Summary
Stroke is the world's leading cause of disability with limited brain repair treatments which effectively improve long‐term neurological deficits. The neuroinflammatory responses persist into the late repair phase of stroke and participate in all brain repair elements, including neurogenesis, angiogenesis, synaptogenesis, remyelination and axonal sprouting, shedding new light on post–stroke brain recovery. Resident brain glial cells, such as astrocytes not only contribute to neuroinflammation after stroke, but also secrete a wide range of trophic factors that can promote post–stroke brain repair. Alternatively, activated microglia, monocytes, and neutrophils in the innate immune system, traditionally considered as major damaging factors after stroke, have been suggested to be extensively involved in brain repair after stroke. The adaptive immune system may also have its bright side during the late regenerative phase, affecting the immune suppressive regulatory T cells and B cells. This review summarizes the recent findings in the evolving role of neuroinflammation in multiple post–stroke brain repair mechanisms and poses unanswered questions that may generate new directions for future research and give rise to novel therapeutic targets to improve stroke recovery.
Keywords: brain repair, immune response, neuroinflammation, regeneration, stroke
1. INTRODUCTION
As the leading cause of disability in adults, cerebral ischemic stroke is frequently accompanied by long‐lasting neurological deficits that significantly impair the quality of life for stroke patients.1 The brain may undergo self‐repair events at late stage of stroke, which allows stroke patients to regain some part of their sensorimotor functions.2, 3, 4 Unfortunately, the self‐repair capacity is very limited, and therapeutic strategies that can sufficiently restore stroke patients’ lost neurological functions are still lacking.2, 5 Neuroinflammation is a promising new brain repair target after stroke for the following four reasons: (a) the long‐lasting cross talk between the peripheral immune system and the ischemic brain provides a wide therapeutic window and thus offers an opportunity to enhance the brain repair during the late phase of stroke; (b) inflammatory responses are fundamentally involved in multiple brain repair mechanisms which work in concert to orchestrate neurological recovery; (c) recent research on neuroinflammation has improved our understanding of the contribution of inflammatory cell infiltration to ischemic brain injury; (d) the finding of alternative phenotypes of microglia/macrophage, neutrophils, and astrocytes after stroke.6, 7
This review introduces the recent findings in the evolving role of neuroinflammation in multiple post–stroke brain repair mechanisms, which hold promise for generating new novel therapeutic targets to improve and accelerate the brain tissue repair and functional recovery.
2. THE ROLE OF NEUROINFLAMMATION IN MULTIPLE BRAIN REPAIR MECHANISMS FOLLOWING CEREBRAL ISCHEMIC INJURY
In response to cerebral ischemic stroke, both resident glial cells in the brain and peripheral immune cells are activated, inducing various immune cells to infiltrate into the injured brain. The cross talk between the peripheral and resident inflammatory cells forms a vicious cycle, leading to a series of detrimental consequences and resulting in a secondary brain damage from augmented neuroinflammation.8, 9, 10 The immune cells participating in neuroinflammation are a large family, consisting of various types of cells and their responses toward acute ischemic attack and late phase brain repair are complex and can persist into late repair phase of cerebral ischemic injury.11, 12 Both innate immune cells such as local microglia, newly infiltrated macrophages and adaptive immune cells, such as CD4+ T cells, CD8+ T cells, remain activated in the late phase of ischemic stroke.11, 12, 13
In the late phase of stroke, multiple endogenous brain repair processes are occurring in the ischemic brain. It is emerging that immune responses in the late phase of stroke mainly affect the functional and structural repair elements, including neurogenesis, new born neuron migration, formation of novel synapses, and neuronal networks, axonal spouting, remyelination, and neurovascular unit remodeling.
2.1. Neuroinflammatory responses are profoundly engaged in post–stroke neurogenesis
2.1.1. Ischemic stroke initiates neurogenesis in the adult brain
Neurogenesis is a physical phenomenon that persists the whole lifetime and is an important and promising step toward the recovery and restoration of brain function after stroke and other neurogenerative diseases.14, 15, 16, 17, 18 The generation and maturation of de novo neurons require multiple steps, including neuronal progenitor cells (NPCs) mobilization, proliferation, neuronal migration, neuron maturation, and synaptic reconstruction. Multipotent NPCs support self‐renewal and differentiation and reside within specialized niches in the adult mammalian CNS.19 One of the best characterized niches is the subventricular zone (SVZ), a layer of cells lying immediately under the ependymal lining of the lateral ventricles.20 Neuron generation mostly happens in the SVZ and the subgranular zone (SGZ) of the hippocampus.21 The SVZ has great potential for neurogenesis, both in rodents and humans, and contains three major stem cell types; B, C, and A. The CNS stem cells (type B cells) display an astrocyte‐like phenotype and express glial fibrillary acid protein (GFAP). They can also give rise to intermediate transit amplifying progenitors (type C cells), which lose the immunoreactivity of GFAP and acquire the expression of the distal‐less homeobox (Dlx)‐2.22, 23 These type C cells can, in turn, differentiate into neuroblasts (type A cells) that express the polysialylated form of neural cell adhesion molecule in addition to doublecortin, and migrate to the olfactory bulb. The cell lineage differentiation pathway proceeds from type B, through type C, to type A cells, with the type B cell believed to be the self‐renewing CNS stem cell.24 After proliferation, the newly born neuroblasts migrate from the generation zone to the injurious areas and differentiate into mature neurons.25, 26
2.1.2. Neuroinflammation is fundamentally engaged in neurogenesis and affects the proneurogenic microenviroment after stroke
Neuroinflammation participates in almost every step of the abovementioned ischemic brain repair, including neurogenesis.27, 28, 29, 30, 31 Microglia activation, and possibly M1 phenotype development, augments post–stroke neuroinflammation and may decrease neurogenesis.32, 33, 34, 35 Persistent brain inflammation also extensively alters the proliferative and migratory properties of SVZ‐resident NPCs in vivo, leading to significant accumulation of nonmigratory neuroblasts within the SVZ germinal niche.29 But injured CNS neurons can benfit from active or passive immunization with CNS myelin‐associated antigens. Peripheral immune cell infiltration and inflammation in the ischemic brain also drastically changes the microenvironment for brain repair and impairs the proneurogenic homeostasis after stroke,36 which is believed to be a prerequisite for the development, and survival of de novo generated neurons.27, 28, 37, 38 In the ischemic stroke model, the neurogenesis after stroke also depends on T lymphocytes subgroups, different subgroup deficient plays different effect on neurogenesis.39 Exogenous stem cell therapy confers superior antiinflammatory effects and the robust antiinflammatory action is considered as an important requirement to improve neurogenesis and other regenerative processes after stroke.40, 41
Therefore, neuroinflammation is crucial for endogenous neurogenesis and it plays dual roles on endogenous neurogenesis after cerebral ischemic stroke. Brain glial cells and distinct components of the peripheral immune system can either promote or impair the post–stroke neurogenesis by their specific phenotypes as discussed below.
2.1.3. Post–stroke responses from resident brain glial cells and neurogenesis
Microglia, resident brain immune cells, are responsible for immune surveillance and clearing up dead/apoptotic neurons through phagocytosis.42, 43, 44 After cerebral ischemic injury, microglia are stimulated and secrete a number of signaling factors into the brain.45 Neurogenesis is thereby influenced through the milieu of secreted factors.28 Microglia can secrete pro‐inflammatory factors such as tumor necrosis factors‐α (TNF‐α)46, 47 and neurotrophic factors such as insulin growth factors (IGF) and brain‐derived neurotrophic factor (BDNF), both of which can reduce inflammation and exert neuroprotective effects.48, 49, 50, 51 The complex role of microglia in neurogenesis is also due to its modulatory nature.27, 49, 52 Under different stimulations, microglia may polarize into either of two phenotypes that conduct drastically different pathophysiological behaviors.53 Although there is a growing concern for the limitations of the terminology of M1/M2 phenotype, the dual role of microglia/macrophage in promoting inflammation and resolution can be mediated by distinct gene expression programs and distinct phenotypes.54, 55 The “classically activated” M1 type is destructive and promotes brain injury by releasing pro‐inflammatory mediators, such as IL‐1, IL‐6, and IL‐12, and nitric oxide.6, 56 M1 microglia have been suggested to inhibit differentiation of neural stem cells (NSCs).57 Inhibiting M1 microglia using minocycline, a selective inhibitor of M1 microglia, is protective through promoting neurogenesis and functional recovery after stroke.58, 59 However, microglia depletion using dual colony‐stimulating factor 1 receptor/c‐kit inhibitor PLX3397 exacerbates neurological deficits,49 indicating that there are different phenotypes of microglia that may promote brain recovery, which is now termed as the “alternatively activated” M2.52, 56, 60, 61 In addition to secreting antiinflammatory factors, M2 cells also support neurogenesis by promoting neuron proliferation and brain repair.27, 62, 63 The M2 polarized microglia are able to produce specific trophic factors that increase neural precursor cell proliferation and neuroblast migration.27, 60, 64
Astrocytes account for nearly half of the brain cells.65 Although astrocytes are not typical immune cells, they can function as inflammatory cells and produce inflammatory factors affecting the post–stroke immune responses.66, 67 In addition, astrocytes provide energy and structural support, glucose metabolism, take up excessive molecules in the extracellular matrix,68, 69 and actively participate in neuronal proliferation, maturation, and survival.37, 38, 65, 67 They stimulate neurogenesis by secreting prodifferentiation factors such as Wnt70 and proproliferation factors such as insulin growth factors (IGF),71, 72 BDNF,73, 74 glia‐derived neurotrophic factor (GDNF), nerve growth factor (NGF), basic fibroblast growth factor (bFGF), vascular endothelial growth factor (VEGF), ciliary neurotrophic factor (CNTF), and erythropoietin (EPO).75, 76, 77 Deletion of astrocytic intermediate filament proteins, GFAP and Vimentin were shown to severely impair stroke recovery.78 Reactive astrocytes also secrete the neuroblast attracting chemokines such as stromal cell‐derived factor‐1 after brain ischemic injury, which contributes to guiding migrating neuroblasts to the infarcted brain area.79 The influence of astrocytes on post–stroke neurogenesis is not limited to the trophic factors as shown in Table 1. Resident astrocyte‐derived neurons could transdifferentiate into morphologically mature and functional neurons, and the newly generated neurons could form new synapses, which possessed typical neuronal morphology and electrophysiological activity.80 It has been shown that striatal astrocytes possess the ability to either produce neuroblasts or acquire NSC‐like properties by expressing NSC related proteins such as nestin, Sox2, and DCX after stroke.80, 81 Reduced Notch1 signaling was essential for triggering striatal astrocytes to enter the neurogenic program.81
Table 1.
