Summary
Aims
Evidence of altered structural and functional connectivity in the frontal‐occipital network is associated with cognitive deficits in patients with schizophrenia. However, the altered patterns of functional connectivity strength (FCS) in individuals with ultra‐high risk (UHR) for psychosis remain unknown. In this study, whole‐brain FCS was assessed to examine the altered patterns of FCS in UHR subjects.
Methods
A total of 34 UHR subjects and 37 age‐ and sex‐matched healthy controls were enrolled to undergo resting‐state functional magnetic resonance imaging. The imaging data were analyzed using the graph theory method.
Results
Compared with healthy controls, UHR subjects showed significantly decreased FCS in the left middle frontal gyrus and significantly increased FCS in the left calcarine cortex. The FCS values in the left middle frontal gyrus were positively correlated to the scores of the Brief Assessments of Cognitionin Schizophrenia Symbol Coding Test (r = 0.366, P = 0.033) in the UHR subjects. A negative correlation was found between the FCS values in the left calcarine cortex and the scores of the Stroop color‐naming test (r = −0.475, P = 0.016) in the UHR subjects. A combination of the FCS values in the 2 brain areas showed an accuracy of 87.32%, a sensitivity of 73.53%, and a specificity of 100% for distinguishing UHR subjects from healthy controls.
Conclusions
Significantly altered FCS in the frontal‐occipital network is observed in the UHR subjects. Furthermore, decreased FCS in the left middle frontal gyrus and increased FCS in the left calcarine have significant correlations with the cognitive measures of the UHR subjects and thus improve our understanding of the underlying pathophysiological mechanisms of schizophrenia. Moreover, a combination of the FCS values in the 2 brain areas can serve as a potential image marker to distinguish UHR subjects from healthy controls.
Keywords: cognitive function, functional connectivity strength, graph theory, resting‐state functional magnetic resonance imaging, support vector machine, ultra‐high risk
1. INTRODUCTION
Ultra‐high risk for psychosis (UHR) is the stage preceding the onset of schizophrenia and is characterized by attenuated psychotic symptoms and a decline in social cognition and function.1 Clinically high‐risk and at‐risk mental states are used to identify participants with a putatively high risk for psychosis.2 Based on a recent meta‐analysis, the rate of transition to frank psychosis in UHR subjects reaches 29% within 2 years and increases to 36% after 3 years of follow‐up.3 Social function, neurocognition, duration of symptoms, depression, and negative symptoms are the risk factors associated with transition.4 However, the pathophysiological mechanism underpinning UHR remains unclear.
Neuroimaging techniques have enhanced our understanding of the pathophysiology of UHR. Memory task‐based functional magnetic resonance imaging (fMRI) studies have shown that UHR subjects exhibit altered function in the prefrontal regions and medial temporal cortex compared with healthy controls.5 Moreover, a positive correlation has been found between structural and functional alterations in the left middle frontal gyrus during task performance in high‐risk subjects.6 Furthermore, a longitudinal study showed that UHR subjects had an increased activation in the left inferior frontal gyrus compared with healthy controls during verbal fluency task performance at baseline.7 After 1 year of follow‐up, no difference was found between UHR subjects and healthy controls, indicating a normalization of regional activity in the prefrontal cortex during the follow‐up. However, the prefrontal cortex neurofunctional response had a direct relationship with 1 year of clinical improvement in the UHR subjects, suggesting that the prefrontal cortex alterations might be important to UHR subjects.7 Whole‐brain structural covariance analysis also revealed that UHR subjects showed subtle changes in the executive control network, default mode network, and other large‐scale networks compared with healthy controls.8, 9 In addition, the altered connectivity of cognitive control network was related to clinical features in UHR subjects.5 Furthermore, a link has been identified between poor cognitive function and risk of psychosis,10 which exhibits a predictive value in the UHR subjects who eventually transfer to frank psychosis.11 These findings suggested that cognitive dysfunction might serve as a specific marker for future degradation in clinical domains.
Working memory and executive function impairments are regarded as core cognitive deficits in schizophrenia.12 Functional imaging studies consistently identify abnormal neural activation and connectivity that involve the dorsolateral prefrontal cortex (DLPFC), such as decreased functional interactions between the bilateral DLPFC and angular gyrus and dysfunction of the left frontoparietotemporal cortex during a working memory task.13, 14 Moreover, Eryilmaz et al12 found that patients with schizophrenia showed reduced connectivity in the frontoparietal control network at rest but normal connectivity during a task. Furthermore, Fang et al15 reported that the dysconnectivity between the right DLPFC and left ventrolateral prefrontal cortex was negatively correlated to working memory deficit in patients with schizophrenia. In a voxel‐based morphometry MRI study, Mechelli et al16 found that UHR subjects had reduced gray matter volume in the bilateral frontal regions. Network abnormalities may also exist in UHR subjects. For example, UHR subjects exhibited altered function in the prefrontal and hippocampal cortices during memory task performance compared with healthy controls.5 A longitudinal fMRI study found that UHR subjects showed reduced activation in the left middle frontal gyrus and other frontal areas during the N‐Back task.6 These findings provided evidence of brain structural and functional changes in UHR subjects during task‐related patterns. However, the alterations of functional connectivity strength (FCS) in UHR at rest remain unclear, which hinder the comprehensive understanding of the pathological mechanisms underlying the cognitive dysfunction and other psychiatric symptoms in UHR subjects.
