Abstract
Alogliptin, a dipeptidyl peptidase‐4 inhibitor, is approved for the treatment of patients with type 2 diabetes (T2DM). EXAMINE was a randomized controlled clinical trial designed to demonstrate the cardiovascular (CV) safety of alogliptin. In the trial, 5380 patients with established T2DM who had a recent acute coronary syndrome event (between 15 and 90 days) were randomized to treatment with either alogliptin or placebo. To better understand and describe the CV safety of alogliptin, we analyzed data from the EXAMINE trial to determine whether treatment with alogliptin affected recurrent and total CV events. Poisson regression analysis compared the total number of occurrences of CV death, MI, stroke, unstable angina, and coronary revascularization between all patients randomized to alogliptin vs placebo groups. Patients with recurrent CV events were older and more likely to have renal disease and history of heart failure. There were 1100 first CV events and an additional 666 recurrent events over a median of 18 months of follow‐up. There were no significant differences with regard to total number of events in patients treated with alogliptin (n = 873) or placebo (n = 893; P = 0.52). Furthermore, there were no differences in the types of events seen in patients treated with alogliptin or placebo. Alogliptin did not increase the risk of either first or recurrent CV events when compared with placebo in patients with T2DM and recent acute coronary syndrome. These data support the CV safety of alogliptin in patients who are at increased risk of future CV events.
Keywords: Acute Coronary Syndrome, Dipeptidyl Peptidase‐4 Inhibitors, Myocardial Infarction, Type 2 Diabetes
1. INTRODUCTION
Alogliptin, a dipeptidyl peptidase‐4 (DPP‐4) inhibitor, is approved for the treatment of patients with type 2 diabetes mellitus (T2DM). The Examination of Cardiovascular Outcomes With Alogliptin vs Standard of Care in patients with type 2 diabetes mellitus and acute coronary syndrome (EXAMINE) study was a randomized, double‐blind, placebo‐controlled, multinational trial of alogliptin in patients with T2DM who were enrolled 15 to 90 days after an acute coronary syndrome (ACS). In the EXAMINE trial, there was neither an increase nor decrease in the risk of time to first cardiovascular (CV) death, myocardial infarction (MI), or stroke (major adverse cardiovascular events).1 To better understand and describe the CV safety of alogliptin by providing a comprehensive picture of total CV events, we sought to determine the number of initial, recurrent, and total CV events (CV death, MI, stroke, unstable angina [UA], and coronary revascularization) that occurred in the EXAMINE trial.
2. METHODS
The full details of the EXAMINE trial have been previously published (http://www.clinicaltrials.gov NCT00968708).2 In brief, patients were eligible for the trial if they had established T2DM, were being treated with pharmacotherapies for T2DM (with the exception of a DPP‐4 inhibitor or glucagon‐like peptide‐1 agonist), had a glycated hemoglobin (HbA1c) level between 6.5% and 11.0% (7.0%–10.0% if on insulin), and had a recent ACS event (either an MI or UA within 15 to 90 days of randomization). Patients with type 1 diabetes mellitus, end‐stage renal disease who had received hemodialysis within 14 days of screening, or unstable CV disorders (New York Heart Association class IV heart failure [HF], refractory angina, uncontrolled arrhythmias, critical valvular heart disease, severe uncontrolled hypertension) were not eligible for the study.
Eligible patients with T2DM and recent ACS were randomly assigned to treatment with either alogliptin or placebo. Patients with an estimated glomerular filtration rate (eGFR) ≥60 mL/min/1.73 m2 were treated with 25 mg of alogliptin or matching placebo. Alogliptin is cleared via renal excretion, so patients with an eGFR of 30 to <60 mL/min/1.73 m2 were treated with 12.5 mg of alogliptin/placebo, and 6.25 mg of alogliptin/placebo was used in patients with an eGFR <30 mL/min/1.73 m2. During the course of the trial, patients were to continue with evidence‐based therapies for their T2DM and risk factors for cardiovascular disease (CVD) prescribed by their healthcare providers. HbA1c was not blinded during the study, and clinicians were encouraged to treat patients based on regional guidelines; however, treatment with glucagon‐like peptide‐1 agonists or DPP‐4 inhibitors during the course of the trial was prohibited. Treatment allocation was blinded to patients and investigators throughout the course of the study. National regulatory authorities and institutional ethics committees at each site approved the study design, and all participants provided written informed consent.