Neurotrophic factors secreted by astrocytes for post–stroke brain repair
| Major findings | Study | Model | Key factors |
|---|---|---|---|
| Neurogenesis | |||
| Improve behavioral impairment | Okoreeh et al, (2017) 66 | Aging female rats with 90 min ischemic stroke | Insulin like growth factor (IGF)‐1 |
| Proliferation factors | Kalani et al, (2008) 70 | Neural stem cells | Wnt signaling |
| Otaegi et al, (2006) 71 | Mouse olfactory bulb stem cells | Insulin‐like growth factor I (IGF‐I) | |
| Shetty et al, (2005) 72 | Hippocampus of aging rats | Fibroblast growth factor‐2 (FGF‐2) | |
| Bartkowska, (2007) 73 | Embryonic cortical precursor cell | TRK signaling | |
| Quesseveur, (2013) 74 | Mice hippocampal astrocytes | Brain‐derived neurotrophic factor (BDNF) | |
| Kang et al, (2013) 76 | Subventricular zone in stoked mice | Ciliary neurotrophic factor (CNTF) | |
| Magnusson et al, (2014) 81 | Striatum of stroked mouse | Notch signaling | |
| Axonal remodeling of corticospinal tract (CST) | Liu et al, (2014) 78 | Cerebral cortical photothrombotic stroke | GFAP/vimentin |
| Transdifferentiate into functional new neurons | Duan et al, (2015) 80 | Striatal astrocytes in MCAO rats | |
| Neurovascular unit remodelling | |||
| Limit neuroinflammation | Cekanaviciute et al, (2014) 115 | Mice where TGFβ signaling is inhibited specifically in astrocytes | Transforming growth factor‐beta (TGFβ) |
| Synaptogenesis | |||
| Synaptogenesis promoter | Tournell et al, (2006) 169 | Astrocytes cultured with hippocampal rat neurons | Agrin |
| Christopherson et al, (2005) 170 and Lin et al, (2003) 171 | Immature astrocytes in vitro and in vivo | Thrombospondins (TSPs)‐1 and ‐2 | |
| Synaptic plasticity modulator | Choi et al, (2016) 166 | Stroke model of rat | Ephrin‐A1 and ephrin‐A5 |
| Axon sprouting | |||
| Axonal sprouting stimulator | Ren et al, (2000) 195 | Rats underwent MCA | Bone morphologic protein(BMP) |
| Choi et al, (2016) 166 | Rose Bengal‐treated rats underwent focal cortical infarcts | Ephrin‐A1 and ephrin‐A5 | |
| Remyelination | |||
| Support myelination | Nash et al, (2011) 233 | Astrocytes plated on poly‐L‐lysine | Ciliary neurotrophic factor (CNTF) |
| Decreased demyelination | Wang et al, (2017) 237 | mouse brain | CD59 |
2.1.4. Peripheral immune cells that participate in neurogenesis after stroke
Apart from resident glial cells, peripheral immune cells, such as T cells, can infiltrate into the ischemic brain via the compromised blood brain barrier (BBB) or choroid plexus82 and indirectly promote neurogenesis and microglia activity by producing IL‐4 or low‐level IFN‐γ, respectively.62, 83 However, the subgroups of T cells that promote neurogenesis were not identified. In fact, there are huge discrepancies with respect to cytokine production from Th1 or Th2 cells and the ratio of Th1/Th2 cells can even affect stroke severity.84 Th17 cells and γδ T cells produce pro‐inflammatory cytokines and chemokines,85, 86 usually impairing neurogenesis.87, 88 However, it is recently suggested that IL‐17A secreted by γδ T cells unexpectedly promoted neuronal differentiation via p38 mitogen‐activated protein kinase in cultured NPCs.89 Regulatory T cells (Tregs) are one special subset of Th2 cells that have been shown to negatively regulate neuroinflammation alleviating post–stroke brain damage.90, 91, 92, 93, 94, 95 Injecting activated Tregs, a special subset of T cells, into the left lateral ventricle of ischemic mouse brain was shown to enhance neural stem cell proliferation in the SVZ.96
Macrophages are another type of peripheral immune cell that can infiltrate into the ischemic brain and promote neurogenesis exhibit a prorecovery profile through their M2 phenotype.97, 98, 99 They produce members of fibroblast growth factor (FGF), transforming growth factor‐α and β (TGF‐α and TGF‐β), insulin‐like growth factor (IGF), and platelet derived growth factor (PDGF) families97 and antiinflammatory cytokines, such as CD206, arginase‐1, IL‐10, and Ym.100 Other than these cytokines, the phagocytic activity of macrophages also helps to provide a microenvironment receptive to brain repair.27 It is now recognized that macrophages play important roles in neurogenesis.101 They can produce neurotrophic factors that promote neuron progenitor proliferation, the migration of neuroblast cells and maturation of the newly born neurons.102, 103 Therefore, the special macrophage M2 phenotype is becoming recognized as an important promotor of post–stroke brain repair.104, 105
Collectively, the impact of neuro‐immune cross talk on the post–stroke neurogenesis process largely depends on the activated immune cell subsets or the specialized phenotypes of the inflammatory cells (Figure 1). Apart from neurogenesis, understanding how the interactions between the neuro‐and immune systems affects the migration and maturation of newly born neurons is still largely unknown and thus warrants further investigation.
Figure 1.
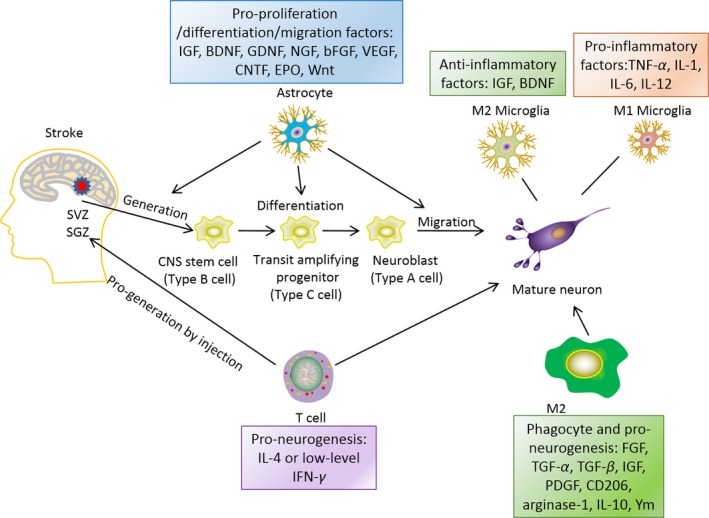
Neuro‐inflammation and neurogenesis. Astrocytes can affect the generation, differentiation and migration of de novo generated neurons by secreting pro‐neurogenic factors, such as insulin growth factor (IGF), glia‐derived neurotrophic factor (GDNF), nerve growth factor (NGF), basic fibroblast growth factor (bFGF), vascular endothelial growth factor (VEGF), ciliary neurotrophic factor (CNTF), erythropoietin (EPO) and Wnt. Microglia can affect neurogenesis in their distinct phenotype, M2 phenotype produces anti‐inflammatory factors, such as IGF, BDNF; while M1 phenotype secrets pro‐inflammatory factors, such as: TNF‐α, IL‐1, IL‐6, and IL‐12. T cells can also promote neurogenesis and maturation of neuroprogenitor cells by producing IL‐4 or low level IFN‐γ. M2 macrophages in the periphery can produce factors, such as fibroblast growth factor (FGF), TGF‐α (transforming growth factor‐α), TGF‐β, IGF, PDGF (platelet drived growth factor), CD206 (cluster of differentiation 206, mannose receptor), arginase‐1, IL‐10, and Ym, all above factors have been shown to promote maturation of newly generated neurons
2.2. Neuroinflammation and neurovascular unit remodeling
2.2.1. Neurovascular unit remodeling after ischemic stroke
The neurovascular unit a complex interplay of biochemical and molecular mechanisms involving practically any cell type of the brain.106 After ischemic stroke, disruption of the normal vasculature leads to neuronal ischemia and cascades of pro‐inflammatory responses that eventually exacerbates ischemic brain injury.107, 108 Angiogenesis, the growth of new blood vessels, could be interpreted as a natural defense mechanism helping to restore oxygen and nutrient supply to the affected brain tissue.109 In this context, angiogenesis and structure reconstruction are necessary and important steps towards function restoration.110, 111, 112 Soon after ischemic attack, endothelial cells proliferate and migrate to the impaired region under the regulation of angiogenic growth factors to form a new microvasculature structure,113 while the perilesional vascular reactivity remains elevated until 21 days after stroke.108 Angiogenesis relies heavily on the coordination of proangiogenic and antiangiogenic factors,7, 75, 76, 114, 115, 116, 117 in which the neuro‐immune crosstalk by microglia, astrocytes, and peripheral immune cells is actively involved (Figure 2).
Figure 2.
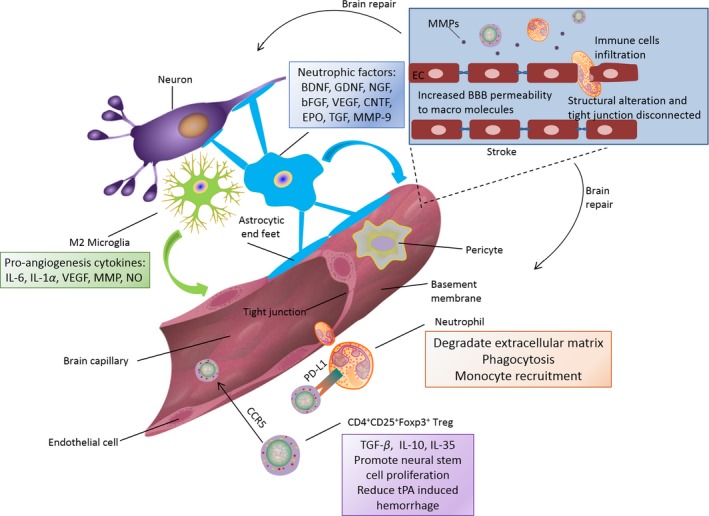
Neurovascular unit remodeling after stroke. Microglia in M2 phenotype can generate proangiogenesis factors such as IL‐6, interleukin‐1α, vascular endothelial growth factor (VEGF), matrix metalloproteinase (MMP) and nitric oxide. In chronic phase of stroke, astrocytes can promote neuronal survival and neurovascular unit remodeling by producing brain‐derived neurotrophic factor (BDNF), gliaderived neurotrophic factor (GDNF), nerve growth factor (NGF), basic fibroblast growth factor (bFGF), vascular endothelial growth factor (VEGF), ciliary neurotrophic factor (CNTF), erythropoietin (EPO), transforming growth factors (TGF) and matrix metalloproteinase 9 (MMP9). Neutrophils assist neurovascular unit repair through extracellular matrix degradation, phagocytosis, monocyte recruitment. CD4+CD25+Foxp3+ Tregs can release anti‐inflammatory cytokines of TGF‐β, IL‐10 and IL‐35. They can also promote neural stem cell proliferation and reduce tissue plasminogen activator (tPA)‐induced haemorrhage. CD4+CD25+Foxp3+ Tregs can be recruited to ischemic blood brain barrier by CCR5 and alleviate BBB disruption through the expression of PD‐L1
2.2.2. Resident glial cells and post–stroke neurovascular unit remodeling
Local resident microglia can secrete proangiogenesis cytokines such as interleukin‐6 (IL‐6),118 IL‐1α,119 vascular endothelial growth factor(VEGF),120 matrix metalloproteinase (MMP)121, 122 and nitric oxide.123, 124 The role of microglia on the post–stroke neurovascular unit largely depends on the phenotype of microglia. Microglia can interact with endothelial cells through integrin Mac1 and the endocytic receptor, low density lipoprotein receptor‐related protein 1 (LRP1) and induce BBB permeability in the thrombotic stroke model.125 On the other hand, induction of the M2 phenotype in activated microglia is effective in promoting BBB remodeling, possibly by enhancing tight junction protein expression during stroke recovery.7
Astrocytes are a major component of BBB and thus play critical roles in supporting neurovascular unit remodeling during the BBB repair phase after cerebral ischemia.79, 126, 127, 128 Activated astrocytes can persistently produce neurotrophic factors, including BDNF, GDNF, NGF, bFGF, VEGF, CNTF, EPO, TGF and so on to support neuronal survival and neurovascular unit remodeling in the chronic phase of stroke.7, 75, 76, 114, 115, 116, 129 Deletion of GFAP and Vimentin, the two major astrocytic intermediate filament proteins, were shown to severely impair stroke recovery.78 Astrocytes may also be an important cellular source of MMP‐9 essential for the remodeling of neurovascular structure and functional recovery in the late phase of stroke (7‐14 days after stroke).130
2.2.3. Peripheral immune cells and post–stroke neurovascular unit remodeling
In addition to the resident glial cells, certain subsets of peripheral immune cells also contribute to BBB repair after stroke. The M2 macrophages also produce proangiogenesis factors such as VEGF, IL‐8,131 IGF‐1, and TGF‐β.132 Therefore, M2 macrophages are also a promising target to enhance angiogenesis and neurovascular unit remodeling after stroke.
