Abstract
Treatment with intravenous ferric carboxymaltose (FCM) has been shown to improve symptoms, functional capacity, and quality of life in patients with heart failure and iron deficiency. However, the underlying mechanisms for these beneficial effects remain undetermined. The aim of this study is to quantify cardiac magnetic resonance changes in myocardial iron content after administration of intravenous FCM in patients with heart failure and iron deficiency and contrast them with parameters of heart failure severity. This is a multicenter, double‐blind, randomized study. Fifty patients with stable symptomatic heart failure, left ventricular ejection fraction <50%, and iron deficiency will be randomly assigned 1:1 to receive intravenous FCM or placebo. Intramyocardial iron will be evaluated by T2* and T1 mapping cardiac magnetic resonance sequences before and at 7 and 30 days after FCM. After 30 days, patients assigned to placebo will receive intravenous FCM in case of persistent iron deficiency. The main endpoint will be changes from baseline in myocardial iron content at 7 and 30 days. Secondary endpoints will include the correlation of these changes with left ventricular ejection fraction, functional capacity, quality of life, and cardiac biomarkers. The results of this study will add important knowledge about the effects of intravenous FCM on myocardial tissue and cardiac function. We hypothesize that short‐term (7 and 30 days) myocardial iron content changes after intravenous FCM, evaluated by cardiac magnetic resonance, will correlate with simultaneous changes in parameters of heart failure severity. The study is registered at http://www.clinicaltrials.gov (NCT03398681).
Keywords: Cardiac Magnetic Resonance, Ferric Carboxymaltose, Heart Failure, Iron Deficiency, Myocardial Iron
1. INTRODUCTION
Iron deficiency (ID) is a common finding in patients with heart failure (HF). It is usually associated, even in the absence of anemia, with decrease in functional capacity, quality of life, and with increased risk of mortality and readmission.1, 2, 3, 4, 5, 6, 7 Treatment with intravenous (IV) ferric carboxymaltose (FCM) in patients with HF and ID has shown to improve symptoms, functional capacity, and quality of life, and to decrease hospitalizations under an acceptable safety profile.8, 9 Such benefit has been consistent in patients with and without anemia,10, 11 suggesting additional pathophysiological pathways besides anemia resolution. In addition, recent studies suggested that myocardial ID could play a direct role in the pathogenesis and progression of HF.12, 13, 14
Despite the fact that T2‐star (T2*) cardiac magnetic resonance (CMR) sequencing has been shown to provide a reliable assessment of myocardial iron content,15, 16, 17, 18 newer techniques, such as T1 mapping, have emerged as an alternative tool for myocardial iron assessment.19 Changes in myocardial T1 mapping are more linear and have fewer artifacts than with the T2* sequence, which translates into a more reproducible and sensitive technique.20 Thus, in this work we aim to evaluate the utility of T1 mapping for detecting myocardial iron changes after IV iron administration. In a recent pilot study, our group reported an association between IV FCM administration and myocardial iron repletion assessed by T2* CMR. Interestingly, myocardial iron changes were strongly related to an improvement in left ventricular ejection fraction (LVEF).21
We sought to determine whether short‐term (7‐day and 30‐day) myocardial iron content changes after IV FCM, evaluated by T1* CMR, would correlate with simultaneous changes in parameters of HF severity.
2. METHODS
2.1. Overall study design
Changes in Myocardial Iron After Iron Administration (Myocardial‐IRON) is a multicenter, double‐blind, randomized, placebo‐controlled study designed to test the effect of IV FCM (Ferinject; Vifor Pharma, Glattbrugg, Switzerland) on myocardial iron content assessed by CMR in 5 academic centers in Spain (Hospital Clínico Universitario de Valencia, Hospital de Manises, Hospital General Universitari de Castelló, Hospital Universitario y Politécnico La Fe, and Consorci Hospital General Universitari de Valencia). After signing the informed consent, patients will be randomized 1:1 to receive FCM or placebo. Intramyocardial iron will be evaluated before its administration, and 7 and 30 days after. At 30 days, patients assigned to placebo will receive FCM if ID persists (Figure 1).
Figure 1.
