Summary
Aims
Deep brain stimulation of the subthalamic nucleus (STN‐DBS) has become an effective treatment strategy for patients with Parkinson's disease. However, the biological mechanism underlying DBS treatment remains poorly understood.
Method
In this study, we investigated how STN‐DBS modulated the brain network using a bimodal positron emission tomography (PET)/functional magnetic resonance imaging (fMRI) dataset. We first performed an activation likelihood estimation meta‐analysis of 13 PET/SPECT studies concerning STN‐DBS effects on resting‐state brain activity in Parkinson's disease. Additionally, using a functional connectivity analysis in resting‐state fMRI, we investigated whether these STN‐DBS‐affected regions were functionally connected to constitute an effective network.
Results
The results revealed that STN‐DBS reduced brain activity in the right thalamus, bilateral caudal supplementary area, and the left primary motor cortex, and it increased brain activity in the left thalamus during rest. Second, these STN‐DBS‐affected areas were functionally connected within an STN‐DBS effective network.
Conclusion
Deep brain stimulation of the subthalamic nucleus (STN‐DBS) may deactivate the motor cortex as a remote and network effect, affecting the target and the neighboring subcortical areas. These areas may constitute an effective network of STN‐DBS modulation. Our results shed light on the mechanisms of STN‐DBS treatment from a network perspective and highlight the potential therapeutic benefits of targeted network modulation.
Keywords: deep brain stimulation, meta‐analysis, Parkinson's disease, resting‐state fMRI, subthalamic nucleus
1. INTRODUCTION
Parkinson's disease (PD) is one of the most common neurodegenerative diseases and is characterized by bradykinesia, rigidity, and resting tremor. Parkinson's disease (PD) affects approximately 1% of individuals older than 60 years.1 The pathological hallmarks of PD involve dopaminergic deficiency and Lewy body deposits in many brain regions, especially in the substantia nigra. Dopaminergic medication is the most basic and effective treatment for controlling PD symptoms. However, the long‐term administration of dopaminergic medication may lead to the development of motor complications. Deep brain stimulation (DBS) is another highly effective therapy for PD, particularly mid‐ or late‐stage PD. Within the past 20 years, the subthalamic nucleus (STN) has become the most frequently used target of DBS in the treatment of PD. STN‐DBS has been shown to effectively restore motor function, reduces the levodopa dosage and motor complications, and significantly improves quality of life for patients with PD.2
Despite the significant therapeutic effects of STN‐DBS, an important but unanswered question is how STN‐DBS modulates brain activity, thereby leading to its therapeutic effects in PD. In the early 1990s, it was proposed that bradykinesia of PD was associated with an abnormal striato‐thalamo‐cortical (STC) pathway, in which hyperactivity in the STN and globus pallidus internus (GPi) enhanced striatal inhibition of the thalamo‐cortex ensemble.3, 4 According to the STC model, STN‐DBS was initially thought to reduce activity in the target (ie, the STN), relieve striatal inhibition of the thalamo‐cortex, and subsequently restore motor function in patients (the inhibition hypothesis). In contrast, the excitation hypothesis, in which DBS is thought to activate local neuronal elements in the stimulated site (eg, the STN), has also been proposed because increased output from the target during DBS was observed based on neural recording and neural transmitter studies.5, 6 However, it was further observed that DBS may play a more complicated role than simple inhibition or excitation. The benefit of STN‐DBS may be associated with the disruption of the pathologic beta‐band oscillation and the information flow within the STC pathway (the disruption hypothesis).7, 8 More investigations are warranted to elucidate the biological mechanisms of STN‐DBS in the treatment of PD.
Compared with the previously mentioned neural recording and neural transmitter techniques, functional neuroimaging techniques using positron emission tomography (PET), single‐photon emission computed tomography (SPECT), and functional magnetic resonance imaging (fMRI) have provided unique opportunities to ascertain the effects of DBS on brain activity in vivo at a systems level. However, several functional imaging studies reported contradictory results regarding the effects of STN‐DBS (increased or decreased activity) in many different regions, including subcortical (eg, the thalamus, GPi, and STN) and cortical areas [the supplementary motor area (SMA), primary motor cortex (M1), and prefrontal cortex], as well as the cerebellum.9, 10, 11, 12, 13, 14, 15, 16 Limited sample sizes, patient sample heterogeneity, various imaging modalities, and different task designs could contribute to these discrepancies in results across studies. Therefore, how STN‐DBS modulates brain activity in the treatment of PD remains to be elucidated.
Thus, a quantitative meta‐analysis of STN‐DBS functional imaging studies would greatly help explain the inconsistent results across studies with various imaging modalities and yield a statistically defensible conclusion by computing a pooled effect of STN‐DBS on brain activity. Based on the available published PET/SPECT studies and meta‐analytic approaches, we first explored how STN‐DBS modulates brain activity during rest. Additionally, using a functional connectivity analysis in resting‐state fMRI (R‐fMRI), we investigated whether these STN‐DBS‐affected regions were functionally connected to constitute an effective network, which may explain the potential biological mechanisms of STN‐DBS treatment from a network perspective.