Although the detrimental role of neutrophils on the BBB damage in the acute phase of stroke is well established,133, 134, 135, 136, 137 neutrophils may also have a beneficial effect in neurovascular repair after stroke. The degradation of extracellular matrix by neutrophils should induce the release of growth factors, which are normally bound to the extracellular matrix in the zymogen form, such as vascular endothelial growth factor (VEGF) and transforming growth factor (TGF)‐β.138 Neutrophils clear away dead cells, debris, and bacteria through phagocytosis, thus providing a better microenvironment for repair.139, 140 Moreover, neutrophils promote monocyte recruitment into the extracellular matrix and phagocytotic function, leading to the clearance of their own.141, 142 It has recently been highlighted that neutrophils can polarize toward the beneficial N2 phenotypes in the setting of stroke by activating the nuclear peroxisome proliferator‐activated receptor (PPAR)‐γ.143 So far the mechanism of neutrophil‐promoted neurovascular repair after stroke is largely unknown. It will be intriguing to investigate whether neutrophils may transform into a protective phenotype to promote the neural network remodeling after stroke. In addition to the direct role of neutrophils on brain recovery after stroke, the ability of neutrophils to infiltrate the brain makes it a potential candidate for drug delivery into the brain.144
CD4+CD25+Foxp3+ Tregs are a special subset of regulatory T cells that have been identified as an intrinsic protective factor after cerebral ischemic injury.12, 93, 136, 145, 146 CD4+CD25+Foxp3+ Treg cells constitute 10% of peripheral CD4+ T cells147 and are essential for maintaining immunological self‐tolerance and promote immunosuppressive features through releasing antiinflammatory cytokines, such as TGF‐β, IL‐10 and IL‐35.148 In response to cerebral ischemic injury, CD4+CD25+Foxp3+ Tregs can be directly activated at the surface chemokine receptor CCR5, allowing Tregs to be recruited to the BBB. At the BBB, these Tregs exert early protection against BBB disruption through interacting with neutrophils through the surface expression of PD‐L1,12, 90, 136 and secreting IL‐10 to protect against neuroinflammatory responses.93, 149 The protection of Tregs on BBB disruption after stroke can also be utilized to ameliorate tissue plasminogen activator‐induced brain hemorrhage after stroke by inhibiting neutrophil‐derived MMP‐9 and endothelial derived‐CCL2.150, 151 In addition, CD4+CD25+Foxp3+Tregs have also been regarded as a promising regulatory candidate of brain repair processes by dampening excessive immune responses and also promote the survival of newly generated neuroblasts.148 Brain infiltration of endogenous CD4+CD25+Foxp3+Tregs after cerebral ischemia corresponds with the brain repair phase which occurs about 7 days after stroke.27, 93 Injecting Tregs into the left lateral ventricle of the ischemic mouse brain promoted neural stem cell proliferation via secreting IL‐10, suggesting a brain repair effect of Tregs in the late phase of stroke.96 However, due to the complex function of regulatory cells in immune homeostasis,146 divergent findings have been described for the role of Tregs in stroke models.152
Despite considerable findings on the association of peripheral immune cells and the BBB disruption after stroke, it still remains largely unknown how the immune cells participate in the remodeling of neurovascular unit in the late phase of stroke.
2.3. Neuroinflammation and post–stroke synaptogenesis
2.3.1. Synaptogenesis and synaptic rearrangement after cerebral ischemic stroke
Transient global ischemia or hypoxia can induce impairment of spatial learning and memory, which may result from gradual loss of synapses, dysregulation of synaptic adhesion, apoptotic neuronal cell death, and insufficient synaptic repair in the dorsal hippocampus CA1 area.153, 154 Synaptic degradation begins after global ischemic attack and proceeds during the reperfusion period, less than half of the synapses survive 7 days after ischemic stroke.153 The loss of synapses is accompanied by reduction in axon terminal volumes and synaptic vesicle numbers which is initiated as early as 2 hours after the cerebral ischemia induced by left carotid occlusion.155
2.3.2. Inflammatory regulation of synaptogenesis by brain glial cells after stroke
Synaptogenesis is an important form of brain repair after cerebral ischemic stroke.156, 157 It involves formation of a neurotransmitter release site in the presynaptic neuron, a receptive field at the postsynaptic partners and the precise alignment of pre‐ and postsynaptic specializations.158, 159, 160 The regulation of post–stroke synaptogenesis is complicated and still not fully understood. Recent research suggests that the resident glial cells are essential regulators for synapse formation and modulation during CNS development,79, 161, 162, 163, 164 after traumatic brain injury165 and cerebral ischemic stroke.166 The interaction of astrocytic processes with the pre‐ and postsynaptic terminals forms a “tripartite synapse”, which is important for synaptic dysfunction and neuronal death.167 Ischemic neurons express lipocalin‐2, which functions as a “help me” signal to transform the glial cells into their prorecovery phenotypes.168 Hippocampal neurons grew more synapses when cocultured with astrocytes, which is possibly mediated by agrin—a known synaptogenesis promoter derived from astrocytes.169 Physiologically, thrombospondins (TSPs)‐1 and ‐2 are two major factors produced by immature astrocytes to promote synaptogenesis during brain development170 and their expression is usually reduced in the adult brain. However, elevation of TSP‐1 and TSP‐2 were observed in a rat ischemic stroke model, and this might be one of the mechanisms underlying post‐ischemic astrocyte induced synaptogenesis.171 During synaptogenesis, an impressive degree of plasticity is retained through morphological and molecular rearrangements in the pre‐ and postsynaptic compartments that underlie the strengthening or weakening of synaptic pathways, which is termed as synaptic plasticity.172 The upregulation of ephrin‐A1 and ephrin‐A5 in activated astrocytes of the corticospinal tract 2 weeks after stroke is believed to modulate synaptic plasticity in stroke recovery.166 In response to ischemic injury in adult brains, striatal resident reactive astrocytes can differentiate from GABAergic and cholinergic neurons into functional mature neurons, and form synapses to receive inputs from surrounding neurons.80, 173 The above evidence suggests that astrocytes have pleiotropic mechanisms to enhance synaptogenesis after stroke (Figure 3).
Figure 3.
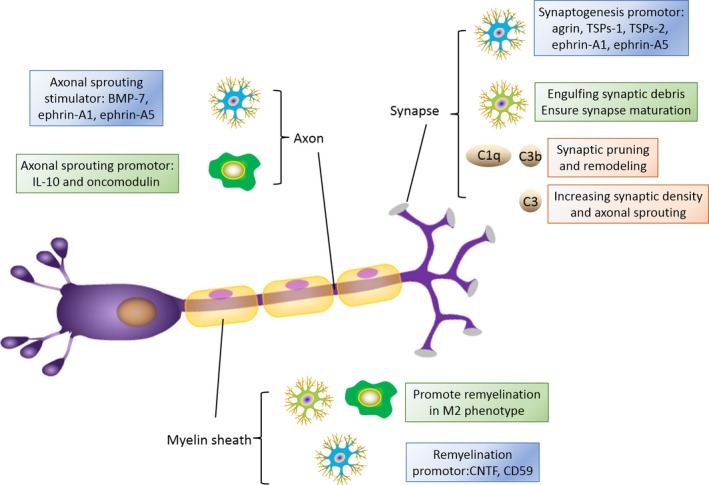
Interaction of immune cells with synaptogenesis, axonal regrowth and myelin sheath repair. In terms of synaptogenesis, astrocytes are beneficial by secreting agrin, thrombospondins (TSPs)‐1 and ‐2, ephrin‐A1 and ephrin‐A5. Microglia is important in synaptic pruning by engulfing synaptic debris and ensure synapse maturation. Complement system exert their positive effects on synaptic pruning and remodeling, along with increasing synaptic density and axonal sprouting. For axonal sprouting, astrocytes can produce stimulating factors like bone morphologic protein 7 (BMP 7), ephrin‐A1 and ephrin‐A5. Macrophages promote axonal sprouting through IL‐10 and oncomodulin release. M2 microglia and macrophage have been found to promote remyelination and may help to repair white matter injury. Astrocytes may also promote remyelination by expressing CD 59 and ciliary neurotrophic factor (CNTF)
Microglia are also actively involved in brain development, especially in synaptic remodeling.174, 175, 176 Microglia play an essential role in synaptic pruning by engulfing synaptic debris.177 Deprivation of microglia resulted in abnormal synapse maturation in mice.178 Microglia not only regulate synaptic pruning in the developing brain,178 but also the functional state of synapses at the ischemic terminals.179 However, whether different microglial phenotypes affect synaptic remodeling after ischemic stroke needs to be clarified.
2.3.3. The emerging role of complement system in post–stroke synaptogenesis
The emerging role of the complement system in synaptogenesis and neural network plasticity opens new conceptual avenues for considering complement interception as a potential therapeutic modality for improving brain repair after stroke.180, 181 The complement system is the innate immune response to foreign objects triggering a proteolytic cleavage cascade. It is activated by the classical, lectin, or alternative pathways.182, 183 The classical and lectin pathways can be activated by antibodies, apoptotic cells, or polysaccharides. C1q is the target recognition protein of the classical complement pathway.183 The alternative pathway can be activated spontaneously and functions to amplify the other pathways. All pathways converge with the formation of a C3 convertase and the cleavage of C3 to produce C3a and C3b. C3 cleavage also leads to cleavage of C5 to yield C5a and C5b.182 Distinct complement effectors appear to play multifaceted roles in brain homoeostasis by regulating synaptic pruning in the retinogeniculate system and sculpting functional neural circuits in the brain.184 C1q can bind neuronal pentraxins, a family of acute immune response proteins involved in clustering α‐amino‐3‐hydroxy‐5‐methyl‐4‐isoxazolepropionic acid (AMPA) receptors at the postsynaptic cleft. This activation removes their stabilizing ability on dendritic spine receptors. This would ultimately decrease the amount of communication between the pre‐ and postsynapses resulting in synaptic weakening.185 Astrocyte‐mediated activation of C1q results in downstream activation of C3b, which then deposits on neurites, thus “tagging” them for elimination. Mice lacking either C1q or C3 show increased synaptic connectivity and enhanced epileptiform activity due to failed synaptic pruning.81, 186 Unlike the detrimental impact of C1q and C3b on the brain plasticity, C3a was recently shown to enhance neural plasticity by increasing synaptic density and axonal sprouting.187 Consistent with the beneficial role of C3a in neural plasticity, genetic ablation of complement receptor 3(CR3) and C3 or pharmacological perturbations of these proteins results in microglial dysfunction and deficits in synaptic remodeling.174 Furthermore, disrupting microglia‐specific CR3/C3 signaling resulted in sustained deficits in synaptic connectivity.174
With the above evidence showing close relationships of the complement system with the synaptic connectivity and the pathogenesis of cerebral ischemic injury (Figure 3),182 it is reasonable to expect that the complement system may potentially have crucial impacts on the post–stroke brain function recovery.
