The neuromuscular junction (NMJ) is a classic chemical synapse formed between motoneurons and muscles.1 The postsynaptic myogenesis and clustering of nicotinic acetylcholine cholinergic receptors (AChRs) play critical roles for NMJ formation.1 During embryogenesis and a short period after birth, AChRs contain pentamer complexes including two α1 and single β, δ, γ subunits. The transcription of γ subunit can be regulated by neurotrophic factor ARIA and neuronal activity.2, 3 At the adult stage, the γ subunit is replaced by the ε subunit, and AChR is distributed on the center of muscle fibers in mammals.1, 3 In addition, we previously identified postsynaptic muscle‐derived Slit2 acts downstream of Wnt/β‐catenin signaling as a retrograde signal is essential for presynaptic differentiation during NMJ formation,4, 5 suggesting morphogens have conserved roles in mammalian synaptogenesis.
Notch1 signaling plays essential roles in various cellular processes, including stem cell maintenance, cell fate determination, proliferation, migration, differentiation, and cell‐cell communication during development.6, 7 The intracellular domain of Notch1 (NICD) performs its main function upon ligand inducing, which subsequently translocates into the nucleus and actives Hairy and Enhancer of split (Hes) and Hes‐related (Hey) genes.7 Previous studies have demonstrated that Foxo/Notch1 signaling suppresses myogenic differentiation and fiber‐type specification,8 and a temporal switch from Notch to Wnt signaling is essential for normal adult myogenesis.9 Notch1 gain of function inhibits the transcription of myogenic transcription factor MyoD and therefore suppresses the differentiation of C2C12 myoblasts in vitro.10 However, so far, it still lacks genetic evidence whether and how Notch1 gain of function affects the myogenesis and the NMJ formation in vivo.
To investigate the role of Notch1 signaling in NMJ formation, we first generated a muscle‐specific Notch1 gain of function (GOF) transgenic mouse line by conditional overexpression of the activated form of Notch1 intracellular domain (NICD) driven by a human skeletal α‐actin (HSA) Cre with specific expression in skeletal muscles (Figure 1A, Figure S1). The overexpression of NICD as well as its downstream genes was confirmed by Western blot and quantitative PCR (Figure 1B‐C). The expression of NICD in the skeletal muscle of HSA‐NICD+ mice shows over 5‐fold increase compared to that of R26NICD control littermates (P = 0.00017) (Figure 1B). Analysis of downstream target genes of Notch1 signaling showed significantly increased expression of Hes1, Hes5, and Hey1 (Figure 1C). Intriguingly, we found the most striking phenotypes of HSA‐NICD+ mice were the failure to move and neonatal death. Compared to wild‐type mice, HSA‐NICD+ mice appear thin and weak with visible cyanosis, the diaphragm turns to be slim and translucent, and the lung size reduced with pulmonary dysfunction (Figure 1D). Normally, embryos or neonatal pups lacking fetal breathing movements are majorly due to either the absence of skeletal and/or diaphragm muscles or the absence of NMJ. Therefore, we next checked the development of the skeletal muscles by histological and ultrastructural studies. We found that the average areas of cross‐sectional skeletal muscle fibers from hindlimb in HSA‐NICD+ mice were significantly reduced (Figure 1E), and the expression of fast and slow type of myosin was both significantly decreased in HSA‐NICD+ mice (Figure S2), suggesting the muscular hypoplasia in NICD overexpressed mice. This notion was further confirmed by electron microscope study, which shows the density of diaphragm muscle fibers was less and irregularly arranged with increased extracellular matrix between muscle fibers, and the Z lines were also abnormally twisted in HSA‐NICD+ mice (Figure 1F).
Figure 1.

