Abstract
Cardiovascular disease remains the leading cause of death in women in the United States and is a major public health issue for all women, but it is of increasing concern to breast cancer survivors. Advancements in early detection and breast cancer therapy have resulted in over 90% of women surviving 5 years past their diagnosis of breast cancer. Nonetheless, with increased survivorship from breast cancer, there has been an increase in cardiovascular disease in these women. The consequences of the treatments for breast cancer may increase the risk for cardiovascular disease. Additionally, there is an overlap of risk factors common to both breast cancer and cardiovascular disease. The increased risk of cardiovascular disease in women who survive breast cancer must be recognized, with a focus on the prevention and early detection of cardiovascular disease.
Keywords: Acute Coronary Care, Acute Coronary Syndrome, Breast Cancer, Cardiovascular Disease
1. INTRODUCTION
Cardiovascular disease remains the leading cause of death in women in the United States and is a major public health issue for all women,1 but it is of increasing concern to breast cancer survivors. Advancements in early detection and breast cancer therapy have resulted in over 90% of women surviving 5 years past their diagnosis of breast cancer, with a related mortality reduction of ~2% per year over the last decade.2, 3 Nonetheless, with increased survivorship from breast cancer there has been an increase in cardiovascular disease in these women.4 For breast cancer survivors, deaths due to cardiovascular diseases account for 35% of the non–cancer‐related deaths in those age 50 years and older, and cardiovascular mortality is the greatest single non–cancer‐related cause of death.5
Based on the most recent statistics available, 236 968 women were diagnosed with breast cancer in 2014 and 41 211 women died from breast cancer in the United States.6 The Surveillance, Epidemiology and End Results data and numerous other population studies have demonstrated an increased incidence in cardiovascular disease in breast cancer survivors, compared to other women without breast cancer.4, 5, 7
Although cardiovascular morbidity and mortality is common in breast cancer survivors, earlier research focused primarily on the consequences of treatments for breast cancer as a cause for cardiovascular outcomes. Nonetheless, a recent assessment beyond the effect of cancer therapies has demonstrated an increase in cardiovascular diseases, irrespective of treatment, with an overlap of risk factors common to both diseases. Those individuals with breast cancer have been shown to have a greater prevalence of cardiovascular risk factors.8 Given that increased cardiovascular risk appears to manifest approximately 5 to 7 years after the initial diagnosis of breast cancer,4, 7 a potential window of opportunity exists for possible preventive intervention strategies, although there are limited intervention trials available to assess effectiveness of specific interventions to date.
2. CARDIOVASCULAR EFFECTS OF BREAST CANCER THERAPY
There are a number of potential ways that the treatment of breast cancer can result in an increase in cardiovascular disease–specific risk factors or cardiovascular disease. Degree of susceptibility to adverse cardiovascular effects appears to be related to the presence or absence of cardiovascular risk factors or overt cardiovascular disease prior to the onset of breast cancer diagnosis and treatment. This has been described by Jones et al9 as a “multiple‐hit” hypothesis (Figure 1).
Figure 1.