2. METHODS
2.1. Search criteria and data extraction
A literature search was conducted in PubMed (www.pubmed.org) for articles reporting STN effects on brain activity in patients with PD. We used the following strings: (“Parkinson's disease” OR “Parkinson disease” OR “PD”) AND “deep brain stimulation” AND (“STN” OR “subthalamic nucleus”) AND (“PET” OR “SPECT” OR “fMRI” OR “functional magnetic resonance imaging” OR “positron emission tomography” OR “single‐photon emission computed tomography”). This strategy resulted in 121 studies on November 1, 2016. We also searched review papers and the references of the retrieved articles to ensure that additional articles were not missed. Studies included in the meta‐analysis met the following criteria: (i) Intact coordinate information was included, (ii) bilateral/unilateral STN stimulation was performed, (iii) studies that contrasted brain activity during STN‐DBS on vs STN‐DBS off or post‐DBS vs pre‐DBS with medication washed out, and (iv) involved a resting‐state design concerning the primary STN‐DBS effect. The exclusion criteria were as follows: (i) a case study or less than 5 patients with PD, (ii) analyses based on regions of interest (ROIs) or a region‐specific receptor (ie, not whole‐brain analyses), (iii) subjects who were not human beings or patients with PD, (iv) studies that did not measure the effects of DBS on brain activity, (v) studies in which the time interval between the neurosurgery and scanning was <1 month to rule out the microlesion effects of electrodes, and (vi) articles not published in English. If two articles had the same study design with an overlapping dataset, only one study with the larger sample size was included. The data extraction included the following domains: author, date of publication, number of patients with PD, age of patients with PD, experimental design, result and coordinates, mean UPDRS III score at baseline and during the experiment, the mean disease duration and stimulation duration, and the DBS parameters (voltage, frequency, and pulse width).
2.2. Meta‐analysis based on activation likelihood estimation
The STN‐DBS meta‐analyses were conducted using GingerALE version 2.3.3 (http://brainmap.org/ale/). An ALE analysis represents a coordinate‐based meta‐analysis of neuroimaging studies, and this type of analysis treats the reported foci as an uncertainty distribution.17 In the ALE, foci were modeled as a spatial 3D Gaussian probability distribution using various full‐width‐at‐half‐maximum (FWHM) values that were different from the sample sizes across the studies. Thus, for each study, we generated a modeled activation map by converting foci into a probability distribution. In the map, the value of every voxel represented the probability distribution of the peak coordinates obtained from the study. Next, the convergence of all the modeled activation maps across studies was used to obtain voxel‐wise ALE scores by estimating the uncertain peaks, which reflect the union of the activation probabilities across experiments. Finally, the ALE score of each voxel was compared with the null hypothesis distribution through a permutation analysis (N = 5000). A multiple comparison correction was conducted with P < 0.001 (uncorrected) as a voxel‐level threshold and a cluster‐level threshold of P < 0.05. Importantly, prior to the meta‐analysis, the peak foci reported in the MNI space were first transformed into the stereotactic Talairach atlas using the icbm2tal (Lancaster) transformation.18
2.3. Processing of R‐fMRI dataset and functional connectivity analysis
To further explore whether STN‐DBS modulates functional brain activity in patients with PD on a network level, we performed a four‐step procedure as follows. Briefly, (i) we first extracted the clusters synthesized in the previous ALE meta‐analysis as seed ROIs, generating five sphere‐shaped ROIs with 5 mm in diameter; (ii) for each ROI, we conducted a seed‐based functional connectivity analysis based on the R‐fMRI data from a group of 55 young healthy adults (male/female: 29/26; age: 19‐30 years) (for details of the R‐fMRI data, see Data S1). In this analysis, we first extracted the mean time course of each ROI (indicating an STN‐DBS‐affected brain area) and then calculated Pearson's correlations between the ROI and the whole brain in a voxel‐wise manner. The correlation coefficients were transformed with a Fisher Z transformation to improve the normality; (iii) for each ROI, we performed a voxel‐wise one‐sample t test on individual z‐transformed correlation maps, and significant connectivity was identified using a Bonferroni correction in voxel level (P < 10−4); (iv) we also computed the functional correlations among the STN‐DBS‐affected brain areas by extracting the average time courses of the affected areas within these ROIs; and (v) ROI‐based functional connectivity maps were overlapped to obtain the effective network of STN‐DBS. Importantly, prior to the functional connectivity analysis, the R‐fMRI data were preprocessed using the DPARSFA toolbox (DPARSF, http://www.restfmri.net/forum/DPARSF)19 as follows: removal of the first 10 time points for each functional volume, slice‐timing correction, realignment, registration to the T1 images and segmentation to the gray matter, white matter and cerebrospinal fluids, normalization using T1 image unified segmentation and resampling to a 3 × 3×3 mm3 voxel, spatial smoothing with a 4‐mm FWHM Gaussian kernel, linear detrending, regressing out global signal regression and six head‐motion parameters, and temporal band pass filtering (0.01‐0.1 Hz).
