Neuromyelitis optica (NMO) spectrum disorder (SD) is an autoimmune relapsing demyelinating disease of the central nervous system that is mainly mediated by anti‐aquaporin 4 (AQP4) antibodies and preferentially affects the spinal cord and optic nerve.1 It is believed that these antibodies bind to AQP4 protein on astrocytes and initiate the complement cascade to induce astrocyte necrosis.1 Secondary granulocyte and macrophage infiltration lead to oligodendrocyte death, demyelination, and neuron loss.1 Therapeutic interventions designed to modulate complement activation and neutrophil and macrophage infiltration have been proven to be efficacious in murine models of NMO.2, 3, 4
Hydroxychloroquine, a drug widely used in the treatment of rheumatoid arthritis and systemic lupus erythematosus, has been reported to have beneficial effects in a wide array of diseases, including immune, cardiovascular, and hematological disorders; malignancies; and infections.5 In addition to inhibiting the chemotaxis, phagocytosis and cytokine production of neutrophils and macrophages,5, 6, 7 hydroxychloroquine has been found to suppress complement activation recently.8 However, whether hydroxychloroquine can be used as a therapy for NMO remains to be determined.
First, we verified the effect of hydroxychloroquine on complement in vitro. Briefly, Chinese hamster ovary cells transfected with M23‐AQP4‐overexpressing lentiviruses were cultured in 24‐well plates. When confluent, the cells were incubated with or without different dilutions of the mixed serum of 5 patients with NMOSD (serumNMO) with or without different dilutions of human complement for an hour; some of the wells were treated simultaneously with hydroxychloroquine at a concentration of 1.5 × 10−6 mol/L, a steady‐state plasma concentration reported in a clinical study.9 Then, the medium was collected, and C3a levels were examined with a BD OptEIA™ Human C3a ELISA Kit. Some cells were stained with a LIVE/DEAD Viability/Cytotoxicity Kit for cytotoxicity assay, and other cells were fixed and immunostained for human immunoglobin G (hIgG) binding and complement activation tests.
As shown in Figure 1A, the serumNMO group showed positive staining for hIgG, and the control group showed negative staining for hIgG, which indicates that AQP4 was successfully overexpressed in the former group and was capable of binding with AQP4‐IgG from the serum. The serumNMO + complement and serumNMO + complement + hydroxychloroquine groups showed positive staining for C1q, C3b, and C5b‐9, but the control and serumNMO groups showed negative staining for C1q, C3b and C5b‐9, which suggests that the complement cascade was activated in the former groups. There was no apparent difference in immunofluorescence intensity between the serumNMO + complement and serumNMO + complement + hydroxychloroquine groups, which suggests that hydroxychloroquine did not inhibit AQP4‐IgG binding or complement activation. As shown in Figure 1B, the concentration of C3a in the medium was higher in the serumNMO + complement groups treated with or without hydroxychloroquine than in the control and serumNMO groups (P < .05), but there was no significant difference in the concentration of C3a between the serumNMO + complement groups treated with or without hydroxychloroquine (P > .05). These results suggest that hydroxychloroquine likely has no effect on complement activation. The live/dead staining results showed that fewer live cells (green) and more dead cells (red) were present in the serumNMO + complement groups treated with or without hydroxychloroquine than in the control and serumNMO groups (Figure 1C). The cell viability results, namely, the percentages of live cells, for the groups treated with different dilutions of serumNMO with or without different dilutions complement are summarized in Figure 1D and 1E, which showed that hydroxychloroquine failed to increase cell viability in the complement and serumNMO dilution gradients (P > .05).
Figure 1.