Specific activation of Notch1 signaling in muscles leads to neonatal death. (A) The schematic diagram for generation of HSA‐NICD + GOF mice. The transgenic mice (R26NICD) were generated using a knock‐in allele of NICD cDNA with a flox‐STOP cassette inserted into the Rosa26 locus. 2.2 Kb HSA‐Cre that is specifically expressed in skeletal muscles activates the transcription of single‐copy NICD in HSA‐NICD + mice. (B) Western blot analysis was used to verify the transgenic NICD expression, which was significantly increased over 5‐fold in HSA‐NICD + mice compared to R26NICD control (***P < 0.001, n = 4, Student's t test). (C) Quantitative RT‐PCR analysis of the downstream target genes of Notch1 in RNA isolated from R26NICD control and HSA‐NICD + mice (**P < 0.01, ***P < 0.001, n = 3, Student's t test). (D) In contrast to neonatal wild‐type pups showing motile activity, HSA‐NICD + mice are cyanotic, do not move, and die soon after birth. The diaphragm muscles are thin and translucent, and the lung lobes are unable to floating on the water in HSA‐NICD + mice. (E) Hematoxylin‐eosin staining of the cross‐sections of hindlimb skeletal muscles. Compared to wild‐type controls, HSA‐NICD + mice showed significantly decreased cross‐sectional area (Bar = 1 mm). (F) Ultrastructure analysis of diaphragm muscles using electron microscopy. Abnormalities of the disorganized diaphragmatic sarcomere were observed in HSA‐NICD + mice
In addition to myogenesis, we further asked whether the NMJ formation was affected in HSA‐NICD+ mice. To address this question, we checked the NMJ formation by α‐bungarotoxin (α‐BTX) whole‐mount staining of the neonatal diaphragms. It shows that the AChR clusters are highly distributed in the central region of the diaphragm in wild‐type mice; however, there are only very few dispersed immature AChR clusters formed in the diaphragms of HSA‐NICD+ mice (Figure 2A). The total number of AChR clusters was quantified in 1 mm2 segments of left ventral diaphragms to include clusters distributed inside the central area. The number of AChR clusters was significantly decreased from 1346 ± 91/mm2 in control to 78 ± 26/mm2 in HSA‐NICD+ diaphragms (P < 0.001, n = 5) (Figure 2B). Whole‐mount staining of AChR clusters (α‐BTX) and presynaptic phrenic nerve terminals with antibodies against neurofilament (NF) and synaptophysin (Syn) showed that the phrenic nerve branches were extensively disorganized and axon terminals failed to differentiate to form presynaptic terminals and were unable to be merged with morbid AChR clusters in HSA‐NICD+ mice (Figure 2C), suggesting the impaired formation of the NMJ in Notch1 GOF mice.
Figure 2.
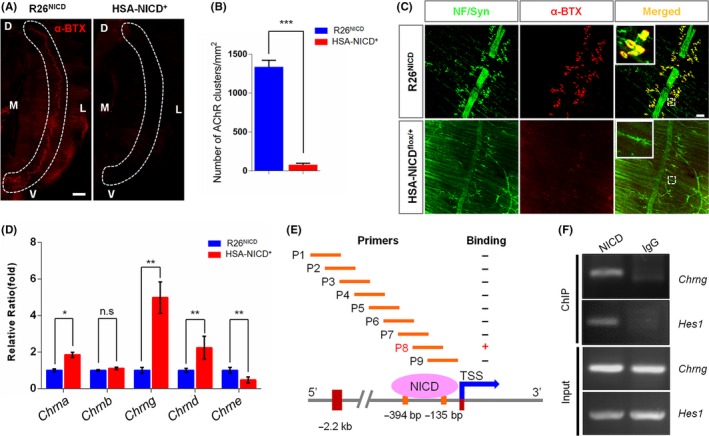
Aberrant activation of Notch1 signaling impairs the neuromuscular junction (NMJ) formation. (A) P0 diaphragms from control and HSA‐NICD + mice were whole‐mount stained with Alexa‐Flour 594‐conjugated α‐BTX (red) to label AChR clusters. The representative left side of hemidiaphragm is shown. M, medial; L, lateral; D, dorsal; V, ventral. Dashed lines indicate the AChR clusters‐enriched regions (Bar = 500 μm). (B) Quantitative analysis of the number of AChR clusters in 1 mm2 segments within the dashed lines in R26NICD control and HSA‐NICD + mice (***P < 0.001, n = 5, Student's t test). (C) Aberrant NMJ formation in HSA‐NICD + mice. Whole‐mount staining of P0 diaphragm shows significant reduction in AChR clusters and increased length of secondary branches in HSA‐NICD + mice (Bar = 50 μm). (D) Quantitative RT‐PCR analysis of the transcriptional expression of each AChR subunits in diaphragms from control and HSA‐NICD + mice (*P < 0.05, **P < 0.01, n = 6, Student's t test). (E) Schematic representation of Chrng promoter spanning ‐2.2 kb to +1 bp of the transcription start site (STT) for ChIP assay using NICD antibody. P1‐P9 indicates the regions amplified by PCR, among which, only P8 (‐394 bp to ‐135 bp) shows positive immunoprecipitation with NICD. (F) NICD associates with the promoter of Chrng gene in differentiated C2C12 myotubes. Hes1 and IgG serve as positive and negative controls, respectively
