Abstract
Background
Sauna bath has potential as a lifestyle treatment modality for heart failure (HF). It is important to analyze the current evidence to help suggest paths of future study and potential for clinical application.
Hypothesis
Sauna bath has a positive effect on HF patients.
Methods
PubMed, Cochrane Library, and CINAHL databases were searched to identify randomized and nonrandomized controlled studies to compare effects of sauna bath with no sauna bath. Studies were searched for both infrared sauna bath and Finnish sauna bath. The strength of evidence was rated using a modified GRADE approach. Out of 1444 studies, nine met the inclusion criteria and were included in this review. Seven of these nine studies were included in the meta‐analysis. Only studies with infrared sauna bath met the inclusion criteria.
Results
In the meta‐analysis, exposure to an infrared sauna bath in 60°C for 15 minutes, followed by a 30‐minute rest in warm environment, five times a week for 2 to 4 weeks, was associated with a significant reduction in B‐type natriuretic peptide, cardiothoracic ratio, and an improvement in left‐ventricular ejection fraction. There was no significant effect on left‐ventricular end‐diastolic diameter, left atrial diameter, systolic blood pressure, or diastolic blood pressure. The strength of evidence varied from moderate to insufficient.
Conclusion
Infrared sauna bath was associated with short‐term improvement in cardiac function. More evidence is needed about long‐term effects of sauna bath and the effects of a Finnish sauna on cardiovascular health among patients with HF or other cardiovascular diseases.
Keywords: heart failure, sauna bath, Waon therapy
1. INTRODUCTION
The prevalence of heart failure (HF) is increasing at the same time as treatment strategies are improving and the mean age of death from HF has risen.1, 2 Aging of the population, worsening risk factor profile, and improved treatments and diagnostic technology have led to an increase in the prevalence of HF.3, 4, 5 This suggests that there will be an increase in the number of patients with HF in the more advanced stages of the disease. HF is associated with high morbidity and mortality and will impose a significant economic burden on healthcare systems in the future.6 Thus, beyond pharmacologic and other relatively expensive interventional therapies, for example, cardiac resynchronization therapy, it will be important to search for additional possible strategies to manage the symptoms of these patients. Although several established cardiovascular disease risk factors such as hypertension, diabetes, and history of coronary heart disease explain a large proportion of the risk of HF, its pathogenesis is still not fully understood, as several other factors appear to be involved. Multidisciplinary and easily applicable treatment strategies should be focused on improving the quality of life of HF patients, and therefore the value of life‐style factors such as exercise training and sauna bathing among HF patients should be further investigated.7
