Abstract
Background
Sodium‐glucose linked transporter 2 inhibition recently emerged as a promising therapy for reducing the risk of heart failure (HF) in patients with type 2 diabetes mellitus (T2DM). However, there is a lack of data endorsing its role in symptomatic HF patients. We sought to evaluate the short‐term effects of empagliflozin on maximal exercise capacity in these patients.
Hypothesis
We postulate tretament with empagliflozin may improve functional capacity in patients with T2DM and established HF.
Methods
Nineteen T2DM patients with symptomatic HF were prospectively included and underwent cardiopulmonary exercise testing before and 30 days after initiation of empagliflozin therapy. A mixed‐effects model for repeated measures was used.
Results
Median patient age was 72 years (interquartile range, 60–79 years); 42.1% were in New York Heart Association class III. Baseline mean (± SD) peak oxygen consumption (peak VO2) was 10.9 ± 4.0 mL/min/kg. Peak VO2 increased significantly at 30 days (∆: +1.21 [0.66 to 1.76] mL/min/kg; P < 0.001). A significant improvement in ventilatory efficiency during exercise, 6‐minute walking distance, and quality of life, and a reduction in antigen carbohydrate 125, were also found. Estimated glomerular filtration rate and natriuretic peptides did not significantly change.
Conclusions
In this pilot study, empagliflozin was associated with 1‐month improvement in exercise capacity in T2DM patients with symptomatic HF. This beneficial effect was also found for other surrogates of severity.
Keywords: Empagliflozin, Exercise Capacity, Heart Failure
1. INTRODUCTION
Sodium‐glucose linked transporter 2 (SGLT2) inhibition has recently emerged as a promising therapy in patients with coexisting type 2 diabetes mellitus (T2DM) and heart failure (HF).1, 2 Indeed, in patients with T2DM and high cardiovascular risk, the Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes (EMPA‐REG OUTCOME) trial1 showed that treatment with empagliflozin resulted in a 35% reduction in the risk of hospitalizations for HF compared with placebo, with curves diverging early during follow‐up. However, to date, there are insufficient data endorsing the clinical role of empagliflozin or other SGLT2 inhibitors in patients with T2DM and symptomatic HF.
In this work, we sought to evaluate the early short‐term effects of empagliflozin on maximal exercise capacity evaluated by peak oxygen uptake (peak VO2) in T2DM patients with symptomatic HF. Secondary endpoints included changes in ventilatory efficiency during exercise (VE/VCO2 slope), distance walked in 6‐minute walking test (6MWT), quality of life (QoL), and cardiac/renal biomarkers.
2. METHODS
2.1. Study design and patients
Patients were prospectively enrolled from March 1, 2017, to July 1, 2017, at a structured HF clinic in a tertiary center in Spain. The inclusion criteria were (1) an established diagnosis of T2DM; (2) diagnosis of HF evidenced by structural or functional abnormality of the heart at rest, defined as systolic left ventricular (LV) dysfunction (left ventricular ejection fraction [LVEF] <40%), or LV hypertrophy (defined as septum or LV posterior wall thickness ≥ 12 mm or LV mass index >104 g/m2 in women or 116 g/m2 in men), or E/e' ratio > 15, or significant valvular heart disease (moderate to severe); (3) plasma N‐terminal pro brain natriuretic peptide (NT‐proBNP) >125 pg/mL; (4) previous history of symptomatic chronic HF of New York Heart Association (NYHA) functional class ≥II with optimal medical treatment and clinical stability, without hospital admission or decompensation in the past 3 months; (5) ability to perform a valid baseline exercise test; and (6) signs of fluid overload despite treatment with loop diuretics, including the presence of ≥1 of the following: peripheral edema, jugular ingurgitation, crackles, or elevated values of carbohydrate antigen‐125 (CA125), defined as >35 U/mL. Exclusion criteria were (1) inability to perform a cardiopulmonary exercise test (CPET), and (2) estimated glomerular filtration rate (eGFR) <30 mL/min/1.73 m2 at baseline.
Eligible patients received treatment with empagliflozin at 10 mg/d without changes in concomitant medications. All patients provided informed consent, and the protocol was approved by the research ethics committee in accordance with the principles of the Declaration of Helsinki and national regulations.
2.2. Procedures
At each visit (baseline assessment and after 30‐day initiation of empagliflozin) we registered demographic information, medical history, vital signs, 12‐lead electrocardiogram, CPET, QoL (through the Minnesota Living with Heart Failure Questionnaire [MLHF]), standard laboratory data, and pharmacological treatments. During the study period, the dose of empagliflozin was 10 mg in all patients. By protocol, no treatment changes occurred between the 2 visits.
