Abstract
Subvalvular aortic stenosis (SAS) is one of the common adult congenital heart diseases, with a prevalence of 6.5%. It is usually diagnosed in the first decade of life. Echocardiography is the test of choice to diagnose SAS. Surgical correction is the best treatment modality, and the prognosis is usually excellent. In this review, we describe the pathophysiology, diagnosis, prognosis, and management of SAS with a focus on different pathophysiologic mechanisms, diagnostic approach, and prognosis of the disease by reviewing the current literature.
Keywords: Review, Subaortic Stenosis, Subvalvular Aortic Stenosis
1. INTRODUCTION
Subvalvular aortic stenosis (SAS) is the second most common type of aortic stenosis, accounting for 14% of left ventricular outflow tract (LVOT) obstruction, with valvular aortic stenosis being the most common cause (70%).1 The prevalence of SAS is 6.5% of all the adult congenital heart diseases.2 It predominantly involves males, with a male‐to‐female ratio of 2:1. SAS is associated with defects such as VSD, AVSD, or conotruncal anomalies in 60% of cases and may develop after patch closure of a perimembranous or malaligned VSD or AVSD.3, 4
SAS is considered an acquired disease. It is rarely diagnosed during infancy, but it often manifests in the first decade of life with features of progressive LVOT obstruction, left ventricular hypertrophy (LVH), and aortic regurgitation (AR).5 A familial form of this disease, Shone syndrome, has also been described.6
2. ANATOMY AND PATHOPHYSIOLOGY
SAS encompasses a variety of anatomic lesions that can occur either alone or in combination. The following discrete entities have been described in literature7, 8 (Figure 1):
Thin, crescent‐shaped membrane just below the aortic valve: discrete SAS. This represents 75% to 85% of SAS cases.
Thick fibromuscular ridge.
Tunnel or tubular: long, narrow, fibromuscular channel along the LVOT.
Figure 1.
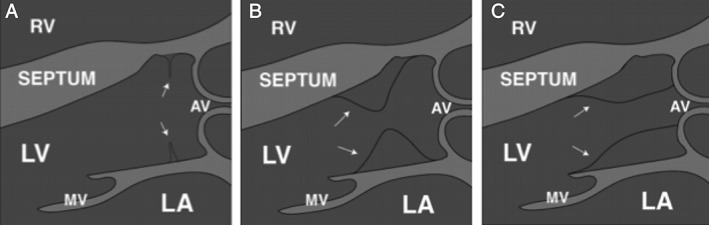
Conceptual diagram describing each of these lesions. (A) Discrete subaortic membrane (arrows). (B) Thick fibromuscular ridge (arrows). (C) Tunnel or tubular (arrows). Abbreviations: AV, aortic valve; LA, left atrium; LV, left ventricle; MV, mitral valve; RV, right ventricle
Rarely, abnormal mitral valve chords can cause outflow tract obstruction mimicking SAS.7 SAS caused by a thin fibrous membrane is more focal.9 In contrast, a fibromuscular ridge causes more diffuse obstruction and often results in a tunnel‐type lesion that is associated with a greater degree of stenosis.9
Additionally, SAS due to a misaligned VSD with posterior deviation of the outlet septum into the LVOT has been described in literature.10 This is usually in association with coarctation or interruption of the aortic arch.10
2.1. Possible theories explaining development of anatomic lesions
In 1979, Rosenquist et al11 described 22 heart specimens with SAS and found out that the mean mitral aortic separation in patients with SAS was more than twice that in normal hearts. Based on this particular finding, they speculated that an increase in mitral aortic separation could contribute to the etiology of SAS if this alters the angle at which blood is ejected from the left ventricle during a critical period of early heart development. This, in turn, could cause the embryonic cells near the crest of the ventricular septum to accumulate and eventually differentiate into a ridge or band of fibroelastic tissue.