The access to saunas in many parts of the world, along with the relatively low cost of sauna bathing, gives it a great potential as a lifestyle treatment modality. It is therefore important to summarize the evidence and identify gaps of knowledge that can help suggest paths of future study and potential for clinical application.
The aim of this study was to assemble the evidence through a systematic review and meta‐analysis of the effects of sauna bath in patients with HF, evaluated by symptoms, blood pressures, biomarkers, cardiac dimensions and function, endothelial function, and mortality.
2. METHODS
2.1. Search strategy
The search strategy was based on the PICO approach (Population, Intervention, Control, Outcome) recommended by the Cochrane Handbook for Systematic reviews.8 We searched the databases of PubMed,9 Cochrane library,10 and CINAHL11 with search terms “sauna,” “saunas,” “steam bath,” “steam baths,” and “waon therapy”. The aim was to identify all literature of the health effects of sauna bath on cardiovascular effects. The studies with cardiovascular effects were reviewed in more detail, including reference lists, to identify the studies that met the inclusion criteria. The searches were done during 2 February 2016 to 19 April 2016, with a final updated search 27 April 2017 identifying one additional study (Figure 1).
Figure 1.
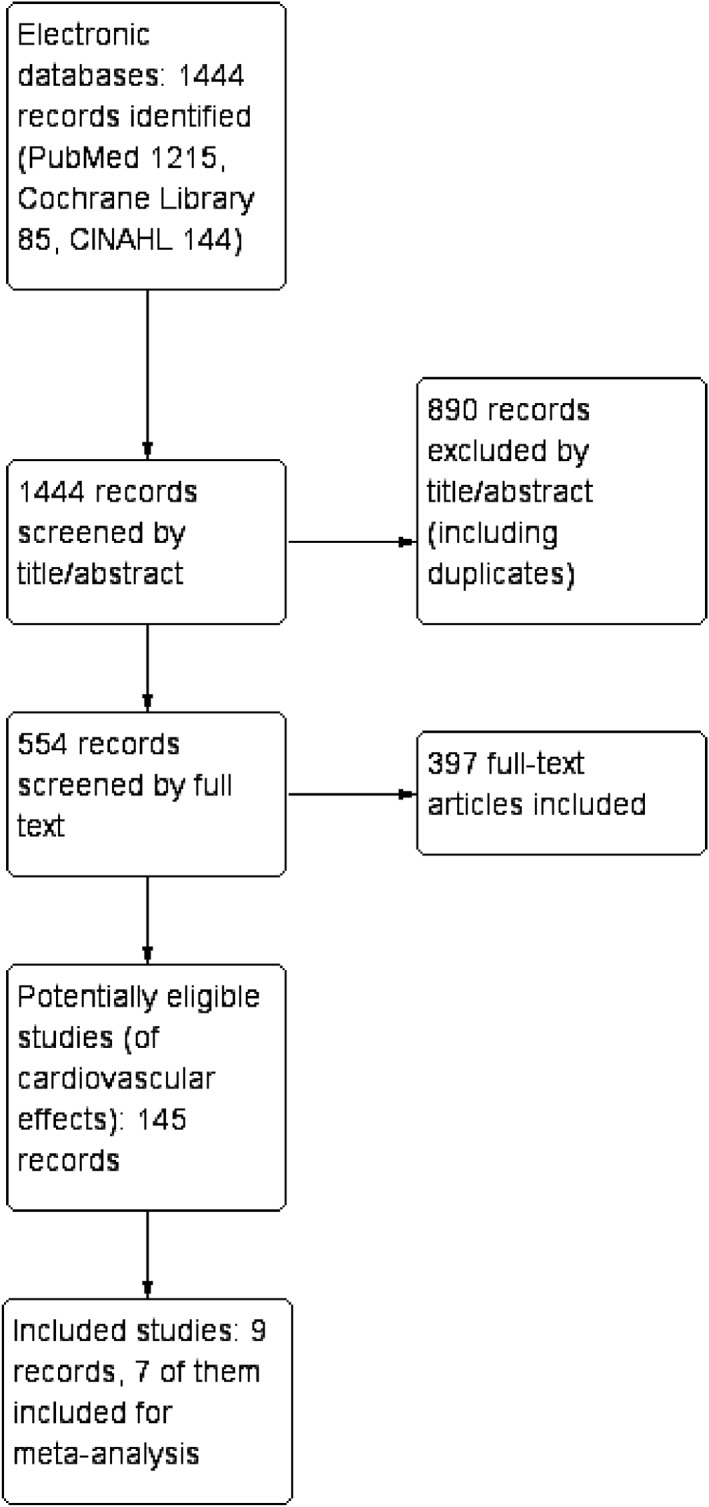
Flow chart
2.2. Inclusion criteria
The search results were independently reviewed by two authors (M.K. and H.H.). Studies with abstract in English were included.
Studies that met the following criteria were included in the review: (a) Randomized and nonrandomized controlled studies. (b) Population (P): Heart failure, men and women, ≥16 years of age. (c) Types of sauna bath (I = Intervention): moist (>10% relative humidity), dry (0%‐10% relative humidity), or infrared. Temperature of sauna bath: 60 to 100°C. (d) Sauna bath compared to no sauna bath. (C = control) Studies comparing different types of sauna baths were excluded. (e) Types of outcome measures (O = Outcome): I) Symptoms and signs: New York Heath Association (NYHA) class, systolic blood pressure (SBP), diastolic blood pressure (DBP); II) Blood biomarkers: B‐type natriuretic peptide (BNP); III) Imaging: left atrial diameter (LAD), left‐ventricular end‐diastolic diameter (LVEDD), left‐ventricle ejection fraction (EF), cardiothoracic ratio (CTR), endothelial function. IV) Mortality; V) safety/tolerance (Figures 2 and 3).
Figure 2.
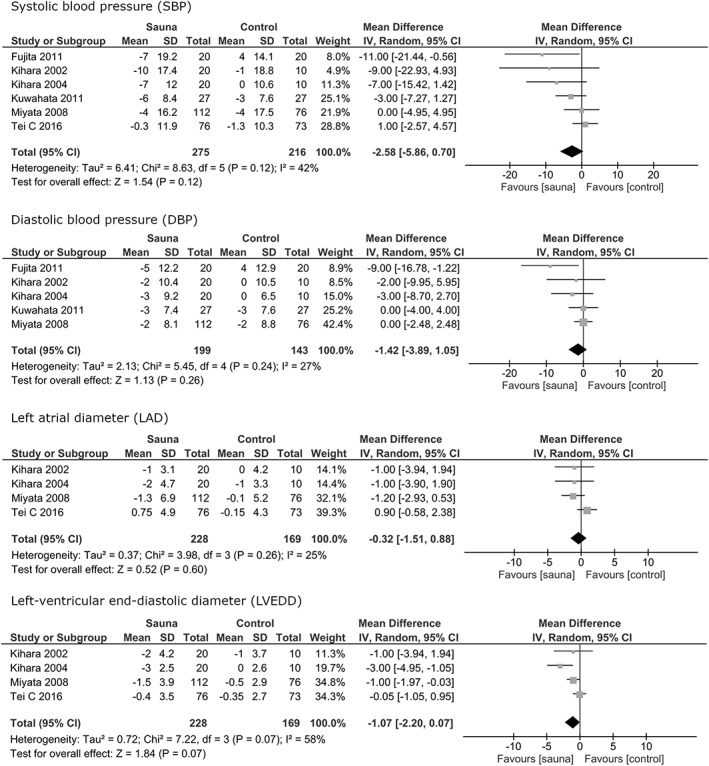
Forest plots of the results of the meta‐analysis for the outcomes systolic blood pressure (SBP), diastolic blood pressure (DBP), left atrial diameter (LAD) and left‐ventricular end‐diastolic diameter (LVEDD). CI, confidence interval
Figure 3.