Over the last decade, the importance of graph theoretical analysis combined with resting‐state fMRI has been emphasized.17, 18, 19 Graph theoretical analysis, originated from the discovery of small‐world and scale‐free networks, is an unbiased method to examine whole‐brain functional connectome. The human brain networks are characterized by small‐world property, modularity, and the distribution of highly connected hubs using the graph theoretical analysis.20 In patients with schizophrenia, the small‐world topological property is disrupted in many brain regions such as the prefrontal lobe, and parietal and temporal lobes.21 FCS, a graph theory metric, is applied to identify the hubs of the human brain networks based on the graph theory analysis. Different from the independent component analysis or the seed‐based analysis, graph‐based FCS measures the relationships between a given voxel and the entire connectivity matrix of the whole‐brain functional connectome instead of the relationships with individual regions or networks.22 Additionally, previous study indicated that increased FCS at rest was predominantly located at the default mode network and visual cortex, and the distribution of FCS showed a strong spatial correlation with regional cerebral blood flow in the human brain functional networks.23
In this study, graph theoretical analysis was employed to investigate the altered FCS in voxel‐based functional networks between UHR subjects and healthy controls. Based on prior studies in schizophrenia,24 we hypothesized that UHR subjects would show significantly disrupted FCS in certain brain regions, especially in the DLPFC. Moreover, we hypothesized that altered FCS could be significantly correlated with clinical variables. We also hypothesized that altered FCS might sever as a potential image marker for distinguishing UHR subjects from healthy controls using support vector machine (SVM).
2. MATERIALS AND METHODS
2.1. Subjects
A total of 34 UHR subjects were recruited from the Department of Psychiatry, the Second Xiangya Hospital of Central South University in China, and 37 healthy controls were simultaneously recruited from the local community. UHR subjects were recruited using the structured interview for prodromal syndromes (SIPS)25 and the scale of prodromal syndromes (SOPS).26 The SIPS/SOPS contains the definitions for the 3 types of prodromal syndromes: brief intermittent psychotic syndrome, attenuated positive symptom syndrome, and genetic risk and deteriorated syndrome. The SIPS is used to diagnose the 3 types of prodromal syndromes,27 which includes a well‐defined anchor version of the Global Assessment of Function scale,28 a checklist for the criteria of DSM‐IV schizotypal personality disorders,29 and a family history of mental illness. The SOPS (19 items) is designed to measure the severity of 4 prodromal symptoms: positive symptoms, negative symptoms, disorganization symptoms, and general symptoms. There are 5 positive symptom items, 6 negative symptom items, 4 disorganization symptom items, and 4 general symptom items in the SOPS. Each item contains a severity scale rating from 0 (“absent”) to 6 (“severe and psychotic” for the positive symptom items and “extreme” for the 3 other items).
Healthy controls were screened by the nonpatient edition of Structured Clinical Interview for DSM‐IV.29 Potential controls were excluded if they had a first‐degree relative with a psychiatric illness.
All subjects were right‐handed and aged 16‐30 years old. Exclusion criteria for all subjects were severe physical diseases (such as cardiovascular diseases, liver and kidney diseases, or any other organic diseases), neurological diseases, history of drug abuse, and pregnancy, any head trauma, or any contradictions for MRI scan.
All participants were evaluated using the Brief Assessments of Cognition in Schizophrenia Symbol Coding Test (BACS‐SC),30 Trail Making Test A (TMT‐A),31 Hopkins Verbal Learning Test‐Revised (HVLT‐R),32 Continuous Performance Test‐Identical Pairs (CPT‐IP),33 BVMT‐R,34 and Stroop Color‐Word Test35 to measure the severity of cognitive functions, such as speed of processing, verbal learning, attention/vigilance, visual learning and memory, and verbal processing speed and executive function.
This study was approved by the Ethics Committee of the Second Xiangya Hospital of Central South University. All subjects gave a written informed consent.
2.2. Data acquisition
A 3.0 T Siemens scanner was used to obtain the fMRI images in the Second Xiangya Hospital of Central South University. All subjects were told to keep still with their eyes closed during data acquisition. Echo‐planar imaging sequence was used to collect functional images with the following parameters: repetition time/echo time = 2000 milliseconds/30 milliseconds, 33 slices, 64 × 64 matrix, 90° flip angle, 22 cm field of view, 4 mm slice thickness, no slice gap, and 240 volumes (480 seconds).
2.3. Data processing
Statistical parametric mapping (SPM8, http://fil.ion.ucl.ac.uk/spm/) was used to preprocess the functional images. The fMRI images were first corrected for slice timing and head motion. All subjects met the criteria of head movement not exceeding 2.0 mm displacement in x, y, or z and 2° of rotation in any directions. Mean motion, mean head displacement, and rotation were calculated to consider the comparability of head motion between groups. Next, the images were spatially normalized using the Montreal Neurological Institute (MNI) template in SPM8 and resampled to 3 × 3 × 3 mm3. Afterward, the images were linearly detrended and temporally band‐pass filtered (0.01‐0.08 Hz) to reduce the effects of high‐frequency physiological noises and low‐frequency drifts. After these procedures, several nuisance signals were removed, including 6 motion parameters, white matter signal, and ventricular regions of no interest.