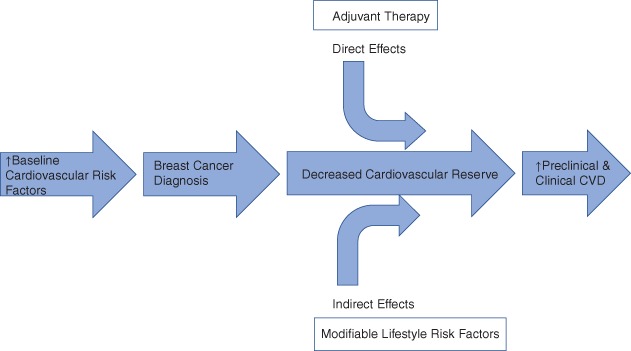
A schematic representation describing the “multiple‐hit” hypothesis. At diagnosis, a significant proportion of early breast cancer patients present with preexisting or heightened cardiovascular disease (CVD) risk factors, which increase the risk of adjuvant therapy–associated cardiovascular injury. Independently, many adjuvant therapies used in breast cancer are associated with unique and varying degrees of direct adverse effects on the cardiovascular system. These direct effects occur in the context of concomitant lifestyle perturbations (indirect effects) that combine to reduce cardiovascular reserve. Collectively, these changes may leave the patient more susceptible to further cardiovascular insults and at higher risk of premature death due to cardiovascular mortality. From: Jones et al.,9 with permission
3. CHEMOTHERAPY AND CARDIOVASCULAR CONSEQUENCES
There is a well‐established link between anthracycline therapy and development of congestive heart failure.10 High cumulative doses of anthracyclines resulted in a risk of heart failure ranging from 3% to 26%.11 As a result of this knowledge, anthracycline doses have decreased, and the cardiotoxicity effects have been reduced to 2% to 3%.12 Doxorubicin is the most commonly used chemotherapeutic agent used in breast cancer and is an anthracycline that directly affects cardiovascular function, with the potential to cause type 1 cardiotoxicity, which is irreversible. A more recently developed and very commonly used therapy for breast cancer, trastuzumab, a monoclonal antibody that targets the human epidermal growth factor receptor‐2 (HER‐2) and other targeted HER‐2 therapies, can similarly affect cardiac function. However, trastuzumab may cause type 2 cardiotoxicity, which differs from type 1, in that it is potentially reversible if discovered early. It is estimated that trastuzumab results in a significant reduction of left ventricular (LV) ejection fraction in 7.1% to 18.6% of patients, based on 4 major clinical trials, but clinical heart failure results in only 1.7% to 4.1% of subjects.13 Outside of clinical trials, the rates of heart failure with trastuzumab use have been much higher, with 1 large registry cohort showing rates of heart failure in 12.1% of those treated with trastuzumab alone, and 20.1% of those treated with trastuzumab in conjunction with an anthracycline.14 There are great limitations in determining the incidence and prevalence of chemotherapy‐ and radiotherapy‐ induced cardiac abnormalities, or chemotherapy‐related cardiac dysfunction (CRCD) given differences in definitions, lack of consistent data reporting of consequences of therapies, and selection bias in studies of breast cancer treatment clinical trials. Nonetheless, for patients who undergo a myocardial biopsy to determine the cause of cardiac dysfunction, those with chemotherapy‐induced cardiomyopathy have the poorest prognosis of all cardiomyopathies.15 Different models to predict the risk of heart failure prior to initiation of trastuzumab have been proposed with age, sex, anthracycline use, known coronary artery disease, atrial fibrillation, renal failure, and cardiovascular risk factors appearing to affect the risk of heart failure development.16, 17
4. RADIATION THERAPY AND CARDIOVASCULAR CONSEQUENCES
Radiation to the breast may have direct effects to the myocardium, but the data are conflicting regarding the association of breast radiation and accelerated atherosclerosis. It is estimated that the heart receives 1 to 5 Gy of radiation when undergoing radiation therapy for breast cancer. In a case control study from Sweden and Denmark of women with breast cancer who underwent radiation therapy from 1958 to 2001, the rates of major coronary events were linearly associated with the mean radiation dose to the heart, where for every 1 Gy of radiation, the risk for coronary events increased by 7.4% (P < 0.001).18 The increased risk started within the first 5 years after radiation therapy, and those with more cardiovascular risk factors had an increased risk of cardiovascular events, but the proportional increase of cardiovascular events by radiation dosage was the same. Older studies have shown an increased risk of cardiac morbidity and mortality with radiation, particularly left‐sided breast cancer, but this was observed with higher doses of radiation than are used in our current era.19 More recent studies have had conflicting results, with many single institution studies and large data registries showing no increased cardiovascular risk,20, 21, 22 whereas others support an increased risk for cardiovascular disease associated with radiation.23, 24 Nonetheless, a recent meta‐analysis spanning the years 2010 to 2015 demonstrated a mean radiation dose to the heart of 4.4 Gy and a hazard ratio for cardiovascular deaths of 1.30 (P < 0.001) for those who received radiation compared with those who did not, with a proportional increase in cardiac mortality of 0.04 per Gy.25
5. SHARED RISK FACTORS FOR BREAST CANCER AND CARDIOVASCULAR DISEASE
There is significant overlap in risk factors for cardiovascular disease and breast cancer (Figure 2). This overlap of risk factors for 2 very different diseases is often overlooked, and should be a consideration for the treatment provider when addressing future health risks beyond the necessary treatment for breast cancer. The recognition of this overlap may help women breast cancer survivors address modifiable risk factors for heart disease after breast cancer treatments are completed.