The primary endpoint of the EXAMINE trial was the time to the first incidence of CV death, nonfatal MI, or nonfatal stroke. For the purposes of this analysis, in which we sought to better understand the total burden of CV events in patients with T2DM following a recent ACS, we evaluated a broader composite endpoint of CV death, nonfatal MI, nonfatal stroke, or coronary revascularization (either percutaneous coronary intervention or coronary artery bypass grafting). HF has been the topic of a prior analysis and publication; it was not included in this analysis.3
All clinical endpoints evaluated in the trial were adjudicated by a clinical events committee using prespecified definitions based on the guidelines from the US Food and Drug Administration (FDA).4 Fatal events were counted as a single event, such that, if a patient experienced an MI and then had CV death with the cause of death adjudicated as being due to the MI, the event was considered 1 fatal MI event.
2.1. Statistical analysis
Comparisons between baseline characteristics were made with the χ2 test (categorical variables) and Kruskal‐Wallis test (continuous variables). Poisson regression analysis was performed to compare the total number of occurrences of CV death, MI, stroke, UA, and coronary revascularization between all patients in the alogliptin and placebo groups. All statistical analyses were performed by independent academic statisticians using SAS software, version 9.4 (SAS Institute, Inc., Cary, NC) at the Baim Institute for Clinical Research (Boston, MA).
3. RESULTS
Of the 5380 patients randomized in the EXAMINE trial following an ACS event, 1100 patients had ≥1 post‐randomization CV event over a median follow‐up period of 18 months (Table 1). Over this follow‐up period, in the 1100 patients with a CV event during the trial, 380 patients had an additional CV event (resulting in a total of 666 recurrent CV events; Table 1). The majority of patients randomized in the trial did not have an additional CV event (4280 [79.6%]), whereas 720 (13.4%) had a single recurrent CV event and 380 (7.1%) had multiple recurrent CV events. Patients with recurrent CV events were older; had had T2DM for a longer duration; had higher prevalence of HF, peripheral arterial disease, and prior stroke; and were more likely to have renal disease (Table 1).
Table 1.
Baseline characteristics stratified by number of post‐randomization CV events
| Characteristics | 0 Events, n = 4280 | 1 Event, n = 720 | Multiple Events, n = 380 | P Value |
|---|---|---|---|---|
| Alogliptin | 2156 (50.4) | 350 (48.6) | 195 (51.3) | 0.62 |
| Age, y | 60 (54–68) | 62 (55–70) | 62 (55–68) | <0.001 |
| Male sex | 2926 (68.4) | 477 (66.3) | 248 (65.3) | 0.28 |
| Duration of T2DM, y | 6.7 (2.5–13.0) | 9.2 (3.5–15.3) | 9.9 (4.8–16.2) | <0.001 |
| Baseline HbA1c concentration, % | 7.9 (7.2–8.7) | 7.9 (7.2–8.7) | 8.0 (7.3–8.8) | 0.42 |
| BMI, kg/m2 | 28.7 (25.6–32.4) | 28.7 (25.3–32.8) | 29.3 (26.0–33.8) | 0.01 |
| Race | <0.001 | |||
| American Indian/Alaska Native | 91 (2.1) | 16 (2.2) | 3 (0.8) | |
| Asian | 872 (20.4) | 166 (23.1) | 51 (13.4) | |
| Black/African American | 153 (3.6) | 42 (5.8) | 21 (5.5) | |
