Abstract
Background
Hypothyroidism has been known to be associated with hyperlipidemia, endothelial dysfunction and atherosclerosis. Elevation of thyroid‐stimulation hormone (TSH) is a gold standard to detect these conditions. However, no large studies have investigated the association between TSH elevation and long‐term clinical outcomes in patients with acute myocardial infarction (AMI).
Hypothesis
Hypothyroidism is associated with higher mortality in patients with AMI.
Methods
A total of 4748 AMI patients undergoing percutaneous coronary intervention (PCI) with drug‐eluting stents were consecutively enrolled. We analyzed 1977 patients whose thyroid function data available after the exclusion of hyperthyroidism and possible central hypothyroidism. Patients were divided into two groups; euthyroid group (n = 1846) with normal TSH and normal free thyroxine (FT4); hypothyroidism group (n = 131) with elevated TSH and normal or low FT4. The two groups were subsequently compared with their all‐cause and cardiac mortalities.
Results
Median follow‐up duration was 3.5 years. Hypothyroidism group were older, included in more females, and had higher incidences of atrial fibrillation, stroke, and renal dysfunction. Elevated TSH was associated with significantly higher all‐cause mortality (26.0% vs 11.7%, P < 0.0001) and cardiac mortality (9.2% vs 4.6%, P = 0.014). The multivariate Cox proportional hazards model identified that TSH elevation was a significant predictor of all‐cause mortality (adjusted hazard ratio 1.560, 95% confidence interval 1.017 to 2.392, P = 0.041).
Conclusions
Our data suggest that AMI patients with TSH elevation had worse clinical outcome. Moreover, TSH elevation was a predictor of all‐cause mortality in patients with AMI.
Keywords: acute myocardial infarction, hypothyroidism, thyroid stimulation hormone
1. INTRODUCTION
Alteration of thyroid function may adversely affect the heart and cardiovascular system, which has a direct effect on the cardiovascular disease and is caused by a complex relationship with risk factors for the cardiovascular disease.1, 2 Hyperthyroidism is associated with systolic hypertension, atrial fibrillation, and hypercoagulability, while hypothyroidism can lead to hyperlipidemia and vascular inflammation.3, 4, 5, 6, 7
In several clinical studies, subclinical hypothyroidism (SH), defined by elevated thyroid‐stimulating hormone (TSH) levels and normal serum free thyroxine (FT4) levels, has also been associated with dyslipidemia, hypertension, impaired renal function, accelerated atherosclerosis and coronary artery disease.8, 9, 10
Although there is a study of thyroid hormonal change in the early period of acute myocardial infarction (AMI) and thyroid hormone has been recently reported to play an important role during cardiac remodeling after AMI,11, 12 research on the clinical outcome of AMI according to thyroid function is lacking. Accordingly, we sought to examine the association between hypothyroidism and clinical outcomes in patients who treated with drug‐eluting stent (DES) implantation for AMI.
2. METHODS
2.1. Study population and COREA‐AMI registry
The convergent REgistry of cAtholic and chonnAm university for AMI) (COREA‐AMI) is a retrospective multicenter registry of demographic, clinical, and procedural data, and has long‐term clinical outcome of all AMI patients underwent percutaneous coronary intervention (PCI) with the use of DES from nine major cardiovascular centers in Korea between January 2004 and December 2009. All participated hospitals are located throughout the country, and they all have performed high‐volume PCI more than 500 cases per year. There was no industry involvement in the design, conduct, or analysis of the study. The study protocol was approved by institutional review boards at each participating institution. This registry was registered on ClinicalTrial.gov (study ID: NCT02385682).
For the present study, 2693 out of total 4748 registered patients were able to identify TSH and FT4 level. Of these, patients except hyperthyroidism, subclinical hyperthyroidism, and possible central hypothyroidism were divided into normal and elevated TSH groups. The two groups were subsequently compared with respect to their all‐cause and cardiac mortalities.