Another explanation for it to be considered as an acquired lesion is that it is associated with abnormalities in the LVOT and also requires some preexistent morphologic substrate for development.4 Sigfússon et al. have demonstrated that steepened aortoseptal angle may be a risk factor for the development of SAS (see Supporting Information, Figure 1, in the online version of this article). Fluid modeling studies have shown that the steepened angle results in altered shear forces.12 Altered fluid shear stress has been shown to induce vascular endothelial‐cell turnover in vitro and has been connected to the development of vascular obstruction in animal models.13
There is a possible genetic component that has yet to be fully understood. Additionally, there is a possible association with other congenital cardiac defects, as prevalence of congenital cardiac defects has been reported to be as high as 6.5% in adult patients with SAS.2
2.2. Progression and hemodynamics
The progressive nature of LVOT obstruction caused by SAS in children has been well documented in literature.3, 14, 15 However, discrete SAS progresses slowly in adulthood. In particular, patients with associated coronary heart disease are at risk for faster progression and should be monitored closely.16 Oliver et al.,2 in their analysis of 134 adults diagnosed with SAS, found that the gradient across LVOT measured by Doppler echocardiography increased from a mean of 39 mmHg to 46 mmHg over an average follow‐up of 4.8 years.
Factors associated with rate of progression of LVOT obstruction are not completely clear. It is thought that abnormal fluid dynamic forces at the LVOT level can cause septal shear stress, causing cellular growth factors to engineer regional cellular proliferation contributing to the worsening of LVOT obstruction.4, 12 Why the rate of progression is different in children compared with adults is not completely understood at this time. Perhaps, the earlier in life the septal shear stress is increased above a threshold, the more intense the response and the more rapid the progression of the LVOT obstruction.
The primary hemodynamic effect on the left ventricle is increased afterload. The ventricle hypertrophies in an attempt to reduce wall stress. Also, SAS is associated with AR in 30% to 80% of the patients due to damage of the leaflets from high‐velocity jets caused by the stenosis.2, 16, 17
3. DIAGNOSIS
Most adult patients with SAS are asymptomatic. Some patients will not have symptoms until they pursue activities that cause physical stress, such as exercise or pregnancy. Symptoms may include pre‐syncope, shortness of breath, or fatigue. As the obstruction worsens, some patients may develop chest pain or syncope during exertion. Others may develop palpitations; rarely, it can lead to congestive heart failure. The diagnosis of SAS starts with the auscultation of a systolic ejection murmur, which is loudest at the left mid‐sternal border radiating to the upper sternal border.18 This leads to the suspicion for the presence of LVOT obstruction. The differential diagnosis of such a murmur includes aortic stenosis, supravalvular aortic stenosis, SAS, and hypertrophic obstructive cardiomyopathy (HOCM). More detailed physical examination can help to distinguish subaortic stenosis from the other causes of LVOT obstruction, as highlighted in Table 1. A concurrent diastolic murmur may indicate the presence of AR that can be associated with SAS.18
Table 1.
Physical examination findings to differentiate various causes of LVOT obstruction
| Discrete Subvalvular | Valvular | Supravalvular | HOCM | |
|---|---|---|---|---|
| Carotid pulse | Normal or pulsus parvus et tardus | Normal or pulsus parvus et tardus | Unequal | Brisk, jerky, systolic rebound |
| Ejection click | No | Yes | No | Uncommon or none |
| Murmur of aortic regurgitation | Sometimes | Common after age 40 years | Rare | No |
| Valsalva effect on systolic murmur | Decreased | Decreased | Decreased | Increased |
| Fourth heart sound (S4) | Uncommon | If severe | Uncommon | Common |
| Presence of paradoxical splitting | No | Sometimes | No | Common |
| Location on maximal thrill and murmur | Second RIS | Second RIS | First RIS, suprasternal notch | Fourth LIS |
Abbreviations: HOCM, hypertrophic obstructive cardiomyopathy; LIS, left intercostal space; LVOT, left ventricular outflow tract; RIS, right intercostal space.
Echocardiography is the test of choice to diagnose SAS. It is used to characterize the anatomy of the subaortic lesion, to assess LVOT involvement and dimensions and function of the LV, as well as the integrity of the aortic and mitral valves. However, often it is difficult to assess the degree of obstruction of outflow in SAS on a 2‐dimensional echocardiogram, and thus Doppler examination is indicated.