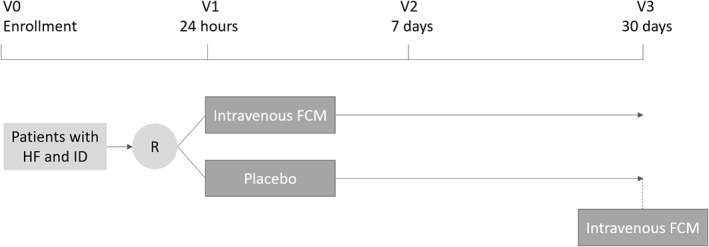
Study design. Abbreviations: FCM, ferric carboxymaltose; HF, heart failure; ID, iron deficiency; R, randomization; V, visit
The study will be carried out in accordance with the principles of the Declaration of Helsinki (1996) and the Guideline for Good Clinical Practice of the International Council for Harmonisation. The study protocol was approved by Agencia Española del Medicamento y Productos sanitarios (AEMPS) on December 6, 2016, and by Comité Ético de Investigación Clínica (CEIC) del Hospital Clínico Universitario de Valencia on January 26, 2017, with an amendment on June 22, 2017. CMR studies will be performed by ERESA (Valencia, Spain), and laboratory parameters will be analyzed in local laboratories. The study is registered at http://www.clinicaltrials.gov (NCT03398681). For a complete list of Myocardial‐IRON investigators, see Supporting Information, Appendix 1, in the online version of this article.
2.2. Study population
Eligible patients are those with stable chronic HF (New York Heart Association [NYHA] class II–III), LVEF <50%, and ID, the latter defined as serum ferritin <100 μg/L or 100 to 299 μg/L with transferrin saturation (TSAT) <20% and hemoglobin (Hb) <15 g/dL. All patients must meet all inclusion and exclusion criteria (Table 1).
Table 1.
Inclusion and exclusion criteria
| Inclusion criteria |
| Outpatients with chronic HF |
| Age > 18 years |
| NYHA class II–III with optimal medical treatment in the last 4 weeks, without dose changes of HF treatment in the last 2 weeks (except for diuretics) |
| NT‐proBNP >400 pg/mL |
| LVEF <50% in the last 12 months |
| ID, defined as serum ferritin <100 μg/L, or 100–299 μg/L if TSAT <20% and Hb <15 g/dL |
| Willing and able to give informed consent for participation in the study |
| Exclusion criteria |
| Known intolerance to FCM |
| History of acquired iron overload |
| Severe valve disease or cardiac surgery scheduled in the next 30 days |
| ACS, TIA, or ictus in the previous 3 months |
| CABG, major surgery, or cardiac, cerebrovascular, or aortic percutaneous intervention (diagnostic angiography is allowed) in the previous 3 months |
| Scheduled revascularization in the next 30 days |
| Scheduled CRT device implantation in the next 30 days |
| Active bleeding in the last 30 days |
| Active infection or malignancy |
| Immediate need for transfusion or Hb ≥15 g/dL |
| Anemia for reasons other than ID |
| Immunosuppressive therapy or dialysis |
| History of treatment with EPO, IV iron, or transfusion in the previous 12 weeks |
| Treatment with oral iron at doses >100 mg/d in the previous week |
| Contraindications to CMR, including noncompatible pacemakers or defibrillators, cochlear implants, cerebral aneurysm clips, claustrophobia, or large body size that does not allow the performance of the test |
| Pregnant or lactating females |
| Subject of childbearing age who is unwilling to use adequate contraceptive measures during the study and up to 5 half‐lives after the administration of study treatment |
| Participation in another trial at the time of inclusion or in the previous 30 days |
| Any disorder that compromises the ability to sign informed consent and/or comply with study procedures |
Abbreviations: ACS, acute coronary syndrome; CABG, coronary artery bypass grafting; CMR, cardiac magnetic resonance; CRT, cardiac resynchronization therapy; EPO, erythropoietin; FCM, ferric carboxymaltose; Hb, hemoglobin; HF, heart failure; ID, iron deficiency; IV, intravenous; LVEF, left ventricular ejection fraction; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; NYHA, New York Heart Association; TIA, transient ischemic attack; TSAT, transferrin saturation.
2.3. Randomization
Patients will be randomly allocated into 1:1 ratio to receive FCM or placebo by means of a web‐based computer‐generated block sequence. Investigators and patients will be blinded to treatment allocations.
2.4. Study procedures
Study procedures are detailed in Table 2.