2.2. Assessment and definition of thyroid function
Blood samples were obtained at the time of their arrival in the hospital prior to PCI and were used to perform the standard battery of hematological and biochemical tests. The serum TSH and FT4 levels were measured by enzyme immunoassay using a commercially available kit (ADVIA Centaur XP (Seimens, Washington, DC, USA). The reference ranges of TSH and FT4 were 0.35 to 5.50 mIU/L and 0.93 to 1.70 ng/dL, respectively.
Overt hypothyroidism (OH) was defined as a documented history of OH in the patients' clinical record or elevated TSH (>5.50 mIU/L) and low FT4 (<0.93 ng/dL) levels. SH was defined as a documented history of SH in the patients' clinical record or elevated TSH and normal FT4 levels. Euthyroidism (ET) was defined as no documented history of hypothyroidism in the patients' clinical record and/or normal TSH and FT4 levels. In this study, hypothyroidism refers to the combination of SH and OH.
2.3. Percutaneous coronary intervention procedure and medical treatment
Before the PCI, all patients received loading doses of dual‐antiplatelet agents including aspirin with 250 to 500 mg and clopidogrel with 600 mg. Newer antiplatelet agents, such as prasugrel, ticagrelor were not available during the period in which this study was conducted. The procedure was performed through femoral or radial artery after administration of unfractionated heparin (100 U/kg). During the procedure, patients received unfractionated heparin to maintain an activated clotting time between 250 and 300 seconds. A glycoprotein IIb/IIIa inhibitor was administered at the discretion of a physician. The choice of stent, pre‐stenting balloon dilatation, post‐stenting adjuvant balloon inflation, and the use of glycoprotein IIb/IIIa inhibitors was at each physician's discretion. After the procedure, antiplatelet therapy consists of using 100 mg of aspirin and 75 mg of clopidogrel for 1 year and then using only one.
2.4. Study definitions and clinical follow‐up
The records of cardiovascular risk factors, past history, and laboratory findings were mainly dependent on patients' medical record. All‐cause mortality was considered to be cardiac deaths after the exclusion of noncardiac mortality. Cardiac mortality was caused by myocardial infarction, heart failure, and arrhythmia including sudden cardiac death.
The clinical, angiographic, procedural or operative, and outcome data were collected in the dedicated PCI and surgical databases by independent research personnels. All the outcomes of interest were confirmed by source document and they were centrally adjudicated by a local events committee of the Cardiovascular Center of Seoul St. Mary's Hospital, Seoul, Korea, whose members were unaware of patients' status. For validation of complete follow‐up data, information on censored survival data was obtained through 31 July 2013 from a telephone interview with the corresponding patients and also from the National Population Registry of the Korea National Statistical Office with the use of unique personal identification number.
2.5. Statistical analysis
Continuous variables were expressed as mean ± SD and compared with the Student's t test or the Mann‐Whitney U test. Discrete variables were expressed as percentages and compared with the χ 2 test or Fisher's exact test. A multivariable Cox regression analysis (after confirming the appropriateness of the proportional hazards assumption) was carried out to identify independent predictors for all‐cause and cardiac mortalities. Variables which were evaluated in the multivariable Cox regression analysis included using those with a statistical P value less than <0.05 in the baseline characteristics (Table 1) and also those without statistical significance, but with prognostic impact demonstrated in previous studies. The effect of each variable in developing models was assessed using the Wald test and described as hazard ratios (HR) with 95% confidence intervals (CI). The cumulative survival was estimated by the Kaplan‐Meier survival curves, and compared using the log‐rank tests. All analyses were two‐tailed, with clinical significance defined as values of P < 0.05. All statistical analyses were done with Statistical Analysis Software package (SAS version 9.1, SAS Institute, Cary, North Carolina).
Table 1.