2.4. Whole‐brain voxel‐wise functional connectivity analysis
Pearson's correlations between time series of any pair of brain voxels of the whole brain were computed, and a whole‐brain connectivity matrix was generated for each subject. The FCS value referred to the mean value of functional connectivity between a given voxel and all other voxels of the whole brain. The FCS maps were transformed to z‐scores, as illustrated in a previous study.36
2.5. Statistical analysis
Two‐sample t tests and chi‐square tests were applied to compare the clinical and demographic data (P < 0.05). Voxel‐based two‐sample t tests were performed on the functional connectivity maps between UHR subjects and healthy controls. The significant level was corrected at P < 0.01 for multiple comparisons using the Gaussian random field theory (voxel significance P < 0.001; cluster significance P < 0.01) with the REST software. Age and years of education were used as covariates in the analyses. As the resting‐state FCS might be influenced by head motions, the framewise displacement (FD) value was computed for each subject, and the mean FD was also applied as a covariate in the analyses.
After brain regions with significant group differences were identified, the mean z‐values of these regions were extracted to evaluate the correlations between abnormal FCS values and symptom severity or cognitive function in the UHR subjects. The significance level was set at P < 0.05 (Bonferroni corrected).
2.6. Classification analysis using SVM
Support vector machine was conducted to evaluate the ability of combination the FCS values in the 2 brain areas for discriminating UHR subjects from healthy controls using the LIBSVM software package (http://www.csie.ntu.edu.tw/~cjlin/libsvm/) in the Matlab. To acquire the optimal sensitivity and specificity, function kernels of Gaussian radial basis and the grid search method were applied to carry out a parameters optimization with a “leave‐one‐subject‐out” cross‐validation.
3. RESULTS
3.1. Demographics and clinical characteristics
The demographic and clinical data are present in Table 1. Except for years of education (12.06 ± 3.44 vs 14.65 ± 2.09, P < 0.001), no significant differences were observed between UHR subjects and healthy controls in sex ratio (P = 0.341), age (P = 0.346), the mean FD values (P = 0.386), and the TMT‐A (P = 0.159). Compared with healthy controls, UHR subjects exhibited poor performance in the BACS‐SC (P = 0.003), HVLT‐R (P < 0.001), CPT‐IP (P = 0.005), BVMT‐R (P = 0.019), and Stroop Color‐Word test including reading colored words (P = 0.03), color‐naming scores (P < 0.001), and color‐word scores (P < 0.001).
Table 1.
Demographic and clinical characteristics of ultra‐high risk (UHR) subjects and healthy controls
| Variables | UHR subjects (n = 34) | Healthy controls (n = 37) | χ2/t value | P‐value |
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | |||
| Sex, male/female | 21/13 | 18/19 | 1.231 | 0.341a |
| Age, y | 21.5 ± 3.5 | 20.8 ± 3.1 | −0.948 | 0.346b |
| Education, y | 12.0 ± 3.4 | 14.7 ± 2.1 | 3.867 | <0.001b |
| FD (mm) | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.873 | 0.386b |
| SIPS | ||||
| Positive symptom score | 9.5 ± 3.1 | NA | ||
| Negative symptom score | 12.8 ± 6.6 | NA | ||
| Disorganized symptom score | 6.1 ± 3.2 | NA | ||
| General symptom score | 6.0 ± 3.5 | NA | ||
| Total score | 34.3 ± 11.3 | NA | ||
| BACS‐SC | 53.6 + 9.1 | 61.0 + 10.7 | 3.135 | 0.003b |
| TMT‐A | 42.7 ± 21.6 | 36.9 ± 11.2 | −1.425 | 0.159b |
| HVLT‐R | 22.4 ± 6.6 | 27.1 ± 3.9 | 3.668 | <0.001b |
| CPT‐IP | 2.4 ± 0.7 | 2.8 ± 0.6 | 2.877 | 0.005b |
| BVMT‐R | 23.9 ± 7.4 | 27.7 ± 5.8 | 2.397 | 0.019b |
| Stroop Color‐Word Test | n = 26c | n = 33c | ||
| Word reading (W) | 81.4 ± 32.5 | 96.1 ± 17.7 | 2.296 | 0.03b |
| Color naming (C) | 50.8 ± 23.2 | 71.7 ± 14.5 | 4.167 | <0.001b |
| Color‐word (CW) | 27.8 ± 15.4 | 43.6 ± 8.1 | 4.875 | <0.001b |
NA, not applicable; FD, framewise displacement; SIPS, the structured interview for prodromal syndromes; BACS‐SC, Brief Assessments of Cognition in Schizophrenia: Symbol Coding Test; TMT‐A, Trail Making Test A; HVLT‐R, Hopkins Verbal Learning Test‐Revised; CPT‐IP, Continuous Performance Test‐Identical Pairs; BVMT‐R, Brief Visuospatial Memory Test‐Revised.
The P‐value for sex difference between the two groups was obtained by a chi‐squared test.
The P‐values were obtained by two‐sample t tests.
Eight individuals of UHR and 4 healthy participants did not finish the Stroop Color‐Word Test.
3.2. Differences in FCS between UHR subjects and healthy controls
Compared with the control group, the UHR group exhibited significantly decreased FCS in the left middle frontal gyrus (t value = −5.0714), corresponding to the MNI coordinate x, y, and z of −36, 39, and 18. Moreover, UHR subjects showed significantly increased FCS in the left calcarine cortex (t value = 3.8481). The MNI coordinate x, y, and z of the left calcarine cortex was −12, −84, and 12 (Table 2 and Figure 1). No other differences were found between the 2 groups.