Table 2.
Study procedures
| Visit 0, Enrollment | Visit 1, 24 Hours | Visit 2, 7 Days | Visit 3, 30 Days | Additional Visits | |
|---|---|---|---|---|---|
| Informed consent form | X | ||||
| Medical history | X | ||||
| Concomitant medications | X | ||||
| Physical examination | X | X | X | X | X |
| Vital signs | X | X | X | X | X |
| Review of inclusion and exclusion criteria | X | ||||
| Randomization | X | ||||
| ECG | X | X | |||
| Echocardiography | X | X | |||
| Laboratory tests | X | X | X | ||
| NYHA functional class | X | X | X | X | X |
| 6MWT | X | X | X | ||
| KCCQ | X | X | X | ||
| CMR | X | X | X | ||
| Adverse clinical events | X | X | X | X | |
| Changes in treatment | X | X | X | X |
Abbreviations: 6MWT, 6‐minute walking test; CMR, cardiac magnetic resonance; ECG, electrocardiography; KCCQ, Kansas City Cardiomyopathy Questionnaire; NYHA, New York Heart Association.
2.4.1. Cardiac magnetic resonance
CMR data will be blindly acquired and quantified offline by 2 experienced cardiologists (M.P.L.L. and J.V.M., both with 15 years' experience in CMR imaging) on a 1.5‐Tesla MR scanner (Magnetom Essenza or Avanto; Siemens, Erlangen, Germany). The 3 consecutive CMR studies of each patient will be analyzed by the same operator. No contrast media are used. All images are obtained with electrocardiographic gating and breathholding.
Cine images are acquired at rest in short‐axis views every 1 cm with steady‐state free precession imaging sequences (time resolution: 37 ms; voxel size: 1.7 × 1.7 × 7 mm). Right ventricular (RV) ejection fraction and LVEF (%), left ventricular (LV) end‐diastolic and end‐systolic volume index (mL/m2), and LV mass (g/m2) are calculated by semiautomatic planimetry of endocardial and epicardial borders in short‐axis‐view cine images.
The basic T2* pulse sequence is a breathhold, multiecho gradient echo T2* sequence (voxel size: 1.6 × 1.6 × 8 mm) with 8 echo times from 2.65 to 21 ms, in mid‐ventricular short axis. For T2* analysis, a region of interest (ROI) is chosen in the mid‐LV septum. The mean signal intensities of the ROI are measured in the series of increasing echo time images to give an exponential decay curve. The monoexponential decay model and the nonlinear curve fitting algorithm are used to fit the curve to obtain T2* measurement.
T1 mapping is performed with modified Look‐Locker inversion recovery (MOLLI) sequences with motion correction (voxel size: 1.5 × 1.5 × 7 mm) in 3 short axes (basal, medial, and apical). After T1 maps are generated, a ROI is chosen in the mid‐LV septum in the 3 short axes and the average T1 values are calculated.
For details of the CMR sequences used, see Supporting Information, Appendix 3, in the online version of this article. All measurements were made on the Syngo MR C15 (Siemens) platform. The same protocol will be repeated at 7 and 30 days.
2.4.2. Six‐minute walking test
The 6MWT is performed in a place well‐equipped for cardiopulmonary resuscitation. Subjects are advised not to have undertaken vigorous exercise within the previous 2 hours and are instructed to cover the maximum distance possible in 6 minutes, at a self‐graded walking speed. Pausing to rest will be allowed when needed.
2.4.3. Kansas City Cardiomyopathy Questionnaire
The KCCQ is a self‐administered instrument designed to evaluate health‐related quality of life in patients with chronic HF. It is composed of 23 items (15 questions) that form 7 domains: physical limitations, symptoms (frequency, severity, and change over time), self‐efficacy and knowledge, social interference, and quality of life. It is scored by assigning each response from 1 to 5, 6, or 7 points, with 1 the lowest. The sum of these items is subsequently converted to a scale of 0 to 100 points (see Supporting Information, Appendix 2, in the online version of this article).22 The Spanish version of the KCCQ23 will be completed by patients with the support of trained nurses.