Baseline patient demographic, clinical, angiographic, and procedural data according to TSH level
| Variables | Normal TSH (n = 1846) | Elevated TSH (n = 131) | P value |
|---|---|---|---|
| Demographics | |||
| Age (year) | 61.4 ± 12.8 | 67.8 ± 11.1 | <0.001 |
| Age ≥ 65 years | 934 (50.6) | 100 (76.3) | <0.001 |
| Female gender | 471 (22.5) | 59 (45.0) | <0.001 |
| Risk factors | |||
| BMI (kg/m2) | 24.3 ± 3.2 | 23.8 ± 3.2 | 0.101 |
| Diabetes mellitus | 628 (34.0) | 45 (34.4) | 0.938 |
| Hypertension | 935 (50.7) | 74 (56.5) | 0.196 |
| Atrial fibrillation | 71 (3.9) | 17 (13.0) | <0.001 |
| Current smoking | 781 (42.3) | 50 (38.2) | 0.354 |
| Family history of CAD | 119 (6.4) | 3 (2.3) | 0.056 |
| Prior history of stroke | 117 (6.3) | 16 (12.2) | 0.009 |
| Prior history of myocardial infarction | 40 (3.8) | 8 (6.1) | 0.188 |
| Prior history of PCI | 60 (3.3) | 7 (5.3) | 0.206 |
| Prior history of CABG | 30 (1.6) | 3 (2.3) | 0.478 |
| Hemodynamic status | |||
| Initial systolic blood pressure (mmHg) | 129.1 ± 30.5 | 126.5 ± 30.4 | 0.350 |
| Initial diastolic blood pressure (mmHg) | 78.4 ± 18.8 | 77.1 ± 19.1 | 0.470 |
| Initial heart rate | 76.6 ± 20.1 | 78.3 ± 21.2 | 0.337 |
| Killip classes II‐IV | 342 (18.5) | 27 (20.6) | 0.558 |
| Primary diagnosis at admission | 0.001 | ||
| STEMI | 1175 (63.7) | 65 (49.6) | |
| NSTEMI | 671 (36.3) | 66 (50.4) | |
| Discharge medication | |||
| Statin | 1708/1802 (94.8) | 113/123 (91.9) | 0.167 |
| Beta‐blocker | 1301/1802 (72.2) | 89/123 (72.4) | 0.977 |
| ACEI/ARB | 1372/1802 (76.1) | 98/123 (79.7) | 0.372 |
| Laboratory data | |||
| LVEF (%) | 53.3 ± 11.0 | 51.6 ± 11.4 | 0.103 |
| LVEF <40% | 191/1781 (10.7) | 20/121 (16.5) | 0.049 |
| FT4 | 1.19 ± 0.20 | 1.08 ± 0.29 | <0.001 |
| TSH | 1.28 ± 0.88 | 11.52 ± 33.42 | 0.001 |
| Glucose (mg/dL) | 170.1 ± 79.2 | 175.2 ± 81.5 | 0.511 |
| Creatinine (mg/dL) | 1.15 ± 0.88 | 1.19 ±0.66 | 0.468 |
| eGFR, mL/min/1.73 m2 | 76.6 ± 26.4 | 68.7 ± 27.8 | 0.001 |
| eGFR <60 mL/min/1.73 m2 | 400 (21.7) | 53 (40.5) | <0.001 |
| Hs‐CRP (mg/L) | 2.20 ± 4.04 | 1.72 ± 2.70 | <0.001 |
| CK‐MB (ng/mL) | 114.8 ± 144.4 | 96.0 ± 142.1 | 0.150 |
| Troponin‐I (ng/mL) | 26.8 ± 42.7 | 21.1 ± 33.2 | 0.563 |
| Total cholesterol (mg/dL) | 180.9 ± 40.3 | 181.1 ± 32.8 | 0.960 |
| Triglycerides (mg/dL) | 123.5 ± 88.6 | 133.5 ± 123.0 | 0.239 |
| HDL cholesterol (mg/dL) | 41.6 ± 10.1 | 41.4 ± 11.6 | 0.848 |
| LDL cholesterol (mg/dL) | 114.8 ± 35.0 | 112.6 ± 37.0 | 0.529 |
| Angiographic and procedural data | |||
| Glycoprotein IIb‐IIIa inhibitor | 93 (5.0) | 8 (6.1) | 0.591 |
| IABP | 47 (2.5) | 3 (2.3) | 1.000 |
| ECMO | 2 (0.1) | 0 (0) | 1.000 |