Table 2.
Abnormal functional connectivity strength between ultra‐high risk (UHR) subjects and healthy controls
| Cluster location | Peak (MNI) | Number of voxels | T value | ||
|---|---|---|---|---|---|
| x | y | z | |||
| UHR Subjects > controls | |||||
| Left calcarine cortex | −12 | −84 | 12 | 60 | 3.848 |
| UHR subjects < controls | |||||
| Left middle frontal gyrus | −36 | 39 | 18 | 54 | −5.071 |
MNI, Montreal Neurological Institute.
Figure 1.
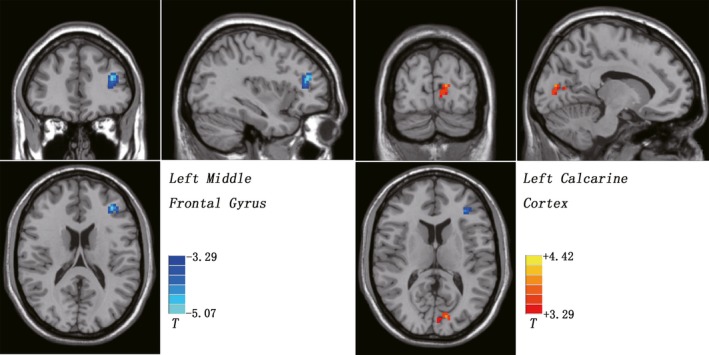
Functional connectivity strength (FCS) differences between ultra‐high risk (UHR) subjects and healthy controls. Hot color represents increased FCS, and cold color represents decreased FCS in the UHR subjects. The color bar represents the t value of the group analysis
3.3. Correlation analysis
In the UHR group, the z‐values of the left middle frontal gyrus were positively correlated to the BACS‐SC scores (r = 0.366, P = 0.033). Moreover, the FCS values of the left calcarine cortex were negatively correlated to the Stroop color‐naming scores (r = −0.475, P = 0.016; Figure 2). No significant correlations were observed between abnormal FCS and symptom severity in the UHR subjects.
Figure 2.
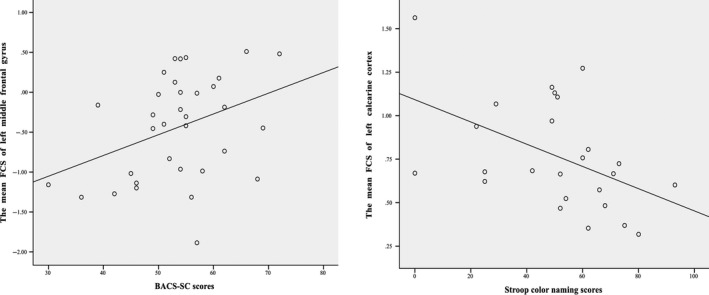
Correlations between the mean FCS values of left middle frontal gyrus/left calcarine cortex and BACS‐SC scores/Stroop color‐naming scores in the UHR subjects. FCS, functional connectivity strength; BACS‐SC, Brief Assessments of Cognition in Schizophrenia: Symbol Coding Test; UHR, ultra‐high risk
3.4. Distinguishing UHR subjects from healthy controls
The SVM analysis was performed to determine whether the combination of the FCS values in the 2 brain areas could discriminate the UHR subjects from the healthy controls with satisfactory accuracy. The results showed that the ability of the combination of the FCS values in the left middle frontal gyrus and left calcarine cortex for discriminating the UHR subjects from the healthy controls was optimal with an accuracy of 87.32% (62 of 71 in the 2 groups), a sensitivity of 73.53% (25 of 34 in the UHR group), and a specificity of 100% (37 of 37 in the control group; Figure 3).
Figure 3.
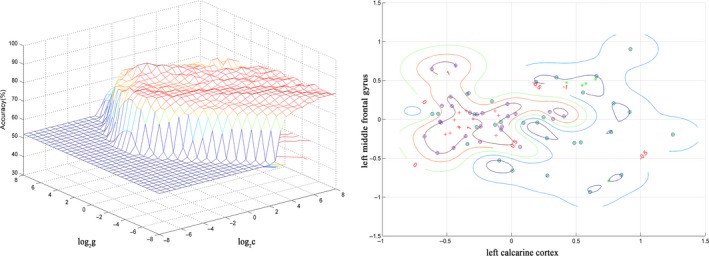
Visualization of classification by support vector machine (SVM) using the combination of the functional connectivity strength values in the abnormal brain regions. Left: SVM parameters selection result (3D visualization) [Grid Search Method]: Best c = 1.4142; Best g = 22.6274; CV accuracy = 84.507%; Right: the visualization of classification: a combination of the left middle frontal gyrus and left calcarine cortex
4. DISCUSSION
To our knowledge, this study is the first to investigate the whole‐brain FCS with graph theory in UHR subjects at rest. The results revealed that UHR subjects showed altered FCS in the frontal‐occipital network. Importantly, the FCS values in the frontal‐occipital network were significantly correlated to cognitive deficits in the UHR subjects. Moreover, the ability of combination of the FCS values in the 2 abnormal brain regions for discriminating the UHR subjects from the healthy controls was satisfactory.