| Native Hawaiian/other Pacific Islander | 10 (0.2) | 1 (0.1) | 0 (0.0) | |
| White | 3116 (72.8) | 489 (67.9) | 304 (80.0) | |
| Multiracial | 38 (0.9) | 6 (0.8) | 1 (0.3) | |
| Region | <0.001 | |||
| US, Canada | 647 (15.1) | 117 (16.3) | 89 (23.4) | |
| Western Europe, Australia, New Zealand, Middle East | 467 (10.9) | 80 (11.1) | 69 (18.2) | |
| Central/South America, Mexico | 1141 (26.7) | 176 (24.4) | 76 (20.0) | |
| Eastern Europe, Africa | 1216 (28.4) | 193 (26.8) | 99 (26.1) | |
| Asia, Pacific Islands | 809 (18.9) | 154 (21.4) | 47 (12.4) | |
| CV risk factors and history | ||||
| Current smoker | 592 (13.8) | 90 (12.5) | 52 (13.7) | 0.32 |
| HTN | 3484 (81.4) | 642 (89.2) | 343 (90.3) | <0.001 |
| MIa | 0 (0.0) | 137 (19.0) | 227 (59.7) | <0.001 |
| PCIa | 0 (0.0) | 206 (28.6) | 253 (66.6) | <0.001 |
| CABGa | 0 (0.0) | 58 (8.1) | 58 (15.3) | <0.001 |
| CHF | 1137 (26.6) | 251 (34.9) | 113 (29.7) | <0.001 |
| CVA | 262 (6.1) | 87 (12.1) | 39 (10.3) | <0.001 |
| PAD | 369 (8.6) | 84 (11.7) | 61 (16.1) | <0.001 |
| eGFR, mL/min/1.73 m2 | ||||
| Mean ± SD | 72.0 ± 21.0 | 65.9 ±22.1 | 68.1 ±22.7 | <0.001 |
| Median (IQR) | 72.4 (58.1–86.1) | 65.8 (50.7–80.8) | 67.3 (53.2–84.7) | |
| Range (min, max) | 181.9 (4.2, 186.1) | 131.1 (5.0, 136.1) | 155.9 (13.7, 169.6) | |
| <60 mL/min/1.73 m2 | 1157 (27.0) | 286 (39.7) | 122 (32.1) | <0.001 |
| ≥60 mL/min/1.73 m2 | 3123 (73.0) | 434 (60.3) | 258 (67.9) | |
| Index ACS event | 0.07 | |||
| MI | 3273 (76.7) (n = 4267) | 574 (79.7) | 305 (80.5) (n = 379) | |
| UA | 994 (23.3) (n = 4267) | 146 (20.3) | 74 (19.5) (n = 379) | |
| Time from index ACS event to randomization, d | ||||
| Mean ± SD | 48.5 ± 22.1 (n = 4267) | 45.5 ± 21.5 | 44.2 ±21.2 (n = 379) | <0.001 |
| Median (IQR) | 45.0 (30.0–65.0) | 41.0 (28.0–61.0) | 41.0 (27.0–59.0) | |
| Range (min, max) | 133 (8.0, 141.0) | 87 (10.0, 97.0) | 91 (10.0, 101.0) |
Abbreviations: ACS, acute coronary syndrome; BMI, body mass index; CABG, coronary artery bypass grafting; CHF, congestive heart failure; CV, cardiovascular; CVA, cerebrovascular accident; eGFR, estimated glomerular filtration rate; HbA1c, glycated hemoglobin; HTN, hypertension; IQR, interquartile range; max, maximum; MI, myocardial infarction; min, minimum; PAD, peripheral arterial disease; PCI, percutaneous coronary intervention; SD, standard deviation; T2DM, type 2 diabetes mellitus; UA, unstable angina; US, United States.
Data are presented as n (%), mean ±SD, median (IQR), or range.
Prior to randomization and before the index ACS event.
In the patients who had multiple CV events during follow‐up, the burden of recurrent events was high, with the mean number of CV events being 1.6 ±1.2 events per patient (Table 2). There were a considerable number of patients who had multiple events, including 12 patients who had ≥6 events (Figure 1). In the 2701 patients treated with alogliptin, there were 545 initial events and 328 recurrent events; in the 2679 patients treated with placebo, there were 555 initial events and 338 recurrent events (Figure 2A). There was no difference in the number or type of CV events that occurred during follow‐up in the alogliptin or placebo groups (P = 0.52, Poisson; Figure 2B).