| Culprit vessel, n (%) | 0.605 | ||
| Left main | 47 (2.5) | 4 (3.1) | |
| Left anterior descending artery | 894 (48.4) | 68 (51.9) | |
| Left circumflex artery | 291 (15.8) | 20 (15.3) | |
| Right coronary artery | 614 (33.3) | 39 (29.8) | |
| Involved vessels, n (%) | 0.114 | ||
| One vessel | 833 (45.1) | 64 (48.9) | |
| Two vessels | 593 (32.1) | 31 (23.7) | |
| Three vessels | 420 (22.8) | 36 (27.5) | |
| Pre‐PCI TIMI flow grade 0, n (%) | 741 (43.2) | 49 (42.2) | 0.839 |
| Post‐PCI TIMI flow grade III, n (%) | 1583 (88.8) | 107 (84.3) | 0.118 |
Abbreviations: ACEI/ARB, angiotensin‐converting enzyme inhibitor/angiotensin II receptor blocker; AMI, acute myocardial infarction; BMI, body mass index; CABG, coronary artery bypass graft; CAD, coronary artery disease; CK‐MB, creatine kinase‐MB fraction; ECMO, extracorporeal membrane oxygenator; eGFR, estimated glomerular filtration rate; FT4, free thyroxine; HDL, high‐density lipoprotein; Hs‐CRP, high‐sensitivity C‐reactive protein; IABP, intra‐aortic balloon pulsation; LDL, low‐density lipoprotein; LVEF, left ventricular ejection fraction; NSTEMI, non‐ST‐segment elevation myocardial infarction; PCI, percutaneous coronary intervention; STEMI,ST‐segment elevation myocardial infarction; TIMI, thrombolysis in myocardial infarction; TSH, thyroid‐stimulating hormone.
Data are presented as the mean ± standard deviation or n (%).
3. RESULTS
3.1. Characteristics of the study populations
The study flowchart was briefly presented in Figure 1. Among 4748 patients registered, we selected 1977 subjects could be analyzed serum TSH and FT4 levels. All patients were divided into two groups according to TSH level; a normal TSH group (n = 1846) and an elevated TSH group (n = 131) composed of SH (n = 106) and OH (n = 25).
Figure 1.
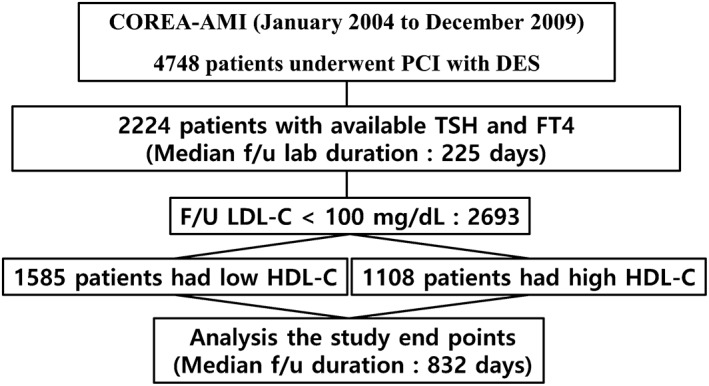
Study flow chart. COREA‐AMI, Convergent REgistry of cAtholic and chonnAm university for acute myocardial infarction; DES, drug‐eluting stent; FT4, free thyroxine; TSH, thyroid‐stimulating hormone
Baseline demographic, clinical, laboratory, angiographic, and procedural characteristics between the two groups are shown in Tables 1. Elevated TSH group were older, and had more female gender, higher prevalence of AF (atrial fibrillation), and old stroke. These patients with elevated TSH were more likely to have lower estimated glomerular filtration rate (eGFR) and high‐sensitivity C‐reactive protein (hs‐CRP), and higher presentation of non‐ST‐segment elevation myocardial infarction (NSTEMI). Lipid profiles, hemodynamic status, peak cardiac enzyme, and angiographic findings were not different between the two groups.