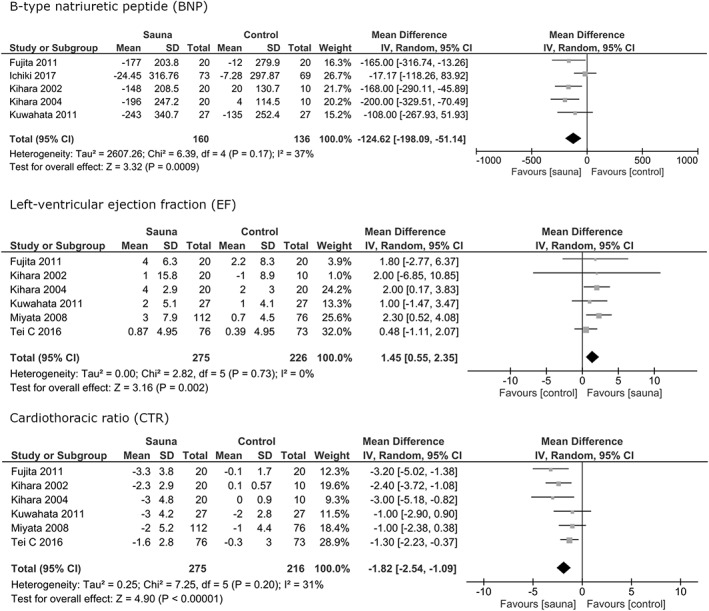
Forest plots of the results of the meta‐analysis for the outcomes B‐type natriuretic peptide (BNP), left ventricular ejection fraction (EF) and cardiothoracic ratio (CTR). CI, confidence interval
2.3. Assessment of study quality
The quality assessment of the randomized and nonrandomized observational studies was done using the Downs and Black checklist.12 The last point (27) was modified to “did the study report a power calculation?” with points given as: no = 0; yes, power ≥ 70% = 1 point, ≥75% = 2 points, ≥80% = 3 points, ≥85% = 4 points and ≥ 90% = 5 points. Thus, the maximum score for quality assessment was 32. Total points by study are displayed in Table 1. The quality of the studies was broadly divided into three categories: 0 to 10 low, 11 to 21 moderate, 22 to 32 high. This scale was not included in the original article.12
Table 1.
List of studies included and patient characteristics
| Study | Study design, blinding | Number of participants (sauna/control) | NYHA classes at baseline for all groups. If numerical = at average | Mean ejection fraction: sauna/control | Mean age: sauna /control | Male % | Ischemic cardiomyopathy (sauna/control, % of respective group) | Medications, % of sauna/control | Implantable cardioverter‐defibrillator, cardiac resynchronisation therapy‐device (% of sauna/control) | Location | Quality assesment: Downs and Black score (of 32) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ACE (Angiotensin Converting Enzyme)‐inhibitors or (ACE‐I) Angiotensine II receptro‐blockers (ARB) | B‐blockers | Diuretics | |||||||||||
| Kihara 2002 23 | Controlled trial, no | 20/10 | II, III | 38/32 | 62/64 | 60 | 15/unknown | “All patients” | “Most patients” | “All patients” | Not listed | Japan | 16 |
| Kihara 2004 24 | Randomized controlled trial (RCT), no | 20/10 | II, III | 29/29 | 59/59 | 70 | 20/20 | 95/90 | 55/40 | 95/100 | Not listed | Japan | 19 |
| Miyata 2008 25 | RCT, no | 112/76 | 2.61, 2.51 | 31.6/36.6 | 63/66 | 66 | 15/9 | Data not available | Data not available | Data not available | Not listed | Japan | 20 |
| Kihara 2009 16 | Retrospective study, no | 64/65 | 2.6 | 38.5/35.8 | 61.9/64.6 | 64 | 26/21 | 69/65 | 61/57 | 73/83 | Not listed | Japan | 19 |
| Kuwahata 2011 26 | RCT, no | 27/27 | II, III, IV | 31/33 | 63/64 | 70 | 37/37 | ACE‐I 52/63 ARB 48/37 | 93/93 | 100/100 | — | Japan | 19 |
| Fujita 2011 27 | RCT, no | 20/20 | II, III | 31.8/34.3 | 64/65 | 83 | 30/35 | 100/95 | 90/95 | Not listed | — | Japan | 19 |
| Tei 2016 28 | RCT, no | 76/73 | II, III, IV | 30.2/30.9 | 66/66 | 61 | 26/30 | ACE‐I 58/55 ARB 24/27 | 86/88 | 97/99 | CRT‐P 1/4 CRT‐D 18/22, ICD 2/4 | Japan | 21 |
| Ichiki 2017 29 (substudy of Tei 2016) | RCT, no | 76/73 | II, III, IV | 30.2/30.9 | 66/66 | 61 | 26/30 | ACE‐I 58/55 ARB 24/27 | 86/88 | 97/99 | CRT‐P 1/4 CRT‐D 18/22, ICD 2/4 | Japan | 21 |
| Basford 2009 30 | Crossover, no | 9‐crossover | II, III, IV | 20 | 72 | 67 | Not listed | Not listed | Not listed | Not listed | Excluded from the study | USA | — |
2.4. Data synthesis and analysis
Studies that met the inclusion criteria and were similar in follow‐up length and study design were pooled to conclude a meta‐analysis. The criteria for inclusion in the meta‐analysis were not prespecified and were formed after the search results. For continuous data, mean differences between the groups and SDs of the change were used and presented as random effects 95% confidence intervals (CIs) in forest plots. The data was constructed with the statistical software RevMan 5.3.13, 14
Few of the studies that met the inclusion criteria reported the differences of the means or the SDs of the changes. In accordance with Cochrane Handbook for Systematic Reviews of Interventions, SD of the change for each outcome was calculated by using the P‐values and sample sizes. In studies reporting the P‐value as (P < x), the upper limit (x) was used as the P‐value. In studies not reporting P‐values for all outcomes, we imputed the SDs for changes from baseline according to Cochrane Handbook of Systematic Reviews.8 This was done by calculating the correlation coefficient for both the treatment and control measurements, and the mean of those values was used for the matching outcome. The difference had to be <0.25, values and the mean coefficient >0.5, otherwise the coefficient was not used. The coefficients were calculated from Tei 2016, Kihara 2002, Kuwahata 2011, and Miyata 2008 studies. SEs were transformed to SDs. As neither means nor SDs for BNP results were presented for the studies by Ichiki 2017 and Tei 2016 (interpreted to have the same data), the authors of the Ichiki 2017 study were contacted, and they provided us with the means and SDs of changes for the BNP.