Table 2.
No. of events per patient with alogliptin or placebo during follow‐up of the EXAMINE trial
| Endpoints | Alogliptin, n = 2701 | Placebo, n = 2679 | Total, N = 5380 | P Value |
|---|---|---|---|---|
| No. of events per patient | ||||
| Mean ±SD | 1.6 ±1.1 (n = 545) | 1.6 ±1.2 (n = 555) | 1.6 ±1.2 (N = 1100) | 0.54 |
| Median (IQR) | 1.0 (1.0–2.0) | 1.0 (1.0–2.0) | 1.0 (1.0–2.0) | |
| Range (min, max) | 10.0 (1.0, 11.0) | 13.0 (1.0, 14.0) | 13.0 (1.0, 14.0) | |
| CV death | 112 (4.1) | 130 (4.9) | 242 (4.5) | 0.21 |
| Death | 153 (5.7) | 173 (6.5) | 326 (6.1) | 0.223 |
| No. of nonfatal events per patient | ||||
| Mean ± SD | 1.6 ± 1.1 (n = 462) | 1.7 ±1.3 (n = 449) | 1.7 ±1.2 (N = 911) | 0.91 |
| Median (IQR) | 1.0 (1.0–2.0) | 1.0 (1.0–2.0) | 1.0 (1.0–2.0) | |
| Range (min, max) | 10.0 (1.0, 11.0) | 13.0 (1.0, 14.0) | 13.0 (1.0, 14.0) |
Abbreviations: ACS, acute coronary syndrome; CV, cardiovascular; EXAMINE, Examination of Cardiovascular Outcomes With Alogliptin vs Standard of Care in patients with T2DM and ACS; IQR, interquartile range; max, maximum; min, minimum; SD, standard deviation; T2DM, type 2 diabetes mellitus.
Data are presented as n (%), mean ±SD, median (IQR), or range.
Figure 1.
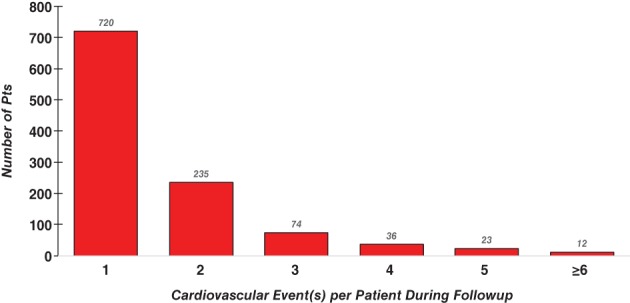
Number of CV events per patient during follow‐up of the EXAMINE trial. Abbreviations: CV, cardiovascular; EXAMINE, EXamination of cArdiovascular outcoMes with alogliptIN versus standard of carE in patients with type 2 diabetes mellitus and acute coronary syndrome; pts, patients
Figure 2.
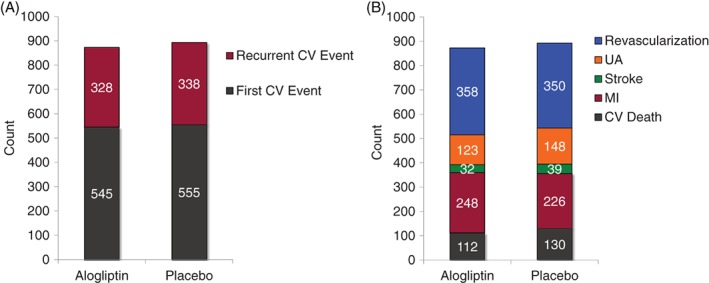
(A) Incidence and (B) number and type of CV events in patients treated with alogliptin or placebo. Abbreviations: CV, cardiovascular; MI, myocardial infarction; UA, unstable angina
Predictors for multiple CV events included having had MI or revascularization prior to the index ACS event that led to study entry (Table 3).
Table 3.