3. RESULTS
3.1. Studies included in the meta‐analysis
The literature search and study selection process are shown in Figure 1. The literature search yielded 13 functional imaging studies that reported STN‐DBS effects on brain activities. A total of 147 PD patients were included. In the study of Cilia et al,15 one of the reported coordinates in the thalamus showed inconsistent spatial location with the brain region described by the author. Therefore, we performed the meta‐analysis with the foci excluding the inaccurately described coordinate. Given that tremor may have a potential influence on neuroimaging results, we also summarized the tremor information of the patients in the included studies (Table 1).
Figure 1.
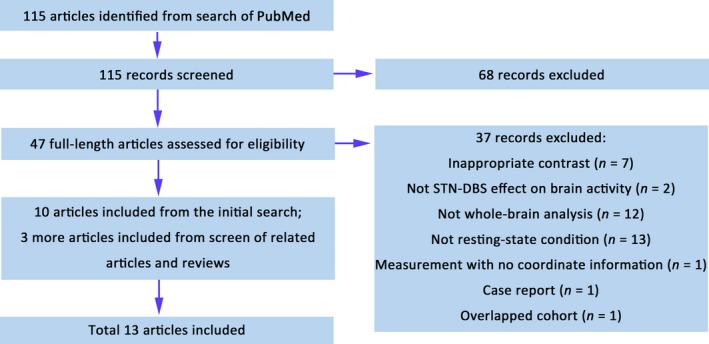
Flow diagram of the selection of studies
Table 1.
Resting‐state studies included in the meta‐analysis
| Study | Modality | Number of PD | Stimulated STN | Mean duration of DBS treatment (month) | Baseline UPDRS III | DBS‐on UPDRS III | Mean disease duration (years) | Mean age of PD (years) | Contrast | LEDD (mg) | Tremor information |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sestini et al.9 | rCBF SPECT | 10 | Bilateral | 5 | 69 | 48 | 15 | 64 | Post‐DBS on vs pre‐DBS | 1440a, 897b | Tremor was not excluded. |
| Sestini et al.35 (2002) | rCBF SPECT | 10 | Bilateral | 4.8 | 67 | 34.8 | 12 | 59 | Post‐DBS on vs pre‐DBS | 1445a, 867b | Tremor was not excluded |
| Vafaee et al.10 | CMRO2 PET | 7 | Bilateral | 18.3 | 44.7 | 17.1 | 16.4 | 58.4 | DBS on vs DBS off | 1382a, 740b | Head tremor was excluded. |
| Hilker et al.11 | FDG PET | 8 | Bilateral | 3.8 | 45.6 | 13.5 | 15 | 61.8 | DBS on vs DBS off | 750a (levodopa), 406b (levodopa) | Severe resting hand tremor was included as inclusion criteria |
| Hershey et al.12 | rCBF PET | 9 | Bilateral | 5.7 | 41.4 | 19.4 | 12 | 56 | DBS on vs DBS off | 419b | Observable tremor was excluded |
| Le Jeune et al.36 (2010) | FDG PET | 20 | Bilateral | 3 | 30.03 | 15.6 | 10.6 | 57.9 | Post‐DBS on vs pre‐DBS | 1106a, 751b | Tremor was not excluded. |
| Wang et al.14 (2010) | FDG PET | 5 | Bilateral | 6.2 | 46.6 | 20 | 7.3 | 63 | Post‐DBS on vs pre‐DBS | 1030a, 700b | Tremor was not excluded |
| Cilia et al.15 | rCBF SPECT | 40 | Bilateral | 12 | 42.1 | 19.3 | 13.4 | 59.9 | Post‐DBS on vs pre‐DBS | 911a, 575b | Tremor was not excluded. |
| Geday et al.37 (2009) | rCBF PET | 9 | Bilateral | 12 | 35.4 | 11 | 13.2 | 59.9 | DBS on vs DBS off | ‐ | No subject experienced significant tremor or dystonia |
| Nagaoka et al.38 (2007) | FDG PET | 8 | Bilateral | 1 | 38 | 14 | 11.9 | 61.9 | DBS on vs DBS off | 708a, 588b | Tremor was not excluded. |
| Li et al.39 (2006) | FDG PET | 5 | Bilateral | 1 | 46.6 | 20 | 7.3 | 61.6 | Post‐DBS on vs pre‐DBS | 1030a, 700b | Tremor was not excluded. |
| Ceballos‐Baumann et al13 | rCBF PET | 8 | Contralateral to the side of initial symptom | ≥4 | 35.75 | 19 | 11 | 53.8 | DBS on vs DBS off | 549a, 199b | Tremor was not excluded. |
| Arai et al31 | FDG PET | 8 | Contralateral to mostly affected side | 13 | 32 | 15.9 | 9.8 | 54 | DBS on vs DBS off | ‐ | Tremor was not excluded. |
LEDD, levodopa equivalent daily dosage.
Measured at surgery.
Measured at experience. UPDRS III was measured with medication off. The baseline represents pre‐DBS condition or DBS off condition based on contrast design.
3.2. STN‐DBS effect on brain activity in the resting state
The ALE meta‐analysis revealed that STN‐DBS elevated cerebral blood flow or metabolism in the left thalamus and STN (Figure 2A and Table 2) and decreased cerebral blood flow or metabolism in the bilateral caudal SMA, right GPi/thalamus, and left M1 area (Figure 2B and Table 2).