2.4. Axonal sprouting and regrowth after ischemic stroke and the role of neuroinflammatory responses
Axonal sprouting is a process in which sprouts grow out of axons and is an important step toward recovery after ischemic stroke.188, 189 It can be detected in the penumbra three weeks after the ischemic attack,190 and last for several months.191, 192 Ischemia and reperfusion injury leads to production of numerous cytokines and free radicals, which thereafter activate immune cells and subsequent destructive cascades. Activated astrocytes are fundamentally involved in post‐ischemic repair, not only in angiogenesis and synaptogenesis, but also in induction of axonal sprouting.193 There are several special cytokines that have been identified to involve in post‐ischemic axonal sprouting. Bone morphologic proteins (BMP) are a family of growth factors that promotes cell differentiation. It is also a powerful stimulator for dendritic development.194 Ren et al found that intracisternal injection of BMP‐7 was associated with better behavior performance in mice and that the underlying mechanism may be axonal sprouting stimulated by BMP‐7.195 Growth and differentiation factor(GDF) is another important factor which enhances axonal sprouting through TGF‐β signaling.192 The upregulation of ephrin‐A1 and ephrin‐A5 in activated astrocytes of the corticospinal tract 2 weeks post injury is also believed to modulate axonal reorganization in stroke recovery.166
Activated macrophages and microglia after stroke are also important players in axonal sprouting (Figure 3).196, 197 The M1 phenotype inhibits axonal growth by secreting destructive signals such as interferon‐γ (IFN‐γ)198 and promoting dendrite retraction.199 M2 cells, in particular the M2 macrophage, on the other hand, is a potent promoter of axonal sprouting by secreting protective factors such as IL‐10 and oncomodulin.200, 201 However, the role of microglia on axonal sprouting after cerebral ischemic stroke still warrants further investigation due to the conflicting evidence suggesting that microglia are irrelevant for neuronal degeneration and axonal regeneration after acute neuronal injury.202
2.5. Interaction between post–stroke inflammation and remyelination
Myelin sheath is an important cellular component that mediates conductance of action potentials in the central nervous system. It also has important functions in regulating axon diameters and influencing axonal transport.203, 204 Cerebral ischemic stroke causes long‐lasting demyelination, which contributes significantly to long‐term sensorimotor and cognitive dysfunction.205, 206, 207 Remyelination is a repair process to restore myelin sheath around axons after demyelination.208, 209 This process is initiated by oligodendrocytes.210, 211 It relies on proliferation and maturation of oligodendrocyte progenitor cells to become functional oligodendrocytes, which produces the myelin sheath to conduct action potentials from neurons to neurons.212, 213 Improving remyelination has been repetitively shown to improve post–stroke neural behavioral function.214, 215, 216, 217, 218, 219, 220, 221 Although the role of immune cells on post–stroke demyelination and remyelination are still obscure, studies in experimental allergic encephalomyelitis (EAE) and other demyelination disease models provide evidence showing correlation of demyelination and remyelination with the infiltration of different immune cells, including neutrophils, mast cells, microglia, macrophages, and nature killer (NK) cells.222, 223, 224, 225, 226, 227 Neutrophils and mast cells appear to promote demyelination through degranulation.222, 223 While microglia and NK cells may contribute to remyelination.224, 225, 226, 227 Oligodendrocyte differentiation was promoted when cocultured with M2 microglia/macrophage in vitro, and was inhibited in M2 microglia/macrophage‐depleted EAE animals.227 Demyelination was exacerbated in EAE animals when microglia M2 polarization was inhibited.228 However, the M1 type macrophage, together with the Th17 cells are involved in the demyelination in the animal model of sclerosis.229 The evidence of macrophage's impact on white matter intergrity is also conflicting. It may attack oligodendrocyte precursor cells thus hinder remyelination.230 However, macrophage depletion in female rats resulted in significant decrease in oligodendrocyte remyelination following lysolecithin‐induced demyelination.226 Simply depleting peripheral immune cells by splenectomy did not change immune responses to myelin basic protein and stroke outcome.4, 231 Therefore, the influence of peripheral immune cells, such as macrophages and resident microglia on remyelination is highly dependent on the dominant phenotype.
Astrocytes can affect post–stroke remyelination by secreting different extracellular matrix proteins, including laminin, vitronectin, fibronectin (Fn) and tenascin‐C (TnC).232 It has been shown that astrocytes grown on TnC exhibit a quiescent phenotype and are unable to support myelination in vitro.233 The production of Fn by astrocytes, although not directly toxic to oligodendrocyte progenitor cells (OPCs) or oligodendrocytes, has a negative impact on OPC differentiation.234 On the other hand, lipids synthesized by astrocytes are essential for oligodendroglial myelination.235 Astrocytes inhibition has been shown to exacerbate demyelination and delay remyelination processes after ischemic brain injury.236 Downregulating CD59 in astrocytes increases demyelination in the mouse brain.237 However, the role of astrocyte in remyelination is controversial.238 In addition to extracellular matrix proteins, astrocytes are also important producers of a myriad of factors that can hinder OPC differentiation and remyelination,239 such as high molecular weight hyaluronan (HA).240 HA accumulation has been identified in remyelination failure in MS mouse models.241 Astrocytes are also engaged in myelin phagocytosis in diseases with characteristic myelin injury such as multiple sclerosis. The myelin debris taken up by astrocytes may increase the nuclear localization of NF‐κB and chemokine expression which in turn results in recruitment of immune cells.238
3. SUMMARY AND CONCLUSIONS
Recent studies on neuroinflammation and the exquisitely coordinated regenerative events have led to a better understanding of their interaction. Neuroinflammation, including glial cell activation and peripheral immunological changes persist through the late stage of stroke, when multiple brain repair processes take place and thus contributes significantly to functional neurological recovery after stroke. Pleiotropic factors can be secreted from both resident glial cells and peripheral immune cells in response to ischemic stroke and change the microenvironmental cues for brain regenerative processes. Distinct phenotypes of microglia/macrophage or neutrophils may have distinct effects on brain repair. However, the engagements of neuro‐immune cross talk in neural plasticity after cerebral ischemic stroke are just beginning to be understood and merit further investigation. Understanding these biological events may provide a great opportunity for developing novel stroke recovery therapies, which may substantially reduce the clinical and societal burden of stroke in those disabled survivors.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ACKNOWLEDGEMENTS
P.L is supported by the National Natural Science Foundation of China (NSFC) grant 81400956 and 81722017, and the Shanghai Rising‐Star Program (16QA1402600), the Shanghai Natural Science Program (13ZR1452200) by the Science and Technology Commission of Shanghai Municipality. W. Y is supported by the NSFC (81370513, 81571048). This work is also supported by the Shanghai Municipal commission of Health and Family Planning Funding for Key Developing Disciplines (2015ZB0101).
Wang X, Xuan W, Zhu Z‐Y, et al. The evolving role of neuro‐immune interaction in brain repair after cerebral ischemic stroke. CNS Neurosci Ther. 2018;24:1100–1114. 10.1111/cns.13077
Wang and Xuan are contributed equally to this work.
Contributor Information
Pei‐Ying Li, Email: peiyingli.md@gmail.com.
Wei‐Feng Yu, Email: ywf808@yeah.net.
REFERENCES
- 1. Maldonado NJ, Kazmi SO, Suarez JI. Update in the management of acute ischemic stroke. Crit Care Clin. 2014;30(4):673‐697. [DOI] [PubMed] [Google Scholar]
- 2. Cramer SC. Repairing the human brain after stroke. II. Restorative therapies. Ann Neurol. 2008;63(5):549‐560. [DOI] [PubMed] [Google Scholar]
- 3. Titova EM, Ghosh N, Valadez ZG, Zhang JH, Bellinger DL, Obenaus A. The late phase of post–stroke neurorepair in aged rats is reflected by MRI‐based measures. Neuroscience. 2014;283:231‐244. [DOI] [PubMed] [Google Scholar]
- 4. Yang B, Hamilton JA, Valenzuela KS, et al. Multipotent adult progenitor cells enhance recovery after stroke by modulating the immune response from the spleen. Stem Cells. 2017;35(5):1290‐1302. [DOI] [PubMed] [Google Scholar]
- 5. Chen J, Leak RK, Yang GY. Perspective for stroke and brain injury research: mechanisms and potential therapeutic targets. CNS Neurosci Ther. 2015;21(4):301‐303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hu X, Leak RK, Shi Y, et al. Microglial and macrophage polarization‐new prospects for brain repair. Nat Rev Neurol. 2015;11(1):56‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yang Y, Salayandia VM, Thompson JF, Yang LY, Estrada EY, Yang Y. Attenuation of acute stroke injury in rat brain by minocycline promotes blood‐brain barrier remodeling and alternative microglia/macrophage activation during recovery. J Neuroinflammation. 2015;12:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. An C, Shi Y, Li P, et al. Molecular dialogs between the ischemic brain and the peripheral immune system: dualistic roles in injury and repair. Prog Neurogibol. 2014;115:6‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jones KA, Maltby S, Plank MW, et al. Peripheral immune cells infiltrate into sites of secondary neurodegeneration after ischemic stroke. Brain Behav Immun. 2017;67:299–307. [DOI] [PubMed] [Google Scholar]
- 10. Liu ZJ, Chen C, Li FW, et al. Splenic responses in ischemic stroke: new insights into stroke pathology. CNS Neurosci Ther. 2015;21(4):320–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hu X, Li P, Guo Y, et al. Microglia/macrophage polarization dynamics reveal novel mechanism of injury expansion after focal cerebral ischemia. Stroke. 2012;43(11):3063–3070. [DOI] [PubMed] [Google Scholar]
- 12. Mracsko E, Liesz A, Stojanovic A, et al. Antigen dependently activated cluster of differentiation 8‐positive T cells cause perforin‐mediated neurotoxicity in experimental stroke. J Neurosci. 2014;34(50):16784–16795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jin WN, Yang X, Li Z, et al. Non‐invasive tracking of CD4+ T cells with a paramagnetic and fluorescent nanoparticle in brain ischemia. J Cereb Blood Flow Metab. 2016;36(8):1464–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gu W, Brannstrom T, Wester P. Cortical neurogenesis in adult rats after reversible photothrombotic stroke. J Cereb Blood Flow Metab. 2000;20(8):1166–1173. [DOI] [PubMed] [Google Scholar]
- 15. Liu XS, Zhang ZG, Zhang RL, et al. Stroke induces gene profile changes associated with neurogenesis and angiogenesis in adult subventricular zone progenitor cells. J Cereb Blood Flow Metab. 2007;27(3):564–574. [DOI] [PubMed] [Google Scholar]