Statistical tests for variance were also done with the RevMan 5.3 software.13 χ 2 statistic was used as a test for heterogeneity and I 2 statistic was used to quantify heterogeneity. Statistical heterogeneity was observed in varying degrees. As the outcomes still came basically from the same studies, all outcomes were reported with the random effects model. Narrative synthesis was used with outcomes that were found in only one study (endothelial function, mortality) and for the results that were retrieved from the studies that were not included in the meta‐analysis (Basford 2009).
The statistical analysis was done by two authors (M.K. and L.B.).
2.5. Quality of the evidence
To summarize the quality and strength of the evidence in support of the intervention, we used a modified version of the GRADE (The Grades of Recommendation, Assessment, Development, and Evaluation) approach.8 The quality of evidence was rated with a scale of 4 to 1 as following: 4 = high, 3 = moderate, 2 = low, and 1 = insufficient. The assessment was modified so that the quality of evidence of a group of studies could be reduced by: (a) Moderate risk for bias (total points 11‐21, reduces by 1) and high risk for bias (total points 0‐10) reduces by 2); (b) Inconsistency/heterogeneity (significant heterogeneity = I 2 ≥ 50%, reduces by 1); (c) Indirectness (if interventions of interest were directly applied to the population of interest),15 reduces 1 if not; (d) Imprecision (mean not in favor, 95% CI not totally in favor of intervention, both reduce by 1); (e) Publication bias (reduces by 1).
3. RESULTS
Of the 1444 studies, 553 studied the health effects of sauna bath. A full‐text review of those studies identified 145 studies on cardiovascular effects of sauna bath. These studies were chosen as possibly eligible and checked for identification of additional records from the reference lists. A deeper review of these studies resulted in the identification of nine studies that fulfilled the inclusion criteria. A list of included studies and patient characteristics is presented in Table 1. Of those studies, seven were included in the meta‐analysis. Two studies, Kihara 2009 and Basford 2009, were excluded from the meta‐analysis because of different follow‐up period, outcome measure, and study design compared to the other included studies.
In the meta‐analysis, the seven studies reported a total of 491 patients with HF, 275 in the sauna groups, 216 in the control groups. All the seven studies evaluated an infrared sauna therapy (Waon therapy). The frequency of sauna bath was five times a week and the follow‐up periods varied from 2 to 4 weeks. The severity of HF according to the NYHA classification varied from grade II to IV at baseline, though the majority of patients (>95%) were classed II or III. The mean EF at baseline was found to be <40% in all of the studies included. As our search strategy produced a large number of records to be reviewed by full text, we have not provided a full list of excluded records.
Of the seven studies included in the meta‐analysis, six were randomized. The risk for bias for each of the included studies (total points) was rated moderate by our modified version of the Downs and Black checklist.
3.1. Risk for bias in studies included
Generally, the risk of bias in the included studies was considered moderate on a scale of 0 to 32 points, each of the studies scoring in the moderate region (11‐21 total points) (Table 1). Tei 2016 reported 73 and 73 patients at baseline but in the results we could only see 73 and 69 patients (BNP), 71 and 66 (NYHA), and 71 and 69 (CTR). No source of funding was reported for any of the studies.
3.2. Effects of interventions
3.2.1. Symptoms and signs
Blood pressure
Six studies in the meta‐analysis assessed the effects of sauna bath on SBP (mm Hg). There was no significant effect of sauna bath compared to control (mean difference [MD] −2.58, 95% CI: −5.86 to 0.70, I 2 = 42%, P = 0.12).
Five studies in the meta‐analysis reported effects of sauna bath on DBP (mm Hg). There was no significant effect of sauna bath compared to control group (MD = −1.42; 95% CI: −3.89 to 1.05, I 2 = 27%, P = 0.26).
NYHA class
Four studies reported NYHA classifications at baseline and at the end of the follow‐up. Three of them were reported as number of patients in each class. One study reported NYHA class as means with SDs. There were some improvements in the NYHA classifications of the patients in the sauna groups compared to the controls. No studies were included in the meta‐analysis, as none of the studies reported the criteria used to judge NYHA class.
3.2.2. Blood biomarkers
BNP
Five studies reported effects on BNP (pg/mL). Sauna bath lowered the BNP value significantly in the sauna group in comparison with the control group. (MD = −124.62; 95% CI = −198.09 to −51.14, I 2 = 37%, P = 0.0009).
The different assays for measuring BNP in the five studies are listed in Table 2.
Table 2.
BNP assays reported in the studies
| Study | Source | Reported BNP assay type |
|---|---|---|
| Fujita 2011 | Plasma | Chemiluminescent enzyme immunoassay using a commercially available kit (PATHFAST; Mitsubishi Chemical Medience Co., Ltd., Tokyo, Japan) |
| Kuwahata 2011 | Plasma | Chemiluminescent enzyme immunoassay using a commercially available kit (PATHFAST, Mitsubishi Chemical Medience Co., Ltd.) |
| Kihara 2002 | Plasma | Radioimmunoassay |
| Kihara 2004 | Plasma | Radioimmunoassay |
| Ichiki 2017 | Plasma (EDTA) | 2‐site immunoenzymatic sandwich assay on a Beckman Coulter DXI 800 (Beckman Coulter, Inc.) |
Abbreviation: BNP, B‐type natriuretic peptide.
