/1517 | 0.96 (0.83 to 1.10) | ||
| Diabetes | 402 | −0.27 (−0.41 to −0.31) | 75/286 | 0.90 (0.71 to 1.12) | ||
All models adjusted for age, sex, income, occupation, smoking, physical activity score, diet quality score, BMI, diabetes, systolic blood pressure, baseline eGFR, and ACR (for the eGFR decline model); estimates are per 5 ng/mL higher plasma 25(OH)D. Positive slope represents faster eGFR decline.
Negative difference represents slower eGFR decline.
DBP genotype nomenclature rs4588 and rs7041 are labeled in accordance with their most common designation in the literature; however, the 1000 Genomes platform from which they were imputed labeled the alleles as G/T for rs4588 and A/C for rs7041.
Overall effects not reported due to significant interactions.
DBP genotype
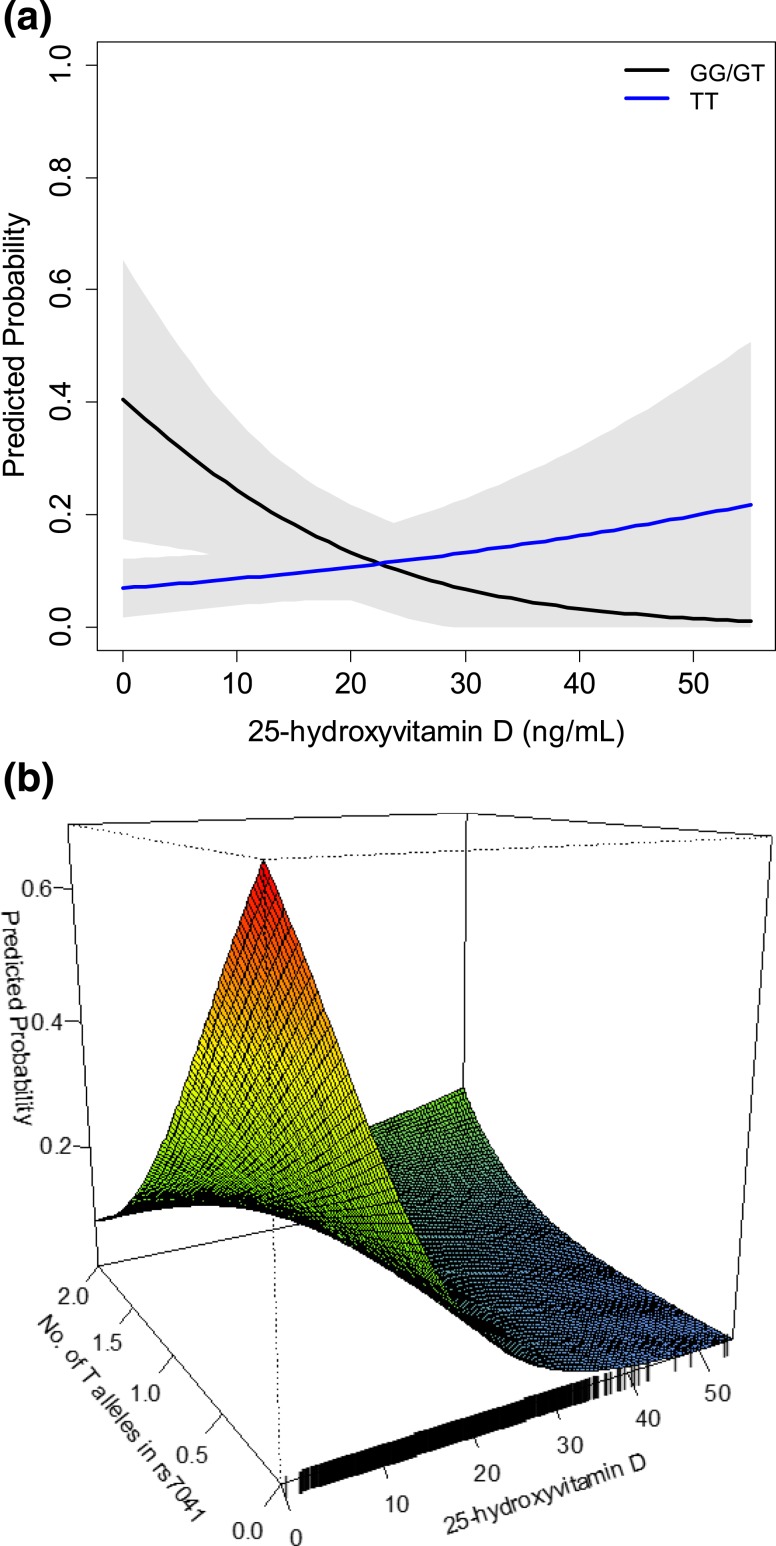
Adjusted for demographics, lifestyle, comorbidity, and baseline eGFR, higher 25(OH)D was associated with lower odds of incident CKD among participants with the GG or GT genotype for the rs7041 locus (n = 293 out of 979 participants; OR, 0.69 for each 5 ng/mL higher; 95% CI, 0.51 to 0.93). Participants with the more common TT genotype (n = 686 out of 979 participants) demonstrated no association between 25(OH)D and incident CKD (P-interaction = 0.005) [Table 3; Fig. 2(a)]. Only 10 participants with genetic data were excluded from this model because of an uncertain genotype. An additive genetic model comprising all individuals, and using continuous SNP dosage from the imputation algorithm, revealed consistent evidence of an interaction between higher 25(OH)D, number of T alleles in rs7041 and the odds of incident CKD [Fig. 2(b)]. The model predicted probabilities of incident CKD in Fig. 2 represents the predictions for an otherwise “average” participant in the JHS. The model predicted probabilities based on other covariate values are demonstrated pictorially in the supplement (30), demonstrating clinically meaningful risk differences across levels of 25(OH)D among individuals with GT or GG genotype and other high-risk features, such as diabetes mellitus. Similar findings between 25(OH)D, DBP, and incident CKD were found after additional adjustment for genetic admixture (30) and after accounting for familial correlation (data not shown).
Figure 2.
Model-predicted probability of incident CKD according to 25(OH)D levels and rs7041 single nucleotide polymorphism genotype. (a) rs7041 modeled as a dichotomous variable (GG or GT vs TT). (b) rs7041 modeled as a continuous variable using “dose” of T alleles in an additive genetic model as a sensitivity analysis to assess robustness of the dichotomous coding of rs7041 to uncertainty of imputation of some genotypes. Probabilities are predicted from a logistic regression model including age, sex, income, occupation, smoking, physical