3.2. Clinical outcomes for the study populations
The median duration of follow‐up period was 1281 days. Complete follow‐up data for clinical outcomes were obtained in 100% of the overall cohort for the duration of this study.
In the elevated TSH group, all‐cause mortality occurred in a total of 34 (26.0%) while in the normal TSH group, 216 (11.7%) during long‐term follow‐up. The incidence of cardiac mortality and in‐hospital mortality were significantly higher in patients with elevated TSH. Based on analysis of the study population, the elevated TSH showed significant association with all‐cause mortality (unadjusted HR 2.44, 95% CI 1.70 to 3.50, P < 0.001) (Table 2). However, there were no differences between two groups in terms of nonfatal stroke, nonfatal myocardial infarction, and revascularization.
Table 2.
Clinical events in patients with normal thyroid‐stimulating hormone compared with elevated thyroid‐stimulating hormone
| Normal TSH (n = 1846) | Elevated TSH (n = 131) | Unadjusted HR (95% CI) | P value | |
|---|---|---|---|---|
| All‐cause mortality | 216 (11.7) | 34 (26.0) | 2.44 (1.70‐3.50) | <0.001 |
| Cardiac mortality | 84 (4.6) | 12 (9.2) | 2.10 (1.15‐3.85) | 0.016 |
| In‐hospital mortality | 44 (2.4) | 8 (6.1) | 2.63 (1.24‐5.58) | 0.012 |
| Nonfatal stroke | 38 (2.1) | 5 (3.8) | 1.98 (0.78‐5.03) | 0.151 |
| Nonfatal myocardial infarction | 35 (1.9) | 1 (0.8) | 0.43 (0.06‐3.13) | 0.404 |
| Revascularization | 259 (14.0) | 15 (11.5) | 0.85(0.51‐1.44) | 0.549 |
CI, confidence interval; HR , hazard ratio; TSH, thyroid‐stimulating hormone.
Because the study population was relatively small and difference of sample size between the two groups was large, multivariate Cox regression was performed in several models (Table 3). The elevated TSH group had a significant association with all‐cause mortality in model 1 through 5, but not in model 6 with left ventricular ejection fraction (LVEF) added.
Table 3.
Multivariate Cox proportional hazard models of elevated thyroid‐stimulating hormone for all‐cause mortality
| HR (95% CI) | P value | |
|---|---|---|
| Model 1: Age, gender | 1.856 (1.289‐2.672) | 0.001 |
| Model 2: Model 1 + AF, DM, HTN, smoking, BMI, STEMI | 1.681 (1.140‐2.479) | 0.009 |
| Model 3: model 2 + Hs‐CRP, CK‐MB | 1.795 (1.172‐2.748) | 0.007 |
| Model 4: model 3 + eGFR | 1.560 (1.017‐2.392) | 0.041 |
| Model 5: model 3 + angiographic findingsa | 1.779 (1.157‐2.735) | 0.009 |
| Model 6: Model 3 + LVEF | 1.358 (0.845‐2.182) | 0.206 |
AF, atrial fibrillation; BMI, body mass index; CI, confidence interval; CK‐MB, creatine kinase‐MB fraction; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; Hs‐CRP, high‐sensitivity C‐reactive protein; HR, hazard ratio; HTN, hypertension; LVEF, left ventricular ejection fraction; STEMI, ST‐segment elevation myocardial infarction; TSH, thyroid‐stimulating hormone.
Multivessel disease, post‐PCI TIMI flow grade, and LAD culprit vessel.
The Kaplan‐Meier survival curves (Figure 2) showed that elevated TSH showed significantly worse outcomes than normal TSH as determined by the log‐rank test; all‐cause mortality and cardiac mortality (P = <0.001 and P = 0.014, respectively). This survival curve shows that the survival difference becomes even bigger depending on the time.
Figure 2.