3.2.3. Imaging
Left atrial and ventricular diameter
Four studies reported effects on LAD (millimeters, mm) and LVEDD (mm) measured by echocardiography. There was no significant difference between sauna bath and control in LAD (MD = −0.32; 95% CI: −1.51 to 0.88, I 2 = 25%, P = 0.60) or LVEDD (MD = −1.07; 95% CI: −2.20 to 0.07, I 2 = 58%, P = 0.07) (Table 3).
Table 3.
Types of intervention and schedule of exposure in the studies included
| Study | Type of sauna | Temperature | Duration of single sauna bath | Frequency | Follow‐up(s) |
|---|---|---|---|---|---|
| Kihara 2002 | Far‐infrared‐ray dry sauna | 60°C | 15 min | 1×/day, 7 times a week | 2 weeks |
| Kihara 2004 | Far‐infrared‐ray dry sauna | 60°C | 15 min | 1×/day, 5 times a week | 2 weeks |
| Miyata 2008 | Far‐infrared‐ray dry sauna | 60°C | 15 min | 1×/day, 5 times a week | 2 weeks |
| Kihara 2009 | Far‐infrared‐ray dry sauna | 60°C | 15 min | 5 days after admission, at least 2 times a week after hospital discharge | 5 years |
| Kuwahata 2011 | Far‐infrared‐ray dry sauna | 60°C | 15 min | 1×/day, 5 days a week for 4 weeks | 4 weeks |
| Fujita 2011 | Far‐infrared‐ray dry sauna | 60°C | 15 min | 1×/day | 4 weeks |
| Tei 2016 | Far‐infrared‐ray dry sauna | 60°C | 15 min | 1×/day, 5 times a week for 2 weeks | 2 weeks |
| Ichiki 2017 | Far‐infrared‐ray dry sauna | 60°C | 15 min | 1×/day, 5 times a week for 2 weeks | 2 weeks |
| Basford 2009 | Far‐infrared‐ray dry sauna | 60°C | 15‐20 min | 3 times a week for 4 weeks | 4 and 4 weeks |
EF
Six studies reported the effects of sauna bath on EF (%), which was significantly higher in patients with sauna intervention compared to controls (MD = 1.45; 95% CI: 0.55‐2.35, I 2 = 0%, P = 0.002).
CTR
Six studies reported the effects on CTR (= ratio of maximal horizontal cardiac diameter to maximal horizontal thoracic diameter on x‐ray). Sauna bathing lowered the CTR significantly in comparison to control (MD = −1.82, 95% CI = −2.54 to −1.09, I 2 = 31%, P < 0.00001).
Endothelial function
One nonrandomized crossover study (Kihara 2002) reported changes in endothelial function in vessel percent flow‐mediated dilation and percent nitroglycerin, which was defined as “the percent change in diameter between 4 min after administration of sublingual nitroglycerin spray (300 μg) and that on the initial scan.” Flow‐mediated dilation improved in the treatment group from baseline 4.4 (±2.5) to 5.7 (±2.5) P = 0.0006. There was no significant difference in the control group. The NTG‐induced (Nitroglycerin‐induced) dilation change from baseline to after sauna was not different in any of the groups.
3.2.4. Mortality
One study16 reported mortality as a part of cardiac events during a 5‐year follow‐up of sauna bathing (Waon therapy) vs only standard medical treatment. The patients were compared to a control group matched for age, sex and etiology, and severity of HF. The study population was 129 patients admitted to a Japanese hospital, of which 64 patients were treated daily with for 5 days after admission, and Waon therapy was continued at least twice a week in an out‐patient clinic after hospital discharge. A cardiac event was defined as cardiac death or rehospitalization due to HF. The cardiac event rate was 68.7% in the control group and 31.3% in the sauna bathing group. The authors calculated a 38% reduction of cardiac events by sauna bathing.
3.3. Safety/tolerance
Two studies reported adverse events. Tei 2016 reported seven Waon therapy‐related minor adverse events: decrease in blood pressure, hypovolemia, increase in urine volume, decrease in body weight, and bleeding after tooth extraction. In the Basford 2009 study no adverse events were observed.
3.4. Quality of evidence
The quality of evidence was rated moderate to insufficient. There was moderate evidence for an effect on BNP, EF, and CTR, low evidence for outcomes SBP, DBP, and LAD, and insufficient evidence for LVEDD. The results of the assessment are presented in Table 4.
Table 4.