activity and diet quality score, BMI, diabetes, systolic blood pressure (SBP), and baseline eGFR. Graphed probability is for an average participant with age 55 years, female sex, lower-middle income status, indoor occupation (professional/management, service, sales, production, and student), nonsmoker, poor categories for physical activity and diet quality, BMI 32 kg/m2, no diabetes, SBP 128 mm Hg, and baseline eGFR of 94 mL/min/1.73 m2.
Genetic variation at rs7041 did not statistically modify the association between 25(OH)D and annual eGFR decline (P = 0.3). Genetic variation in rs4588 did not modify associations between 25(OH)D and either CKD outcome (Table 3).
Diabetes mellitus
Diabetes status also modified the association between 25(OH)D levels and annual eGFR decline (P < 0.001). In fully adjusted models, each 5 ng/mL higher 25(OH)D level was associated with 0.27 mL/min/1.73 m2 (95% CI, 0.13 to 0.41) slower annual eGFR decline among participants with diabetes. Diabetes status did not modify the association between 25(OH)D and incident CKD (Table 3).
Dietary sodium intake, use of RAAS inhibitors, and obesity
Dietary sodium intake (P-interaction = 0.8 and 0.7), RAAS inhibitor use (P-interaction = 0.1 and 0.4), and obesity measures (BMI, P-interaction = 0.9 and 0.9; waist circumference, P-interaction = 0.4 and 0.6) also did not modify the associations between 25(OH)D with eGFR decline and incident CKD, respectively.
Sensitivity analyses
In sensitivity analyses using alternative definitions of incident CKD, including (i) eGFR-only criteria and (ii) both eGFR and macroalbuminuria, interactions between 25(OH)D, number of T alleles, and incident CKD were no longer statistically significant although power was markedly reduced. Models with case status imputed at the extremes (i.e., all missing individuals at exam 3 classified as cases or noncases) revealed point estimates in the same direction as the primary analysis with marginal evidence of interaction (P < 0.10) (30).
Discussion
In this large, community-based cohort of black Americans who were prospectively followed for a median of 8 years, higher levels of plasma 25(OH)D at baseline was associated with slower kidney function decline in those with diabetes mellitus despite adjustments for demographics, lifestyle factors, comorbidities, and baseline kidney function. Higher levels of 25(OH)D were also associated with lower odds of incident CKD among those with DBP genotypes that may confer higher levels of circulating DBP. These associations were robust to additional adjustment for genetic admixture and familial correlation. Thus, amid conflicting prior reports from studies conducted in heterogeneous populations, our data support a potential role of 25(OH)D in CKD development and progression in a subset of black Americans.