Predictors of recurrent events in patients with T2DM
| Predictors | OR (95% CI) | P Value |
|---|---|---|
| Central/South America, Mexico (as compared with US/Canada) | 1.04 (0.76–1.43) | 0.81 |
| Western Europe, Australia, New Zealand, Middle East (as compared with US/Canada) | 1.26 (0.89–1.79) | 0.19 |
| Eastern Europe, Africa (as compared with US/Canada) | 0.86 (0.63–1.16) | 0.31 |
| Asia, Pacific Islands (as compared with US/Canada) | 0.62 (0.44–0.88) | 0.01 |
| MIa | 119.72 (72.49–197.72) | <0.001 |
| PCIa | 138.79 (85.69–224.80) | <0.001 |
| CABGa | 80.58 (41.52–156.39) | <0.001 |
Abbreviations: ACS, acute coronary syndrome; CABG, coronary artery bypass grafting; CI, confidence interval; MI, myocardial infarction; OR, odds ratio; PCI, percutaneous coronary intervention; T2DM, type 2 diabetes mellitus; US, United States.
Prior to randomization and before the index ACS event.
4. DISCUSSION
In this analysis of the EXAMINE trial, we found that the burden of CV events is high and recurrent events are common in patients with T2DM and recent ACS. Although clinical effectiveness studies, including both randomized controlled clinical trials and observational studies, traditionally focus on the incidence of the first CV event, this underestimates the true burden of CV events, as it does not take into account those patients who have multiple recurrent events.5, 6 Second, we found that those patients with recurrent events are at increased risk of mortality, and the presence of known CVD (prior MI or revascularization) identifies patients with T2DM in whom recurrent events are more common.7 In those patients with these characteristics, intensive medical therapy and close clinical monitoring should be considered in an attempt to minimize the risk of future CV events and death. Importantly, alogliptin neither increased nor decreased the risk of initial or recurrent CV events in patients with T2DM, even after accounting for all the initial and recurrent events that occurred during the study.
During clinical trial follow‐up, data on all CV events of interest are collected; although when performing comparative effectiveness analyses, the reporting is typically performed by analyzing only the first occurrence of a CV event. However, patients, clinicians, and payers have considerable interest in the total burden of CV events.8, 9 Prior studies have found that intensive lipid control with high‐dose statins and more potent antiplatelet therapies can reduce the overall burden of CV events.10, 11 These studies highlight the importance of assessing recurrent events to provide an overall picture of benefit.
The EXAMINE trial was designed to demonstrate the CV safety of alogliptin, a DPP‐4 inhibitor, as required by guidance from the FDA.12 This current analysis of all ischemic CV events that occurred in the EXAMINE trial allows for a more complete assessment of the overall CV safety of alogliptin and provides additional evidence supporting the safety of alogliptin for use in the treatment of patients with T2DM and CVD. We found that 7.1% of the patients enrolled in the EXAMINE trial had multiple CV events following the initial ACS event. Prior studies have found that a small proportion of patients account for the majority of healthcare expenditures in the United States.13 In the EXAMINE study, those patients with recurrent/multiple events were older, had had T2DM for a prolonged period of time, or had HF or prior atherothrombotic disorders (peripheral arterial disease, stroke). Prior ischemic events with either a coronary revascularization or MI were independent predictors of having multiple CV events in our population of patients with T2DM. Identification of patient characteristics associated with a higher likelihood of recurrent events over the short and intermediate term following an ACS are needed to allow for the development of strategies to identify these high‐risk patients. Prior efforts to identify patients who are at high risk of readmission following an HF admission with intensification of follow‐up and therapy have been successful in reducing readmission rates.14 Thus, it is possible that the design of interventions to increase the intensity of therapies in patients with T2DM who are at high risk for additional events could have the potential to improve outcomes while minimizing costs and result in reduced overall healthcare costs.