- 16. He X, Lu Y, Lin X, et al. Optical inhibition of striatal neurons promotes focal neurogenesis and neurobehavioral recovery in mice after middle cerebral artery occlusion. J Cereb Blood Flow Metab. 2017;37(3):837–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhang R, Zhang Z, Chopp M. Function of neural stem cells in ischemic brain repair processes. J Cereb Blood Flow Metab. 2016;36(12):2034–2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liu TT, Ye XL, Zhang JP, et al. Increased adult neurogenesis associated with reactive astrocytosis occurs prior to neuron loss in a mouse model of neurodegenerative disease. CNS Neurosci Ther. 2017;23(11):885–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Doetsch F. The glial identity of neural stem cells. Nat Neurosci. 2003;6(11):1127–1134. [DOI] [PubMed] [Google Scholar]
- 20. Martino G, Pluchino S. The therapeutic potential of neural stem cells. Nat Rev Neurosci. 2006;7(5):395–406. [DOI] [PubMed] [Google Scholar]
- 21. Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132(4):645–660. [DOI] [PubMed] [Google Scholar]
- 22. Adlaf EW, Mitchell‐Dick A, Kuo CT. Discerning Neurogenic vs. non‐neurogenic postnatal lateral ventricular astrocytes via activity‐dependent input. Front Neurosci. 2016;10:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Heinrich C, Gotz M, Berninger B. Reprogramming of postnatal astroglia of the mouse neocortex into functional, synapse‐forming neurons. Methods Mol Biol. 2012;814:485–498. [DOI] [PubMed] [Google Scholar]
- 24. Alvarez‐Buylla A, Garcia‐Verdugo JM. Neurogenesis in adult subventricular zone. J Neurosci. 2002;22(3):629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kaneko N, Sawada M, Sawamoto K. Mechanisms of neuronal migration in the adult brain. J Neurochem. 2017;141(6):835–847. [DOI] [PubMed] [Google Scholar]
- 26. Fujioka T, Kaneko N, Ajioka I, et al. beta1 integrin signaling promotes neuronal migration along vascular scaffolds in the post–stroke brain. EBioMedicine. 2017;16:195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Xiong XY, Liu L, Yang QW. Functions and mechanisms of microglia/macrophages in neuroinflammation and neurogenesis after stroke. Prog Neurogibol. 2016;142:23–44. [DOI] [PubMed] [Google Scholar]
- 28. Yu TS, Washington PM, Kernie SG. Injury‐induced neurogenesis: mechanisms and relevance. Neuroscientist. 2016;22(1):61–71. [DOI] [PubMed] [Google Scholar]
- 29. Pluchino S, Muzio L, Imitola J, et al. Persistent inflammation alters the function of the endogenous brain stem cell compartment. Brain. 2008;131(Pt 10):2564–2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tobin MK, Bonds JA, Minshall RD, Pelligrino DA, Testai FD, Lazarov O. Neurogenesis and inflammation after ischemic stroke: what is known and where we go from here. J Cereb Blood Flow Metab. 2014;34(10):1573–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Moraga A, Gomez‐Vallejo V, Cuartero MI, et al. Imaging the role of toll‐like receptor 4 on cell proliferation and inflammation after cerebral ischemia by positron emission tomography. J Cereb Blood Flow Metab. 2016;36(4):702–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ekdahl CT, Zhu C, Bonde S, Bahr BA, Blomgren K, Lindvall O. Death mechanisms in status epilepticus‐generated neurons and effects of additional seizures on their survival. Neurobiol Dis. 2003;14(3):513–523. [DOI] [PubMed] [Google Scholar]
- 33. Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302(5651):1760–1765. [DOI] [PubMed] [Google Scholar]
- 34. Meng HL, Li XX, Chen YT, et al. Neuronal soluble fas ligand drives M1‐microglia polarization after cerebral ischemia. CNS Neurosci Ther. 2016;22(9):771–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Huang M, Wan Y, Mao L, et al. Inhibiting the migration of M1 microglia at hyperacute period could improve outcome of tMCAO rats. CNS Neurosci Ther. 2017;23(3):222–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kipnis J, Yoles E, Schori H, Hauben E, Shaked I, Schwartz M. Neuronal survival after CNS insult is determined by a genetically encoded autoimmune response. J Neurosci. 2001;21(13):4564–4571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Liu B, Teschemacher AG, Kasparov S. Astroglia as a cellular target for neuroprotection and treatment of neuro‐psychiatric disorders. Glia. 2017;65(8):1205–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nonose Y, Gewehr PE, Almeida RF, et al. Cortical Bilateral adaptations in rats submitted to focal cerebral ischemia: emphasis on glial metabolism. Mol Neurobiol. 2017;55(3):2025–2041. [DOI] [PubMed] [Google Scholar]
- 39. Saino O, Taguchi A, Nakagomi T, et al. Immunodeficiency reduces neural stem/progenitor cell apoptosis and enhances neurogenesis in the cerebral cortex after stroke. J Neurosci Res. 2010;88(11):2385–2397. [DOI] [PubMed] [Google Scholar]
- 40. Stonesifer C, Corey S, Ghanekar S, Diamandis Z, Acosta SA, Borlongan CV. Stem cell therapy for abrogating stroke‐induced neuroinflammation and relevant secondary cell death mechanisms. Prog Neurogibol. 2017;158:94–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cunningham CJ, Redondo‐Castro E, Allan SM. The therapeutic potential of the mesenchymal stem cell secretome in ischaemic stroke. J Cereb Blood Flow Metab. 2018;38(8):1276–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gemma C, Bachstetter AD. The role of microglia in adult hippocampal neurogenesis. Front Cell Neurosci. 2013;7:229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Colonna M, Butovsky O. Microglia function in the central nervous system during health and neurodegeneration. Ann Rev Immunol. 2017;35:441–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kim JY, Kim N, Yenari MA. Mechanisms and potential therapeutic applications of microglial activation after brain injury. CNS Neurosci Ther. 2015;21(4):309–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sierra A, Encinas JM, Deudero JJ, et al. Microglia shape adult hippocampal neurogenesis through apoptosis‐coupled phagocytosis. Cell Stem Cell. 2010;7(4):483–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Keohane A, Ryan S, Maloney E, Sullivan AM, Nolan YM. Tumour necrosis factor‐alpha impairs neuronal differentiation but not proliferation of hippocampal neural precursor cells: Role of Hes1. Mol Cell Neurosci. 2010;43(1):127–135. [DOI] [PubMed] [Google Scholar]
- 47. Madsen PM, Clausen BH, Degn M, et al. Genetic ablation of soluble tumor necrosis factor with preservation of membrane tumor necrosis factor is associated with neuroprotection after focal cerebral ischemia. J Cereb Blood Flow Metab. 2016;36(9):1553–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ziv Y, Schwartz M. Immune‐based regulation of adult neurogenesis: implications for learning and memory. Brain Behav Immun. 2008;22(2):167–176. [DOI] [PubMed] [Google Scholar]
- 49. Jin WN, Shi SX, Li Z, et al. Depletion of microglia exacerbates postischemic inflammation and brain injury. J Cereb Blood Flow Metab. 2017;37(6):2224–2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. De Geyter D, De Smedt A, Stoop W, De Keyser J, Kooijman R. Central IGF‐I receptors in the brain are instrumental to neuroprotection by systemically injected IGF‐I in a rat model for ischemic stroke. CNS Neurosci Ther. 2016;22(7):611–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. De Magalhaes Filho CD, Kappeler L, Dupont J, et al. Deleting IGF‐1 receptor from forebrain neurons confers neuroprotection during stroke and upregulates endocrine somatotropin. J Cereb Blood Flow Metab. 2017;37(2):396–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zhang M, Wu X, Xu Y, et al. The cystathionine beta‐synthase/hydrogen sulfide pathway contributes to microglia‐mediated neuroinflammation following cerebral ischemia. Brain Behav Immun. 2017;66:332–346. [DOI] [PubMed] [Google Scholar]
- 53. Truettner JS, Bramlett HM, Dietrich WD. Posttraumatic therapeutic hypothermia alters microglial and macrophage polarization toward a beneficial phenotype. J Cereb Blood Flow Metab. 2017;37(8):2952–2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ransohoff RM. A polarizing question: do M1 and M2 microglia exist? Nat Neurosci. 2016;19(8):987–991. [DOI] [PubMed] [Google Scholar]
- 55. Amici SA, Dong J, Guerau‐de‐Arellano M. Molecular mechanisms modulating the phenotype of macrophages and microglia. Front Immunol. 2017;8:1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wang J, Zhao H, Fan Z, et al. Long noncoding RNA H19 promotes neuroinflammation in ischemic stroke by driving histone deacetylase 1‐dependent M1 microglial polarization. Stroke. 2017;48(8):2211–2221. [DOI] [PubMed] [Google Scholar]
- 57. Wang C, Yeo S, Haas MA, Guan JL. Autophagy gene FIP200 in neural progenitors non‐cell autonomously controls differentiation by regulating microglia. J Cell Biol. 2017;216(8):2581–2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kim BJ, Kim MJ, Park JM, et al. Reduced neurogenesis after suppressed inflammation by minocycline in transient cerebral ischemia in rat. J Neurol Sci. 2009;279(1–2):70–75. [DOI] [PubMed] [Google Scholar]
- 59. Kobayashi K, Imagama S, Ohgomori T, et al. Minocycline selectively inhibits M1 polarization of microglia. Cell Death Dis. 2013;4:e525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Yuan J, Ge H, Liu W, et al. M2 microglia promotes neurogenesis and oligodendrogenesis from neural stem/progenitor cells via the PPARgamma signaling pathway. Oncotarget. 2017;8(12):19855–19865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Shu ZM, Shu XD, Li HQ, et al. Ginkgolide B protects against ischemic stroke via modulating microglia polarization in mice. CNS Neurosci Ther. 2016;22(9):729–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Butovsky O, Ziv Y, Schwartz A, et al. Microglia activated by IL‐4 or IFN‐gamma differentially induce neurogenesis and oligodendrogenesis from adult stem/progenitor cells. Mol Cell Neurosci. 2006;31(1):149–160. [DOI] [PubMed] [Google Scholar]
- 63. Cacci E, Ajmone‐Cat MA, Anelli T, Biagioni S, Minghetti L. In vitro neuronal and glial differentiation from embryonic or adult neural precursor cells are differently affected by chronic or acute activation of microglia. Glia. 2008;56(4):412–425. [DOI] [PubMed] [Google Scholar]
- 64. Thored P, Heldmann U, Gomes‐Leal W, et al. Long‐term accumulation of microglia with proneurogenic phenotype concomitant with persistent neurogenesis in adult subventricular zone after stroke. Glia. 2009;57(8):835–849. [DOI] [PubMed] [Google Scholar]
- 65. Song H, Stevens CF, Gage FH. Astroglia induce neurogenesis from adult neural stem cells. Nature. 2002;417(6884):39–44. [DOI] [PubMed] [Google Scholar]
- 66. Okoreeh AK, Bake S, Sohrabji F. Astrocyte‐specific insulin‐like growth factor‐1 gene transfer in aging female rats improves stroke outcomes. Glia. 2017;65(7):1043–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Becerra‐Calixto A, Cardona‐Gomez GP. The role of astrocytes in neuroprotection after brain stroke: potential in cell therapy. Front Mol Neurosci. 2017;10:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Chen Y, Swanson RA. Astrocytes and brain injury. J Cereb Blood Flow Metab. 2003;23(2):137–149. [DOI] [PubMed] [Google Scholar]
- 69. Valdebenito R, Ruminot I, Garrido‐Gerter P, et al. Targeting of astrocytic glucose metabolism by beta‐hydroxybutyrate. J Cereb Blood Flow Metab. 2016;36(10):1813–1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Kalani MY, Cheshier SH, Cord BJ, et al. Wnt‐mediated self‐renewal of neural stem/progenitor cells. Proc Natl Acad Sci USA. 2008;105(44):16970–16975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Otaegi G, Yusta‐Boyo MJ, Vergano‐Vera E, et al. Modulation of the PI 3‐kinase‐Akt signalling pathway by IGF‐I and PTEN regulates the differentiation of neural stem/precursor cells. J Cell Sci. 2006;119(Pt 13):2739–2748. [DOI] [PubMed] [Google Scholar]
- 72. Shetty AK, Hattiangady B, Shetty GA. Stem/progenitor cell proliferation factors FGF‐2, IGF‐1, and VEGF exhibit early decline during the course of aging in the hippocampus: role of astrocytes. Glia. 2005;51(3):173–186. [DOI] [PubMed] [Google Scholar]