Inconsistencies regarding associations of 25(OH)D with outcomes in prior studies may reflect differences in factors that affect 25(OH)D metabolism, including different genetic and clinical profiles of individuals. In our study, low 25(OH)D was not associated with incident CKD in those homozygous for the T allele at the rs7041 locus in the gene encoding DBP, a variant that is associated with lower levels of circulating DBP in the general population (12, 13). In contrast, low 25(OH)D was associated with an increased odds of incident CKD in those with the GG or GT genotype at rs7041. Our result is consistent with prior work that demonstrates a greater risk of cardiovascular disease, diabetes, and fracture-related hospitalizations in individuals with low 25(OH)D and the GG or GT (vs TT) genotype at rs7041 (14–16).
Several biologic mechanisms might explain our finding of interaction between DBP genotype and plasma 25(OH)D level on incident CKD. Due to the sterol binding capacity of DBP, higher circulating DBP levels may lower the bioavailability of 25(OH)D (14–16). Under this hypothesis, the association of low 25(OH)D with outcomes specifically in individuals with the GG/GT genotype at rs7041, which confers higher levels of circulating DBP, could be attributed to low levels of bioavailable, as opposed to total, 25(OH)D in this group. However, there have been concerns (31) regarding the validity of DBP immunoassays used in studies (12) that shaped this hypothesis. Nonetheless, studies that assessed DBP levels using mass spectrometry–based methods demonstrate consistent differences in DBP levels according to genotype (13). Additionally, irrespective of DBP levels, binding affinity of DBP for 25(OH)D may also vary by DBP genotype and contribute to bioavailability (32). DBP may have other biologic functions (33), distinct from sterol binding, that might also explain interactions between 25(OH)D and DBP genotypes. For example, high DBP levels may confer risk for interstitial and endothelial inflammation (34–37), which has been shown to predict the development of microalbuminuria (34, 35). Consistent with this hypothesis, the association of higher (vs lower) 25(OH)D levels with lower risk of CKD development specifically in individuals with DBP variants (GT or GG in rs7041) that confer higher DBP levels could indicate a mitigating effect of 25(OH)D sufficiency on inflammatory risk related to high DBP. Thus, future investigations should directly measure DBP using gold-standard methods to evaluate the potential role of DBP levels on CKD outcomes.
Our data also support a role of low 25(OH)D as an independent risk for faster decline in kidney function in black Americans with diabetes. This finding corroborates, in an exclusively black cohort, a growing body of evidence that suggests low 25(OH)D confers greater risk factor for adverse CKD outcomes among individuals with diabetic kidney disease (1, 19). In one randomized controlled trial (19), for example, activation of the vitamin D receptor with paricalcitol lowered albuminuria in individuals with diabetic CKD. However, this finding was not replicated in another similarly designed trial (38) that enrolled individuals with nondiabetic CKD. Taken together with our finding, the data consistently support relationships between low 25(OH)D and kidney outcomes in diabetes specifically. This finding may support RAAS stimulation as a potential mechanism linking low 25(OH)D with adverse CKD outcomes due to the prominent role of RAAS activation in diabetic kidney disease.
Our study had noteworthy limitations. Because the JHS did not measure levels of bioavailable 25(OH)D and circulating DBP, which can be used to estimate bioavailable 25(OH)D (39), we are not able to determine the mechanisms underlying the effect modification by DBP genotype. Furthermore, the number of participants in the interaction models for 25(OH)D with DBP genotype was very small, which might have underpowered our analysis or led to imprecise estimates. Additionally, the interaction between DBP genotypes and 25(OH)D was only robust for incident CKD definitions that include albuminuria. Although we posit that this might be because albuminuria is a proximate consequence of renal inflammation, we cannot confirm that this association with albuminuria would translate to clinically meaningful differences in eGFR decline or eGFR-based events. Finally, the JHS was observational; therefore, residual confounding cannot be excluded. Despite these limitations, our study had several strengths. We accounted for key covariates that are known to affect 25(OH)D levels and its downstream biologic activity, including comorbidities, environment, and lifestyle factors. For instance, we used a robust method to account for seasonal variation in 25(OH)D levels, taking into consideration the month of blood draw. We also accounted for occupation and physical activity, both of which affect cutaneous synthesis of vitamin D via duration of sunlight exposure, and adiposity, which may provide a storage depot for 25(OH)D. Geographic differences in sunlight exposure were not a concern in our analysis because all participants are from the Jackson, MS, area. Our study is the largest to examine the relation between 25(OH)D, kidney outcomes, and potential effect modifiers in a cohort of community-dwelling black Americans.