Figure 2.
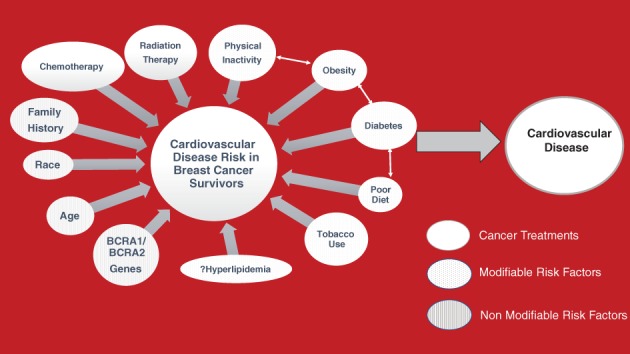
The impact of cancer treatment and cardiovascular risk factors on breast cancer survivors impacting the development of heart disease. Cancer treatment that can impact cardiovascular risk depicted in white. Nonmodifiable cardiovascular risk factors depicted by lines. Modifiable cardiovascular risk factors depicted by dots
Breast cancer and cardiovascular disease are associated with strong evidence supporting inflammation as a mediator of both disease processes.26, 27 Chronic inflammation is associated with oxidative stress, and this too is associated with both disease processes, so it is not surprising that certain diseases and exposures that increase inflammation and oxidative stress may be risk factors for both diseases. Tobacco use and poor diet affect the risk of both diseases.28 There is significant epidemiological data showing that increased physical activity is associated with a reduced risk for both cardiovascular disease and cancer.29 Diabetes is a significant risk factor for cardiovascular disease, but its impact on breast cancer is also established. Insulin resistance, as seen in type 2 diabetes mellitus, promotes estrogen and testosterone release in women, and these sex steroids may be causal in the relationship between diabetes and breast cancer.30 There is evidence linking breast cancer in postmenopausal women with obesity, making obesity another risk factor for both diseases.31 Hyperlipidemia, particularly elevated levels of low‐density lipoprotein (LDL), is a well‐established risk factor for atherosclerosis, whereas it has been suggested that elevated LDL may be associated with the development of breast cancer (in contrast with all other cancers).32 A well‐established nonmodifiable risk factor for breast cancer is both the BRCA1 and BRCA2 gene mutation. Interestingly, these BRCA genes are also involved in preservation of cardiac function, and abnormalities may affect susceptibility to cardiac damage. It has been observed that the presence of either of these gene abnormalities is associated with a higher risk of heart failure in patients receiving anthracycline‐based therapies, irrespective of duration and dosing of anthracycline exposure.33, 34
Breast cancer survivorship has been increasing and comprises the largest group of cancer survivors in the United States.35 Despite the increased risk of cardiovascular disease in breast cancer survivors, there are no specific guidelines for the assessment and prevention of cardiovascular risk in these patients. In fact, the current American survivorship guidelines endorsed by all major cancer societies do not recommend any specific cardiovascular screening beyond the usual cardiovascular risk monitoring for the general population.36 Recently, recommendations for preferential echocardiographic baseline and surveillance imaging of breast cancer patients before and during therapy have evolved to improve early detection of CRCD.37
6. CARDIOVASCULAR DISEASE PREVENTION IN BREAST CANCER SURVIVORS: RECOMMENDATIONS
6.1. Risk Assessment and Monitoring
Over the last decade, guidelines for risk assessment and monitoring of breast cancer patients have been evolving. Before the initiation of breast cancer therapy, it is recommended that patients undergo a thorough history and physical examination to determine baseline cardiovascular risk and potential for cardiac toxicity. A cardiovascular risk assessment tool has been developed (Table 1), which provides a scoring system and approaches to proactive management for risk mitigation.38 Baseline assessment and surveillance cardiac monitoring during cancer therapy has been recommended in women undergoing breast cancer treatment. Initial monitoring protocols were based on biomarker (troponin) and radionuclide