2.4.4. Biomarkers
The results from laboratory data will be reviewed and signed by the investigator, who will record in the case‐report form whether results are normal, abnormal, and clinically significant. The following parameters will be assessed at baseline, 7, and 30 days: (1) hematology: Hb, hematocrit, red‐cell distribution width, mean corpuscular volume, and mean corpuscular Hb; (2) serum electrolytes: sodium, potassium, and chloride; (3) ID parameters: ferritin, TSAT, soluble transferrin receptor, and hepcidin; (4) renal function parameters: cystatin C, serum creatinine, blood urea nitrogen, and estimated glomerular filtration rate; (5) liver function parameters: alanine aminotransferase and aspartate aminotransferase; and (6) HF biomarkers: carbohydrate antigen 125, N‐terminal pro‐brain natriuretic peptide, galectin‐3, ST‐2, and high‐sensitivity troponin T.
2.4.5. Clinical visits
A summary of study procedures performed at each visit is detailed in Table 2. Visit 0 is the screening and eligibility assessment; after signing and dating the informed consent, the study procedures will be initiated. Scheduled follow‐up visits will be performed at 24 hours and at 7 and 30 days after randomization. Patients will be censored if they withdraw from the informed consent or die. Optional additional visits are permitted. The main reason for each optional visit, and for any laboratory test or procedure additionally performed, must be recorded on the case‐report form. Information on concomitant medications and clinical adverse events will be recorded.
2.5. Trial intervention
Eligible patients will be randomized to receive FCM or placebo.
2.5.1. Intravenous ferric carboxymaltose
FCM solution (Ferinject) will be given as an IV perfusion of 20 mL (equivalent to 1000 mg of iron) diluted in a sterile saline solution (0.9% NaCl) and administered over ≥15 minutes.
Because FCM is a dark‐brown solution and easily distinguishable, the personnel responsible for its preparation and administration will not be involved in any study assessments. To ensure that patients will be unaware of the study drug, the materials used in drug administration will be covered with aluminum foil or other opaque material and the injection site will be shielded from patient view.
2.5.2. Placebo
Normal saline (0.9% NaCl) will be administered as per the instructions in the placebo group.
2.5.3. Concomitant drugs
The indication for other HF drugs will be followed according to the current recommendations for clinical practice.
2.6. Endpoints
2.6.1. Primary endpoint
The main endpoint will be change in myocardial iron content from baseline at 7 and 30 days, assessed by T2* and T1‐mapping CMR sequences. The statistical comparisons for the primary efficacy objective will test the null hypotheses of no differences in changes in myocardial iron content from baseline as assessed by T2* and T1‐mapping CMR; the alternative hypotheses will indicate differences in either direction. Strictly speaking, the primary objective will be the 30‐day evaluation; the 7‐day evaluation will be considered a co–primary endpoint.
2.6.2. Secondary endpoints
The study has 3 secondary endpoints.
1. On the entire sample, to correlate these changes with the following clinical markers of HF disease severity: LVEF, functional capacity (6MWT and NYHA class), quality of life (KCCQ), and cardiac biomarkers.
2. On the sample stratified into 3 prespecified subgroups: age > 70 years vs ≤70 years; anemia vs no anemia (according to World Health Organization criteria); and ischemic vs nonischemic etiology.
3. On the entire sample, to correlate these changes with blood markers specific to iron biology/deficiency (ferritin, TSAT, soluble transferrin receptor, and hepcidin).
2.6.3. Safety endpoints
Based on previous studies,8, 9 a safety surveillance will be specifically focused on (1) general disorders and administration‐site conditions; (2) skin and subcutaneous tissue disorders; (3) nervous system disorders; (4) gastrointestinal disorders; (5) vascular disorders; (6) ear and labyrinth disorders; (7) injury, poisoning, and procedural complications; and (8) cardiac disorders.
2.7. Sample‐size calculation
The sample size was calculated based on the expected changes in T2*, according to the following parameters: (1) 2 treatment arms; (2) statistical power of the primary endpoint of 80%; and (3) α error of 0.05. We used repeated‐measures ANOVA using the Lawley‐Hotelling test to evaluate the effect of treatment. Based on studies from our group,21 we predict a mean difference of 9.25 ±8.69 in T2* at 30 days after treatment, and a correlation of 0.38 between T2* measurements at baseline and 1 month later. The correlation of T2* at baseline and 7 days would be 0.40, because we expect the correlation to decrease with time. For a desired power of 0.80 and a type I error of 0.025, we need to include 42 participants to detect a mean difference of 9.25 on T2* at 30 days, assuming no differences with placebo. Assuming a loss of 10% of patients, we increased the sample size to 50 patients (25 patients per arm).