In summary, higher levels of plasma 25(OH)D were associated with slower kidney function decline in black Americans with diabetes. Higher 25(OH)D was also associated with lower odds of incident CKD among those with DBP genotypes that may confer higher levels of circulating DBP. These findings elucidate a potential role of higher 25(OH)D as a protective factor for CKD in a subset of blacks and shed light on the underlying reasons for differences in 25(OH)D-associated risks that could be relevant for a wide variety of clinical outcomes.
Acknowledgments
The authors thank the staff and participants of the JHS.
Financial Support: The JHS is supported and conducted in collaboration with Jackson State University (HHSN268201300049C and HHSN268201300050C), Tougaloo College (HHSN268201300048C), and the University of Mississippi Medical Center (HHSN268201300046C and HHSN268201300047C) contracts from the National Heart, Lung, and Blood Institute (NHLBI) and the National Institute for Minority Health and Health Disparities (NIMHD). Additional support for this work was provided by National Institutes of Health Grant R01DK111952 (to J.J.S.) and Duke O’Brien Center for Kidney Research Grants P30DK096493 and UL1TR001117 (to C.A.D.) from the National Center for Advancing Translational Sciences. A preliminary version of this work was reported in abstract form at the American Society of Nephrology Annual Kidney Week in November 2017. The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Institutes of Health; or the US Department of Health and Human Services.
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- 25(OH)D
25-hydroxyvitamin D
- ACR
albumin-to-creatinine ratio
- BMI
body mass index
- BP
blood pressure
- CKD
chronic kidney disease
- DBP
vitamin D binding protein
- eGFR
estimated glomerular filtration rate
- JHS
Jackson Heart Study
- RAAS
renin-angiotensin-aldosterone system
References
- 1. de Boer IH, Katz R, Chonchol M, Ix JH, Sarnak MJ, Shlipak MG, Siscovick DS, Kestenbaum B. Serum 25-hydroxyvitamin D and change in estimated glomerular filtration rate. Clin J Am Soc Nephrol. 2011;6(9):2141–2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Keyzer CA, Lambers-Heerspink HJ, Joosten MM, Deetman PE, Gansevoort RT, Navis G, Kema IP, de Zeeuw D, Bakker SJ, de Borst MH; PREVEND Study Group . Plasma vitamin D level and change in albuminuria and eGFR according to sodium intake. Clin J Am Soc Nephrol. 2015;10(12):2119–2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. de Boer IH, Ioannou GN, Kestenbaum B, Brunzell JD, Weiss NS. 25-hydroxyvitamin D levels and albuminuria in the Third National Health and Nutrition Examination Survey (NHANES III). Am J Kidney Dis. 2007;50(1):69–77. [DOI] [PubMed] [Google Scholar]
- 4. Melamed ML, Astor B, Michos ED, Hostetter TH, Powe NR, Muntner P. 25-hydroxyvitamin D levels, race, and the progression of kidney disease. J Am Soc Nephrol. 2009;20(12):2631–2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Aloia J, Mikhail M, Dhaliwal R, Shieh A, Usera G, Stolberg A, Ragolia L, Islam S. Free 25(OH)D and the vitamin D paradox in African Americans. J Clin Endocrinol Metab. 2015;100(9):3356–3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hsu CY, Lin F, Vittinghoff E, Shlipak MG. Racial differences in the progression from chronic renal insufficiency to end-stage renal disease in the United States. J Am Soc Nephrol. 2003;14(11):2902–2907. [DOI] [PubMed] [Google Scholar]
- 7. Murphy D, McCulloch CE, Lin F, Banerjee T, Bragg-Gresham JL, Eberhardt MS, Morgenstern H, Pavkov ME, Saran R, Powe NR, Hsu CY; Centers for Disease Control and Prevention Chronic Kidney Disease Surveillance Team . Trends in prevalence of chronic kidney disease in the United States. Ann Intern Med. 2016;165(7):473–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Robinson-Cohen C, Hoofnagle AN, Ix JH, Sachs MC, Tracy RP, Siscovick DS, Kestenbaum BR, de Boer IH. Racial differences in the association of serum 25-hydroxyvitamin D concentration with coronary heart disease events. JAMA. 2013;310(2):179–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gutiérrez OM, Farwell WR, Kermah D, Taylor EN. Racial differences in the relationship between vitamin D, bone mineral density, and parathyroid hormone in the National Health and Nutrition Examination Survey. Osteoporos Int. 2011;22(6):1745–1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Scialla JJ, Astor BC, Isakova T, Xie H, Appel LJ, Wolf M. Mineral metabolites and CKD progression in African Americans. J Am Soc Nephrol. 