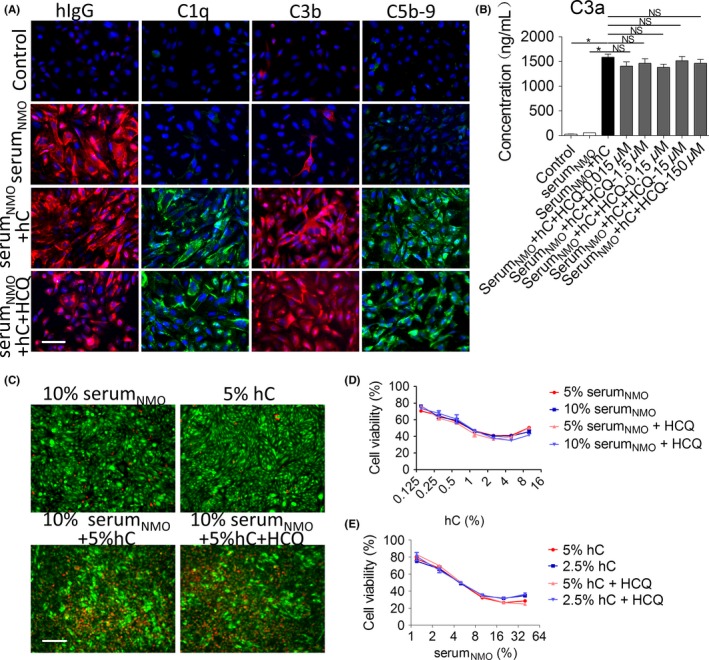
Hydroxychloroquine (HCQ) failed to inhibit complement activation in vitro. Chinese hamster ovary cells transfected with M23‐AQP4‐overexpressing lentiviruses were cultured to confluence and then incubated with or without different dilutions of the mixed serum of 5 patients with NMOSD (serumNMO) with or without different dilutions of complement (hC) for an hour; some wells were treated with 1.5 × 10−6 mol/L HCQ simultaneously. A, Immunostaining for hIgG, C1q, C3b, and C5b‐9 in the treated Chinese hamster ovary cells. Scale bar = 50 μm. B, The concentration of C3a in the medium was detected by ELISA. Data are expressed as the mean ± SE. of 4 different cell batches analyzed in triplicate. * P < .05 vs NMOIgG group by one‐way ANOVA. NS, no significance. C, Live (green)/dead (red) staining in the treated Chinese hamster ovary cell population. Scale bar = 200 μm. Cell viability in the groups treated with different dilutions of hC (D) and serumNMO (E) was calculated based on the staining results. NS vs individual control by one‐way ANOVA. (mean ± SE., n = 3)
Second, we studied the effect of hydroxychloroquine on lesions in a mouse model of NMO. The protocols were previously reported.1 Briefly, total IgG was isolated from the serum of 5 patients with NMOSD with protein‐A resin and concentrated to 15 mg/mL (termed IgGNMO). Then, weight‐matched female C57BL/6 mice (2‐3 months old) were pretreated with intraperitoneal injections of hydroxychloroquine or sterile distilled water. The hydroxychloroquine dosage of 20 mg/kg/d was chosen based on the results of a study of a clinical treatment regimen designed to elicit a faster clinical response.9 On the 23rd day of treatment, the 2 groups of mice were intracranially injected with 6 μL of IgGNMO and 4 μL of complement; they were sacrificed 1 or 5 days later. Then, the brains were collected and processed for sectioning and then immunostaining.