Maximal functional capacity was evaluated with incremental and symptom‐limited CPET (Metamax 3B; Cortex, Leipzig, Germany) on a bicycle ergometer, beginning with a workload of 10 W and increasing stepwise at 10‐W increments every 1 minute. Gas‐exchange data and cardiopulmonary variables were averaged every 10 seconds. Peak VO2 was considered the highest value of VO2 during the last 20 seconds of exercise. The VE/VCO2 slope was determined by measuring the slope across the entire course of exercise.
2.3. Endpoints
The primary endpoint of the study was the comparison of peak VO2 changes at baseline and at 30 days (Δ‐peak VO2). Secondary endpoints included 30‐day changes in VE/VCO2 slope (Δ‐VE/VCO2), 6MWT (Δ‐6MWT), MLHF (Δ‐MLHF), NT‐proBNP (Δ‐NT‐proBNP), CA125 (Δ‐CA125), and eGFR (Δ‐eGFR).
2.4. Statistical analysis
Continuous variables were expressed as mean ± SD or median (interquartile range [IQR]), as appropriate; discrete variables are expressed as numbers and percentages. In an ANCOVA design, changes in the exposures were tested with a mixed‐effects model for repeated measures. The model included as covariates the baseline value of the exposure. A 2‐sided P value of <0.05 was set as the criterion for statistical significance.
3. RESULTS
A total of 54 consecutive T2DM patients with HF were screened for eligibility, and 19 were finally included in this study. Main reasons for ineligibility were no signs of fluid overload (n = 15), eGFR <30 mL/min/1.73 m2 (n = 8), and orthopedic/neurological inability to perform a valid CPET (n = 8).
The median age of the patients was 72 years (95% confidence intervals, 60–79 years), 73.7% were male, 68.4% displayed prior ischemic heart disease, 47.4% showed LVEF <40%, 36.8% were on treatment with insulin, and 42.1% were in NYHA functional class III.
Mean (± SD) baseline peak VO2, VE/VCO2 slope, 6MWT, and MLHF score were 10.9 ± 4.0 mL/min/kg, 41.7 ± 9.1, 303.1 ± 100.6 m, and 34.1 ± 20.6, respectively. The median NT‐proBNP, CA125, and eGFR were 1382 pg/mL (95% confidence intervals, 639–3340 pg/mL), 27 U/mL (95% confidence intervals, 12–64 U/mL), and 48.1 mL/min/1.73 m2 (95% confidence intervals, 38.6–63.4 mL/min/1.73 m2), respectively. Detailed baseline characteristics are presented in the Table. At 30 days, 16 of 19 patients (84.2%) performed the second CPET (1 patient refused and 2 patients withdrew from the empagliflozin early due to side effects (gastrointestinal intolerance).
Table 1.
Baseline characteristics (N = 19)
| Variable | Value |
|---|---|
| Demographics and medical history | |
| Median age, y | 72 (60–79) |
| Male sex | 14 (73.7) |
| BMI, kg/m2 | 30.6 ± 5.5 |
| HTN | 16 (84.2) |
| DM | 19 (100) |
| Insulin‐dependent DM | 7 (36.8) |
| Dyslipidemia | 15 (78.9) |
| Smoker | 2 (10.5) |
| Former smoker | 7 (36.8) |
| Ischemic heart disease | 13 (68.4) |
| Valvular heart disease | 5 (26.3) |
| COPD | 3 (15.8) |
| Stroke | 1 (5.3) |
| CKD | 12 (63.2) |
| NYHA class >II | 8 (42.1) |
| Vital signs | |
| Heart rate, bpm | 73 ± 15 |
| SBP, mm Hg | 119 ± 15 |
| DBP, mm Hg | 65 ± 9 |
| ECG | |
| LBBB | 5 (26.3) |
| AF | 11 (57.9) |
| Laboratory data | |
| Hb, g/dL | 13.7 ± 2.0 |
| Cr, mg/dL | 1.44 ± 0.48 |
| eGFRa, mL/min/1.73 m2 | 48.1 (38.6–63.4) |
| Serum NA, mEq/L | 138 ± 3 |
| NT‐proBNP, pg/mL | 1382 (639–3340) |
| CA125, U/mL | 27 (12–64) |
| Echocardiography | |
| LVEF, % | 45 ± 17 |
| LVEF ≤40% | 9 (47.4) |
| TAPSE, mm | 18 ± 5 |
| PASPb, mm Hg | 52 ± 12 |
| Medical treatment | |
| Loop diuretics | 19 (100) |
| Thiazides | 7 (36.8) |
| Furosemide equivalent doses, mg/d | 80 (40–160) |
| Aldosterone receptor blockers | 8 (42.1) |
| β‐Blockers | 16 (84.2) |
| ACEIs/ARBs | 10 (52.6) |
| Sacubitril/valsartan | 2 (10.5) |
| Metformin | 16 (84.2) |
| DPP4 inhibitors | 3 (15.8) |
| Digoxin | 2 (10.5) |
| Statins | 121 (63.2) |
Abbreviations: ACEI, angiotensin‐converting enzyme inhibitor; AF, atrial fibrillation; ARB, angiotensin II receptor blocker; BMI, body mass index; CA125, carbohydrate antigen‐125; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; Cr, creatinine; DBP, diastolic blood pressure; DM, diabetes mellitus; DPP4, dipeptidyl peptidase‐4; ECG, electrocardiography; eGFR, estimated glomerular filtration rate; Hb, hemoglobin; HTN, hypertension; IQR, interquartile range; LBBB, left bundle branch block; LVEF, left ventricular ejection fraction; NA, sodium; NT‐proBNP, N‐terminal pro brain natriuretic peptide; NYHA, New York Heart Association; PASP, pulmonary artery systolic pressure; SBP, systolic blood pressure; SD, standard deviation; TAPSE, tricuspid annular plane systolic excursion.