2013;24(1):125–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shieh A, Aloia JF. Assessing vitamin D status in African Americans and the influence of vitamin D on skeletal health parameters. Endocrinol Metab Clin North Am. 2017;46(1):135–152. [DOI] [PubMed] [Google Scholar]
- 12. Powe CE, Evans MK, Wenger J, Zonderman AB, Berg AH, Nalls M, Tamez H, Zhang D, Bhan I, Karumanchi SA, Powe NR, Thadhani R. Vitamin D-binding protein and vitamin D status of black Americans and white Americans. N Engl J Med. 2013;369(21):1991–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rebholz CM, Grams ME, Lutsey PL, Hoofnagle AN, Misialek JR, Inker LA, Levey AS, Selvin E, Hsu CY, Kimmel PL, Vasan RS, Eckfeldt JH, Coresh J; Chronic Kidney Disease Biomarkers Consortium . Biomarkers of vitamin D status and risk of ESRD. Am J Kidney Dis. 2016;67(2):235–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lutsey PL, Michos ED, Misialek JR, Pankow JS, Loehr L, Selvin E, Reis JP, Gross M, Eckfeldt JH, Folsom AR. Race and vitamin D binding protein gene polymorphisms modify the association of 25-hydroxyvitamin D and incident heart failure: the ARIC (Atherosclerosis Risk in Communities) study. JACC Heart Fail. 2015;3(5):347–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Reis JP, Michos ED, Selvin E, Pankow JS, Lutsey PL. Race, vitamin D-binding protein gene polymorphisms, 25-hydroxyvitamin D, and incident diabetes: the Atherosclerosis Risk in Communities (ARIC) Study. Am J Clin Nutr. 2015;101(6):1232–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Takiar R, Lutsey PL, Zhao D, Guallar E, Schneider AL, Grams ME, Appel LJ, Selvin E, Michos ED. The associations of 25-hydroxyvitamin D levels, vitamin D binding protein gene polymorphisms, and race with risk of incident fracture-related hospitalization: twenty-year follow-up in a bi-ethnic cohort (the ARIC Study). Bone. 2015;78:94–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shapses SA, Lee EJ, Sukumar D, Durazo-Arvizu R, Schneider SH. The effect of obesity on the relationship between serum parathyroid hormone and 25-hydroxyvitamin D in women. J Clin Endocrinol Metab. 2013;98(5):E886–E890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jorde R, Sneve M, Hutchinson M, Emaus N, Figenschau Y, Grimnes G. Tracking of serum 25-hydroxyvitamin D levels during 14 years in a population-based study and during 12 months in an intervention study. Am J Epidemiol. 2010;171(8):903–908. [DOI] [PubMed] [Google Scholar]
- 19. de Borst MH, Vervloet MG, ter Wee PM, Navis G. Cross talk between the renin-angiotensin-aldosterone system and vitamin D-FGF-23-klotho in chronic kidney disease. J Am Soc Nephrol. 2011;22(9):1603–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. de Borst MH, Navis G. Sodium intake, RAAS-blockade and progressive renal disease. Pharmacol Res. 2016;107:344–351. [DOI] [PubMed] [Google Scholar]
- 21. de Zeeuw D, Agarwal R, Amdahl M, Audhya P, Coyne D, Garimella T, Parving HH, Pritchett Y, Remuzzi G, Ritz E, Andress D. Selective vitamin D receptor activation with paricalcitol for reduction of albuminuria in patients with type 2 diabetes (VITAL study): a randomised controlled trial. Lancet. 2010;376(9752):1543–1551. [DOI] [PubMed] [Google Scholar]
- 22.Taylor Jr HA. The Jackson Heart Study of the future. Ethn Dis. 2012;22(3 Suppl 1):S1-49–S1-54. [PubMed] [Google Scholar]
- 23.Fuqua SR, Wyatt SB, Andrew ME, Sarpong DF, Henderson FR, Cunningham MF, Taylor HA Jr. Recruiting African-American research participation in the Jackson Heart Study: methods, response rates, and sample description. Ethn Dis. 2005;15(4 Suppl 6):S6-18–S6-29. [PubMed] [Google Scholar]