2.8. Statistical analysis
All statistical comparisons will be made under an intention‐to‐treat principle. Continuous variables will be presented as mean ± SD for normally distributed variables and as median (interquartile range) otherwise. Discrete data will be expressed as percentages.
The primary and secondary endpoints will be tested using an analysis of covariance (ANCOVA) design within a framework of linear mixed model. The analysis will include a between (FMC vs placebo) and within comparison (changes at 7 and 30 days). The interaction term Tx*visit will be included if the omnibus P value is ≤0.05. The ANCOVA model for the primary analysis will include as dependent variable the myocardial T2* CMR values; the contrast among treatment groups at 30 days and 7 days will test the primary and co‐primary endpoint, respectively. As a prespecified analysis, no adjustment for multiple comparisons will be made. Baseline value of myocardial T2* CMR will be included as an obligated covariate. The use of other covariates will be dictated if important differences among treatment groups are observed after randomization. Based on the normality of residuals, a decision about transforming the outcome variable will be made. A similar approach will be taken for the secondary endpoints where LV and RV systolic function, KCCQ, NYHA class, and serum biomarkers will be the outcome variables.
A 2‐sided P value of 0.05 will be considered statistically significant for all analyses. Stata release 15.1 (StataCorp LP, College Station, TX) will be used for the analysis.
2.9. Current status
Patient enrollment started in May 2017. As of December 31, 2017, twenty‐five patients had been enrolled in the study (50% of the target). Baseline characteristics of these patients are described in Table 3.
Table 3.
Baseline characteristics (n = 25)
| Variable | Value |
|---|---|
| Demographics and medical history | |
| Age, years | 72.5 (67–78.5) |
| Male sex | 17 (68.0) |
| Hypertension | 16 (64) |
| Dyslipidemia | 15 (60) |
| DM | 12 (48) |
| Smoker | 3 (12.0) |
| Former smoker | 13 (52.0) |
| CAD | 9 (36.0) |
| Hospital admission for AHF in the last year | 14 (56.0) |
| COPD | 6 (24.0) |
| CKD | 8 (32.0) |
| Stroke | 5 (20.0) |
| NYHA functional class | |
| II | 23 (92.0) |
| III | 2 (8.0) |
| Vital signs | |
| Heart rate, bpm | 70 (60–79) |
| SBP, mm Hg | 118 (106–130) |
| ECG and echocardiography | |
| AF | 9 (36.0) |
| LVEF, % | 40 (34–44) |
| Laboratory tests | |
| Hb, g/dL | 12 (12.1–13.3) |
| Anemia (WHO criteria)a | 8 (32.0) |
| TSAT, % | 14.9 (11–18.9) |
| Ferritin, ng/mL | 78 (42–148) |
| Absolute ID | 14 (56) |
| Relative ID | 11 (44.0) |
| Lymphocyte count, ×103 cells/mL | 1720 (1210–2130) |
| Sodium, mEq/L | 140 (139–142) |
| Potassium, mEq/L | 4.6 (4.3–4.9) |
| Urea, mEq/L | 62 (50–82) |
| sCr, mg/dL | 1.17 (0.94–1.57) |
| eGFR <60 mL/min/1.73 m2 | 62 (44–83) |
| NT‐proBNP, pg/mL | 1690 (1117–2836) |
| Medical treatment | |
| Diuretic | 23 (92.0) |
| β‐Blocker | 22 (80.0) |
| ACEI | 6 (24.0) |
| ARB | 6 (24.0) |
| Sacubitril/valsartan | 6 (24.0) |
| MRI | 13 (52.0) |
Abbreviations: ACEI, angiotensin‐converting enzyme inhibitor; AF, atrial fibrillation; AHF, acute decompensated heart failure; ARB, angiotensin II receptor blocker; CAD, coronary artery disease; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; DM, diabetes mellitus; ECG, electrocardiography; eGFR, estimated glomerular filtration rate; Hb, hemoglobin; ID, iron deficiency; IQR, interquartile range; LOS, length of stay; LVEF, left ventricular ejection fraction; MI, myocardial infarction; MRI, mineralocorticoid receptor inhibitor; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; NYHA, New York Heart Association; SBP, systolic blood pressure; sCr, serum creatinine; TSAT, transferrin saturation; WHO, World Health Organization. Data are presented as n (%) or median (IQR).