In a study by Oliver et al., Doppler examination has aided in the precise identification of the cardiac abnormality leading to LVOT obstruction, which leads to the correct assessment of the different anatomic patterns.2 It is used to estimate the gradient and the extent of obstruction across the LVOT. In addition, by using Doppler, it was possible to diagnose small subaortic membranes causing acceleration of the LVOT flow, but without a hemodynamically significant pressure gradient.2
Differentiating SAS from other causes of LVOT obstruction, especially HOCM, can prove difficult at times.19 This is because some patients with SAS develop asymmetrical septal hypertrophy and secondary dynamic subaortic obstruction.20
Severe septal hypertrophy and dynamic obstruction of the LVOT can mask the existence of a subaortic membrane, leading to a false diagnosis of HOCM.2 In such cases where conventional Doppler examination may be inconclusive, transesophageal echocardiography is more reliable for the accurate diagnosis of a subaortic membrane that is masked by the hypertrophied and prominent ventricular septum.2
AR can be present in >50% of patients with SAS.2 There are no published guidelines particularly addressing the hemodynamic effect of AR on SAS. We believe that, similar to valvular aortic stenosis, when severe AR accompanies SAS, measures of SAS severity remain accurate, including maximum velocity and mean gradient.21 However, because of the high transaortic volume flow rate, maximum velocity and mean gradient will be higher than expected for a given valve area. As per the recent update of the American Society of Echocardiography guidelines on aortic stenosis, reporting accurate quantitative data for the severity of both stenosis and regurgitation is helpful for clinical decision‐making.21
Cardiac catheterization is sometimes performed to further clarify the mechanism and extent of subaortic obstruction. This provides hemodynamic data such as the gradient across the valve, measurement of cardiac output, and estimates of the degree of AR. However, cardiac catheterization is not typically indicated in the diagnosis of SAS, but it can be utilized for preoperative hemodynamic evaluation and for preoperative workup before surgical repair to rule out significant coronary artery disease.
Cardiac magnetic resonance imaging (CMR) and cardiac computed tomography (see Supporting Information, Figure 2, in the online version of this article)18, 22 are the up‐and‐coming imaging modalities being used to diagnose different etiologies of LVOT. CMR can be used to clarify anatomy and quantify flow velocity. This can be done using T1‐weighted images with 3‐ to 5‐mm slice thickness; but the images are usually inferior to those of transesophageal echocardiography.23 Another limitation of CMR is that the spin dephasing artifact usually obscures the area of interest. This, along with the fact that the SAS membrane is often thin, makes it difficult to visualize. Cardiac computed tomography, on the other hand, is typically used in a role that complements transthoracic echocardiography.22 To date, it has not replaced echocardiography in standard practice because of its limitations: it is more complicated, more expensive, and exposes patients to radiation and iodinated contrast.22
Figure 2.
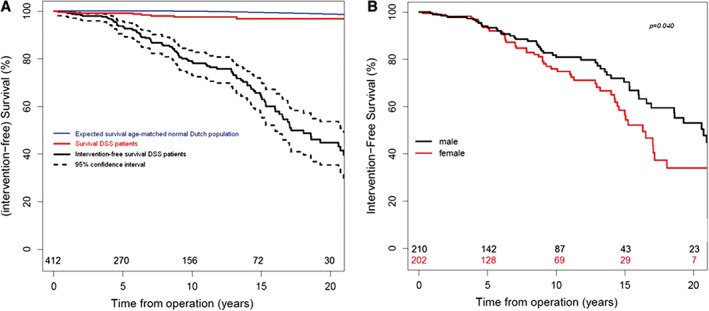
Kaplan–Meier plots. (A) Survival and intervention‐free survival for patients with DSS and expected survival for the normal age‐matched Dutch population. (B) By sex.1 Abbreviations: DSS, discrete subvalvular aortic stenosis
4. PROGNOSIS
Survival after SAS surgery has been shown to be excellent (Figure 2A). However, the LVOT gradient still increases slowly over time. In most patients, follow‐up can be done at 2‐ to 4‐year intervals due to the slow nature of the progression of the LVOT obstruction.24 Two groups that have been found to have a higher rate of progression are females (Figure 2B) and patients age > 30 years at time of diagnosis; thus, these patient groups should be monitored more frequently, but there is no set time for echocardiography follow‐up intervals.24 Eventually, most patients will require reoperation for recurrent SAS at some point in their lifetime.24
Reoperation for recurrent discrete subaortic stenosis is common; the reoperation rate is reported between 6% and 30%.25 Most of the reports discussing the risk of reoperation in patients undergoing relief of subaortic obstruction have focused on anatomic subtypes.26 Two high‐risk subgroups for recurrence and reoperation were clearly identified: first was the group of patients with tunnel SAS, and the other was the group with multilevel LVOT obstruction.27 It was suggested that patients with a residual left ventriculo‐aortic gradient >30 mmHg at the end of bypass should undergo reoperation with a more aggressive subaortic resection during the same operating session.25 Table 2 highlights the predictors of reoperation.