The left middle frontal gyrus, a part of the DLPFC, exhibited decreased FCS in the UHR subjects in our study. As a key hub of the central executive network, the DLPFC plays a crucial role in working memory.37 Recently, cross‐sectional neuroimaging studies suggested that the prefrontal cortex was the site with the most robust abnormalities in UHR subjects38 and patients with schizophrenia.39 Reduced connectivity between the right inferior frontal gyrus and right posterior hippocampus was described in UHR subjects relative to healthy controls in a cross‐sectional study using delayed matching to sample task.40 Other studies provided evidence of reduced connectivity between the middle frontal gyrus and superior temporal gyrus during working memory task performance in UHR subjects.41 Moreover, reduced activation in the prefrontal cortex42 was found in the UHR subjects who would later transfer to frank psychosis. Our result of decreased FCS in the left middle frontal gyrus is in line with these findings,39 suggesting that altered functional connectivity in the frontal cortex may partly reflect genetic vulnerability to psychopathology. Moreover, some studies have linked neurocognitive deficits with the prefrontal cortex atrophy in patients with schizophrenia,43 indicating that dysfunction in the prefrontal cortex may be correlated to neurocognitive function. Both functional and structural MRI studies have consistently shown deficits in the DLPFC as an important medicating factor in explaining working memory deficits in schizophrenia.15, 43, 44 Furthermore, UHR subjects in the present study exhibit increased FCS in the left calcarine cortex, a part of occipital lobe. Our findings generally support a recent multicenter neuroimaging study which reported increased local gyrification index in the occipital and other cortical regions in UHR subjects.45
Our finding of decreased FCS in the left middle fontal gyrus in UHR subjects is consistent with neuroimaging findings in patients with schizophrenia, which may represent deficits in attention and working memory. Working memory impairments have been identified as primary deficits in patients with schizophrenia.15 Several studies have identified FCS deficits in the left middle frontal gyrus (part of the DLPFC), which are important in explaining deficits in working and episodic memory and related cognitive functions.37 A recent positron emission tomographic (PET) study showed that decreased dopamine release capacity was related to decreased activation in the DLPFC during a working memory task.46 Other studies have shown that deficits in the DLPFC are important for working memory deficits in schizophrenia.37 A previous study5 exhibited decreased activation in the left middle frontal gyrus, bilateral medial prefrontal corex, and hippocampal gyrus during verbal episodic memory in UHR subjects relative to healthy controls. Decreased activation in the middle frontal gyrus was correlated to poor performances on some measures related to episodic memory in schizophrenia.47 Consistent with these previous findings, the correlation between the FCS values in the left middle fontal gyrus and the BACS‐SC scores suggests that abnormal FCS is related to deficits in the attention and information processing. Attention and information processing have a close relationship with working memory. For example, selective attention can enable efficient information processing during early perceptual and semantic processing, and attention can be considered as a “gatekeeper” for working memory.48 Converging evidence suggests that selective attention and working memory show a striking overlap in the information processing, and top‐down modulation may mediate the connection between selective attention process and working memory operations.49 Moreover, working memory is a subsystem of the information processing model, which enables short‐term information storage needed for integration and processing.50 Impairment in the speed of information processing has been identified as a potential predictor of transition to frank psychosis.51 Therefore, FCS deficits in the middle frontal gyrus may be a potential endophenotype for the prediction of UHR subjects who are at increased probability of transition to frank psychosis.
The calcarine cortex is a part of occipital pole and mainly functions as a primary visual region. Evidence suggests that the calcarine cortex is involved in visual processing of emotional stimuli, and increased activation of the visual cortical regions has been observed when people experiencing emotional stimuli.52 The results of increased FCS in the left calcarine cortex are in line with a recent finding of increased amplitude of low‐frequency fluctuations in the bilateral calcarine sulcus in subjects with genetic high risk for schizophrenia.53 Previous structural and functional MRI studies in patients with schizophrenia found reduced visual cortex thickness and impaired dorsal stream visual region activation, and dysfunction in these brain regions were linked with neurocognitive dysfunction, such as masking task performance deficits and some other perceptual processing tests impairment.54, 55 Therefore, dysfunction in early‐stage visual process could contribute to impairments in the prefrontal cortex, and subsequent hippocampus and ventral visual stream. These findings emphasize the importance of dysfunction within the occipital‐frontal‐hippocampal network in the pathophysiology of schizophrenia. Early‐stage visual cortical processing dysfunction has been found in patients with schizophrenia56 and UHR subjects.57 In addition, the cortical processing deficits may be involved in the perceptual processing deficits in patients with schizophrenia.58 The present result of increased FCS in the left calcarine cortex is in line with previous findings in patients with schizophrenia and thus highlights the importance of aberrant visual cortex in the pathophysiology of UHR subjects. The correlation between increased FCS in the left calcarine cortex and the Stroop color‐naming scores suggests that FCS changes are related to deficits in executive function (selective attention and inhibition control). Recent systematic review also suggests that pervasive cognitive deficits exist in UHR subjects,59 and some cognition domains are strongly related to the risk of psychotic conversion, such as executive function, working memory, verbal memory, and processing speed. Taken together, increased FCS in the left calcarine cortex in UHR subjects may be linked with executive dysfunction, such as selective attention and inhibition control.