WHO criteria for anemia: adult male, Hb 13 g/dL; adult nonpregnant female, Hb 12 g/dL, adult pregnant female, Hb 11 g/dL. Absolute ID, ferritin <100 ng/mL; relative ID, ferritin 100–299 ng/mL and TSAT <20%.
2.10. Planned substudies
The following substudies are planned:
The correlation of basal T1 mapping and T2* with basal ferritin and TSAT.
Correlations of changes in T1 mapping and T2* with changes in ferritin and TSAT.
The effect of FCM on RV function.
The effect of FCM on LV tissue Doppler.
3. RESULTS
Once the study is finished, the changes in T2* and T1 mapping after FCM administration at 7 and 30 days will be documented. Furthermore, we will describe changes in laboratory data, functional capacity (6MWT, NYHA class), quality of life (KCCQ), LVEF, and ventricular diameters and volumes in echocardiography and CMR. Finally, we will relate the changes in T2* and T1 mapping with secondary endpoints mentioned above. We expect the results to be available in October 2018.
4. DISCUSSION
The prevalence of ID in chronic HF is approximately 50%. It is commonly associated with decreased functional capacity and quality of life, and increased risk of mortality and readmission, even in the absence of anemia.1, 2, 3, 4, 5, 6, 7 Indeed, the administration of FCM has been shown to reverse these changes within an acceptable safety profile.8, 9 Several studies have demonstrated clinical improvement after IV iron administration in patients with and without anemia, suggesting that its beneficial effect includes additional mechanisms independent of the erythropoietic pathway.10, 11
4.1. Iron and myocardial function
Iron plays a crucial role in oxygen transport, through the production of Hb; oxygen storage, through myoglobin; and as a component of the mitochondrial respiratory chain involved in energy production.1
An experimental study has shown that rats with iron‐deficiency anemia developed LV hypertrophy and dilation due to mitochondrial ultrastructural damage.12 Another study in nonanemic iron‐deficient mice showed that iron content in cardiomyocytes and mitochondrial function was restored by iron repletion.13 In humans, a small study showed a reduction in the iron content of cardiomyocytes in patients with HF and reduced ejection fraction (HFrEF) as compared with controls.14 More recently, Toblli et al., in a small randomized trial including 60 patients with HFrEF, ID, and chronic kidney disease, showed that iron sucrose administration translated into a significant 6‐month improvement in LVEF.24 More recently, findings from a cohort of 232 patients undergoing renal transplantation showed an increase of LVEF that was particularly notable in those with systolic dysfunction.25
This preliminary evidence has led us to postulate that myocardial ID may play a direct role in the pathogenesis and progression of HF. However, the clinical impact of myocardial ID on HF has not been thoroughly evaluated, mainly because of the lack of reliable and widely available noninvasive techniques for myocardial iron quantification.
4.2. CMR and myocardial iron assessment
CMR has emerged as a noninvasive accurate technique for evaluation of cardiac anatomy, function, and risk stratification.26, 27 More recently, this technique has been used to assess myocardial iron content.15 The T2* CMR sequence has been considered a reliable tool for myocardial iron overload assessment.16, 17 Nagao et al., in a small case–control study, found a significant decrease in myocardial iron concentration, assessed by T2* CMR, particularly in nonischemic HF patients.18 Later, these authors also reported that T2* CMR was related to an increased risk of adverse outcomes.18 In a pilot study of 8 patients with HFrEF, our group found that treatment with FCM was associated with significant 30‐day changes in T2* CMR, and they were associated with marked improvement in LVEF.21 Some new CMR techniques, such as T1 mapping, have emerged as potential alternatives for myocardial iron quantification.19 We postulate that the T1 mapping CMR sequence, a more sensitive and reproducible technique,20 could also identify myocardial ID and quantify changes in myocardial iron content after FCM administration.
In summary, preliminary evidence suggests that myocardial iron content plays a key pathophysiological role in HF. We speculate that with the new CMR sequences we will be able to reliably assess changes in myocardial iron content after IV iron administration, and, thereby, open a new modality of treatment for care of HF patients. In addition, these results will add new insights about the role of iron in the physiopathology of the disease. A randomized clinical trial is a necessary step forward to advance the knowledge in this area.