Data are presented as n (%), mean ± SD, or median (IQR).
Calculated using the Modification of Diet in Renal Disease formula.
Data available in 15 patients.
3.1. Primary endpoint
Compared with baseline, peak VO2 increased significantly at 30 days (∆: +1.21 [0.66 to 1.76] mL/min/kg; P < 0.001), which accounts for an 11.10% increase from the baseline value (Figure 1A). A 30‐day peak VO2 increase ≥10% was found in 14 patients.
Figure 1.
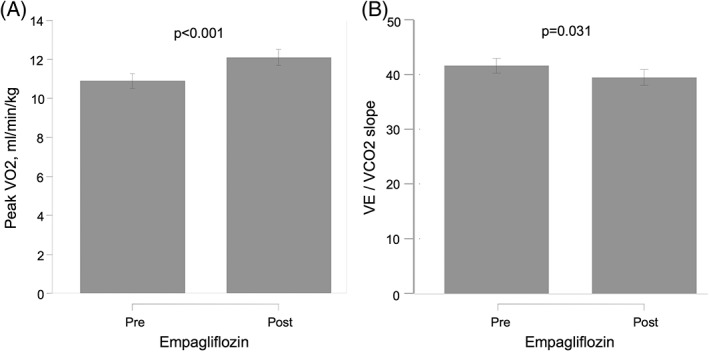
Effect of empagliflozin on CPET parameters in T2DM patients with symptomatic HF at 30 days, showing (A) changes in peak VO2 and (B) changes in VE/VCO2 slope. Abbreviations: CPET, cardiopulmonary exercise testing; HF, heart failure; peak VO2, peak oxygen consumption; T2DM, type 2 diabetes mellitus; VE/VCO2 slope, ventilatory efficiency
3.2. Secondary endpoints
A significant improvement in VE/VCO2 slope was also found at 30 days (∆‐VE/VCO2: –2.17 [−4.14 to −0.20]; P = 0.031), which accounts for a 5.21% reduction, as shown in Figure 1B. In parallel, significant improvements in MLHF (∆‐MLHF: 39.97% reduction; P < 0.001) and 6MWT (∆‐6MWT: 8.67% increase; P < 0.001) were registered (Figure 2A and 2B, respectively).
Figure 2.
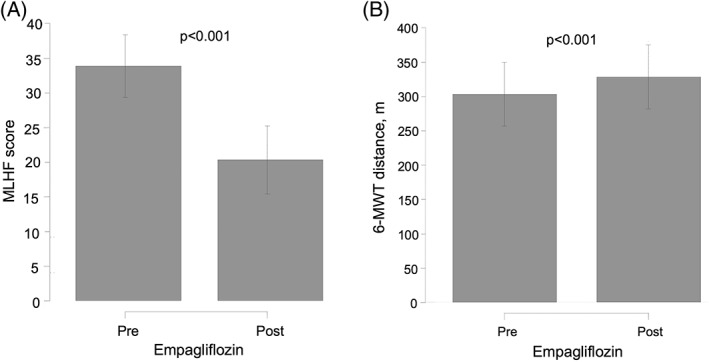
Effect of empagliflozin on QoL and submaximal functional capacity in T2DM patients with symptomatic HF at 30 days, showing (A) changes in MLHF score and (B) changes in 6‐MWT. Abbreviations: 6‐MWT, 6‐minute walk test; HF, heart failure; MLHF, Minnesota Living with Heart Failure Questionnaire; QoL, quality of life; T2DM, type 2 diabetes mellitus
Biomarkers showed divergent results to empagliflozin treatment. A reduction in the congestion biomarker CA125 was observed (∆‐logCA125: −0.32 [−0.51 to −0.13]; P = 0.001), which corresponded to a 9.04% reduction compared with baseline (Figure 3). No significant changes were found for NT‐proBNP (∆‐logNT‐proBNP: −0.05 [−0.27 to 0.18]; P = 0.679) and eGFR [∆‐logGFR: −0.05 [−0.104 to −0.013]; P = 0.129).