Summary of findings table
| Summary of findings | ||||||
|---|---|---|---|---|---|---|
| Population: HF patients | ||||||
| Intervention: sauna bath | ||||||
| Control: no sauna bath | ||||||
| Mean difference | 95% CI | No. of participants (sauna/control) | Heterogeneity | Quality of evidence (modified GRADE) supporting the intervention | Comments | |
|---|---|---|---|---|---|---|
| Outcomes | ||||||
| SBP | ‐2.58 | ‐5.86 to 0.70 | 275/216 | I 2 = 42% | 2 Low |
Moderate risk for bias −1, imprecision −1 |
| DBP | −1.42 | −3.89 to 1.05 | 199/143 | I 2 = 27% | 2 Low |
Moderate risk for bias −1, imprecision −1 |
| LVEDD | −1.07 | −2.20 to 0.07 | 228/169 | I 2 = 58% | 1 Insufficient |
Moderate risk for bias −1, heterogeneity −1, imprecision −1 |
| EF | 1.45 | 0.55 to 2.35 | 275/226 | I 2 = 0% | 3 Moderate |
Moderate risk for bias −1 |
| LAD | −0.32 | −1.51 to 0.88 | 228/169 | I 2 = 25% | 2 Low |
Moderate risk for bias −1, imprecision −1 |
| BNP | −124.62 | −198.09 to −51.14 | 160/136 | I 2 = 37% | 3 Moderate |
Moderate risk for bias −1 |
| CTR | −1.82 | −2.54 to −1.09 | 275/216 | I 2 = 31% | 3 Moderate |
Moderate risk for bias −1 |
Abbreviations: HF, heart failure; SBP, systolic blood pressure; DBP, diastolic blood pressure; LVEDD, left‐ventricular end‐diastolic diameter; EF, left‐ventricular ejection fraction; LAD, left atrial diameter; BNP, b‐type natriuretic peptide; CTR, cardiothoracic ratio.
4. DISCUSSION
This was a comprehensive systematic review, which resulted in a meta‐analysis of seven studies including 491 patients with HF. In all of the seven studies, the patients received infrared sauna bath, called Waon therapy, in 60°C for 15 minutes followed by a 30‐minute rest in warm environment, five times a week, for 2 to 4 weeks. Out of seven outcomes, three (EF, BNP, and CTR) showed significance in favor of sauna bath compared to control patients (no sauna). All patients were receiving standard medications according to the original publications.
The quality of evidence varied from moderate to insufficient. The main factors that reduced the quality were risk for bias and imprecision. This means that the evidence presented by this review does support a therapeutic effect of sauna bathing on HF, and more studies are thus needed to be able to draw definitive conclusions.
Two of the studies included observed adverse effects and reported only minor adverse effect, which might mean that sauna bath is a safe activity for this group of cardiac patients. Notably, this systematic review was not designed to assess the general safety of sauna bathing among HF patients. A possible source for identification of additional studies on safety could be inclusion of studies with other languages than English.
We could observe that no single parameter was consequently followed in all of the seven studies in the meta‐analysis. Only the outcome LVEDD showed heterogeneity over the set limit (I 2 > 50%) to affect the strength of evidence. We could observe that if the Kihara 2004 study was removed, which led to reduced heterogeneity, the outcome still did not show significance. Removing Kihara 2004 from other outcomes did not change the significance of the result in any of the outcomes.
NYHA class was assessed in four of the studies included, but none of those presented the methods they used to differentiate between classes. According to previous studies, NYHA class has a high level of inter‐observer variability, especially in discriminating NYHA class II and III, in which most of the patients in this review were rated.17 Thus we refrained from separate analyses of NYHA class II and III.
A recent systematic review from Brazil only identified five studies on Waon therapy for patients with HF and showed favorable effects on natriuretic peptides and blood pressure.18 No significant difference in blood pressure was seen in our analysis including two additional studies, instead a significant difference in EF was found. These differences in results are most likely explained by our wider search strategy resulting in 1444 compared to 247 identified studies, and 554 compared to 10 full‐text reviews, respectively, in this study compared to that of the Brazilian study.
Interestingly, only Waon therapy studies were included in our study but not any other sauna bath studies including Finnish sauna bath. In 1991, Dr. Tei originally started the first pilot chronic sauna study, which is now called Waon therapy, for HF patients.19 The sauna temperature of Waon therapy was set at 60°C which was a lower temperature than general sauna or Finnish sauna. It was given for 15 minutes followed by 30‐minute rest in warm environment so as not to cause any side effects on HF patients and performed five times per week for several weeks.
The possible beneficial mechanistic pathways of sauna bathing have been discussed in a recent systematic review by Laukkanen et al20 Possible underlying mechanisms on vascular function listed in the review are reduction in systemic blood pressure, improvement in endothelial function, reduction in oxidative stress and inflammation, beneficial modulation of the autonomic nervous system, and positive alteration in levels of circulating vascular risk factors such as natriuretic peptides and lipids, hormonal changes; improved vascular function and improvement in the cardiorespiratory system as well as cardiovascular function. A response to a sauna bath has been compared to physical activity such as walking, which has, as mentioned in the introduction, found to improve the quality of life and HF related hospitalization in patients with systolic heart failure.7
4.1. Limitations
As the majority of patients had EF < 40% in all studies in the meta‐analysis the results are primarily applicable for patients with HF and reduced EF. Different effects in HF patients with a preserved EF could be expected as studies have shown that these patients react differently to medical therapies and have a different pathophysiology.21, 22
The follow‐up of the studies in the meta‐analysis was relatively short, with a mean duration of 2 to 4 weeks. Therefore, more studies with longer follow‐up are needed to provide evidence of the long‐term cardiac effects.
The studies included in the meta‐analysis were interpreted to have been conducted by partly the same research groups, which must also be taken into consideration when interpreting the data.
Accurate statistical tests were not presented appropriately in every study. This might lower the accuracy of a portion of the results. We also included both randomized and nonrandomized studies, which could be assumed to lower the scientific confidence of the results in this review. However, the only nonrandomized study did not influence the results enough to affect the quality of evidence.
As only studies with infrared sauna baths met the inclusion criteria, this review cannot show any definite supporting evidence of a positive value of Finnish sauna bathing on HF patients, although Finnish sauna might have physiological effects similar to those of an infrared sauna that operates with a lower temperature.