Furthermore, SVM analyses show that the combination of the FCS values in the left middle frontal gyrus and left calcarine cortex yields an accuracy of more than 80% for discriminating UHR subjects from healthy controls. The specificity is particularly remarkable, because every healthy control was correctly classified. UHR subjects are identified correctly with a sensitivity of 73%. Therefore, the combination of decreased FCS value in the left middle frontal gyrus and increased FCS in the left calcarine cortex may serve as a potential image marker for distinguishing the UHR subjects from the controls. Our classification results suggest that the combination of abnormal FCS in these 2 brain regions may be used to early detect individuals at risk for psychosis. These findings correspond to previous imaging studies assessing whether the neuroimaging pattern classification can be used to separate UHR subjects from controls using the SVM methods.60 Our results suggest that the combination measures of left middle frontal gyrus and left calcarine cortex may be used as an fMRI‐based diagnostic aid to improve early detection, and may have clinical utility.
The study has several limitations aside from the relatively small sample size. First, this study did not recruit patients with schizophrenia to compare their FCS values with those of UHR subjects, and this issue may limit our understanding of the disease progression between UHR subjects and patients with schizophrenia. Second, a longitudinal study is needed to test whether functional deficits in the left middle frontal gyrus and left calcarine cortex can be used as a potential biomarker to predict the transition to psychosis. Finally, this study is cross‐sectional, and it is unclear whether part of the UHR subjects will subsequently convert to psychosis. A follow‐up study is meaningful to compare the differences between UHR subjects who convert to psychosis and those who do not convert to psychosis.
5. CONCLUSIONS
In conclusion, significantly altered FCS in the frontal‐occipital network is found in the UHR subjects. Furthermore, decreased FCS in the left middle frontal gyrus and increased FCS in the left calcarine have significant correlations with the cognitive measures of UHR subjects, and thus improve our understanding of the underlying pathophysiological mechanisms of schizophrenia. Moreover, a combination of the FCS values in the 2 brain areas can serve as a potential image marker to distinguish UHR subjects from healthy controls.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ACKNOWLEDGMENTS
This study was supported by grants from the National Key Research and Development Program of China (2016YFC1307100 and 2016YFC1306900) and the National Natural Science Foundation of China (Grant Nos. 81571310, 81630033, 81771447, and 81471363). The founders of the study had no role in the study design, data collection, data analysis, data interpretation, and writing of the report. The corresponding authors had full access to all the data in the study.
Li R‐R, Lyu H‐L, Liu F, et al. Altered functional connectivity strength and its correlations with cognitive function in subjects with ultra‐high risk for psychosis at rest. CNS Neurosci Ther. 2018;24:1140–1148. 10.1111/cns.12865
Contributor Information
Jing‐Ping Zhao, Email: zhaojingping@csu.edu.cn.
Wen‐Bin Guo, Email: guowenbin76@csu.edu.cn.
REFERENCES
- 1. Shim G, Oh JS, Jung WH, et al. Altered resting‐state connectivity in subjects at ultra‐high risk for psychosis: an fMRI study. Behav Brain Funct. 2010;6:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fusar‐Poli P, Borgwardt S, Bechdolf A, et al. The psychosis high‐risk state: a comprehensive state‐of‐the‐art review. JAMA Psychiatry. 2013;70:107‐120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fusar‐Poli P, Bonoldi I, Yung AR, et al. Predicting psychosis: meta‐analysis of transition outcomes in individuals at high clinical risk. Arch Gen Psychiatry. 2012;69:220‐229. [DOI] [PubMed] [Google Scholar]
- 4. Nelson B, Yuen HP, Wood SJ, et al. Long‐term follow‐up of a group at ultra high risk (“prodromal”) for psychosis: the PACE 400 study. JAMA Psychiatry. 2013;70:793‐802. [DOI] [PubMed] [Google Scholar]
- 5. Allen P, Seal ML, Valli I, et al. Altered prefrontal and hippocampal function during verbal encoding and recognition in people with prodromal symptoms of psychosis. Schizophr Bull. 2011;37:746‐756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fusar‐Poli P, Broome MR, Woolley JB, et al. Altered brain function directly related to structural abnormalities in people at ultra high risk of psychosis: longitudinal VBM‐fMRI study. J Psychiatr Res. 2011;45:190‐198. [DOI] [PubMed] [Google Scholar]
- 7. Fusar‐Poli P, Broome MR, Matthiasson P, et al. Prefrontal function at presentation directly related to clinical outcome in people at ultrahigh risk of psychosis. Schizophr Bull. 2011;37:189‐198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Heinze K, Reniers RL, Nelson B, et al. Discrete alterations of brain network structural covariance in individuals at ultra‐high risk for psychosis. Biol Psychiatry. 2015;77:989‐996. [DOI] [PubMed] [Google Scholar]