Figure 2.
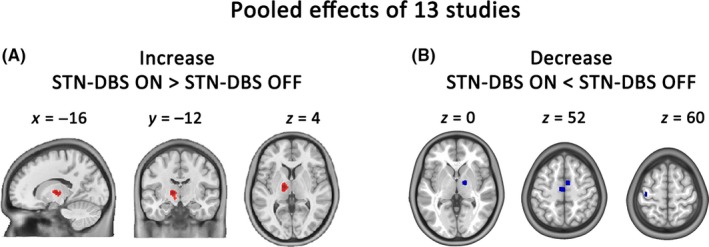
ALE meta‐analysis of resting‐state PET/SPECT studies. (A) Brain areas showing increased activity caused by STN‐DBS. (B) Brain areas showing decreased activity caused by STN‐DBS. Coordinates are given in MNI space (Voxel‐wise threshold P < 0.001[uncorrected]; cluster‐level threshold P < 0.05 with 5000 permutations). GPi, globus pallidus internus; STN, subthalamic nucleus; THA, thalamus; SMA, supplementary motor area
Table 2.
Meta‐analytic results of STN‐DBS effect on brain activity in patients with PD
| Volume(mm3) | Peak ALE value | MNI coordinates | Label | |||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Increase | 1160 | 0.01146 | −16 | −12 | 4 | L. Thalamus |
| 0.00811 | −12 | −12 | −6 | L. subthalamic nucleus | ||
| Decrease | 712 | 0.01456 | −4 | −14 | 52 | L. caudal supplementary motor area |
| 504 | 0.01493 | 6 | −2 | 52 | R. caudal supplementary motor area | |
| 432 | 0.01359 | 14 | −6 | 0 | R. lentiform nucleus, globus pallidus interna | |
| 256 | 0.01493 | −42 | −18 | 60 | L. precentral gyrus | |
R, right; L, left.
All the coordinates are denoted by MNI space coordinates.
3.3. Functional connectivity analysis of R‐fMRI dataset
An R‐fMRI functional connectivity analysis in healthy subjects was performed to explore whether these clusters, identified by a meta‐analysis of 13 studies, were densely connected with each other. The within‐group results showed that each cluster had strong functional connectivity to the other regions (P < 10−4, Bonferroni corrected; Figure S1). Figure 3 shows that five clusters were strongly connected with each other within the same network.
Figure 3.
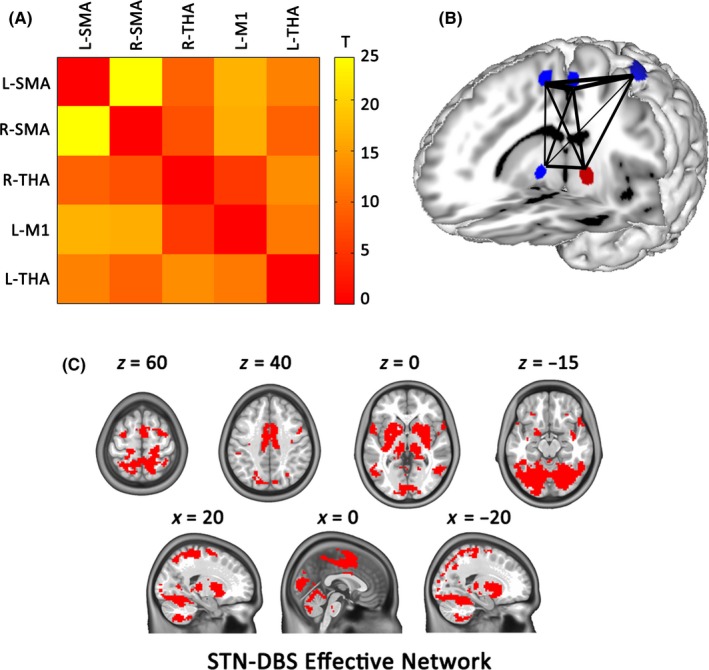
Functional connectivity among the STN–DBS‐affected brain regions at rest. (A) The mean correlation matrices of five clusters obtained from the above meta‐analysis in the healthy subjects at rest. The color code indicates z‐transformed correlation coefficients between two clusters in each cell of the matrix. (B) Surface rendering visualizing the functional connectivity between clusters. Red clusters and green clusters represent brain regions with an increase and decrease in brain activity, respectively. Line thickness corresponds to the t scale. (C) Illustration of STN‐DBS effective network. THA, thalamus; SMA, supplementary motor area
4. DISCUSSION
In our study, an ALE‐based meta‐analysis revealed that STN‐DBS of patients with PD impacted five brain regions (decreased activity: left SMA, right SMA, left M1 area, and the right thalamus; increased activity: left thalamus), which also constituted a tightly correlated STN‐DBS effective network. We postulate that the STN‐DBS effective network may underlie the neural modulation by STN‐DBS treatment and provides clinical suggestions for treatment assessments.