angiographic LV function assessments. An increase in cardiac troponin39 or decrease in left ventricular ejection fraction (LVEF)40 has been shown to be associated with clinical heart failure. More recently, echocardiography has emerged to be a preferential imaging modality in breast cancer patients, not only because this technique can assess LVEF without radiation exposure, a particular concern in women with breast cancer, but moreover, the highly sensitive echocardiographic strain modality can detect reductions in subclinical LV systolic function before a reduction in LVEF is observed.41, 42 Echocardiographic strain is a measure of regional myocardial deformation obtained by angle‐independent 2‐dimensional (2D) speckle tracking, and is a standard feature now provided by vendors of all commercially available cardiac ultrasound machines. Thus, it is currently recommended that all women undergoing breast cancer treatment have baseline 2D echocardiography with strain imaging, with follow‐up routine surveillance imaging during therapy, at intervals determined by the specific cancer therapeutic regimen.37, 43 If a reduction in strain or LVEF is noted, appropriate interventions with cardioprotective medications (β‐blockers and/or angiotensin‐converting enzyme inhibitors) and/or adjustments to cancer treatment schedule can be implemented. Multidisciplinary patient‐centric cardio‐oncology teams have evolved to provide optimal management of breast cancer patients, and include oncologists, radiotherapists, and cardiologists. Although trastuzumab and related agents have no late cardiac toxicities, this is not true for anthracyclines. At the current time, the precise interval for long‐term imaging follow‐up of breast cancer survivors has not been determined. Clinical practice suggests follow‐up at 6 months and 1 year of cancer therapy completion, and then at least at 5‐year intervals, in asymptomatic patients who have received anthracycline therapies, but this may need to be adjusted according to individual prognosis, cardiovascular risks, and comorbidity profile.
Table 1.
Risk assessment and monitoring associated with left ventricular dysfunction
| Patient‐Related Risk Factors | Medication‐Related Risk Factorsa |
|---|---|
| One point for each risk factor present | High (risk score 4): anthracyclines, trastuzumab, ifosfamide, cyclophosphamide, clofarabine |
| Age (bimodal distribution): <15 or >65 years | Intermediate (risk score 2): docetaxel, pertuzumab, sunitinib, sorafenib |
| Female | Low (risk score 1): bevacizumab, imatinib, lapatinib, dasatinib |
| Hypertension | Rare (risk score 0): etoposide, rituximab, thalidomide |
| Diabetes mellitus | |
| Atherosclerosis (coronary artery disease, cerebrovascular disease, peripheral artery disease) | |
| Preexisting heart disease or heart failure | |
| Prior anthracycline | |
| Prior radiation therapy to the chest | |
| Cardiotoxicity risk score | |
| Medication‐related risk score + number of patient‐related risk factors = CRS >6: very high; CRS 5–6: high; CRS 3–4: intermediate; CRS 1–2: low; CRS 0: very low | |
| Mayo Clinic monitoring recommendations | |
| Very high risk: echocardiogram with GLS before every (other) cycle, end, 3–6 months, and 1 year; optional ECG, cTn with echocardiogram during chemotherapy | |
| High risk: echocardiogram with GLS every 3 cycles, end, 3–6 months, and 1 year after treatment; optional ECG, cTn with echocardiogram during chemotherapy | |
| Intermediate risk: echocardiogram with GLS, midterm, end, and 3–6 months after treatment; optional ECG, cTn midterm of chemotherapy | |
| Low risk: Optional echocardiogram with GLS and/or ECG; cTn at the end of treatment | |
| Very low risk: None |
Abbreviations: cTn, serum cardiac troponin. ECG, electrocardiogram; GLS, global longitudinal strain.