- 9. Wang H, Guo W, Liu F, et al. Patients with first‐episode, drug‐naive schizophrenia and subjects at ultra‐high risk of psychosis shared increased cerebellar‐default mode network connectivity at rest. Sci Rep. 2016;6:26124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brewer WJ, Francey SM, Wood SJ, et al. Memory impairments identified in people at ultra‐high risk for psychosis who later develop first‐episode psychosis. Am J Psychiatry. 2005;162:71‐78. [DOI] [PubMed] [Google Scholar]
- 11. Kim HS, Shin NY, Jang JH, et al. Social cognition and neurocognition as predictors of conversion to psychosis in individuals at ultra‐high risk. Schizophr Res. 2011;130:170‐175. [DOI] [PubMed] [Google Scholar]
- 12. Eryilmaz H, Tanner AS, Ho NF, et al. Disrupted working memory circuitry in schizophrenia: disentangling fMRI markers of core pathology vs other aspects of impaired performance. Neuropsychopharmacol. 2016;41:2411‐2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chahine G, Richter A, Wolter S, et al. Disruptions in the left frontoparietal network underlie resting state endophenotypic markers in schizophrenia. Hum Brain Mapp. 2017;38:1741‐1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wu S, Wang H, Chen C, et al. Task performance modulates functional connectivity involving the dorsolateral prefrontal cortex in patients with schizophrenia. Front Psychol. 2017;8:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fang X, Wang Y, Cheng L, et al. Prefrontal dysconnectivity links to working memory deficit in first‐episode schizophrenia. Brain Imaging Behav. 2017;12:335‐344. [DOI] [PubMed] [Google Scholar]
- 16. Mechelli A, Riecher‐Rossler A, Meisenzahl EM, et al. Neuroanatomical abnormalities that predate the onset of psychosis: a multicenter study. Arch Gen Psychiatry. 2011;68:489‐495. [DOI] [PubMed] [Google Scholar]
- 17. Xia M, He Y. Magnetic resonance imaging and graph theoretical analysis of complex brain networks in neuropsychiatric disorders. Brain Connect. 2011;1:349‐365. [DOI] [PubMed] [Google Scholar]
- 18. Li M, Wang J, Liu F, et al. Handedness‐ and brain size‐related efficiency differences in small‐world brain networks: a resting‐state functional magnetic resonance imaging study. Brain Connect. 2015;5:259‐265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Su Q, Yao D, Jiang M, et al. Increased functional connectivity strength of right inferior temporal gyrus in first‐episode, drug‐naive somatization disorder. Aust N Z J Psychiatry. 2015;49:74‐81. [DOI] [PubMed] [Google Scholar]
- 20. Bullmore E, Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci. 2009;10:186‐198. [DOI] [PubMed] [Google Scholar]
- 21. Liu Y, Liang M, Zhou Y, et al. Disrupted small‐world networks in schizophrenia. Brain. 2008;131(Pt 4):945‐961. [DOI] [PubMed] [Google Scholar]
- 22. Zuo XN, Ehmke R, Mennes M, et al. Network centrality in the human functional connectome. Cereb Cortex. 2012;22:1862‐1875. [DOI] [PubMed] [Google Scholar]
- 23. Liang X, Zou Q, He Y, et al. Coupling of functional connectivity and regional cerebral blood flow reveals a physiological basis for network hubs of the human brain. Proc Natl Acad Sci USA. 2013;110:1929‐1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ursu S, Kring AM, Gard MG, et al. Prefrontal cortical deficits and impaired cognition‐emotion interactions in schizophrenia. Am J Psychiatry. 2011;168:276‐285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Miller TJ, McGlashan TH, Rosen JL, et al. Prospective diagnosis of the initial prodrome for schizophrenia based on the Structured Interview for Prodromal Syndromes: preliminary evidence of interrater reliability and predictive validity. Am J Psychiatry. 2002;159:863‐865. [DOI] [PubMed] [Google Scholar]
- 26. Miller TJ, McGlashan TH, Rosen JL, et al. Prodromal assessment with the structured interview for prodromal syndromes and the scale of prodromal symptoms: predictive validity, interrater reliability, and training to reliability. Schizophr Bull. 2003;29:703‐715. [DOI] [PubMed] [Google Scholar]
- 27. Miller TJ, McGlashan TH, Woods SW, et al. Symptom assessment in schizophrenic prodromal states. Psychiatr Q. 1999;70:273‐287. [DOI] [PubMed] [Google Scholar]
- 28. Hall RC. Global assessment of functioning. A modified scale. Psychosomatics. 1995;36:267‐275. [DOI] [PubMed] [Google Scholar]
- 29. American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders, 4th edn (DSM‐IV). Washington, D.C.: APA: American Psychiatric Association; 1994. [Google Scholar]
- 30. Keefe RS, Goldberg TE, Harvey PD, et al. The Brief Assessment of Cognition in Schizophrenia: reliability, sensitivity, and comparison with a standard neurocognitive battery. Schizophr Res. 2004;68:283‐297. [DOI] [PubMed] [Google Scholar]
- 31. Reitan RM, Wolfson D. The Halstead‐Reitan Neuropsychological Test Battery: Theory and Clinical Interpretation, 2nd edn South Tucson, AZ: Neuropsychology Press; 1993. [Google Scholar]
- 32. Brandt J. Hopkins Verbal Learning Test, Revised: Professional Manual. Psychological Assessment Resources; 2001.
- 33. Erlenmeyer‐Kimling L, Cornblatt BA. A summary of attentional findings in the New York High‐Risk Project. J Psychiatr Res. 1992;26:405‐426. [DOI] [PubMed] [Google Scholar]
- 34. Benedict RH, Odessa F. Brief Visuospatial Memory Test‐Revised. Psychological Assessment Resources; 1997.