4.1. Alterations of brain activity in cortical areas (caudal SMA and M1) in response to STN‐DBS
We found that STN‐DBS reduced brain activity in the bilateral caudal SMA and left M1 areas at rest. The critical involvement of the motor cortex in PD pathophysiology has been previously demonstrated. For instance, electrophysiological studies have demonstrated excessive cortical beta oscillation in the motor cortex in PD and have found a strong correlation between this beta oscillation and the severity of motor symptoms.20, 21 Moreover, both levodopa and STN‐DBS normalized cortical beta oscillation in association with an improvement in motor function.21 Increased activity in the caudal SMA and M1 in patients with PD during rest or motor execution has also been consistently reported in neuroimaging studies,22, 23, 24, 25 which can also be normalized by levodopa administration and STN‐DBS in association with symptom improvement.11, 26, 27, 28, 29, 30 Therefore, we postulated that reduced brain activity in the caudal SMA and M1 may underlie the therapeutic effect of STN‐DBS. However, this hypothesis still requires testing in future studies. In summary, our results suggest that STN‐DBS may reduce brain activity in the caudal SMA and M1, which may be associated with the therapeutic effect of STN‐DBS.
4.2. Alterations of brain activity in subcortical areas (thalamus) in response to STN‐DBS
The meta‐analysis of 13 studies revealed contradictory responses of the left and the right thalamus/STN/GPi areas to STN‐DBS. These inconsistent alterations may be associated with several facts. First, lateralization effect was considered one of the factors. To date, some evidence has shown that unilateral DBS resulted in opposite brain activity changes in subcortical areas.31 In our study, 11 studies involved bilateral STN‐DBS stimulation, and the other two studies were confined to unilateral STN stimulation (Table 1). The subtype meta‐analysis of unilateral stimulation studies demonstrated that cerebral blood flow or metabolism was elevated in the left thalamus and decreased in the right thalamus/(GPi) (Figure S2), which suggested asymmetrical effects of STN‐DBS on brain activity. Notably, this subtype analysis with a smaller number of studies could result in lower statistical power, and the meta‐analytic results could be easily impacted by a single study. Therefore, we still need to explore the lateralization effect of STN‐DBS on subcortical brain activity with a larger sample size. Second, due to the lower spatial and temporal resolution of PET/SPECT modalities, the mechanisms of STN‐DBS effects on brain activity remain an open question. We believe that influences of STN‐DBS on the stimulated target and the extended areas might be more intricate than simply excitation or inhibition. Considering the effective areas of DBS in the STN, changes in electrical potentials could appear at the soma and terminal synapses of dendrites or axons of STN neurons, the action potential‐initiating segment in the axon hillock or proximal axon of interneurons and efferent neurons residing in the STN as well as the axons passing through or in the vicinity of the STN. In all likelihood, the physiological mechanisms underlying these electrophysiological changes could be quite different. Therefore, STN‐DBS may result in various metabolic or blood perfusion changes in the subcortical regions. Third, in our study, separate meta‐analyses were performed on studies that manifested lower activity and higher activity, and some of the regions were observed to have contradictory activities. This finding was possibly caused by the ALE model, which can estimate only the effect of a single direction. Some newly developed models, such as the effect‐size signed differential mapping approach, provide the ability to combine both positive and negative coordinates to obtain a unique statistical map.32, 33 Future work adopting these models might reduce the controversy by manifesting the regions with both high and low activities detected in the ALE‐based meta‐analysis. Finally, the inconsistent alterations in the thalamus could also have resulted from the smaller sample size. Therefore, additional investigations with larger sample are still needed for the future work.
Taken together, these results lead us to postulate that brain activity in the target and neighboring areas in response to STN‐DBS may be more complicated than simply excitation or inhibition, as revealed by neuroimaging studies. Application of a neurophysiological method, which investigates changes in electrical potentials across cell membranes of neural elements, may provide more information about the influence of STN‐DBS on subcortical areas. Neuroimaging studies with larger sample sizes and a new meta‐analytic approach enabling the fusion of negative and positive coordinates may also be helpful in the future.
4.3. Proposed model for the effective network of STN‐DBS
Overall, STN‐DBS changed brain activity in the bilateral thalamus/STN, the bilateral SMA, and the left M1 areas. The functional connectivity analysis of the R‐fMRI dataset showed that these STN‐DBS‐related areas were connected within the same network. Therefore, we postulate a novel notion named the effective network of STN‐DBS, which represents the underlying functional network affected and modulated by STN‐DBS. In support of this notion, a prior study showed close connections among stimulation sites and their target effective brain regions across diverse psychiatric and neurological disorders,34 namely, the “target‐response” network. This effective network of STN‐DBS may help us understand the neural basis of brain modulation, optimize treatment, and identify new stimulation targets in PD.
Based on the current hypotheses and proposed model regarding the mechanism of DBS, we clarify the action of STN‐DBS on the effective network as follows. The STN‐DBS plays an important role in brain regions ranging from local to remote areas (Figure 4). Specifically, for the local effect, STN‐DBS affects brain activity in the target and the subcortical regions, but the mechanisms underlying this effect remain unclear, considering the multiple facts may influence metabolism and brain perfusion in the stimulated site and nearby areas. For the remote effect, STN‐DBS reduces brain activity in the motor cortex, such as the M1 and caudal SMA, potentially via network modulation. Collectively, the remotely modulated areas (M1 and SMA) and the locally affected areas (thalamus, STN, and GPi) may constitute an STN‐DBS effective network (Figure 4). In summary, we postulate that the modulation of the STN‐DBS effective network may underlie the neural substrate of its therapeutic effect.