Risk assessment, cardiotoxicity risk score at the time of baseline assessment and monitoring for patients undergoing anticancer therapy. From: Herrmann et al.,38 with permission.
Medication‐related risk factor (1–4) was based on the risk for a decline or dysfunction in the ventricular function.
7. CONCLUSION
Breast treatment has evolved rapidly, resulting in over 90% survival, making breast cancer survivors the largest cancer survivorship group in the United States. As a result of common risk factors, the effects of chemotherapy and radiation, and shared genetic and environmental impact on both diseases, a greater risk of cardiovascular disease in these women is observed when compared to the general population. The traditional atherosclerotic cardiovascular disease risk assessment does not account for this increased risk. Additionally, the oncology guidelines to date for survivors of breast cancer do not recommend additional cardiovascular surveillance. It is paramount that we begin to recognize this increased risk of cardiovascular disease in women who survive breast cancer, and focus our work on the prevention of cardiovascular disease and its early detection.
Conflicts of interest
The authors declare no potential conflicts of interest.
Gulati M, Mulvagh SL. The connection between the breast and heart in a woman: Breast cancer and cardiovascular disease. Clin Cardiol. 2018;41:254–258. 10.1002/clc.22886
REFERENCES
- 1. Benjamin EJ, Blaha MJ, Chiuve SE, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics‐2017 update: a report from the American Heart Association. Circulation. 2017;135:e146–e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30. [DOI] [PubMed] [Google Scholar]
- 3. Ryerson AB, Eheman CR, Altekruse SF, et al. Annual report to the nation on the status of cancer, 1975–2012, featuring the increasing incidence of liver cancer. Cancer. 2016;122:1312–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Park NJ, Chang Y, Bender C, et al. Cardiovascular disease and mortality after breast cancer in postmenopausal women: results from the Women's Health Initiative. PLoS One. 2017;12:e0184174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Patnaik JL, Byers T, DiGuiseppi C, Dabelea D, Denberg TD. Cardiovascular disease competes with breast cancer as the leading cause of death for older females diagnosed with breast cancer: a retrospective cohort study. Breast Cancer Res. 2011;13:R64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. U.S. Cancer Statistics Working Group . United States cancer statistics: 1999‐2014 incidence and mortality Web‐based report. Atlanta, Georgia: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, and National Cancer Institute; 2017. Available at: www.cdc.gov/uscs. Accessed December 5, 2017. [Google Scholar]
- 7. Bradshaw PT, Stevens J, Khankari N, Teitelbaum SL, Neugut AI, Gammon MD. Cardiovascular disease mortality among breast cancer survivors. Epidemiology. 2016;27:6–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Weaver KE, Foraker RE, Alfano CM, et al. Cardiovascular risk factors among long‐term survivors of breast, prostate, colorectal, and gynecologic cancers: a gap in survivorship care? J Cancer Surviv. 2013;7:253–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jones LW, Haykowsky MJ, Swartz JJ, Douglas PS, Mackey JR. Early breast cancer therapy and cardiovascular injury. J Am Coll Cardiol. 2007;50:1435–1441. [DOI] [PubMed] [Google Scholar]
- 10. Cardinale D, Colombo A, Lamantia G, et al. Anthracycline‐induced cardiomyopathy: clinical relevance and response to pharmacologic therapy. J Am Coll Cardiol. 2010;55:213–220. [DOI] [PubMed] [Google Scholar]