The BNP levels were measured with different types of assays (Table 2) in the analyzed studies, and two of the studies did not report the manufacturer of the assay used. This might affect the accuracy of the BNP analysis.
5. CONCLUSIONS
This study shows that infrared sauna bath was associated with short‐term improvement in cardiac function among patients with HF. There is moderate evidence that infrared sauna bath improves EF and decreases BNP levels and cardiac size. However, data of effects of sauna bath on SBP, DBP, and LAD are limited without consistent evidence on the relationship between sauna bath and adaptations in LVEDD. The results of this review highlight the paucity of well‐designed large long‐term studies of intermittent heat exposure, in particular Finnish saunas, in patients with HF.
CONFLICTS OF INTEREST
The authors declare no potential conflict of interests.
APPENDIX 1.
Table A1.
Quality assessment results (modified Downs and Black checklist 11). The Ichiki 2017 study was not separately analyzed as the data came from the Tei 2016 study. Questions: green=1 point, red=0 points. Total points: scale: red‐yellow‐green (0 points red, 32 point green)
| Question (modified Downs and Black method) | Kihara 2002 | Kihara 2004 | Miyata 2008 | Kihara 2009 | Kuwahata 2011 | Fujita 2011 | Tei 2016 | Ichiki 2017 (= Tei 2016) |
|---|---|---|---|---|---|---|---|---|
| Reporting | ||||||||
| 1. Is the hypothesis/aim/objective of the study clearly described? | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |
| 2. Are the main outcomes to be measured clearly described in the Introduction or Methods section? | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |
| 3. Are the characteristics of the patients included in the study clearly described? | 0 | 1 | 1 | 1 | 1 | 1 | 1 | |
| 4. Are the interventions of interest clearly described? | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |
| 5. Are the distributions of principal confounders in each group of subjects to be compared clearly described? (0‐2) | 1 | 2 | 2 | 2 | 2 | 2 | 2 | |
| 6. Are the main findings of the study clearly described? | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |
| 7. Does the study provide estimates of the random variability in the data for the main outcomes? | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |
| 8. Have all important adverse events that may be a consequence of the intervention been reported? | 1 | 1 | 1 | 0 | 0 | 0 | 1 | |
| 9. Have the characteristics of patients lost to follow‐up been described? | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |
| 10. Have actual probability values been reported (eg, 0.035 rather than <0.05) for the main outcomes except where the probability value is less than 0.001? | 1 | 0 | 0 | 0 | 0 | 0 | 1 | |
| External validity | ||||||||
| 11. Were the subjects asked to participate in the study representative of the entire population from which they were recruited? | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 12. Were those subjects who were prepared to participate representative of the entire population from which they were recruited? | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 13. Were the staff, places, and facilities where the patients were treated, representative of the treatment the majority of patients receive? | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |
| Internal validity—bias | ||||||||
| 14. Was an attempt made to blind study subjects to the intervention they have received? | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 15. Was an attempt made to blind those measuring the main outcomes of the intervention? | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 16. If any of the results of the study were based on “data dredging,” was this made clear? | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |
| 17. In trials and cohort studies, do the analyses adjust for different lengths of follow‐up of patients? | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |
| 18. Were the statistical tests used to assess the main outcomes appropriate? | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |
| 19. Was compliance with the intervention/s reliable? | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |
| 20. Were the main outcome measures used accurate (valid and reliable)? | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |
| Internal validity—confounding (selection bias) | ||||||||
| 21. Were the patients in different intervention groups (trials and cohort studies) recruited from the same population? | 0 | 0 | 1 | 1 | 1 | 1 | 1 | |
| 22. Were study subjects in different intervention groups (trials and cohort studies) recruited over the same period of time? | 0 | 0 | 0 | 1 | 0 | 1 | 1 | |
| 23. Were study subjects randomized to intervention groups? | 0 | 1 | 1 | 0 | 1 | 1 | 1 | |
| 24. Was the randomized intervention assignment concealed from both patients and healthcare staff until recruitment was complete and irrevocable? | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 25. Was there adequate adjustment for confounding in the analyses from which the main findings were drawn? | 0 | 1 | 1 | 1 | 1 | 0 | 1 | |
| 26. Were losses of patients to follow‐up taken into account? | 1 | 1 | 1 | 1 | 1 | 1 | 0 | |
| Power | ||||||||
| 27. Did the study present a power calculation of significance? (modified) (0‐5) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Total: (of 32) | 16 | 19 | 20 | 19 | 19 | 19 | 21 | 21 |
No = 0, yes = 1, unable to determine = 0.
Källström M, Soveri I, Oldgren J, et al. Effects of sauna bath on heart failure: A systematic review and meta‐analysis. Clin Cardiol. 2018;41:1491–1501. 10.1002/clc.23077
REFERENCES
- 1. Braunwald E. Heart failure. JACC Heart Fail. 2013;1:1‐20. [DOI] [PubMed] [Google Scholar]
- 2. Laribi S, Aouba A, Nikolaou M, et al.; the GREAT network. Trends in death attributed to heart failure over the past two decades in Europe. Eur J Heart Fail. 2012;14:234‐239. [DOI] [PubMed] [Google Scholar]
- 3. Heidenreich PA, Trogdon JG, Khavjou OA, et al.; on behalf of the American Heart Association Advocacy Coordinating Committee, Stroke Council, Council on Cardiovascular Radiology and Intervention, Council on Clinical Cardiology, Council on Epidemiology and Prevention, Council on Arteriosclerosis, Thrombosis and Vascular Biology, Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation, Council on Cardiovascular Nursing, Council on the Kidney in Cardiovascular Disease, Council on Cardiovascular Surgery and Anesthesia, and Interdisciplinary Council on Quality of Care and Outcomes Research.Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation. 2011;123:933‐944. [DOI] [PubMed] [Google Scholar]
- 4. Roger VL. Epidemiology of heart failure. Circ Res. 2013;113:646‐659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Savarese G, Lund LH. Global public health burden of heart failure. Card Fail Rev. 2017;3:7‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McMurray JJ, Stewart S. Epidemiology, aetiology, and prognosis of heart failure. Heart. 2000;83:596‐602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Davies EJ, Moxham T, Rees K, et al. Exercise training for systolic heart failure: cochrane systematic review and meta‐analysis. Eur J Heart Fail. 2010;12:706‐715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. JPT Higgins, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [Updated March 2011]. The Cochrane Collaboration, 2011. Available from: www.cochrane-handbook.org.