Figure 4.
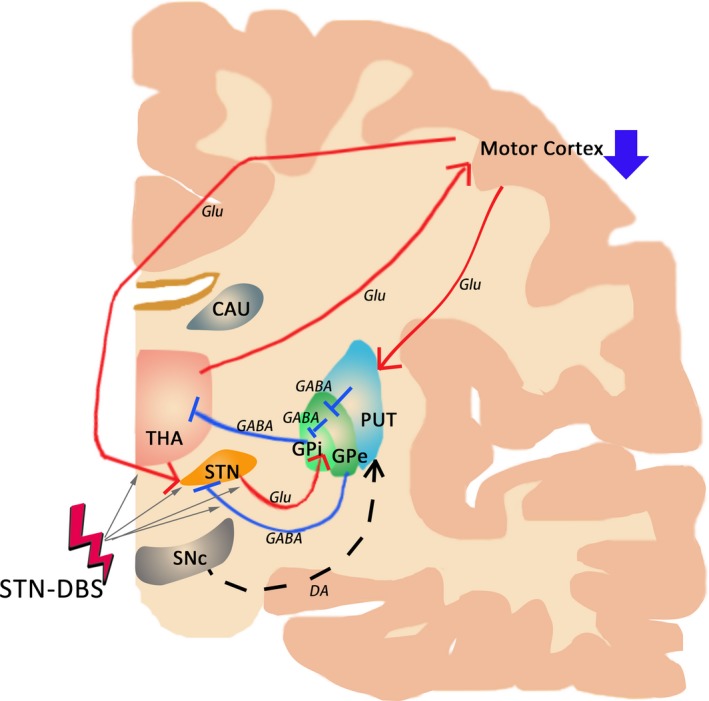
Proposed model of the STN‐DBS effective network. A, Alterations of brain activity by STN‐DBS. STN‐DNS mainly modulates the striato‐thalamo‐cortical pathway, showing remote effects in the cortical regions via network modulation as well as local effects in the target and nearby subcortical regions. For the local effects, STN‐DBS may affect (i) axon terminals synapsing on neurons of the STN, (ii) the dendrites or soma of neurons in the STN, (iii) the action potential‐initiating segment in the axon hillock or proximal axon of interneurons, (iv) efferent neurons residing in the STN, and (v) axons passing through or in the vicinity of the STN (gray arrow). For the remote effects, STN‐DBS reduces brain activity in the motor cortex, such as the M1 and caudal SMA (DOWN arrow in deep blue). Collectively, these data lead us to postulate that the STN‐DBS effective network, which is composed of the bilateral SMA and left M1 areas, and the bilateral thalamus/STN/GPi, may underlie the neural substrate of the therapeutic benefit of STN‐DBS. CAU, caudate nucleus; DA, dopamine; GABA, gamma amino butyric acid; Glu, glutamate; GPe, globus pallidus externus; GPi, globus pallidus internus; PUT, putamen; SNc, substantia nigra pars compacta; STN, subthalamic nucleus; THA, thalamus
4.4. Limitations
Several limitations also existed in our study. First, PD can be classified into different clinical subtypes, which may have different neural substrates. Our analysis did not distinguish between the STN‐DBS effects on different PD subtypes, partly due to the limited number of original papers. It is possible that different phenotypes or different motor symptoms are associated with a specific network. Therefore, more investigation is needed to explore the STN‐DBS effect on more specific motor symptoms. Second, we performed a meta‐analysis to assess brain activity based on PET/SPECT modalities with lower spatial and temporal resolution. Some methods, such as electrophysiological analysis, could sensitively detect rapid and subtle changes in the locus and measure the local field potential, providing more evidence of changes in neurophysiological dynamics. Furthermore, divergent approaches would extend our understanding of the potential neuronal mechanisms of STN‐DBS in the future. Third, in our study, meta‐analyses were separately performed with experiments of increased and decreased activity, and inconsistent changes in the thalamus were shown in our results. This finding may be caused by the shortcomings of the ALE algorithm. Some newly developed approaches, such as effect‐size signed differential mapping, depend on pooled effects with positive and negative coordinates to obtain final statistical results. Future work adopting these models would help compensate for these discrepancies from ALE‐based meta‐analytic findings. Finally, the separate analysis of unilateral stimulation contained only 2 experiments and 16 patients. Thus, the statistical power may be less sufficient, and caution should be taken in interpreting the effect of unilateral STN‐DBS on brain activity. Nevertheless, this separate analysis may also provide additional information.
In summary, we conclude that STN‐DBS decreases brain activity in the motor cortex via network modulation and affects brain activity in subcortical regions in patients with PD. These areas were also functionally connected within the STN‐DBS effective network. These results shed new light on the potential biological mechanisms of STN‐DBS treatment from a network perspective, highlighting the potential therapeutic benefits of targeted brain network modulation.