- 11. Hooning MJ, Botma A, Aleman BM, et al. Long‐term risk of cardiovascular disease in 10‐year survivors of breast cancer. J Natl Cancer Inst. 2007;99:365–375. [DOI] [PubMed] [Google Scholar]
- 12. Eschenhagen T, Force T, Ewer MS, et al. Cardiovascular side effects of cancer therapies: a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2011;13:1–10. [DOI] [PubMed] [Google Scholar]
- 13. Dang CT, Yu AF, Jones LW, et al. Cardiac surveillance guidelines for trastuzumab‐containing therapy in early‐stage breast cancer: getting to the heart of the matter. J Clin Oncol. 2016;34:1030–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bowles EJ, Wellman R, Feigelson HS, et al; Pharmacovigilance Study Team . Risk of heart failure in breast cancer patients after anthracycline and trastuzumab treatment: a retrospective cohort study. J Natl Cancer Inst. 2012;104:1293–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Felker GM, Thompson RE, Hare JM, et al. Underlying causes and long‐term survival in patients with initially unexplained cardiomyopathy. N Engl J Med. 2000;342:1077–1084. [DOI] [PubMed] [Google Scholar]
- 16. Ezaz G, Long JB, Gross CP, Chen J. Risk prediction model for heart failure and cardiomyopathy after adjuvant trastuzumab therapy for breast cancer. J Am Heart Assoc. 2014;3:e000472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Romond EH, Jeong JH, Rastogi P, et al. Seven‐year follow‐up assessment of cardiac function in NSABP B‐31, a randomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel (ACP) with ACP plus trastuzumab as adjuvant therapy for patients with node‐positive, human epidermal growth factor receptor 2‐positive breast cancer. J Clin Oncol. 2012;30:3792–3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Darby SC, Ewertz M, McGale P, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368:987–998. [DOI] [PubMed] [Google Scholar]
- 19. Rutqvist LE, Johansson H. Mortality by laterality of the primary tumour among 55,000 breast cancer patients from the Swedish Cancer Registry. Br J Cancer. 1990;61:866‐868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hojris I, Overgaard M, Christensen JJ, Overgaard J. Morbidity and mortality of ischaemic heart disease in high‐risk breast‐cancer patients after adjuvant postmastectomy systemic treatment with or without radiotherapy: analysis of DBCG 82b and 82c randomised trials. Radiotherapy Committee of the Danish Breast Cancer Cooperative Group . Lancet. 1999;354:1425–1430. [DOI] [PubMed] [Google Scholar]
- 21. Giordano SH, Kuo YF, Freeman JL, Buchholz TA, Hortobagyi GN, Goodwin JS. Risk of cardiac death after adjuvant radiotherapy for breast cancer. J Natl Cancer Inst. 2005;97:419–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Doyle JJ, Neugut AI, Jacobson JS, et al. Radiation therapy, cardiac risk factors, and cardiac toxicity in early‐stage breast cancer patients. Int J Radiat Oncol Biol Phys. 2007;68:82–93. [DOI] [PubMed] [Google Scholar]
- 23. Borger JH, Hooning MJ, Boersma LJ, et al. Cardiotoxic effects of tangential breast irradiation in early breast cancer patients: the role of irradiated heart volume. Int J Radiat Oncol Biol Phys. 2007;69:1131–1138. [DOI] [PubMed] [Google Scholar]
- 24. Harris EE, Correa C, Hwang WT, et al. Late cardiac mortality and morbidity in early‐stage breast cancer patients after breast‐conservation treatment. J Clin Oncol. 2006;24:4100–4106. [DOI] [PubMed] [Google Scholar]
- 25. Taylor C, Correa C, Duane FK, et al.; Early Breast Cancer Trialists' Collaborative Group . Estimating the risks of breast cancer radiotherapy: evidence from modern radiation doses to the lungs and heart and from previous randomized trials. J Clin Oncol. 2017;35:1641–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Libby P. Inflammation and cardiovascular disease mechanisms. Am J Clin Nutr. 2006;83:456S–460S. [DOI] [PubMed] [Google Scholar]