4.3. Study limitations
There is a possibility that large areas of fibrosis may modify T2* and T1 measurement, irrespective of iron status. As CMR are only performed on 1.5‐T machines, and T1 mapping is performed with the MOLLI sequence, the extrapolation of the findings to 3‐T machines or other T1‐mapping protocols is unknown.
Several factors inherent to the study design, such as lower dose (and 1‐time) administration of FCM, short trial duration (endpoint assessment at 30 days), and broad inclusion criteria (LVEF up to 50%; anemia not required), might reduce the expected response to therapy. In addition, the small number of patients leading to inadequate statistical power may become a potential limitation to reliably assess the clinical response.
5. CONCLUSION
We hypothesize that T2* and T1‐mapping CMR sequences will be sensitive enough to detect changes in myocardial iron content following administration of FCM, and that those changes will correlate with surrogates of HF severity.
Conflicts of interest
The authors declare no potential conflicts of interest.
Supporting information
Appendix 1. Complete list of Myocardial‐IRON investigators
Appendix 2. Kansas City Cardiomiopathy Questionnaire
Appendix 3. Details of the CMR sequences used
Miñana G, Cardells I, Palau P, et al. Changes in myocardial iron content following administration of intravenous iron (Myocardial‐IRON): Study design. Clin Cardiol. 2018;41:729–735. 10.1002/clc.22956
Funding information This work was supported in part by an unrestricted grant from Vifor Pharma and Proyectos de Investigación de la Sección de Insuficiencia Cardiaca 2017 from Sociedad Española de Cardiología.
REFERENCES
- 1. Cohen‐Solal A, Leclercq C, Deray G, et al. Iron deficiency: an emerging therapeutic target in heart failure. Heart. 2014;100:1414–1420. [DOI] [PubMed] [Google Scholar]
- 2. Comín‐Colet J, Enjuanes C, González G, et al. Iron deficiency is a key determinant of health‐related quality of life in patients with chronic heart failure regardless of anaemia status. Eur J Heart Fail. 2013;15:1164–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Núñez J, Domínguez E, Ramón JM, et al. Iron deficiency and functional capacity in patients with advanced heart failure with preserved ejection fraction. Int J Cardiol. 2016;207:365–367. [DOI] [PubMed] [Google Scholar]
- 4. Jankowska EA, Rozentryt P, Witkowska A, et al. Iron deficiency predicts impaired exercise capacity in patients with systolic chronic heart failure. J Card Fail. 2011;17:899–906. [DOI] [PubMed] [Google Scholar]
- 5. Jankowska EA, Rozentryt P, Witkowska A, et al. Iron deficiency: an ominous sign in patients with systolic chronic heart failure [published correction appears in Eur Heart J 2011;32:1054]. Eur Heart J. 2010;31:1872–1880. [DOI] [PubMed] [Google Scholar]
- 6. Klip IT, Comin‐Colet J, Voors AA, et al. Iron deficiency in chronic heart failure: an international pooled analysis. Am Heart J. 2013;165:575.e3–582.e3. [DOI] [PubMed] [Google Scholar]
- 7. Núñez J, Comín‐Colet J, Miñana G, et al. Iron deficiency and risk of early readmission following a hospitalization for acute heart failure. Eur J Heart Fail. 2016;18:798–802. [DOI] [PubMed] [Google Scholar]
- 8. Anker SD, Comín‐Colet J, Filippatos G, et al; FAIR‐HF Trial Investigators . Ferric carboxymaltose in patients with heart failure and iron deficiency. N Engl J Med. 2009;361:2436–2448. [DOI] [PubMed] [Google Scholar]
- 9. Ponikowski P, van Veldhuisen DJ, Comín‐Colet J, et al; CONFIRM‐HF Investigators . Beneficial effects of long‐term intravenous iron therapy with ferric carboxymaltose in patients with symptomatic heart failure and iron deficiency. Eur Heart J. 2015;36:657–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Filippatos G, Farmakis D, Colet JC, et al. Intravenous ferric carboxymaltose in iron‐deficient chronic heart failure patients with and without anaemia: a subanalysis of the FAIR‐HF trial. Eur J Heart Fail. 2013;15:1267–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Silverberg DS, Iaina A, Schwartz D, et al. Intravenous iron in heart failure: beyond targeting anemia. Curr Heart Fail Rep. 2011;8:14–21. [DOI] [PubMed] [Google Scholar]