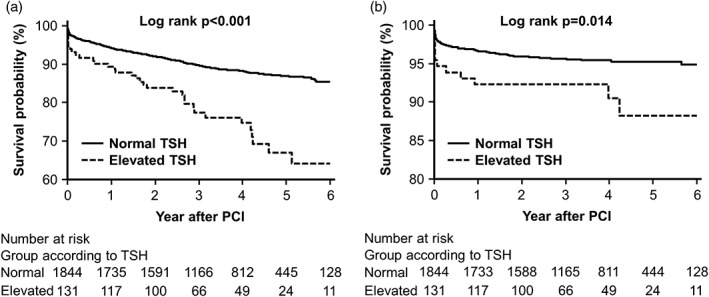
Kaplan‐Meier curves. A, All‐cause mortality. B, Cardiac mortality
In Supporting Information Tables S1 and S2, the elevated TSH group was divided by SH and OH and compared with normal TSH. OH and SH had more incidence rates of all‐cause mortality than ET. SH compared to ET had significant value only in model 1 when adjusted Cox regression was carried out. However, OH had significant values in all six models.
3.3. Subgroup analysis
We calculated the unadjusted HR for all‐cause mortality in various subgroups (Supporting InformationFigure S1). The incidence of the all‐cause mortality was higher in the elevated TSH group than in the normal TSH group in all subgroups, although statistical significance was not found in patients with young age, NSTEMI presentation, eGFR ≥ 60 mL/min/1.73 m2, and LVEF <40%. There were no significant interactions between the TSH level and all‐cause mortality among the subgroups.
4. DISCUSSION
The present study demonstrated that TSH elevation was associated with an increase in all‐cause mortality compared to normal TSH in patients with AMI and may be a risk factor for all‐cause mortality after adjusting various factors. In particular, OH is a significant independent prognostic factor for all‐cause mortality even after all other confounding factors, including risk factors of coronary artery disease, laboratory finding, angiographic finding, and LVEF, have been taken into account. To our knowledge, this study is the first data which show the clinical impact of thyroid function in patients with AMI.
In a meta‐analysis of 55 cohort studies, hypothyroidism is associated with higher risks of cardiac mortality and all‐cause mortality compared with ET in both the general public and cardiac patients.13 Recent study demonstrated that hypothyroidism was an independent predictor of cardiovascular and cerebral events in patients who received PCI; moreover, it highlighted that adequate thyroid replacement treatment could prevent cardiovascular events.14 Another study analyzed that SH is associated with worse clinical events including cardiac mortality and repeat revascularization among the majority of patients with acute coronary syndrome who receive PCI.15 In the present study, OH was significantly associated with mortality and SH was numerically associated with mortality even in AMI patients alike other studies. And mortality was more frequently occurred in OH compared with SH.
There is a small study that reported that improvements in thyroid hormone were associated with improved cardiac function after AMI. In this study, the thyroid hormone tends to decrease in acute phase of AMI.12 This is thought to be an adaptation to acute stress, and thyroid hormone is associated with cardiac contractile function, which suggests that thyroid hormone improvement may be associated with improved cardiac function. In animal models, hypothyroidism induced further increases in cardiac remodeling and replacement of thyroid hormone after AMI was associated with improved cardiac function.16, 17, 18, 19, 20 The increase in carotid intima‐media thickness, a surrogate cardiovascular marker, is associated with hypothyroidism, and the endothelial function assessed by brachial artery flow‐mediated dilatation has improved after the improvement of thyroid hormone, accounting for the increase in cardiovascular disease due to hypothyroidism.21, 22 Changes in coagulation and fibrinolytic cascades may also be associated with stroke because of hypothyroidism.23 Because hypothyroidism is associated with an increase in atherosclerosis, cardiovascular events are considered to be more frequent. However, in this study, elevated TSH was not related with AMI, revascularization and stoke. The cause of these results may be that many patients with SH were included in the present study. Nevertheless, elevated TSH was related with increased mortality in this study. To clarify the relationship between elevated TSH and major adverse cardiovascular and cerebral events, the bigger trial is needed.
Subclinical hypothyroidism, unlike OH, has conflicting results in the occurrence of CHD events, mortality, and HF, and there is a debate in relation to cardiovascular events.9, 24, 25, 26 In the present study, SH, like OH, had more mortality than ET. But SH has lower predictive power than OH after adjustment of other variables. However, there are limitations to this result because of the small number of SH.