- 73. Bartkowska K, Paquin A, Gauthier AS, Kaplan DR, Miller FD. Trk signaling regulates neural precursor cell proliferation and differentiation during cortical development. Development. 2007;134(24):4369–4380. [DOI] [PubMed] [Google Scholar]
- 74. Quesseveur G, David DJ, Gaillard MC, et al. BDNF overexpression in mouse hippocampal astrocytes promotes local neurogenesis and elicits anxiolytic‐like activities. Transl Psychiatry. 2013;3:e253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Carmichael ST. Cellular and molecular mechanisms of neural repair after stroke: making waves. Ann Neurol. 2006;59(5):735–742. [DOI] [PubMed] [Google Scholar]
- 76. Kang SS, Keasey MP, Arnold SA, Reid R, Geralds J, Hagg T. Endogenous CNTF mediates stroke‐induced adult CNS neurogenesis in mice. Neurobiol Dis. 2013;49:68–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Yang X, Geng KY, Zhang YS, et al. Sirt3 deficiency impairs neurovascular recovery in ischemic stroke. CNS Neurosci Ther. 2018;24(9):775–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Liu Z, Li Y, Cui Y, et al. Beneficial effects of gfap/vimentin reactive astrocytes for axonal remodeling and motor behavioral recovery in mice after stroke. Glia. 2014;62(12):2022–2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Liu Z, Chopp M. Astrocytes, therapeutic targets for neuroprotection and neurorestoration in ischemic stroke. Prog Neurogibol. 2016;144:103–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Duan CL, Liu CW, Shen SW, et al. Striatal astrocytes transdifferentiate into functional mature neurons following ischemic brain injury. Glia. 2015;63(9):1660–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Magnusson JP, Goritz C, Tatarishvili J, et al. A latent neurogenic program in astrocytes regulated by Notch signaling in the mouse. Science. 2014;346(6206):237–241. [DOI] [PubMed] [Google Scholar]
- 82. Llovera G, Benakis C, Enzmann G, et al. The choroid plexus is a key cerebral invasion route for T cells after stroke. Acta neuropathol. 2017;134(6):851–868. [DOI] [PubMed] [Google Scholar]
- 83. Wolf SA, Steiner B, Wengner A, Lipp M, Kammertoens T, Kempermann G. Adaptive peripheral immune response increases proliferation of neural precursor cells in the adult hippocampus. FASEB J. 2009;23(9):3121–3128. [DOI] [PubMed] [Google Scholar]
- 84. Wong CH, Jenne CN, Tam PP, et al. Prolonged activation of invariant natural killer T cells and TH2‐skewed immunity in stroke patients. Front Neurol. 2017;8:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Arunachalam P, Ludewig P, Melich P, et al. CCR85 (CC Chemokine Receptor 6) is essential for the migration of detrimental natural interleukin‐17‐producing gammadelta T cells in stroke. Stroke. 2017;48(7):1957–1965. [DOI] [PubMed] [Google Scholar]
- 86. Kwong B, Rua R, Gao Y, et al. T‐bet‐dependent NKp46+ innate lymphoid cells regulate the onset of TH17‐induced neuroinflammation. Nat Immunol. 2017;18(10):1117–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Wang B, Jin K. Current perspectives on the link between neuroinflammation and neurogenesis. Metab Brain Dis. 2015;30(2):355–365. [DOI] [PubMed] [Google Scholar]
- 88. Fisher Y, Strominger I, Biton S, Nemirovsky A, Baron R, Monsonego A. Th1 polarization of T cells injected into the cerebrospinal fluid induces brain immunosurveillance. J Immunol. 2014;192(1):92–102. [DOI] [PubMed] [Google Scholar]
- 89. Lin Y, Zhang JC, Yao CY, et al. Critical role of astrocytic interleukin‐17 A in post–stroke survival and neuronal differentiation of neural precursor cells in adult mice. Cell Death Dis. 2016;7(6):e2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Li P, Wang L, Zhou Y, et al. Chemokine receptor type 5 (CCR90)‐mediated docking of transferred tregs protects against early blood‐brain barrier disruption after stroke. J Am Heart Assoc. 2017; 6:e006387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Rodriguez‐Perea AL, Gutierrez‐Vargas J, Cardona‐Gomez GP, Guarin CJ, Rojas M, Hernandez PA. Atorvastatin modulates regulatory T cells and attenuates cerebral damage in a model of transient middle cerebral artery occlusion in rats. J Neuroimmune Pharmacol. 2017;12(1):152–162. [DOI] [PubMed] [Google Scholar]
- 92. Li P, Mao L, Zhou G, et al. Adoptive regulatory T‐cell therapy preserves systemic immune homeostasis after cerebral ischemia. Stroke. 2013;44(12):3509–3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Liesz A, Suri‐Payer E, Veltkamp C, et al. Regulatory T cells are key cerebroprotective immunomodulators in acute experimental stroke. Nat Med. 2009;15(2):192–199. [DOI] [PubMed] [Google Scholar]
- 94. Lee HT, Liu SP, Lin CH, et al. A crucial role of CXCL14 for promoting regulatory T cells activation in stroke. Theranostics. 2017;7(4):855–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Zhou K, Zhong Q, Wang YC, et al. Regulatory T cells ameliorate intracerebral hemorrhage‐induced inflammatory injury by modulating microglia/macrophage polarization through the IL‐10/GSK3beta/PTEN axis. J Cereb Blood Flow Metab. 2017;37(3):967–979. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 96. Wang J, Xie L, Yang C, et al. Activated regulatory T cell regulates neural stem cell proliferation in the subventricular zone of normal and ischemic mouse brain through interleukin 10. Front Cell Neurosci. 2015;9:361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Nathan CF. Secretory products of macrophages. J Clin Invest. 1987;79(2):319–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Couch Y, Akbar N, Davis S, et al. Inflammatory stroke extracellular vesicles induce macrophage activation. Stroke. 2017;48(8):2292–2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Cheon SY, Kim EJ, Kim JM, Kam EH, Ko BW, Koo BN. Regulation of microglia and macrophage polarization via apoptosis signal‐regulating kinase 1 silencing after ischemic/hypoxic injury. Front Mol Neurosci. 2017;10:261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Wang N, Liang H, Zen K. Molecular mechanisms that influence the macrophage m1–m2 polarization balance. Front Immunol. 2014;5:614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007;10(11):1387–1394. [DOI] [PubMed] [Google Scholar]
- 102. Liu Z, Fan Y, Won SJ, et al. Chronic treatment with minocycline preserves adult new neurons and reduces functional impairment after focal cerebral ischemia. Stroke. 2007;38(1):146–152. [DOI] [PubMed] [Google Scholar]
- 103. Shichita T, Sugiyama Y, Ooboshi H, et al. Pivotal role of cerebral interleukin‐17‐producing gammadeltaT cells in the delayed phase of ischemic brain injury. Nat Med. 2009;15(8):946–950. [DOI] [PubMed] [Google Scholar]
- 104. He Y, Ma X, Li D, Hao J. Thiamet G mediates neuroprotection in experimental stroke by modulating microglia/macrophage polarization and inhibiting NF‐kappaB p65 signaling. J Cereb Blood Flow Metab. 2017;37(8):2938–2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Qi F, Zuo Z, Yang J, et al. Combined effect of BCG vaccination and enriched environment promote neurogenesis and spatial cognition via a shift in meningeal macrophage M2 polarization. J Neuroinflammation. 2017;14(1):32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Dirnagl U. Pathobiology of injury after stroke: the neurovascular unit and beyond. Ann N Y Acad Sci. 2012;1268:21–25. [DOI] [PubMed] [Google Scholar]
- 107. Cai W, Zhang K, Li P, et al. Dysfunction of the neurovascular unit in ischemic stroke and neurodegenerative diseases: an aging effect. Ageing Res Rev. 2017;34:77–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Lake EM, Bazzigaluppi P, Mester J, et al. Neurovascular unit remodelling in the subacute stage of stroke recovery. NeuroImage. 2017;146:869–882. [DOI] [PubMed] [Google Scholar]
- 109. Beck H, Plate KH. Angiogenesis after cerebral ischemia. Acta neuropathologica. 2009;117(5):481–496. [DOI] [PubMed] [Google Scholar]
- 110. Chen JY, Yu Y, Yuan Y, et al. Enriched housing promotes post–stroke functional recovery through astrocytic HMGB1‐IL‐6‐mediated angiogenesis. Cell Death Discov. 2017;3:17054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Li Y, Zhang X, Cui L, et al. Salvianolic acids enhance cerebral angiogenesis and neurological recovery by activating JAK2/STAT3 signaling pathway after ischemic stroke in mice. J Neurochem. 2017;143(1):87–99. [DOI] [PubMed] [Google Scholar]
- 112. Teng H, Zhang ZG, Wang L, et al. Coupling of angiogenesis and neurogenesis in cultured endothelial cells and neural progenitor cells after stroke. J Cereb Blood Flow Metab. 2008;28(4):764–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Krupinski J, Kaluza J, Kumar P, Kumar S, Wang JM. Role of angiogenesis in patients with cerebral ischemic stroke. Stroke. 1994;25(9):1794–1798. [DOI] [PubMed] [Google Scholar]
- 114. Nezhat C, Nezhat F, Nezhat CH, Seidman DS. Severe endometriosis and operative laparoscopy. Curr Opin Obstet Gynecol. 1995;7(4):299–306. [PubMed] [Google Scholar]
- 115. Cekanaviciute E, Fathali N, Doyle KP, Williams AM, Han J, Buckwalter MS. Astrocytic transforming growth factor‐beta signaling reduces subacute neuroinflammation after stroke in mice. Glia. 2014;62(8):1227–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Ohab JJ, Fleming S, Blesch A, Carmichael ST. A neurovascular niche for neurogenesis after stroke. J Neurosci. 2006;26(50):13007–13016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Koster KP, Thomas R, Morris AW, Tai LM. Epidermal growth factor prevents oligomeric amyloid‐beta induced angiogenesis deficits in vitro. J Cereb Blood Flow Metab. 2016;36(11):1865–1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Gertz K, Kronenberg G, Kalin RE, et al. Essential role of interleukin‐6 in post–stroke angiogenesis. Brain. 2012;135(Pt 6):1964–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Galea J, Brough D. The role of inflammation and interleukin‐1 in acute cerebrovascular disease. J Inflamm Res. 2013;6:121–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Greenberg DA, Jin K. Vascular endothelial growth factors (VEGFs) and stroke. Cell Mol Life Sci. 2013;70(10):1753–1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. del Zoppo GJ, Milner R, Mabuchi T, et al. Microglial activation and matrix protease generation during focal cerebral ischemia. Stroke. 2007;38(2 Suppl):646–651. [DOI] [PubMed] [Google Scholar]
- 122. Rempe RG, Hartz AM, Bauer B. Matrix metalloproteinases in the brain and blood‐brain barrier: Versatile breakers and makers. J Cereb Blood Flow Metab. 2016;36(9):1481–1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Seto SW, Chang D, Jenkins A, Bensoussan A, Kiat H. Angiogenesis in ischemic stroke and angiogenic effects of Chinese Herbal Medicine. J Clin Med. 2016;5(6):E56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Talwar T, Srivastava MV. Role of vascular endothelial growth factor and other growth factors in post–stroke recovery. Ann Indian Acad Neurol. 2014;17(1):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Su EJ, Cao C, Fredriksson L, et al. Microglial‐mediated PDGF‐CC activation increases cerebrovascular permeability during ischemic stroke. Acta Neuropathol. 2017;134(4):585–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Lo EH. A new penumbra: transitioning from injury into repair after stroke. Nat Med. 2008;14(5):497–500. [DOI] [PubMed] [Google Scholar]
- 127. Duncombe J, Lennen RJ, Jansen MA, Marshall I, Wardlaw JM, Horsburgh K. Ageing causes prominent neurovascular dysfunction associated with loss of astrocytic contacts and gliosis. Neuropathol Appl Neurobiol. 2017;43(6):477–491. [DOI] [PubMed] [Google Scholar]
- 128. Ezan P, Andre P, Cisternino S, et al. Deletion of astroglial connexins weakens the blood‐brain barrier. J Cereb Blood Flow Metab. 2012;32(8):1457–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. He QW, Li Q, Jin HJ, et al. MiR‐150 regulates poststroke cerebral angiogenesis via vascular endothelial growth factor in rats. CNS Neurosci Ther. 2016;22(6):507–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Zhao BQ, Wang S, Kim HY, et al. Role of matrix metalloproteinases in delayed cortical responses after stroke. Nat Med. 2006;12(4):441–445. [DOI] [PubMed] [Google Scholar]