Table 2.
Independent predictors for increased reoperation rate
| Female sex |
| Peak instantaneous LVOT gradient progression over time |
| Difference between preoperative and postoperative peak instantaneous LVOT gradients |
| Preoperative peak instantaneous LVOT gradient ≥80 mmHg |
| Age > 30 years at diagnosis |
Abbreviations: LVOT, left ventricular outflow tract.
The risk of reoperation may be due to inadequate resection at the first operation, yet recurrent obstruction may appear despite the adequacy of surgical excision.28 One theory suggests that there is a dynamic component that may play a role in residual obstructive LVOT stenosis despite adequate resection.29 This occurs from regrowth of the tissue from the region of the septum to the initial fibromuscular obstruction.30 Another theory suggests that the formation of scar tissue in the subvalvular area during the healing process leads to a fixed size of the LVOT, resulting in localized hypertrophy and fibrosis of the LVOT.31 This may trigger fibromuscular recurrence even though initially it was a discrete membrane.25
Myectomy is another intervention that can be done to help alleviate LVOT obstruction in SAS. However, even after undergoing myectomy, there is still a high chance of recurrence, with reoperation rates between 10% and 20% within 10 years.25 In addition, myectomy is associated with an increased risk of complete heart block. Therefore, given the combination of no long‐term benefit and the risk of heart block, myectomy should not be performed routinely, and it only should be performed if marked LVH is present.24
AR was found in >50% of patients with SAS, but only 20% are considered to be hemodynamically significant.2 If present, the degree of AR can progress in patients who did not have any repair procedure for SAS. Studies have shown that there is a direct relationship between the severity of SAS and AR.32 But it has been shown that there is no significant progression of AR. A study by van der Linde et al. showed that in most patients, AR did not progress over time.24 They also found that 10% of patients who did not have AR before surgery developed mild aortic insufficiency relatively immediately in the postoperative period. Another 10% of patients progressed from mild to moderate AR, and progression to severe AR was found to be very rare (Figure 3). An LVOT gradient ≥80 mmHg was found to be a significant risk factor for developing AR postoperatively (see Supporting Information, Figure 3, in the online version of this article).
Figure 3.
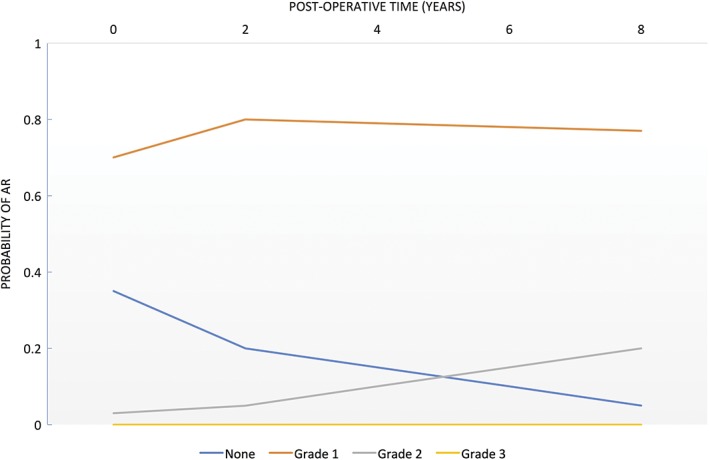
Probability of postoperative AR over time. Abbreviations: AR, aortic regurgitation
Hence, it is recommended to operate before the LVOT gradient reaches 80 mmHg.24 Furthermore, given the possible recurrence and the presence of mild AR, lifelong regular follow‐up with echocardiography is required.24 For a summary of long‐term outcomes after an SAS operation, see Supporting Information, Table 1, in the online version of this article.
5. TREATMENT
Definitive therapy for SAS consists of surgical correction of the obstruction, which may involve simple membrane removal, extensive ring resection with or without myectomy, or a Konno procedure.