Our study has some limitations. First, our findings are subject to selection bias and confounding factors because of its nonrandomized, observational design. Here is a lot of difference between the numbers of participants in both groups. However, to overcome this limitation, we performed rigorous adjustments of our data to reduce the impact of selection bias using six multivariate Cox proportion hazards regression models. Second, we measured the thyroid hormone level only once before the index PCI without tracking follow‐up thyroid function test and information of treatment of impaired thyroid function, so there was no correlation between changes in thyroid function and clinical events. Detailed follow‐up thyroid function may be helpful in further interpreting our findings. Third, investigation of various factors affecting thyroid function including diet and alcohol use was not sufficient. Finally, we could not identify other noncardiac causes of mortality, such as malignancy or infection which may be competing risk against cardiac mortality and should also be considered.
5. CONCLUSIONS
Thyroid‐stimulation hormone elevation was a predictor of all‐cause mortality in patients with AMI. Thyroid function in patients with AMI is associated with prognosis and should be checked.
CONFLICTS OF INTEREST
The authors have no conflicts of interest to report.
Supporting information
Table S1. Association the thyroid function and all‐cause mortality or cardiac mortality
Table S2. Multivariate Cox proportional hazard models of thyroid function for all‐cause mortality
Figure S1. Comparative unadjusted hazard ratios of all‐cause mortality for subgroups
Seo SM, Koh Y‐S, Park H‐J, et al. Thyroid stimulating hormone elevation as a predictor of long‐term mortality in patients with acute myocardial infarction. Clin Cardiol. 2018;41:1367–1373. 10.1002/clc.23062
REFERENCES
- 1. Klein I, Danzi S. Thyroid disease and the heart. Circulation. 2007;116:1725‐1735. [DOI] [PubMed] [Google Scholar]
- 2. Lamprou V, Varvarousis D, Polytarchou K, et al. The role of thyroid hormones in acute coronary syndromes: prognostic value of alterations in thyroid hormones. Clin Cardiol. 2017;40:528‐533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Prisant LM, Gujral JS, Mulloy AL. Hyperthyroidism: a secondary cause of isolated systolic hypertension. J Clin Hypertens (Greenwich). 2006;8:596‐599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stuijver DJ, van Zaane B, Romualdi E, et al. The effect of hyperthyroidism on procoagulant, anticoagulant and fibrinolytic factors: a systematic review and meta‐analysis. Thromb Haemost. 2012;108:1077‐1088. [DOI] [PubMed] [Google Scholar]
- 5. Frost L, Vestergaard P, Mosekilde L. Hyperthyroidism and risk of atrial fibrillation or flutter: a population‐based study. Arch Intern Med. 2004;164:1675‐1678. [DOI] [PubMed] [Google Scholar]
- 6. Duntas LH. Thyroid disease and lipids. Thyroid. 2002;12:287‐293. [DOI] [PubMed] [Google Scholar]
- 7. Cappola AR, Ladenson PW. Hypothyroidism and atherosclerosis. J Clin Endocrinol Metab. 2003;88:2438‐2444. [DOI] [PubMed] [Google Scholar]
- 8. Collet TH, Gussekloo J, Bauer DC, et al. Subclinical hyperthyroidism and the risk of coronary heart disease and mortality. Arch Intern Med. 2012;172:799‐809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rodondi N, den Elzen WPJ, Bauer DC, et al. Subclinical hypothyroidism and the risk of coronary heart disease and mortality. JAMA. 2010;304:1365‐1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chang YC, Chang CH, Yeh YC, et al. Subclinical and overt hypothyroidism is associated with reduced glomerular filtration rate and proteinuria: a large cross‐sectional population study. Sci Rep. 2018;8:2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Friberg L, Werner S, Eggertsen G, et al. Rapid down‐regulation of thyroid hormones in acute myocardial infarction: is it cardioprotective in patients with angina? Arch Intern Med. 2002;162:1388‐1394. [DOI] [PubMed] [Google Scholar]