- 9. PubMed http://www.ncbi.nlm.nih.gov/pubmed.
- 10. Cochrane library http://www.cochranelibrary.com/.
- 11. CINAHL https://health.ebsco.com/products/the-cinahl-database.
- 12. Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non‐randomised studies of health care interventions. J Epidemiol Community Health. 1998;52:377‐384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Review Manager (RevMan) [Computer Program]. Version [5.3]. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration; 2014. [Google Scholar]
- 14. Deeks JJ, Higgins JP, on behalf of the Statistical Methods Group . Statistical algorithms in review manager 5 Review Manager (RevMan) [Computer Program]. Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration; 2010, 2014. [Google Scholar]
- 15. Guyatt GH, Oxman AD, Kunz R, et al.; GRADE Working Group.GRADE guidelines: 8. Rating the quality of evidence—indirectness. J Clin Epidemiol. 2011;64:1303‐1310. [DOI] [PubMed] [Google Scholar]
- 16. Kihara T, Miyata M, Fukudome T, et al. Waon therapy improves the prognosis of patients with chronic heart failure. J Cardiol. 2009;53:214‐218. [DOI] [PubMed] [Google Scholar]
- 17. Raphael C, Briscoe C, Davies J, et al. Limitations of the New York Heart Association functional classification system and self‐reported walking distances in chronic heart failure. Heart. 2007;93:476‐482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rocha Conceicao LS, de Queiroz JG, Neto MG, Martins‐Filho PRS, Carvalho VO. Effect of Waon therapy in individuals with heart failure: a systematic review. J Card Fail. 2018;24:204‐206. [DOI] [PubMed] [Google Scholar]
- 19. Tei C. Waon therapy: soothing warmth therapy. J Cardiol. 2007;49:301‐304. [PubMed] [Google Scholar]
- 20. Laukkanen JA, Laukkanen T, Kunutsor SK. Cardiovascular and other health benefits of sauna bathing: a review of the evidence. Mayo Clin Proc. 2018;93:1111‐1121. [DOI] [PubMed] [Google Scholar]
- 21. Borlaug BA, Paulus WJ. Heart failure with preserved ejection fraction: pathophysiology, diagnosis, and treatment. Eur Heart J. 2011;32:670‐679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schwartzenberg S, Redfield MM, From AM, Sorajja P, Nishimura RA, Borlaug BA. Effects of vasodilation in heart failure with preserved or reduced ejection fraction implications of distinct pathophysiologies on response to therapy. J Am Coll Cardiol. 2012;59:442‐451. [DOI] [PubMed] [Google Scholar]
- 23. Kihara T, Biro S, Imamura M, et al. Repeated sauna treatment improves vascular endothelial and cardiac function in patients with chronic heart failure. J Am Coll Cardiol. 2002;39:754‐759. [DOI] [PubMed] [Google Scholar]
- 24. Kihara T, Biro S, Ikeda Y, et al. Effects of repeated sauna treatment on ventricular arrhythmias in patients with chronic heart failure. Circ J. 2004;68:1146‐1151. [DOI] [PubMed] [Google Scholar]
- 25. Miyata M, Kihara T, Kubozono T, et al. Beneficial effects of Waon therapy on patients with chronic heart failure: results of a prospective multicenter study. J Cardiol. 2008;52:79‐85. [DOI] [PubMed] [Google Scholar]
- 26. Kuwahata S, Miyata M, Fujita S, et al. Improvement of autonomic nervous activity by Waon therapy in patients with chronic heart failure. J Cardiol. 2011;57:100‐106. [DOI] [PubMed] [Google Scholar]
- 27. Fujita S, Ikeda Y, Miyata M, et al. Effect of Waon therapy on oxidative stress in chronic heart failure. Circ J. 2011;75:348‐356. [DOI] [PubMed] [Google Scholar]
- 28. Tei C, Imamura T, Kinugawa K, et al.; WAON‐CHF Study Investigators. Waon therapy for managing chronic heart failure‐ results from a multicenter prospective randomized WAON‐CHF study. Circ J. 2016;80:827‐834. [DOI] [PubMed] [Google Scholar]
- 29. Ichiki T, Burnett JC Jr, Scott CG, et al.; WAON‐CHF Study Investigators. Neurohumoral modulation during Waon therapy in chronic heart failure—subanalysis of Waon‐CHF study. Circ J. 2017. [DOI] [PubMed] [Google Scholar]
- 30. Basford JR, Oh JK, Allison TG, et al. Safety, acceptance, and physiologic effects of sauna bathing in people with chronic heart failure: a pilot report. Arch Phys Med Rehabil. 2009;90:173‐177. [DOI] [PubMed] [Google Scholar]