The timing of the surgery varies. Recommendations range from early operation to longer periods of observation, depending on patient characteristics. Ezon et al. reported that ≥2 studies recommended surgery at diagnosis, regardless of the severity of the obstruction.33 Brauner et al. suggested that early surgery prevents AR.9 However, prevention of AR alone is not a criterion for surgery. According to 2008 American Heart Association (AHA) guidelines, unoperated adults with mean gradient <30 mmHg and without significant LVH are recommended to be followed up annually, because some of these patients will eventually require surgery. In patients with equivocal indications for intervention, stress testing to determine exercise capability, symptoms, electrocardiographic changes or arrhythmias, or increase in LVOT gradient, is reasonable (the 2008 AHA/American College of Cardiology [ACC] guidelines are found in Supporting Information, Table 2, in the online version of this article).34
Currently, there are no established medical therapies to reverse or stop the progression of SAS, including balloon dilation. Thus, the appropriate intervention for patients with significant obstruction is surgical intervention. In those with significant muscular or tunnel‐like obstruction, surgical resection of the subvalvular membrane or fibrous crescent, with or without septal myectomy, is preferred.35 For patients with diffusely narrow LVOTs, the Konno procedure and its modifications may be necessary (details of the Konno procedure can be found in Supporting Information, Figure 4, in the online version of this article).36 Postoperative complications of heart block, mitral valve injury, iatrogenic ventricular septal defect, as well as incomplete relief and/or recurrence of obstruction and infective endocarditis (IE), have been reported. In recent years, enucleation of the fibrous ridge by blunt dissection with myectomy in selected patients has shown promising results.35 In the study by Suri et al., there was a postoperative decline, but ejection fraction stabilized with time after the Konno procedure on the follow‐up echocardiograms.37 Sharma et al. demonstrated that the recovery of ventricular function after the Konno procedure is similar to that seen after aortic valve replacement alone, in contrast to initial studies.38 Pulmonary valve regurgitation can occur as a complication of this procedure and may require pulmonary valve replacement in a minority of patients. In the long‐term follow‐up of the patients who underwent the procedure, New York Heart Association status remained class 1 after initial improvement. Mean follow‐up period was 8.2 ± 5.7 years.37
IE prophylaxis before dental procedures is not recommended as per the 2008 ACC/AHA guidelines unless the patient had prior history of IE or repair with patch or residual defect. IE prophylaxis is recommended only in the initial 6 months after patch repair.34
6. CONCLUSION
SAS is the second most common type of aortic stenosis, accounting for 6.5% of adult congenital disease. It is considered an acquired disease, with different rates of progression among adults and children. Most adult patients with SAS are asymptomatic. Symptoms may include pre‐syncope, shortness of breath, or fatigue with physical stress, such as exercise or pregnancy. Surgical correction is the treatment of choice, and the prognosis is usually excellent, with varied recurrence rates depending on the presence of certain risk factors.
Conflicts of interest
The authors declare no potential conflicts of interest.
Supporting information
Figure S1. Aortoseptal angle measurement in parasternal long axis view in early systole, just before aortic valve opening (2).
Figure S2. (A) MRI image showing subaortic membrane at the junction of the septal leaflet of the mitral valve and interventricular septum [19]. (B) CT coronary angiogram showing aortic cusps (AC) and subaortic membrane and the origin of the left coronary artery [23].
Figure S3. Preoperative LVOT gradient vs postoperative aortic regurgitation. Association between various levels of preoperative peak LVOT gradient and probability of postoperative aortic regurgitation progression over time.
Figure S4. (A) A transverse right infundibulotomy is done and extended with direct visualization of the pulmonary valve annulus. The infundibulotomy provides exposure of the pulmonary valve and the subpulmonary interventricular septum. A diagonal incision is made in the aorta, extended under direct visualization deep into the noncoronary cusp to gain exposure to the aortic valve, and the subaortic interventricular septum. Asc. ao. ‐ ascending aorta; RA ‐ right atrium; RCA ‐ right coronary artery; RV ‐ right ventricle; SVC ‐ superior vena cava. (B) The safely resectable region of the interventricular septum is visualized. A safe approach is to pass 2 sutures through the interventricular septum to delimit and protect the aortic valve from injury. The needle is passed through the septum from the aortic side to the subpulmonary side, marking in 2 sites the location of the right cusp hinge points. This maneuver provides retraction and delimiting suture guides that protect the aortic valve from injury by errant myectomy. LV ‐ left ventricle; RV ‐ right ventricle. (C) A VSD is created from the right‐sided exposure. VSD is enlarged to relieve subaortic stenosis fully without injury to the aortic valve. The VSD is extended sufficiently apically so as to effect complete relief of midcavity or deeper levels of obstruction. (D) Pledgetted VSD sutures are placed around the newly created VSD, assuring purchase of firm tissue. (E) A patch of thin, flexible material, ideally autologous pericardium is used for VSD closure. Inspection and calibration are made through the aortic valve to assure full relief of subaortic obstruction, prior to closure of the aortotomy. (F) A patch is used to repair the right ventriculotomy. RV patch, right ventricular patch.