Figure 3.
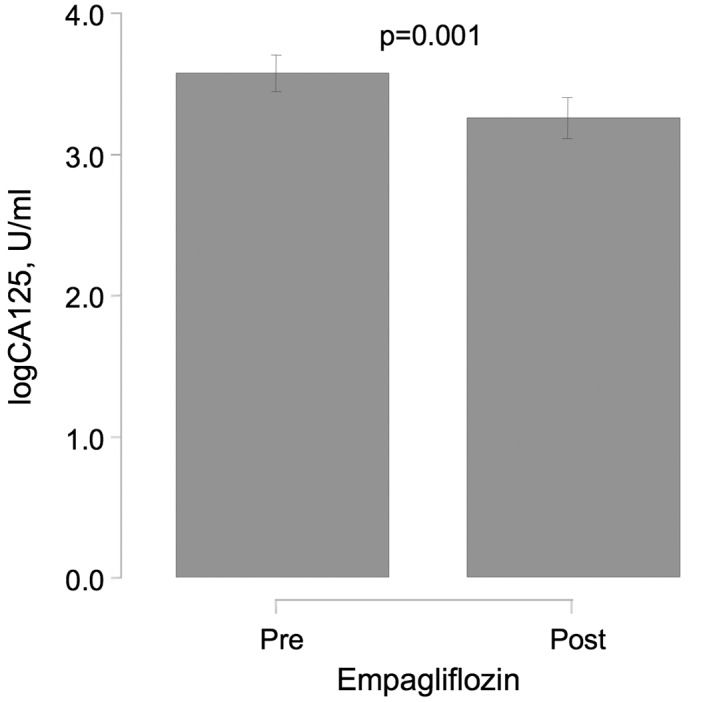
Effect of empagliflozin on congestion biomarker CA125 in T2DM patients with symptomatic HF at 30 days. Abbreviations: CA125, carbohydrate antigen‐125; HF, heart failure; T2DM, type 2 diabetes mellitus
4. DISCUSSION
The main finding of our study was that initiation of empagliflozin over standard HF therapy led to increased short‐term maximal exercise capacity (measured as peak VO2) in T2DM patients with symptomatic HF. Interestingly, the magnitude of the effect on peak VO2 (an increase in 11.1%) is considered to be clinically relevant,3 and the beneficial effect of empagliflozin was also found for other surrogates of exercise tolerance (VE/VCO2 slope, 6MWT, QoL [MLHF], and fluid overload [plasma levels of CA125]).
CPET with incremental workload and symptom‐limited exercise testing is considered the gold standard when studying the cardiovascular, pulmonary, and metabolic adaptations to exercise in HF patients.4 Some mechanisms have been proposed to explain the beneficial effects of SGLT2 inhibitors in HF.2, 5, 6 The most common proposed explanation is the diuretic effect2, 5, 6; however, important differences compared with other diuretic agents need to be addressed. Whereas conventional diuretics used in HF (loop diuretics and thiazides) act on the loop of Henle and the distal tubules, empagliflozin acts on proximal tubules, interacting with sodium/hydrogen exchanger (NHE3), increasing sodium delivery at the distal tubules, enhancing the efficacy of loop and thiazide diuretics.2, 5, 6 In addition, sodium increase at the macula densa promotes, through tubuloglomerular feedback, the vasoconstriction of the afferent artery, decreasing glomerular hyperfiltration and progressive renal function deterioration despite a transient decrease in GFR.2, 5, 6, 7 Beyond diuretic effects, the beneficial effects of empagliflozin have been also attributed to an increase in ketone use by the heart.2, 5, 6, 7, 8 It has been proposed that increased ketone oxidation could maintain cardiac energy supply in situations of limited energy production increasing cardiac efficiency.2, 5, 6, 7, 8 In addition, some authors postulate that inhibition of sodium/hydrogen exchange in the heart may lead to a reduction in cardiac injury, hypertrophy, fibrosis, and remodeling.2
In the present work, we cannot unravel the mechanisms by which empagliflozin improves exercise capacity so early in patients with HF; however, the significant improvement of exercise tolerance together with a reduction of CA125 (a surrogate of congestion)9, 10 suggests a diuretic effect as the major contributor explaining the present findings. The absence of significant NT‐proBNP change may be due to a poor correlation between volume status and natriuretic peptides, especially in elderly patients and in those with renal dysfunction (most patients enrolled in this study).11
4.1. Study limitations
We acknowledge that the main limitations of this study are the lack of a control group and the limited potential for generalizing our results to other populations due to the small sample size and the heterogeneity of the sample. In addition, although improbable, we cannot rule out the “learning effect” as a factor related with functional changes here reported. However, our positive results should be interpreted as a “proof of concept” on the short‐term potential effect of empagliflozin on functional capacity.