- 12. Lymvaios I, Mourouzis I, Cokkinos DV, et al. Thyroid hormone and recovery of cardiac function in patients with acute myocardial infarction: a strong association? Eur J Endocrinol. 2011;165:107‐114. [DOI] [PubMed] [Google Scholar]
- 13. Ning Y, Cheng YJ, Liu LJ, et al. What is the association of hypothyroidism with risks of cardiovascular events and mortality? A meta‐analysis of 55 cohort studies involving 1,898,314 participants. BMC Med. 2017;15:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhang M, Sara JDS, Matsuzawa Y, et al. Clinical outcomes of patients with hypothyroidism undergoing percutaneous coronary intervention. Eur Heart J. 2016;37:2055‐2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lee Y, Lim YH, Shin JH, et al. Impact of subclinical hypothyroidism on clinical outcomes following percutaneous coronary intervention. Int J Cardiol. 2018;253:155‐160. [DOI] [PubMed] [Google Scholar]
- 16. Chen YF, Redetzke RA, Said S, et al. Changes in left ventricular function and remodeling after myocardial infarction in hypothyroid rats. Am J Physiol Heart Circ Physiol. 2010;298:H259‐H262. [DOI] [PubMed] [Google Scholar]
- 17. Pantos C, Mourouzis I, Tsagoulis N, et al. Thyroid hormone at supra‐physiological dose optimizes cardiac geometry and improves cardiac function in rats with old myocardial infarction. J Physiol Pharmacol. 2009;60:49‐56. [PubMed] [Google Scholar]
- 18. Chen YF, Kobayashi S, Chen J, et al. Short term triiodo‐l‐thyronine treatment inhibits cardiac myocyte apoptosis in border area after myocardial infarction in rats. J Mol Cell Cardiol. 2008;44:180‐187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Forini F, Lionetti V, Ardehali H, et al. Early long‐term L‐T3 replacement rescues mitochondria and prevents ischemic cardiac remodeling in rats. J Cell Mol Med. 2011;15:514‐524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Henderson KK, Danzi S, Paul JT, et al. Physiological replacement of T3 improves left ventricular function in an animal model of myocardial infarction‐induced congestive heart failure. Circ Heart Fail. 2009;2:243‐252. [DOI] [PubMed] [Google Scholar]
- 21. Kim SK, Kim SH, Park KS, et al. Regression of the increased common carotid artery‐intima media thickness in subclinical hypothyroidism after thyroid hormone replacement. Endocr J. 2009;56:753‐758. [DOI] [PubMed] [Google Scholar]
- 22. Razvi S, Ingoe L, Keeka G, et al. The beneficial effect of L‐thyroxine on cardiovascular risk factors, endothelial function, and quality of life in subclinical hypothyroidism: randomized, crossover trial. J Clin Endocrinol Metab. 2007;92:1715‐1723. [DOI] [PubMed] [Google Scholar]
- 23. Chaker L, Baumgartner C, den Elzen WP, et al. Subclinical hypothyroidism and the risk of stroke events and fatal stroke: an individual participant data analysis. J Clin Endocrinol Metab. 2015;100:2181‐2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cappola AR, Fried LP, Arnold AM, et al. Thyroid status, cardiovascular risk, and mortality in older adults. JAMA. 2006;295:1033‐1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Iervasi G, Molinaro S, Landi P, et al. Association between increased mortality and mild thyroid dysfunction in cardiac patients. Arch Intern Med. 2007;167:1526‐1532. [DOI] [PubMed] [Google Scholar]
- 26. Rodondi N, Newman AB, Vittinghoff E, et al. Subclinical hypothyroidism and the risk of heart failure, other cardiovascular events, and death. Arch Intern Med. 2005;165:2460‐2466. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Association the thyroid function and all‐cause mortality or cardiac mortality
Table S2. Multivariate Cox proportional hazard models of thyroid function for all‐cause mortality
Figure S1. Comparative unadjusted hazard ratios of all‐cause mortality for subgroups