Table S1. Long‐term outcomes after SAS operation
Table S2. 2008 ACC/AHA guidelines for surgical intervention of SAS
Devabhaktuni S. R., Chakfeh E., Malik A. O., Pengson J. A., Rana J., and Ahsan C. H.. Subvalvular aortic stenosis: a review of current literature. Clin Cardiol. 2018;41:131–136. 10.1002/clc.22775
REFERENCES
- 1. Barekatain A, Fanari Z, Hammami S, et al. Subvalvular aortic stenosis. Del Med J. 2015;87:346–348. [PubMed] [Google Scholar]
- 2. Oliver JM, González A, Gallego P, et al. Discrete subaortic stenosis in adults: increased prevalence and slow rate of progression of the obstruction and aortic regurgitation. J Am Coll Cardiol. 2001;38:835–842. [DOI] [PubMed] [Google Scholar]
- 3. Leichter DA, Sullivan I, Gersony WM. “Acquired” discrete subvalvular aortic stenosis: natural history and hemodynamics. J Am Coll Cardiol. 1989;14:1539–1544. [DOI] [PubMed] [Google Scholar]
- 4. Sigfússon G, Tacy TA, Vanauker MD, et al. Abnormalities of the left ventricular outflow tract associated with discrete subaortic stenosis in children: an echocardiographic study. J Am Coll Cardiol. 1997;30:255–259. [DOI] [PubMed] [Google Scholar]
- 5. Sharma BD, Mittal S, Kasliwal RR, et al. Discrete subvalvular aortic stenosis. J Assoc Physicians India. 2000;48:1103–1106. [PubMed] [Google Scholar]
- 6. Urbach J, Glaser J, Balkin J, et al. Familial membranous subaortic stenosis. Cardiology. 1985;72:214–217. [DOI] [PubMed] [Google Scholar]
- 7. Aboulhosn J, Child JS. Left ventricular outflow obstruction: subaortic stenosis, bicuspid aortic valve, supravalvular aortic stenosis, and coarctation of the aorta. Circulation. 2006;114:2412–2422. [DOI] [PubMed] [Google Scholar]
- 8. Vogt J, Dische R, Rupprath G, et al. Fixed subaortic stenosis: an acquired secondary obstruction? A twenty‐seven‐year experience with 168 patients. Thorac Cardiovasc Surg. 1989;37:199–206. [DOI] [PubMed] [Google Scholar]
- 9. Brauner R, Laks H, Drinkwater DC Jr, et al. Benefits of early surgical repair in fixed subaortic stenosis. J Am Coll Cardiol. 1997;30:1835–1842. [DOI] [PubMed] [Google Scholar]
- 10. Choi JY, Sullivan ID. Fixed subaortic stenosis: anatomical spectrum and nature of progression. Br Heart J. 1991;65:280–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rosenquist GC, Clark EB, McAllister HA, et al. Increased mitral‐aortic separation in discrete subaortic stenosis. Circulation. 1979;60:70–74. [DOI] [PubMed] [Google Scholar]
- 12. Cape EG, Vanauker MD, Sigfússon G, et al. Potential role of mechanical stress in the etiology of pediatric heart disease: septal shear stress in subaortic stenosis. J Am Coll Cardiol. 1997;30:247–254. [DOI] [PubMed] [Google Scholar]
- 13. Langdon TJ, Boerboom LE, Olinger GN, et al. Rheologic genesis of aortic coarctation in a canine model. Am Heart J. 1988;115:489–492. [DOI] [PubMed] [Google Scholar]
- 14. Firpo C, Maitre Azcárate MJ, Quero Jiménez M, et al. Discrete subaortic stenosis (DSS) in childhood: a congenital or acquired disease? Follow‐up in 65 patients. Eur Heart J. 1990;11:1033–1040. [DOI] [PubMed] [Google Scholar]
- 15. Freedom RM, Pelech A, Brand A, et al. The progressive nature of subaortic stenosis in congenital heart disease. Int J Cardiol. 1985;8:137–148. [DOI] [PubMed] [Google Scholar]
- 16. van der Linde D, Takkenberg JJM, Rizopoulos D, et al. Natural history of discrete subaortic stenosis in adults: a multicentre study. Eur Heart J. 2013;34:1548–1556. [DOI] [PubMed] [Google Scholar]
- 17. Sung CS, Price EC, Cooley DA. Discrete subaortic stenosis in adults. Am J Cardiol. 1978;42:283–290. [DOI] [PubMed] [Google Scholar]