Consistent with the in vitro results, we did not observe a remarkable reduction in the deposition of C5b‐9 in hydroxychloroquine‐treated mice at 5 days after surgery (Figure 2A). To investigate the effect of hydroxychloroquine on inflammatory cell infiltration, we performed immunostaining for Gr‐1 to detect neutrophils at 24 hours after surgery; moreover, we performed immunostaining for CD45 and IBA1 to detect leukocytes and activated microglia/macrophages, respectively, at 5 days after surgery. As shown in Figure 2B, we delimitated the areas of neutrophil infiltration and magnified the core regions of the lesions, and then we measured the areas of neutrophil infiltration and determined the cell counts in the core regions. As shown, hydroxychloroquine did not decrease the areas of neutrophil infiltration (P > .05) or neutrophil densities (P > .05) in the core regions. We also demarcated the region of CD45‐positive cell infiltration and magnified the core regions of the lesions, and then we measured the areas of CD45‐positive cell infiltration and determined the counts of CD45‐ and IBA1‐positive cells in the core regions (Figure 2C). We found that hydroxychloroquine did not reduce the areas of CD45‐positive cell infiltration (P > .05) or the counts of CD45‐ (P > .05) or IBA1‐positive cells (P > .05) in the core regions. Taken together, these findings suggested that hydroxychloroquine failed to inhibit neutrophil and microglia/macrophage infiltration. Finally, we evaluated the areas of the lesions by performing immunostaining for AQP4, GFAP, and MBP. As shown in Figure 2D, there were no significant differences in the areas of loss of AQP4 (P > .05), GFAP (P > .05) or MBP (P > .05) staining between the 2 groups, which suggests that hydroxychloroquine could not reduce AQP4 loss, astrocyte death or demyelination in this model.
Figure 2.
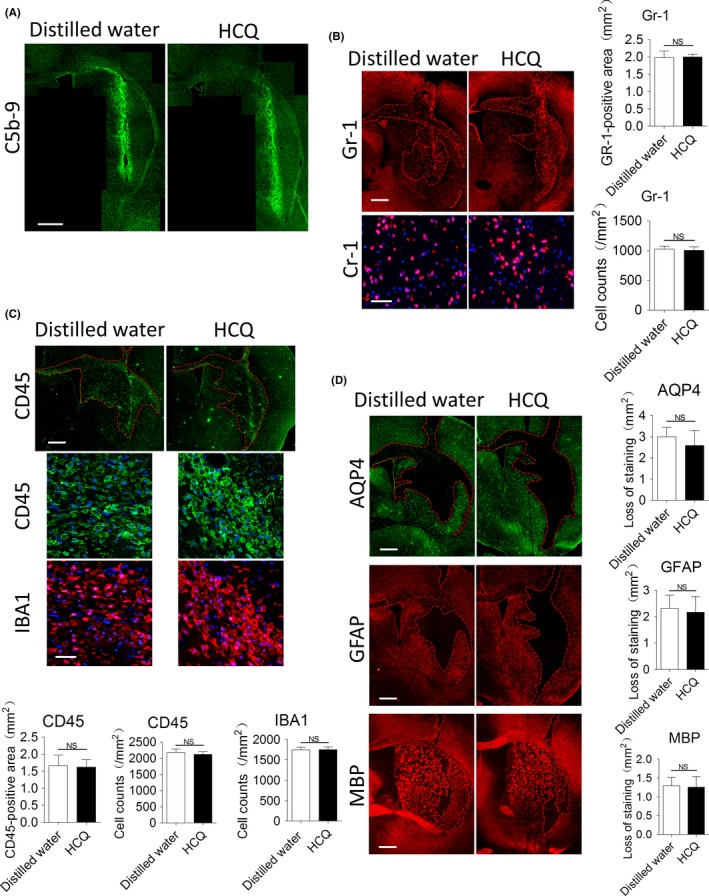
Hydroxychloroquine (HCQ) failed to reduce complement activation, inflammatory infiltration and lesion formation in vivo. Mice were intracranially injected with 6 μL of IgGNMO and 4 μL of complement after being continuously pretreated with or without 20 mg/kg/d HCQ for 23 d. A, Immunostaining for C5b‐9 at 5 d after surgery. B, Representative immunofluorescence staining for Gr‐1 at 1 d after surgery. The red lines delimit the Gr‐1‐positive area. Scale bar = 500 μm in the upper picture and 50 μm in the lower picture; the areas of neutrophil infiltration and the neutrophil counts in the core regions of the lesions were compared by the Mann‐Whitney U test (mean ± SE., n = 5). C, Representative immunofluorescence staining for CD45 and IBA1 at 5 d after surgery. The red lines delimit the areas of positive cells. Scale bar = 500 μm in the upper picture and 50 μm in the lower picture; the areas of cell infiltration and the cell counts in the core regions of the lesions were compared by the Mann‐Whitney U test (mean ± SE., n = 5). D, Representative immunofluorescence staining for GFAP, AQP4, and MBP in both groups at 5 d after surgery. The red lines delimit the lesions with AQP4, GFAP, and myelin loss. Scale bar = 500 μm. Quantification of the lesion sizes was performed by the Mann‐Whitney U test (mean ± SE., n = 5)