- 131. Willenborg S, Lucas T, van Loo G, et al. CCR131 recruits an inflammatory macrophage subpopulation critical for angiogenesis in tissue repair. Blood. 2012;120(3):613–625. [DOI] [PubMed] [Google Scholar]
- 132. Nakamura Y, Sugita Y, Nakashima S, et al. Alternatively activated macrophages play an important role in vascular remodeling and hemorrhaging in patients with brain arteriovenous malformation. J Stroke Cerebrovasc Dis. 2016;25(3):600–609. [DOI] [PubMed] [Google Scholar]
- 133. Amulic B, Hayes G. Neutrophil extracellular traps. Curr Biol. 2011;21(9):R297–R298. [DOI] [PubMed] [Google Scholar]
- 134. Lapergue B, Dang BQ, Desilles JP, et al. High‐density lipoprotein‐based therapy reduces the hemorrhagic complications associated with tissue plasminogen activator treatment in experimental stroke. Stroke. 2013;44(3):699–707. [DOI] [PubMed] [Google Scholar]
- 135. Herz J, Sabellek P, Lane TE, Gunzer M, Hermann DM, Doeppner TR. Role of Neutrophils in exacerbation of brain injury after focal cerebral ischemia in hyperlipidemic mice. Stroke. 2015;46(10):2916–2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Li P, Gan Y, Sun BL, et al. Adoptive regulatory T‐cell therapy protects against cerebral ischemia. Ann Neurol. 2013;74(3):458–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Sladojevic N, Stamatovic SM, Keep RF, et al. Inhibition of junctional adhesion molecule‐A/LFA interaction attenuates leukocyte trafficking and inflammation in brain ischemia/reperfusion injury. Neurobiol Dis. 2014;67:57–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Zlokovic BV. Remodeling after stroke. Nat Med. 2006;12(4):390–391. [DOI] [PubMed] [Google Scholar]
- 139. Corps KN, Roth TL, McGavern DB. Inflammation and neuroprotection in traumatic brain injury. JAMA Neurol. 2015;72(3):355–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. McDonald B, Pittman K, Menezes GB, et al. Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science. 2010;330(6002):362–366. [DOI] [PubMed] [Google Scholar]
- 141. Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013;13(3):159–175. [DOI] [PubMed] [Google Scholar]
- 142. Jickling GC, Liu D, Ander BP, Stamova B, Zhan X, Sharp FR. Targeting neutrophils in ischemic stroke: translational insights from experimental studies. J Cereb Blood Flow Metab. 2015;35(6):888–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Cuartero MI, Ballesteros I, Moraga A, et al. N2 neutrophils, novel players in brain inflammation after stroke: modulation by the PPARgamma agonist rosiglitazone. Stroke. 2013;44(12):3498–3508. [DOI] [PubMed] [Google Scholar]
- 144. Zhang C, Ling CL, Pang L, et al. Direct macromolecular drug delivery to cerebral ischemia area using neutrophil‐mediated nanoparticles. Theranostics. 2017;7(13):3260–3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Bodhankar S, Chen Y, Lapato A, et al. Regulatory CD8(+)CD122 (+) T‐cells predominate in CNS after treatment of experimental stroke in male mice with IL‐10‐secreting B‐cells. Metab Brain Dis. 2015;30(4):911–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Liesz A, Kleinschnitz C. Regulatory T cells in post–stroke immune homeostasis. Transl Stroke Res. 2016;7(4):313–321. [DOI] [PubMed] [Google Scholar]
- 147. Zouggari Y, Ait‐Oufella H, Waeckel L, et al. Regulatory T cells modulate postischemic neovascularization. Circulation. 2009;120(14):1415–1425. [DOI] [PubMed] [Google Scholar]
- 148. Wan YY. Multi‐tasking of helper T cells. Immunology. 2010;130(2):166–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Na SY, Mracsko E, Liesz A, Hunig T, Veltkamp R. Amplification of regulatory T cells using a CD28 superagonist reduces brain damage after ischemic stroke in mice. Stroke. 2015;46(1):212–220. [DOI] [PubMed] [Google Scholar]
- 150. Mao L, Li P, Zhu W, et al. Regulatory T cells ameliorate tissue plasminogen activator‐induced brain haemorrhage after stroke. Brain. 2017;140(7):1914–1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Ishibashi S, Maric D, Mou Y, Ohtani R, Ruetzler C, Hallenbeck JM. Mucosal tolerance to E‐selectin promotes the survival of newly generated neuroblasts via regulatory T‐cell induction after stroke in spontaneously hypertensive rats. J Cereb Blood Flow Metab. 2009;29(3):606–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Kleinschnitz C, Kraft P, Dreykluft A, et al. Regulatory T cells are strong promoters of acute ischemic stroke in mice by inducing dysfunction of the cerebral microvasculature. Blood. 2013;121(4):679–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Nikonenko AG, Radenovic L, Andjus PR, Skibo GG. Structural features of ischemic damage in the hippocampus. Anat Rec. 2009;292(12):1914–1921. [DOI] [PubMed] [Google Scholar]
- 154. Costain WJ, Rasquinha I, Sandhu JK, et al. Cerebral ischemia causes dysregulation of synaptic adhesion in mouse synaptosomes. J Cereb Blood Flow Metab. 2008;28(1):99–110. [DOI] [PubMed] [Google Scholar]
- 155. Ito U, Kuroiwa T, Nagasao J, Kawakami E, Oyanagi K. Temporal profiles of axon terminals, synapses and spines in the ischemic penumbra of the cerebral cortex: ultrastructure of neuronal remodeling. Stroke. 2006;37(8):2134–2139. [DOI] [PubMed] [Google Scholar]
- 156. Chen J, Zacharek A, Cui X, et al. Treatment of stroke with a synthetic liver X receptor agonist, TO901317, promotes synaptic plasticity and axonal regeneration in mice. J Cereb Blood Flow Metab. 2010;30(1):102–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Gutierrez‐Vargas JA, Moreno H, Cardona‐Gomez GP. Targeting CDK5 post–stroke provides long‐term neuroprotection and rescues synaptic plasticity. J Cereb Blood Flow Metab. 2017;37(6):2208–2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Hama K. Studies on fine structure and function of synapses. Prog Brain Res. 1966;21:251–267. [DOI] [PubMed] [Google Scholar]
- 159. Sorra KE, Harris KM. Overview on the structure, composition, function, development, and plasticity of hippocampal dendritic spines. Hippocampus. 2000;10(5):501–511. [DOI] [PubMed] [Google Scholar]
- 160. Szule JA, Jung JH, McMahan UJ. The structure and function of 'active zone material' at synapses. Philos Trans R Soc Lond B Biol Sci. 2015;370(1672):20140189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Haber M, Zhou L, Murai KK. Cooperative astrocyte and dendritic spine dynamics at hippocampal excitatory synapses. J Neurosci. 2006;26(35):8881–8891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Haydon PG. GLIA: listening and talking to the synapse. Nat Rev Neurosci. 2001;2(3):185–193. [DOI] [PubMed] [Google Scholar]
- 163. Mauch DH, Nagler K, Schumacher S, et al. CNS synaptogenesis promoted by glia‐derived cholesterol. Science. 2001;294(5545):1354–1357. [DOI] [PubMed] [Google Scholar]
- 164. Ullian EM, Sapperstein SK, Christopherson KS, Barres BA. Control of synapse number by glia. Science. 2001;291(5504):657–661. [DOI] [PubMed] [Google Scholar]
- 165. Liu M, Zhang C, Liu W, et al. A novel rat model of blast‐induced traumatic brain injury simulating different damage degree: implications for morphological, neurological, and biomarker changes. Front Cell Neurosci. 2015;9:168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Choi DH, Ahn JH, Choi IA, Kim JH, Kim BR, Lee J. Effect of task‐specific training on Eph/ephrin expression after stroke. BMB Rep. 2016;49(11):635–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Diaz A, Merino P, Manrique LG, Cheng L, Yepes M. Urokinase‐type plasminogen activator (uPA) protects the tripartite synapse in the ischemic brain via ezrin‐mediated formation of peripheral astrocytic processes. J Cereb Blood Flow Metab. 2018. 10.1177/0271678X18783653. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Xing C, Wang X, Cheng C, et al. Neuronal production of lipocalin‐2 as a help‐me signal for glial activation. Stroke. 2014;45(7):2085–2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Tournell CE, Bergstrom RA, Ferreira A. Progesterone‐induced agrin expression in astrocytes modulates glia‐neuron interactions leading to synapse formation. Neuroscience. 2006;141(3):1327–1338. [DOI] [PubMed] [Google Scholar]
- 170. Christopherson KS, Ullian EM, Stokes CC, et al. Thrombospondins are astrocyte‐secreted proteins that promote CNS synaptogenesis. Cell. 2005;120(3):421–433. [DOI] [PubMed] [Google Scholar]
- 171. Lin TN, Kim GM, Chen JJ, Cheung WM, He YY, Hsu CY. Differential regulation of thrombospondin‐1 and thrombospondin‐2 after focal cerebral ischemia/reperfusion. Stroke. 2003;34(1):177–186. [DOI] [PubMed] [Google Scholar]
- 172. Gordon‐Weeks PR, Fournier AE. Neuronal cytoskeleton in synaptic plasticity and regeneration. J Neurochem. 2014;129(2):206–212. [DOI] [PubMed] [Google Scholar]
- 173. Perea G, Gomez R, Mederos S et al. Activity‐dependent switch of GABAergic inhibition into glutamatergic excitation in astrocyte‐neuron networks. ELife. 2016;5:e20362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Schafer DP, Lehrman EK, Kautzman AG, et al. Microglia sculpt postnatal neural circuits in an activity and complement‐dependent manner. Neuron. 2012;74(4):691–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Bialas AR, Presumey J, Das A, et al. Microglia‐dependent synapse loss in type I interferon‐mediated lupus. Nature. 2017;546(7659):539–543. [DOI] [PubMed] [Google Scholar]
- 176. Paolicelli RC, Jawaid A, Henstridge CM, et al. TDP‐43 depletion in microglia promotes amyloid clearance but also induces synapse loss. Neuron. 2017;95(2):297–308.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Sellgren CM, Sheridan SD, Gracias J, Xuan D, Fu T, Perlis RH. Patient‐specific models of microglia‐mediated engulfment of synapses and neural progenitors. Mol Psychiatry. 2017;22(2):170–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Paolicelli RC, Bolasco G, Pagani F, et al. Synaptic pruning by microglia is necessary for normal brain development. Science. 2011;333(6048):1456–1458. [DOI] [PubMed] [Google Scholar]
- 179. Wake H, Moorhouse AJ, Jinno S, Kohsaka S, Nabekura J. Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. J Neurosci. 2009;29(13):3974–3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Yuzaki M. The C1q complement family of synaptic organizers: not just complementary. Curr Opin Neurobiol. 2017;45:9–15. [DOI] [PubMed] [Google Scholar]
- 181. Shi Q, Chowdhury S, Ma R, et al. Complement C3 deficiency protects against neurodegeneration in aged plaque‐rich APP/PS1 mice. Sci Transl Med. 2017;9(392):eaaf6295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Elvington A, Atkinson C, Zhu H, et al. The alternative complement pathway propagates inflammation and injury in murine ischemic stroke. J Immunol. 2012;189(9):4640–4647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Kishore U, Gaboriaud C, Waters P, et al. C1q and tumor necrosis factor superfamily: modularity and versatility. Trends Immunol. 2004;25(10):551–561. [DOI] [PubMed] [Google Scholar]
- 184. Ito‐Ishida A, Miyazaki T, Miura E, et al. Presynaptically released Cbln1 induces dynamic axonal structural changes by interacting with GluD2 during cerebellar synapse formation. Neuron. 2012;76(3):549–564. [DOI] [PubMed] [Google Scholar]
- 185. Yuzaki M. Synapse formation and maintenance by C1q family proteins: a new class of secreted synapse organizers. Eur J Neurosci. 2010;32(2):191–197. [DOI] [PubMed] [Google Scholar]
- 186. Chu Y, Jin X, Parada I, et al. Enhanced synaptic connectivity and epilepsy in C1q knockout mice. Proc Natl Acad Sci USA. 2010;107(17):7975–7980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Stokowska A, Atkins AL, Moran J, et al. Complement peptide C3a stimulates neural plasticity after experimental brain ischaemia. Brain. 2017;140(Pt 2):353–369. [DOI] [PubMed] [Google Scholar]