- 18. Qureshi A, Awuor S, Martinez M. Adult presentation of subaortic stenosis: another great hypertrophic cardiomyopathy mimic. Heart Lung Circ. 2015;24:e7–e10. [DOI] [PubMed] [Google Scholar]
- 19. Bruce CJ, Nishimura RA, Tajik AJ, et al. Fixed left ventricular outflow tract obstruction in presumed hypertrophic obstructive cardiomyopathy: implications for therapy. Ann Thorac Surg. 1999;68:100–104. [DOI] [PubMed] [Google Scholar]
- 20. Charles R, Makin C, Coulshed N, et al. Echocardiography in combined discrete and hypertrophic subaortic stenosis. Thorax. 1981;36:126–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Baumgartner H, Hung J, Bermejo J, et al. Recommendations on the echocardiographic assessment of aortic valve stenosis: a Focused Update from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. J Am Soc Echocardiogr. 2017;30:372–392. [DOI] [PubMed] [Google Scholar]
- 22. Mun HS, Wann LS. Noninvasive evaluation of membranous subaortic stenosis: complimentary roles of echocardiography and computed tomographic angiography. Echocardiography. 2010;27:E34–E35. [DOI] [PubMed] [Google Scholar]
- 23. Krieger EV, Stout KK, Grosse‐Wortmann L. How to image congenital left heart obstruction in adults. Circ Cardiovasc Imaging. 2017;10:e004271 10.1161/CIRCIMAGING.116.004271. [DOI] [PubMed] [Google Scholar]
- 24. van der Linde D, Roos‐Hesselink JW, Rizopoulos D, et al. Surgical outcome of discrete subaortic stenosis in adults: a multicenter study. Circulation. 2013;127:1184–1191, e1–e4. [DOI] [PubMed] [Google Scholar]
- 25. Erentug V, Bozbuga N, Kirali K, et al. Surgical treatment of subaortic obstruction in adolescent and adults: long‐term follow‐up. J Card Surg. 2005;20:16–21. [DOI] [PubMed] [Google Scholar]
- 26. Coleman DM, Smallhorn JF, McCrindle BW, et al. Postoperative follow‐up of fibromuscular subaortic stenosis. J Am Coll Cardiol. 1994;24:1558–1564. [DOI] [PubMed] [Google Scholar]
- 27. Ruzmetov M, Vijay P, Rodefeld MD, et al. Long‐term results of surgical repair in patients with congenital subaortic stenosis. Interact Cardiovasc Thorac Surg. 2006;5:227–233. [DOI] [PubMed] [Google Scholar]
- 28. Stassano P, Di Tommaso L, Contaldo A, et al. Discrete subaortic stenosis: long‐term prognosis on the progression of the obstruction and of the aortic insufficiency. Thorac Cardiovasc Surg. 2005;53:23–27. [DOI] [PubMed] [Google Scholar]
- 29. Rayburn ST, Netherland DE, Heath BJ. Discrete membranous subaortic stenosis: improved results after resection and myectomy. Ann Thorac Surg. 1997;64:105–109. [DOI] [PubMed] [Google Scholar]
- 30. Lupinetti FM, Pridjian AK, Callow LB, et al. Optimum treatment of discrete subaortic stenosis. Ann Thorac Surg. 1992;54:467–471. [DOI] [PubMed] [Google Scholar]
- 31. Stewart JR, Merrill WH, Hammon JW Jr, et al. Reappraisal of localized resection for subvalvular aortic stenosis. Ann Thorac Surg. 1990;50:197–203. [DOI] [PubMed] [Google Scholar]
- 32. Rizzoli G, Tiso E, Mazzucco A, et al. Discrete subaortic stenosis: operative age and gradient as predictors of late aortic valve incompetence. J Thorac Cardiovasc Surg. 1993;106:95–104. [PubMed] [Google Scholar]
- 33. Ezon DS. Fixed subaortic stenosis: a clinical dilemma for clinicians and patients. Congenit Heart Dis. 2013;8:450–456. [DOI] [PubMed] [Google Scholar]