Collectively, our results showed that hydroxychloroquine failed to mitigate the development of brain lesions in the mouse model of NMO. The infiltration of neutrophils and microglia/macrophages in vivo, as well as complement activation in vivo and in vitro, could not be inhibited by hydroxychloroquine in our experiment. Our results contradict those of a previous report,8 which found that hydroxychloroquine inhibited complement in an animal model of antiphospholipid syndrome. We do not know the exact reason for the discrepancy and speculate it may be related to differences between the models. However, a recent clinical study found that hydroxychloroquine had no effect on complement,10 a result that supports ours. Our results cannot be directly extrapolated to patients; high‐quality randomized controlled clinical trials should be performed in the future.
CONFLICT OF INTEREST
The authors declare no conflict of interests.
ACKNOWLEDGEMENTS
This work was supported by grants from the National Natural Science Foundation of China (81771300) and the Natural Science Foundation of Guangdong Province, China (2017A030313853).
REFERENCES
- 1. Wang Z, Guo W, Liu Y, et al. Low expression of complement inhibitory protein CD59 contributes to humoral autoimmunity against astrocytes. Brain Behav Immun. 2017;65:173‐182. [DOI] [PubMed] [Google Scholar]
- 2. Phuan PW, Zhang H, Asavapanumas N, et al. C1q‐targeted monoclonal antibody prevents complement‐dependent cytotoxicity and neuropathology in in vitro and mouse models of neuromyelitis optica. Acta Neuropathol. 2013;125:829‐840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Saadoun S, Waters P, MacDonald C, et al. Neutrophil protease inhibition reduces neuromyelitis optica‐immunoglobulin G‐induced damage in mouse brain. Ann Neurol. 2012;71:323‐333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Asavapanumas N, Ratelade J, Verkman AS. Unique neuromyelitis optica pathology produced in naïve rats by intracerebral administration of NMO‐IgG. Acta Neuropathol. 2014;127:539‐551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ben‐Zvi I, Kivity S, Langevitz P, Shoenfeld Y. Hydroxychloroquine: from malaria to autoimmunity. Clin Rev Allergy Immunol. 2012;42:145‐153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rhodes JM, McLaughlin JE, Brown DJ, Nuttall LA, Jewell DP. Inhibition of leucocyte motility and prevention of immune‐complex experimental colitis by hydroxychloroquine. Gut. 1982;23:181‐187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hurst NP, French JK, Gorjatschko L, Betts WH. Studies on the mechanism of inhibition of chemotactic tripeptide stimulated human neutrophil polymorphonuclear leucocyte superoxide production by chloroquine and hydroxychloroquine. Ann Rheum Dis. 1987;46:750‐756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bertolaccini ML, Contento G, Lennen R, et al. Complement inhibition by hydroxychloroquine prevents placental and fetal brain abnormalities in antiphospholipid syndrome. J Autoimmun. 2016;75:30‐38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Browning DJ. Preclinical Foundations: Relevant Anatomy and Physiology. New York, NY: Springer‐Verlag; 2014. [Google Scholar]
- 10. Schreiber K, Breen K, Parmar K, Rand JH, Wu XX, Hunt BJ. The effect of hydroxychloroquine on haemostasis, complement, inflammation and angiogenesis in patients with antiphospholipid antibodies. Rheumatology (Oxford). 2017;57:120‐124. [DOI] [PubMed] [Google Scholar]