CONFLICT OF INTEREST
The article has not been published or submitted elsewhere. The authors declare no conflict of interest.
Supporting information
ACKNOWLEDGMENTS
This research was supported by National Key R&D Program of China (No. 2016YFC1306501), National Natural Science Foundation of China (no. 81571226), Beijing Municipal Science and Technology Commission (no. Z151100003915117, Z171100001017041, and Z151100003915150).
Chen H‐M, Sha Z‐Q, Ma H‐Z, He Y, Feng T. Effective network of deep brain stimulation of subthalamic nucleus with bimodal positron emission tomography/functional magnetic resonance imaging in Parkinson's disease. CNS Neurosci Ther. 2018;24:135–143. 10.1111/cns.12783
The first two authors contributed equally to this work.
Contributor Information
Yong He, Email: yong.he@bnu.edu.cn.
Tao Feng, Email: bxbkyjs@sina.com.
REFERENCES
- 1. Zhang ZX, Roman GC, Hong Z, et al. Parkinson's disease in China: prevalence in Beijing, Xian, and Shanghai. Lancet. 2005;365:595‐597. [DOI] [PubMed] [Google Scholar]
- 2. Kleiner‐Fisman G, Herzog J, Fisman DN, et al. Subthalamic nucleus deep brain stimulation: summary and meta‐analysis of outcomes. Mov Disord. 2006;21(suppl 14):S290‐S304. [DOI] [PubMed] [Google Scholar]
- 3. Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12:366‐375. [DOI] [PubMed] [Google Scholar]
- 4. DeLong MR. Primate models of movement disorders of basal ganglia origin. Trends Neurosci. 1990;13:281‐285. [DOI] [PubMed] [Google Scholar]
- 5. Hashimoto T, Elder CM, Okun MS, Patrick SK, Vitek JL. Stimulation of the subthalamic nucleus changes the firing pattern of pallidal neurons. J Neurosci. 2003;23:1916‐1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Stefani A, Fedele E, Galati S, et al. Subthalamic stimulation activates internal pallidus: evidence from cGMP microdialysis in PD patients. Ann Neurol. 2005;57:448‐452. [DOI] [PubMed] [Google Scholar]
- 7. Zaidel A, Spivak A, Grieb B, Bergman H, Israel Z. Subthalamic span of beta oscillations predicts deep brain stimulation efficacy for patients with Parkinson's disease. Brain. 2010;133:2007‐2021. [DOI] [PubMed] [Google Scholar]
- 8. McIntyre CC, Hahn PJ. Network perspectives on the mechanisms of deep brain stimulation. Neurobiol Dis. 2010;38:329‐337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sestini S, Ramat S, Formiconi AR, Ammannati F, Sorbi S, Pupi A. Brain networks underlying the clinical effects of long‐term subthalamic stimulation for Parkinson's disease: a 4‐year follow‐up study with rCBF SPECT. J Nucl Med. 2005;46:1444‐1454. [PubMed] [Google Scholar]
- 10. Vafaee MS, ØStergaard K, Sunde N, Gjedde A, Dupont E, Cumming P. Focal changes of oxygen consumption in cerebral cortex of patients with Parkinson's disease during subthalamic stimulation. NeuroImage. 2004;22:966‐974. [DOI] [PubMed] [Google Scholar]
- 11. Hilker R, Voges J, Weisenbach S, et al. Subthalamic nucleus stimulation restores glucose metabolism in associative and limbic cortices and in cerebellum: evidence from a FDG‐PET study in advanced Parkinson's disease. J Cereb Blood Flow Metab. 2004;24:7‐16. [DOI] [PubMed] [Google Scholar]
- 12. Hershey T, Revilla FJ, Wernle AR, et al. Cortical and subcortical blood flow effects of subthalamic nucleus stimulation in PD. Neurology. 2003;61:816‐821. [DOI] [PubMed] [Google Scholar]
- 13. Ceballos‐Baumann AO, Boecker H, Bartenstein P, et al. A positron emission tomographic study of subthalamic nucleus stimulation in Parkinson disease: enhanced movement‐related activity of motor‐association cortex and decreased motor cortex resting activity. Arch Neurol. 1999;56:997‐1003. [DOI] [PubMed] [Google Scholar]
- 14. Wang J, Ma Y, Huang Z, Sun B, Guan Y, Zuo C. Modulation of metabolic brain function by bilateral subthalamic nucleus stimulation in the treatment of Parkinson's disease. J Neurol. 2010;257:72‐78. [DOI] [PubMed] [Google Scholar]
- 15. Cilia R, Marotta G, Landi A, et al. Clinical and cerebral activity changes induced by subthalamic nucleus stimulation in advanced Parkinson's disease: a prospective case‐control study. Clin Neurol Neurosurg. 2009;111:140‐146. [DOI] [PubMed] [Google Scholar]
- 16. Boertien T, Zrinzo L, Kahan J, et al. Functional imaging of subthalamic nucleus deep brain stimulation in Parkinson's disease. Mov Disord. 2011;26:1835‐1843. [DOI] [PubMed] [Google Scholar]