- 24. Khan RJ, Gebreab SY, Riestra P, Sims M, Gaye A, Xu R, Davis SK. Associations between vitamin D and cardiovascular disease risk factors in African Americans are partly explained by circulating adipokines and C-reactive protein: The Jackson Heart Study. J Nutr. 2016;146(12):2537–2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Carithers TC, Talegawkar SA, Rowser ML, Henry OR, Dubbert PM, Bogle ML, Taylor HA Jr, Tucker KL, Tucker KL. Validity and calibration of food frequency questionnaires used with African-American adults in the Jackson Heart Study. J Am Diet Assoc. 2009;109(7):1184–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sacco RL. The new American Heart Association 2020 goal: achieving ideal cardiovascular health. J Cardiovasc Med (Hagerstown). 2011;12(4):255–257. [DOI] [PubMed] [Google Scholar]
- 27. Collins TC, Slovut DP, Newton R Jr, Johnson WD, Larrivee S, Patterson J, Johnston JA, Correa A. Ideal cardiovascular health and peripheral artery disease in African Americans: Results from the Jackson Heart Study. Prev Med Rep. 2017;7:20–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF III, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) . A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Aulchenko YS, Struchalin MV, van Duijn CM. ProbABEL package for genome-wide association analysis of imputed data. BMC Bioinformatics. 2010;11(1):134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lunyera J, Davenport CA, Pendergast J, Musani SK, Bhavsar NA, Sims M, Mwasongwe S, Wolf M, Diamantidis CJ, Boulware LE, Scialla JJ. Data from: Modifiers of plasma 25-hydroxyvitamin D and chronic kidney disease outcomes in black Americans: The Jackson Heart Study. DukeSpace Open Access Repository. Accessed 15 December 2018. https://hdl.handle.net/10161/17707. [DOI] [PMC free article] [PubMed]
- 31. Hoofnagle AN, Eckfeldt JH, Lutsey PL. Vitamin D-binding protein concentrations quantified by mass spectrometry. N Engl J Med. 2015;373(15):1480–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chun RF, Peercy BE, Orwoll ES, Nielson CM, Adams JS, Hewison M. Vitamin D and DBP: the free hormone hypothesis revisited. J Steroid Biochem Mol Biol. 2014;144(Pt A):132–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Delanghe JR, Speeckaert R, Speeckaert MM. Behind the scenes of vitamin D binding protein: more than vitamin D binding. Best Pract Res Clin Endocrinol Metab. 2015;29(5):773–786. [DOI] [PubMed] [Google Scholar]
- 34. Mirković K, Doorenbos CR, Dam WA, Lambers Heerspink HJ, Slagman MC, Nauta FL, Kramer AB, Gansevoort RT, van den Born J, Navis G, de Borst MH. Urinary vitamin D binding protein: a potential novel marker of renal interstitial inflammation and fibrosis. PLoS One. 2013;8(2):e55887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shoukry A, Bdeer S-A, El-Sokkary RH. Urinary monocyte chemoattractant protein-1 and vitamin D-binding protein as biomarkers for early detection of diabetic nephropathy in type 2 diabetes mellitus. Mol Cell Biochem. 2015;408(1-2):25–35. [DOI] [PubMed] [Google Scholar]
- 36. Ge L, Trujillo G, Miller EJ, Kew RR. Circulating complexes of the vitamin D binding protein with G-actin induce lung inflammation by targeting endothelial cells. Immunobiology. 2014;219(3):198–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Trujillo G, Habiel DM, Ge L, Ramadass M, Cooke NE, Kew RR. Neutrophil recruitment to the lung in both C5a- and CXCL1-induced alveolitis is impaired in vitamin D-binding protein-deficient mice. J Immunol. 2013;191(2):848–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Keyzer CA, van Breda GF, Vervloet MG, de Jong MA, Laverman GD, Hemmelder MH, Janssen WM, Lambers Heerspink HJ, Kwakernaak AJ, Bakker SJ, Navis G, de Borst MH; Holland Nephrology Study (HONEST) Network . Effects of vitamin D receptor activation and dietary sodium restriction on residual albuminuria in CKD: the ViRTUE-CKD trial. J Am Soc Nephrol. 2017;28(4):1296–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Denburg MR, Bhan I. Vitamin D-binding protein in health and chronic kidney disease. Semin Dial. 2015;28(6):636–644. [DOI] [PubMed] [Google Scholar]