- 28. Koene RJ, Prizment AE, Blaes A, Konety SH. Shared risk factors in cardiovascular disease and cancer. Circulation. 2016;133:1104–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wu Y, Zhang D, Kang S. Physical activity and risk of breast cancer: a meta‐analysis of prospective studies. Breast Cancer Res Treat. 2013;137:869–882. [DOI] [PubMed] [Google Scholar]
- 30. Satija A, Spiegelman D, Giovannucci E, Hu FB. Type 2 diabetes and risk of cancer. BMJ. 2015;350:g7707. [DOI] [PubMed] [Google Scholar]
- 31. Flegal KM, Kit BK, Orpana H, Graubard BI. Association of all‐cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta‐analysis. JAMA. 2013;309:71–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Alexopoulos CG, Blatsios B, Avgerinos A. Serum lipids and lipoprotein disorders in cancer patients. Cancer. 1987;60:3065–3070. [DOI] [PubMed] [Google Scholar]
- 33. van Westerop LL, Arts‐de Jong M, Hoogerbrugge N, de Hullu JA, Maas AH. Cardiovascular risk of BRCA1/2 mutation carriers: a review. Maturitas. 2016;91:135–139. [DOI] [PubMed] [Google Scholar]
- 34. Sajjad M, Fradley M, Sun W, et al. An exploratory study to determine whether BRCA1 and BRCA2 mutation carriers have higher risk of cardiac toxicity. Genes (Basel). 2017;8(2):59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. American Cancer Society . Cancer treatment & survivorship: facts & figures 2016−2017. Atlanta, GA: American Cancer Society; 2016. https://www.cancer.org/content/dam/cancer‐org/research/cancer‐facts‐and‐statistics/cancer‐treatment‐and‐survivorship‐facts‐and‐figures/cancer‐treatment‐and‐survivorship‐facts‐and‐figures‐2016‐2017.pdf. Accessed December 5, 2017. [Google Scholar]
- 36. Runowicz CD, Leach CR, Henry NL, et al. American Cancer Society/American Society of Clinical Oncology breast cancer survivorship care guideline. J Clin Oncol. 2016;34:611–635. [DOI] [PubMed] [Google Scholar]
- 37. Plana JC, Galderisi M, Barac A, et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2014;27:911–939. [DOI] [PubMed] [Google Scholar]
- 38. Herrmann J, Lerman A, Sandhu NP, Villarraga HR, Mulvagh SL, Kohli M. Evaluation and management of patients with heart disease and cancer: cardio‐oncology. Mayo Clin Proc. 2014;89:1287–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cardinale D, Sandri MT, Martinoni A, et al. Left ventricular dysfunction predicted by early troponin I release after high‐dose chemotherapy. J Am Coll Cardiol. 2000;36:517–522. [DOI] [PubMed] [Google Scholar]
- 40. Schwartz RG, McKenzie WB, Alexander J, et al. Congestive heart failure and left ventricular dysfunction complicating doxorubicin therapy. Seven‐year experience using serial radionuclide angiocardiography. Am J Med. 1987;82:1109–1118. [DOI] [PubMed] [Google Scholar]
- 41. Fallah‐Rad N, Walker JR, Wassef A, et al. The utility of cardiac biomarkers, tissue velocity and strain imaging, and cardiac magnetic resonance imaging in predicting early left ventricular dysfunction in patients with human epidermal growth factor receptor II‐positive breast cancer treated with adjuvant trastuzumab therapy. J Am Coll Cardiol. 2011;57:2263–2270. [DOI] [PubMed] [Google Scholar]
- 42. Sawaya H, Sebag IA, Plana JC, et al. Assessment of echocardiography and biomarkers for the extended prediction of cardiotoxicity in patients treated with anthracyclines, taxanes, and trastuzumab. Circ Cardiovasc Imaging. 2012;5:596–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Larsen CM, Mulvagh SL. Cardio‐oncology: what you need to know now for clinical practice and echocardiography. Echo Res Pract. 2017;4:R33–R41. [DOI] [PMC free article] [PubMed] [Google Scholar]