5. CONCLUSION
In this study, treatment onset with empagliflozin was associated with 1‐month improvement in exercise capacity and other functional and biochemical markers in T2DM patients with HF. Further controlled studies are needed to validate the results of the present pilot trial.
Author contributions
Julio Núñez, MD, PhD, and Patricia Palau, MD, PhD, contributed equally.
Conflicts of interest
The authors declare no potential conflicts of interest.
Núñez J, Palau P, Domínguez E, et al. Early effects of empagliflozin on exercise tolerance in patients with heart failure: A pilot study. Clin Cardiol. 2018;41:476–480. 10.1002/clc.22899
Funding information This work was supported in part by grants from CIBER Cardiovascular (16/11/00420, 16/11/00403), FEDER, and PIE (15/00013).
REFERENCES
- 1. Zinman B, Wanner C, Lachin JM, et al; EMPA‐REG OUTCOME Investigators . Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med. 2015;373:2117–2128. [DOI] [PubMed] [Google Scholar]
- 2. Packer M, Anker SD, Butler J, et al. Effects of sodium‐glucose cotransporter 2 inhibitors for the treatment of patients with heart failure: proposal of novel mechanism of action. JAMA Cardiol. 2017;2:1025–1029. [DOI] [PubMed] [Google Scholar]
- 3. Forman DE, Arena R, Boxer R, et al; American Heart Association Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; Council on Quality of Care and Outcomes Research; and Stroke Council . Prioritizing functional capacity as a principal end point for therapies oriented to older adults with cardiovascular disease: a scientific statement for healthcare professionals from the American Heart Association. Circulation. 2017;135:e894–e918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Guazzi M, Arena R, Halle M, et al. 2016 Focused Update: Clinical Recommendations for Cardiopulmonary Exercise Testing Data Assessment in Specific Patient Populations. Circulation. 2016;133:e694–e711. [DOI] [PubMed] [Google Scholar]
- 5. Perrone‐Filardi P, Avogaro A, Bonora E, et al. Mechanisms linking empagliflozin to cardiovascular and renal protection. Int J Cardiol. 2017;241:450–456. [DOI] [PubMed] [Google Scholar]
- 6. Verbrugge FH, Martens P, Mullens W. SGLT‐2 inhibitors in heart failure: implications for the kidneys. Curr Heart Fail Rep. 2017;14:331–337. [DOI] [PubMed] [Google Scholar]
- 7. Wanner C, Inzucchi SE, Lachin JM, et al; EMPA‐REG OUTCOME Investigators . Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375:323–334. [DOI] [PubMed] [Google Scholar]
- 8. Mudaliar S, Alloju S, Henry RR. Can a shift in fuel energetics explain the beneficial cardiorenal outcomes in the EMPA‐REG OUTCOME study? A unifying hypothesis. Diabetes Care. 2016;39:1115–1122. [DOI] [PubMed] [Google Scholar]
- 9. Núñez J, Llàcer P, Bertomeu‐González V, et al; CHANCE‐HF Investigators . Carbohydrate Antigen‐125–Guided Therapy in Acute Heart Failure: CHANCE‐HF, a randomized study. JACC Heart Fail. 2016;4:833–843. [DOI] [PubMed] [Google Scholar]
- 10. Núñez J, Llàcer P, Núñez E, et al. Antigen carbohydrate 125 and creatinine on admission for prediction of renal function response following loop diuretic administration in acute heart failure. Int J Cardiol. 2014;174:516–523. [DOI] [PubMed] [Google Scholar]
- 11. Peacock WF, Soto KM. Current techniques of fluid status assessment. Contrib Nephrol. 2010;164:128–142. [DOI] [PubMed] [Google Scholar]