The NMJ formation depends on the normal aggregation of postsynaptic AChR receptors. Thus, the reason why Notch1 GOF mice failed to form NMJ might be due to the impaired transcription or clustering of AChR subunits. To address this issue, we examined the transcriptional level of various AChR subunits in the muscles from HSA‐NICD+ and control mice. Compared to controls, we found the expression of fetal subunit AChRγ was almost 5‐fold higher in HSA‐NICD+ mice, whereas the expression of adult subunit AChRε in HSA‐NICD+ mice was only near forty percent to normal levels (Figure 2D). Because Notch1 GOF mice showed similar amount of the AChRβ subunit compared to wild‐type mice, the distinction in AChR subunit expression is particular for the γ and ε subunits of AChR. During the NMJ formation, fetal subunit AChRγ was replaced by AChRε subunit. This AChR subunit transition is very interesting but also obscure mechanistically. The high level of γ subunit and decreased level of ε subunits in the muscle of HSA‐NICD+ mice suggesting Notch1 may play certain role in the regulation of AChR γ to ε subunit switch. To test this hypothesis, we further carried out chromatin immunoprecipitation (ChIP) screening assays using differentiated C2C12 myotubes with NICD antibody. We found endogenous NICD and a specific region of the alleles (−394 bp to −135 bp) within the 5′‐UTR of Chrng gene were both in immunoprecipitates from differentiated myotubes (Figure 2E‐F), but not the promoter of Chrne or other subunits (data not shown). Moreover, the binding of NICD to Chrng promoter became weakened during the process of myogenic differentiation (data not shown). This might be due to the downregulated expression of NICD in differentiated C2C12 myotubes (Figure S3).
In sum, this study provides genetic evidence that Notch1 gain of function in muscles leads to neonatal death and NMJ formation defects, suggesting Notch1 plays a substantial role in the regulation of myogenesis and NMJ formation. Particularly, we first demonstrated that Notch1 could regulate AChR γ to ε subunit conversion by direct regulation of the transcription of Chrng genes. Our work provides potential therapeutic applications for neuromuscular disorders in clinic.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supporting information
ACKNOWLEDGMENTS
This work was supported in part by grants from National Natural Science Foundation of China (31522029, 31770929, and 31371149), the State Key Development Program for Basic Research of China (973 Program) (2014CB542203), and Beijing Municipal Science and Technology Commission (Z161100000216154).
REFERENCES
- 1. Wu H, Xiong WC, Mei L. To build a synapse: signaling pathways in neuromuscular junction assembly. Development. 2010;137:1017‐1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Falls DL, Rosen KM, Corfas G, Lane WS, Fischbach GD. ARIA, a protein that stimulates acetylcholine receptor synthesis, is a member of the Neu ligand family. Cell. 1993;72:801‐815. [DOI] [PubMed] [Google Scholar]
- 3. Sanes JR, Lichtman JW. Induction, assembly, maturation and maintenance of a postsynaptic apparatus. Nat Rev Neurosci. 2001;2:791‐805. [DOI] [PubMed] [Google Scholar]
- 4. Wu H, Lu Y, Barik A, et al. Beta‐Catenin gain of function in muscles impairs neuromuscular junction formation. Development. 2012;139:2392‐2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wu H, Barik A, Lu Y, et al. Slit2 as a beta‐catenin/Ctnnb1‐dependent retrograde signal for presynaptic differentiation. Elife. 2015;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Artavanis‐Tsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science. 1999;284:770‐776. [DOI] [PubMed] [Google Scholar]
- 7. Kopan R, Ilagan MX. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell. 2009;137:216‐233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kitamura T, Kitamura YI, Funahashi Y, et al. A Foxo/Notch pathway controls myogenic differentiation and fiber type specification. J Clin Invest. 2007;117:2477‐2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Brack AS, Conboy IM, Conboy MJ, Shen J, Rando TA. A temporal switch from notch to Wnt signaling in muscle stem cells is necessary for normal adult myogenesis. Cell Stem Cell. 2008;2:50‐59. [DOI] [PubMed] [Google Scholar]
- 10. Buas MF, Kabak S, Kadesch T. The Notch effector Hey1 associates with myogenic target genes to repress myogenesis. J Biol Chem. 2010;285:1249‐1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