- 34. Warnes CA, Williams RG, Bashore TM, et al. ACC/AHA 2008 guidelines for the management of adults with congenital heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Develop Guidelines on the Management of Adults With Congenital Heart Disease) . Developed in Collaboration With the American Society of Echocardiography, Heart Rhythm Society, International Society for Adult Congenital Heart Disease, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2008;52:e143–e263. [DOI] [PubMed] [Google Scholar]
- 35. Hirata Y, Chen JM, Quaegebeur JM, et al. The role of enucleation with or without septal myectomy for discrete subaortic stenosis. J Thorac Cardiovasc Surg. 2009;137:1168–1172. [DOI] [PubMed] [Google Scholar]
- 36. Bichell DP. Modified Konno procedure for left ventricular outflow tract obstruction. Oper Tech Thorac Cardiovasc Surg. 2011;16:62–69. [Google Scholar]
- 37. Suri RM, Dearani JA, Schaff HV, et al. Long‐term results of the Konno procedure for complex left ventricular outflow tract obstruction. J Thorac Cardiovasc Surg. 2006;132:1064–1071. [DOI] [PubMed] [Google Scholar]
- 38. Sharma GK, Wojtalik M, Siwińska A, et al. Aortoventriculoplasty and left ventricle function: long‐term follow‐up. Eur J Cardiothorac Surg. 2004;26:129–136. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Aortoseptal angle measurement in parasternal long axis view in early systole, just before aortic valve opening (2).
Figure S2. (A) MRI image showing subaortic membrane at the junction of the septal leaflet of the mitral valve and interventricular septum [19]. (B) CT coronary angiogram showing aortic cusps (AC) and subaortic membrane and the origin of the left coronary artery [23].
Figure S3. Preoperative LVOT gradient vs postoperative aortic regurgitation. Association between various levels of preoperative peak LVOT gradient and probability of postoperative aortic regurgitation progression over time.
Figure S4. (A) A transverse right infundibulotomy is done and extended with direct visualization of the pulmonary valve annulus. The infundibulotomy provides exposure of the pulmonary valve and the subpulmonary interventricular septum. A diagonal incision is made in the aorta, extended under direct visualization deep into the noncoronary cusp to gain exposure to the aortic valve, and the subaortic interventricular septum. Asc. ao. ‐ ascending aorta; RA ‐ right atrium; RCA ‐ right coronary artery; RV ‐ right ventricle; SVC ‐ superior vena cava. (B) The safely resectable region of the interventricular septum is visualized. A safe approach is to pass 2 sutures through the interventricular septum to delimit and protect the aortic valve from injury. The needle is passed through the septum from the aortic side to the subpulmonary side, marking in 2 sites the location of the right cusp hinge points. This maneuver provides retraction and delimiting suture guides that protect the aortic valve from injury by errant myectomy. LV ‐ left ventricle; RV ‐ right ventricle. (C) A VSD is created from the right‐sided exposure. VSD is enlarged to relieve subaortic stenosis fully without injury to the aortic valve. The VSD is extended sufficiently apically so as to effect complete relief of midcavity or deeper levels of obstruction. (D) Pledgetted VSD sutures are placed around the newly created VSD, assuring purchase of firm tissue. (E) A patch of thin, flexible material, ideally autologous pericardium is used for VSD closure. Inspection and calibration are made through the aortic valve to assure full relief of subaortic obstruction, prior to closure of the aortotomy. (F) A patch is used to repair the right ventriculotomy. RV patch, right ventricular patch.
Table S1. Long‐term outcomes after SAS operation
Table S2. 2008 ACC/AHA guidelines for surgical intervention of SAS