- 17. Eickhoff SB, Bzdok D, Laird AR, Kurth F, Fox PT. Activation likelihood estimation meta‐analysis revisited. NeuroImage. 2012;59:2349‐2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lancaster JL, Tordesillas‐Gutierrez D, Martinez M, et al. Bias between MNI and Talairach coordinates analyzed using the ICBM‐152 brain template. Hum Brain Mapp. 2007;28:1194‐1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chao‐Gan Y, Yu‐Feng Z. DPARSF: a MATLAB Toolbox for “Pipeline” Data Analysis of Resting‐State fMRI. Front Syst Neurosci. 2010;4:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pollok B, Krause V, Martsch W, Wach C, Schnitzler A, Südmeyer M. Motor‐cortical oscillations in early stages of Parkinson's disease. J Physiol. 2012;590:3203‐3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Silberstein P, Pogosyan A, Kuhn AA, et al. Cortico‐cortical coupling in Parkinson's disease and its modulation by therapy. Brain. 2005;128:1277‐1291. [DOI] [PubMed] [Google Scholar]
- 22. Yu H, Sternad D, Corcos DM, Vaillancourt DE. Role of hyperactive cerebellum and motor cortex in Parkinson's disease. NeuroImage. 2007;35:222‐233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Eidelberg D, Moeller JR, Dhawan V, et al. The metabolic topography of parkinsonism. J Cereb Blood Flow Metab. 1994;14:783‐801. [DOI] [PubMed] [Google Scholar]
- 24. Huang C, Tang C, Feigin A, et al. Changes in network activity with the progression of Parkinson's disease. Brain. 2007;130:1834‐1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sabatini U, Boulanouar K, Fabre N, et al. Cortical motor reorganization in akinetic patients with Parkinson's disease: a functional MRI study. Brain. 2000;123:394‐403. [DOI] [PubMed] [Google Scholar]
- 26. Ma Y, Tang C, Spetsieris PG, Dhawan V, Eidelberg D. Abnormal metabolic network activity in Parkinson's disease: test‐retest reproducibility. J Cereb Blood Flow Metab. 2007;27:597‐605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Asanuma K, Tang C, Ma Y, et al. Network modulation in the treatment of Parkinson's disease. Brain. 2006;129:2667‐2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Haslinger B, Erhard P, Kampfe N, et al. Event‐related functional magnetic resonance imaging in Parkinson's disease before and after levodopa. Brain. 2001;124:558‐570. [DOI] [PubMed] [Google Scholar]
- 29. Haslinger B, Kalteis K, Boecker H, Alesch F, Ceballos‐Baumann AO. Frequency‐correlated decreases of motor cortex activity associated with subthalamic nucleus stimulation in Parkinson's disease. NeuroImage. 2005;28:598‐606. [DOI] [PubMed] [Google Scholar]
- 30. Mure H, Hirano S, Tang CC, et al. Parkinson's disease tremor‐related metabolic network: characterization, progression, and treatment effects. NeuroImage. 2011;54:1244‐1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Arai N, Yokochi F, Ohnishi T, et al. Mechanisms of unilateral STN‐DBS in patients with Parkinson's disease: a PET study. J Neurol. 2008;255:1236‐1243. [DOI] [PubMed] [Google Scholar]
- 32. Radua J, Mataix‐Cols D. Meta‐analytic methods for neuroimaging data explained. Biol Mood Anxiety Disord. 2012;2:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Radua J, Rubia K, Canales‐Rodriguez EJ, et al. Anisotropic kernels for coordinate‐based meta‐analyses of neuroimaging studies. Front Psychiatry. 2014;5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fox MD, Buckner RL, Liu H, et al. Resting‐state networks link invasive and noninvasive brain stimulation across diverse psychiatric and neurological diseases. Proc Natl Acad Sci U S A. 2014;111:E4367‐E4375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sestini S, Scotto di Luzio A, Ammannati F, et al. Changes in regional cerebral blood flow caused by deep‐brain stimulation of the subthalamic nucleus in Parkinson's disease. J Nucl Med. 2002;43:725‐732. [PubMed] [Google Scholar]
- 36. Le Jeune F, Péron J, Grandjean D, et al. Subthalamic nucleus stimulation affects limbic and associative circuits: a PET study. Eur J Nucl Med Mol Imaging. 2010;37:1512‐1520. [DOI] [PubMed] [Google Scholar]
- 37. Geday J, Østergaard K, Johnsen E, et al. STN‐stimulation in Parkinson's disease restores striatal inhibition of thalamocortical projection. Hum Brain Mapp. 2009;30:112‐121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nagaoka T, Katayama Y, Kano T, et al. Changes in glucose metabolism in cerebral cortex and cerebellum correlate with tremor and rigidity control by subthalamic nucleus stimulation in Parkinson's disease: a positron emission tomography study. Neuromodulation. 2007;10:206‐215. [DOI] [PubMed] [Google Scholar]
- 39. Li D, Zuo C, Guan Y, et al. FDG‐PET study of the bilateral subthalamic nucleus stimulation effects on the regional cerebral metabolism in advanced Parkinson disease. Acta Neurochir Suppl. 2006;99:51‐54. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