- 188. Skibo GG, Nikonenko AG. Brain plasticity after ischemic episode. Vitam Horm. 2010;82:107–127. [DOI] [PubMed] [Google Scholar]
- 189. Cook DJ, Nguyen C, Chun HN, et al. Hydrogel‐delivered brain‐derived neurotrophic factor promotes tissue repair and recovery after stroke. J Cereb Blood Flow Metab. 2017;37(3):1030–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Carmichael ST, Wei L, Rovainen CM, Woolsey TA. New patterns of intracortical projections after focal cortical stroke. Neurobiol Dis. 2001;8(5):910–922. [DOI] [PubMed] [Google Scholar]
- 191. Brown CE, Aminoltejari K, Erb H, Winship IR, Murphy TH. In vivo voltage‐sensitive dye imaging in adult mice reveals that somatosensory maps lost to stroke are replaced over weeks by new structural and functional circuits with prolonged modes of activation within both the peri‐infarct zone and distant sites. J Neurosci. 2009;29(6):1719–1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Chen D, Wei X, Zou J, et al. Contra‐directional expression of serum homocysteine and uric acid as important biomarkers of multiple system atrophy severity: a cross‐sectional study. Front Cell Neurosci. 2015;9:247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Gleichman AJ, Carmichael ST. Astrocytic therapies for neuronal repair in stroke. Neurosci Lett. 2014;565:47–52. [DOI] [PubMed] [Google Scholar]
- 194. Withers GS, Higgins D, Charette M, Banker G. Bone morphogenetic protein‐7 enhances dendritic growth and receptivity to innervation in cultured hippocampal neurons. Eur J Neurosci. 2000;12(1):106–116. [DOI] [PubMed] [Google Scholar]
- 195. Ren J, Kaplan PL, Charette MF, Speller H, Finklestein SP. Time window of intracisternal osteogenic protein‐1 in enhancing functional recovery after stroke. Neuropharmacology. 2000;39(5):860–865. [DOI] [PubMed] [Google Scholar]
- 196. Galatro TF, Holtman IR, Lerario AM, et al. Transcriptomic analysis of purified human cortical microglia reveals age‐associated changes. Nat Neurosci. 2017;20(8):1162–1171. [DOI] [PubMed] [Google Scholar]
- 197. Manso Y, Holland PR, Kitamura A, et al. Minocycline reduces microgliosis and improves subcortical white matter function in a model of cerebral vascular disease. Glia. 2017;66(1):34–46. [DOI] [PubMed] [Google Scholar]
- 198. Horn KP, Busch SA, Hawthorne AL, van Rooijen N, Silver J. Another barrier to regeneration in the CNS: activated macrophages induce extensive retraction of dystrophic axons through direct physical interactions. J Neurosci. 2008;28(38):9330–9341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Kitayama M, Ueno M, Itakura T, Yamashita T. Activated microglia inhibit axonal growth through RGMa. PloS one. 2011;6(9):e25234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Kwon MJ, Kim J, Shin H, et al. Contribution of macrophages to enhanced regenerative capacity of dorsal root ganglia sensory neurons by conditioning injury. J Neurosci. 2013;33(38):15095–15108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Shechter R, London A, Varol C, et al. Infiltrating blood‐derived macrophages are vital cells playing an anti‐inflammatory role in recovery from spinal cord injury in mice. PLoS Med. 2009;6(7):e1000113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Hilla AM, Diekmann H, Fischer D. Microglia are irrelevant for neuronal degeneration and axon regeneration after acute injury. J Neurosci. 2017;37(25):6113–6124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. de Waegh SM, Lee VM, Brady ST. Local modulation of neurofilament phosphorylation, axonal caliber, and slow axonal transport by myelinating Schwann cells. Cell. 1992;68(3):451–463. [DOI] [PubMed] [Google Scholar]
- 204. Lappe‐Siefke C, Goebbels S, Gravel M, et al. Disruption of Cnp1 uncouples oligodendroglial functions in axonal support and myelination. Nat Genet. 2003;33(3):366–374. [DOI] [PubMed] [Google Scholar]
- 205. Shi H, Hu X, Leak RK, et al. Demyelination as a rational therapeutic target for ischemic or traumatic brain injury. Exp Neurol. 2015;272:17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Khodanovich MY, Kisel AA, Akulov AE, et al. Quantitative assessment of demyelination in ischemic stroke in vivo using macromolecular proton fraction mapping. J Cereb Blood Flow Metab. 2018;38(5):919–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Ding G, Chen J, Chopp M, et al. White matter changes after stroke in type 2 diabetic rats measured by diffusion magnetic resonance imaging. J Cereb Blood Flow Metab. 2017;37(1):241–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Mifsud G, Zammit C, Muscat R, Di Giovanni G, Valentino M. Oligodendrocyte pathophysiology and treatment strategies in cerebral ischemia. CNS Neurosci Ther. 2014;20(7):603–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Zhornitsky S, Wee Yong V, Koch MW, et al. Quetiapine fumarate for the treatment of multiple sclerosis: focus on myelin repair. CNS Neurosci Ther. 2013;19(10):737–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. McMurran CE, Jones CA, Fitzgerald DC, Franklin RJ. CNS remyelination and the innate immune system. Front Cell Dev Biol. 2016;4:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Song FE, Huang JL, Lin SH, Wang S, Ma GF, Tong XP. Roles of NG2‐glia in ischemic stroke. CNS Neurosci Ther. 2017;23(7):547–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Gallo V, Armstrong RC. Myelin repair strategies: a cellular view. Curr Opin Neurol. 2008;21(3):278–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Huang JK, Franklin RJ. Current status of myelin replacement therapies in multiple sclerosis. Prog Brain Res. 2012;201:219–231. [DOI] [PubMed] [Google Scholar]
- 214. Chen S, Zhang H, Pu H, et al. n‐3 PUFA supplementation benefits microglial responses to myelin pathology. Sci Rep. 2014;4:7458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Han L, Cai W, Mao L, et al. Rosiglitazone Promotes white matter integrity and long‐term functional recovery after focal cerebral ischemia. Stroke. 2015;46(9):2628–2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Itoh K, Maki T, Lok J, Arai K. Mechanisms of cell‐cell interaction in oligodendrogenesis and remyelination after stroke. Brain Res. 2015;1623:135–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Ramos‐Cejudo J, Gutierrez‐Fernandez M, Otero‐Ortega L, et al. Brain‐derived neurotrophic factor administration mediated oligodendrocyte differentiation and myelin formation in subcortical ischemic stroke. Stroke. 2015;46(1):221–228. [DOI] [PubMed] [Google Scholar]
- 218. Sozmen EG, Rosenzweig S, Llorente IL, et al. Nogo receptor blockade overcomes remyelination failure after white matter stroke and stimulates functional recovery in aged mice. Proc Natl Acad Sci USA. 2016;113(52):E8453–E8462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Stetler RA, Gao Y, Leak RK, et al. APE1/Ref‐1 facilitates recovery of gray and white matter and neurological function after mild stroke injury. Proc Natl Acad Sci USA. 2016;113(25):E3558–E3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Zhang J, Li Y, Zhang ZG, et al. Bone marrow stromal cells increase oligodendrogenesis after stroke. J Cereb Blood Flow Metab. 2009;29(6):1166–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. He X, Li Y, Lu H, Zhang Z, Wang Y, Yang GY. Netrin‐1 overexpression promotes white matter repairing and remodeling after focal cerebral ischemia in mice. J Cereb Blood Flow Metab. 2013;33(12):1921–1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Letourneau R, Rozniecki JJ, Dimitriadou V, Theoharides TC. Ultrastructural evidence of brain mast cell activation without degranulation in monkey experimental allergic encephalomyelitis. J Neuroimmunol. 2003;145(1–2):18–26. [DOI] [PubMed] [Google Scholar]
- 223. Liu L, Belkadi A, Darnall L, et al. CXCR223‐positive neutrophils are essential for cuprizone‐induced demyelination: relevance to multiple sclerosis. Nat Neurosci. 2010;13(3):319–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Johnson FK, Kaffman A. Early life stress perturbs the function of microglia in the developing rodent brain: New insights and future challenges. Brain Behav Immun. 2017;69:18–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Chanvillard C, Jacolik RF, Infante‐Duarte C, Nayak RC. The role of natural killer cells in multiple sclerosis and their therapeutic implications. Front Immunol. 2013;4:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Kotter MR, Setzu A, Sim FJ, Van Rooijen N, Franklin RJ. Macrophage depletion impairs oligodendrocyte remyelination following lysolecithin‐induced demyelination. Glia. 2001;35(3):204–212. [DOI] [PubMed] [Google Scholar]
- 227. Miron VE, Boyd A, Zhao JW, et al. M2 microglia and macrophages drive oligodendrocyte differentiation during CNS remyelination. Nat Neurosci. 2013;16(9):1211–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Sun D, Yu Z, Fang X, et al. LncRNA GAS5 inhibits microglial M2 polarization and exacerbates demyelination. EMBO Rep. 2017;18(10):1801–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Mangalam AK, Rattan R, Suhail H, et al. AMP‐activated protein kinase suppresses autoimmune central nervous system disease by regulating M1‐type macrophage‐Th17 axis. J Immunol. 2016;197(3):747–760. [DOI] [PubMed] [Google Scholar]
- 230. Hayakawa K, Pham LD, Seo JH, et al. CD200 restrains macrophage attack on oligodendrocyte precursors via toll‐like receptor 4 downregulation. J Cereb Blood Flow Metab. 2016;36(4):781–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Zierath D, Shen A, Stults A, Olmstead T, Becker KJ. Splenectomy does not improve long‐term outcome after stroke. Stroke. 2017;48(2):497–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Wiese S, Karus M, Faissner A. Astrocytes as a source for extracellular matrix molecules and cytokines. Front Pharmacol. 2012;3:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Nash B, Thomson CE, Linington C, et al. Functional duality of astrocytes in myelination. J Neurosci. 2011;31(37):13028–13038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Stoffels JM, de Jonge JC, Stancic M, et al. Fibronectin aggregation in multiple sclerosis lesions impairs remyelination. Brain. 2013;136(Pt 1):116–131. [DOI] [PubMed] [Google Scholar]
- 235. Camargo N, Goudriaan A, van Deijk AF, et al. Oligodendroglial myelination requires astrocyte‐derived lipids. PLoS Biol. 2017;15(5):e1002605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Jing L, He Q, Zhang JZ, Li PA. Temporal profile of astrocytes and changes of oligodendrocyte‐based myelin following middle cerebral artery occlusion in diabetic and non‐diabetic rats. Int J Biol Sci. 2013;9(2):190–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Wang Z, Guo W, Liu Y, et al. Low expression of complement inhibitory protein CD59 contributes to humoral autoimmunity against astrocytes. Brain Behav Immun. 2017;65:173–182. [DOI] [PubMed] [Google Scholar]
- 238. Ponath G, Ramanan S, Mubarak M, et al. Myelin phagocytosis by astrocytes after myelin damage promotes lesion pathology. Brain. 2017;140(Pt 2):399–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Moore CS, Abdullah SL, Brown A, Arulpragasam A, Crocker SJ. How factors secreted from astrocytes impact myelin repair. J Neurosci Res. 2011;89(1):13–21. [DOI] [PubMed] [Google Scholar]
- 240. Back SA, Tuohy TM, Chen H, et al. Hyaluronan accumulates in demyelinated lesions and inhibits oligodendrocyte progenitor maturation. Nat Med. 2005;11(9):966–972. [DOI] [PubMed] [Google Scholar]
- 241. Sosunov AA, Guilfoyle E, Wu X, McKhann GM 2nd, Goldman JE. Phenotypic conversions of "protoplasmic" to "reactive" astrocytes in Alexander disease. J Neurosci. 2013;33(17):7439–7450. [DOI] [PMC free article] [PubMed] [Google Scholar]