- 35. Golden CJ. Stroop Color and Word Test. Wood Dale, IL: Stoelting Co.; 1978. [Google Scholar]
- 36. Buckner RL, Sepulcre J, Talukdar T, et al. Cortical hubs revealed by intrinsic functional connectivity: mapping, assessment of stability, and relation to Alzheimer's disease. J Neurosci. 2009;29:1860‐1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lett TA, Voineskos AN, Kennedy JL, et al. Treating working memory deficits in schizophrenia: a review of the neurobiology. Biol Psychiatry. 2014;75:361‐370. [DOI] [PubMed] [Google Scholar]
- 38. Gifford G, Crossley N, Fusar‐Poli P, et al. Using neuroimaging to help predict the onset of psychosis. NeuroImage. 2017;145(Pt B):209‐217. [DOI] [PubMed] [Google Scholar]
- 39. Pettersson‐Yeo W, Allen P, Benetti S, et al. Dysconnectivity in schizophrenia: where are we now? Neurosci Biobehav Rev. 2011;35:1110‐1124. [DOI] [PubMed] [Google Scholar]
- 40. Benetti S, Mechelli A, Picchioni M, et al. Functional integration between the posterior hippocampus and prefrontal cortex is impaired in both first episode schizophrenia and the at risk mental state. Brain. 2009;132(Pt 9):2426‐2436. [DOI] [PubMed] [Google Scholar]
- 41. Crossley NA, Mechelli A, Fusar‐Poli P, et al. Superior temporal lobe dysfunction and frontotemporal dysconnectivity in subjects at risk of psychosis and in first‐episode psychosis. Hum Brain Mapp. 2009;30:4129‐4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Marjoram D, Job DE, Whalley HC, et al. A visual joke fMRI investigation into Theory of Mind and enhanced risk of schizophrenia. NeuroImage. 2006;31:1850‐1858. [DOI] [PubMed] [Google Scholar]
- 43. Ohtani T, Levitt JJ, Nestor PG, et al. Prefrontal cortex volume deficit in schizophrenia: a new look using 3T MRI with manual parcellation. Schizophr Res. 2014;152:184‐190. [DOI] [PubMed] [Google Scholar]
- 44. Potkin SG, Turner JA, Brown GG, et al. Working memory and DLPFC inefficiency in schizophrenia: the FBIRN study. Schizophr Bull. 2009;35:19‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sasabayashi D, Takayanagi Y, Takahashi T, et al. Increased occipital gyrification and development of psychotic disorders in individuals with an at‐risk mental state: a multicenter study. Biol Psychiatry. 2017;82:737‐745. [DOI] [PubMed] [Google Scholar]
- 46. Slifstein M, van de Giessen E, Van Snellenberg J, et al. Deficits in prefrontal cortical and extrastriatal dopamine release in schizophrenia: a positron emission tomographic functional magnetic resonance imaging study. JAMA Psychiatry. 2015;72:316‐324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Achim AM, Lepage M. Episodic memory‐related activation in schizophrenia: meta‐analysis. Br J Psychiatry. 2005;187:500‐509. [DOI] [PubMed] [Google Scholar]
- 48. Awh E, Vogel EK, Oh SH. Interactions between attention and working memory. Neuroscience. 2006;139:201‐208. [DOI] [PubMed] [Google Scholar]
- 49. Awh E, Jonides J. Overlapping mechanisms of attention and spatial working memory. Trends Cogn Sci. 2001;5:119‐126. [DOI] [PubMed] [Google Scholar]
- 50. Baddeley AD, Hitch G. Working memory. The Psychology of Learning and Motivation. 1974;8C:47‐89. [Google Scholar]
- 51. Riecher‐Rossler A, Pflueger MO, Aston J, et al. Efficacy of using cognitive status in predicting psychosis: a 7‐year follow‐up. Biol Psychiatry. 2009;66:1023‐1030. [DOI] [PubMed] [Google Scholar]
- 52. Lang PJ, Bradley MM, Fitzsimmons JR, et al. Emotional arousal and activation of the visual cortex: an fMRI analysis. Psychophysiology. 1998;35:199‐210. [PubMed] [Google Scholar]
- 53. Tang Y, Chen K, Zhou Y, et al. Neural activity changes in unaffected children of patients with schizophrenia: a resting‐state fMRI study. Schizophr Res. 2015;168:360‐365. [DOI] [PubMed] [Google Scholar]
- 54. Reavis EA, Lee J, Wynn JK, et al. Cortical thickness of functionally defined visual areas in schizophrenia and bipolar disorder. Cereb Cortex. 2017;27:2984‐2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sehatpour P, Dias EC, Butler PD, et al. Impaired visual object processing across an occipital‐frontal‐hippocampal brain network in schizophrenia: an integrated neuroimaging study. Arch Gen Psychiatry. 2010;67:772‐782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Butler PD, Javitt DC. Early‐stage visual processing deficits in schizophrenia. Curr Opin Psychiatry. 2005;18:151‐157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lee SY, Namkoong K, Cho HH, et al. Reduced visual P300 amplitudes in individuals at ultra‐high risk for psychosis and first‐episode schizophrenia. Neurosci Lett. 2010;486:156‐160. [DOI] [PubMed] [Google Scholar]
- 58. Sergi MJ, Green MF. Social perception and early visual processing in schizophrenia. Schizophr Res. 2003;59:233‐241. [DOI] [PubMed] [Google Scholar]
- 59. de Paula AL, Hallak JE, Maia‐de‐Oliveira JP, et al. Cognition in at‐risk mental states for psychosis. Neurosci Biobehav Rev. 2015;57:199‐208. [DOI] [PubMed] [Google Scholar]
- 60. Wang S, Wang G, Lv H, et al. Abnormal regional homogeneity as potential imaging biomarker for psychosis risk syndrome: a resting‐state fMRI study and support vector machine analysis. Sci Rep. 2016;6:27619. [DOI] [PMC free article] [PubMed] [Google Scholar]