- 12. Dong F, Zhang X, Culver B, et al. Dietary iron deficiency induces ventricular dilation, mitochondrial ultrastructural aberrations and cytochrome c release: involvement of nitric oxide synthase and protein tyrosine nitration. Clin Sci (Lond). 2005;109:277–286. [DOI] [PubMed] [Google Scholar]
- 13. Haddad S, Wang Y, Galy B, et al. Iron‐regulatory proteins secure iron availability in cardiomyocytes to prevent heart failure. Eur Heart J. 2017;38:362–372. [DOI] [PubMed] [Google Scholar]
- 14. Maeder MT, Khammy O, dos Remedios C, et al. Myocardial and systemic iron depletion in heart failure implications for anemia accompanying heart failure. J Am Coll Cardiol. 2011;58:474–480. [DOI] [PubMed] [Google Scholar]
- 15. Anderson LJ, Holden S, Davis B, et al. Cardiovascular T2‐star (T2*) magnetic resonance for the early diagnosis of myocardial iron overload. Eur Heart J. 2001;22:2171–2179. [DOI] [PubMed] [Google Scholar]
- 16. Kirk P, He T, Anderson LJ, et al. International reproducibility of single breathhold T2* MR for cardiac and liver iron assessment among five thalassemia centers. J Magn Reson Imaging. 2010;32:315–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. He T. Cardiovascular magnetic resonance T2* for tissue iron assessment in the heart. Quant Imaging Med Surg. 2014;4:407–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nagao M, Matsuo Y, Kamitani T, et al. Quantification of myocardial iron deficiency in nonischemic heart failure by cardiac T2* magnetic resonance imaging. Am J Cardiol. 2014;113:1024–1030. [DOI] [PubMed] [Google Scholar]
- 19. Schelbert EB, Messroghli DR. State of the art: clinical applications of cardiac T1 mapping. Radiology. 2016;278:658–676. [DOI] [PubMed] [Google Scholar]
- 20. Kellman P, Hansen MS. T1‐mapping in the heart: accuracy and precision. J Cardiovasc Magn Reson. 2014;16:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Núñez J, Monmeneu JV, Mollar A, et al. Left ventricular ejection fraction recovery in patients with heart failure treated with intravenous iron: a pilot study. ESC Heart Fail. 2016;3:293–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Green CP, Porter CB, Bresnahan DR, et al. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: a new health status measure for heart failure. J Am Coll Cardiol. 2000;35:1245–1255. [DOI] [PubMed] [Google Scholar]
- 23. Comín‐Colet J, Garin O, Lupón J, et al; VALIC‐KC Study Group . Validation of the Spanish version of the Kansas City Cardiomyopathy Questionnaire [article in English, Spanish]. Rev Esp Cardiol. 2011;64:51–58. [DOI] [PubMed] [Google Scholar]
- 24. Toblli JE, Di Gennaro F, Rivas C. Changes in echocardiographic parameters in iron deficiency patients with heart failure and chronic kidney disease treated with intravenous iron. Heart Lung Circ. 2015;24:686–695. [DOI] [PubMed] [Google Scholar]
- 25. Hawwa N, Shrestha K, Hammadah M, et al. Reverse remodeling and prognosis following kidney transplantation in contemporary patients with cardiac dysfunction. J Am Coll Cardiol. 2015;66:1779–1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pilz G, Heer T, Harrer E, et al. Clinical applications of cardiac magnetic resonance imaging. Minerva Cardioangiol. 2009;57:299–313. [PubMed] [Google Scholar]
- 27. Husser O, Monmeneu JV, Bonanad C, et al. Prognostic value of myocardial ischemia and necrosis in depressed left ventricular function: a multicenter stress cardiac magnetic resonance registry. Rev Esp Cardiol (Engl Ed). 2014;67:693–700. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 1. Complete list of Myocardial‐IRON investigators
Appendix 2. Kansas City Cardiomiopathy Questionnaire
Appendix 3. Details of the CMR sequences used