4.1. Study limitations
These data should be considered in the light of several potential limitations. First, the EXAMINE trial had a median follow‐up time of 18 months, which is relatively short given the duration of time in which patients live with T2DM. Thus, it is possible that differences between therapies could emerge over a longer follow‐up period. Second, in this analysis we did not assess events such as total hospitalizations, serious adverse events, or total healthcare expenditures, which are also important when considering the overall burden related to T2DM. Finally, there could be differences between patients who died and were censored because of the occurrence of a fatal event or concomitant treatments among patients who experienced a nonfatal event during the trial; however, we found no overall differences in the total number of CV events, including death, in patients treated with alogliptin or placebo, making this concern unlikely to be relevant.
5. CONCLUSION
Patients with T2DM and recent ACS commonly have a high burden of recurrent events, and these events seem to be concentrated in a minority of patients. Alogliptin neither increased nor decreased the risk of either first or recurrent CV events when compared with placebo in patients with T2DM and recent ACS. These data support the CV safety of alogliptin in patients with T2DM and increased risk of future CV events. Further studies are needed to identify pharmacotherapies and interventions that can reduce the continued burden of CVD in high‐risk populations of patients with T2DM and ACS.
ACKNOWLEDGMENTS
The authors express their appreciation to the 898 EXAMINE investigators and the 5380 patients who participated in the trial.
Conflicts of interest
Dr. Cavender reports consulting fees from AstraZeneca, Chiesi, Merck, Sanofi‐Aventis, Boehringer Ingelheim, Janssen, and Novo Nordisk; he has received research funding (nonsalary) from Abbott Laboratories, AstraZeneca, Chiesi, GlaxoSmithKline, Merck, Novartis, and Takeda. Dr. White reports personal fees from Takeda USA, during the conduct of the study, and personal fees from AstraZeneca, Novartis, AbbVie, Sanofi‐Aventis, GlaxoSmithKline, and Pfizer Consumer Healthcare; he has received nonfinancial support from Roche Inc. and Wolters Kluwer, outside the submitted work. Dr Bergenstal has received research support, consulted, or has been on the scientific advisory board for Boehringer Ingelheim, Bristol‐Myers Squibb, AstraZeneca, Eli Lilly, Merck, Novo Nordisk, Roche, Sanofi, and Takeda; his employer, the nonprofit HealthPartners Institute, contracts for his services, and no personal income goes to Dr Bergenstal; also, he has inherited Merck stock. Dr. Zannad reports personal trial oversight committees and/or consulting fees from Amgen, AstraZeneca, Janssen, Bayer, Boehringer, Boston Scientific, CVRx, General Electric, Quantum Genomics, LivaNova, Mitsubishi, Novartis, Novo Nordisk, and Vifor Fresenius, and being the founder of CardioRenal and Cardiovascular Clinical Trialists. Dr. Heller has received personal fees from Takeda Development Center, Novo Nordisk, Eli Lilly, and Boeringher Ingelheim, and has served on speaker bureaus for Eli Lilly, Novo Nordisk, and AstraZeneca. Dr. Cushman has provided uncompensated consulting to Takeda and Novartis. Dr. Cannon reports research grants from Amgen, Arisaph, Boehringer‐Ingelheim, Bristol‐Myers Squibb, Daiichi Sankyo, Janssen, Merck, and Takeda. He reports consulting fees from Alnylam, Amgen, Amarin, Arisaph, AstraZeneca, Boehringer Ingelheim, Bristol‐Myers Squibb, Eisai, GlaxoSmithKline, Kowa, LipimetiX, Merck, Pfizer, Regeneron, Sanofi, and Takeda. The authors declare no other potential conflicts of interest.
Cavender MA, White WB, Liu Y, et al. Total cardiovascular events analysis of the EXAMINE trial in patients with type 2 diabetes and recent acute coronary syndrome. Clin Cardiol. 2018;41:1022–1027. 10.1002/clc.22960
Funding information Takeda Pharmaceuticals North America
REFERENCES
- 1. White WB, Cannon CP, Heller SR, et al; EXAMINE Investigators . Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med. 2013;369:1327–1335. [DOI] [PubMed] [Google Scholar]
- 2. White WB, Bakris GL, Bergenstal RM, et al. EXamination of cArdiovascular outcoMes with alogliptIN versus standard of carE in patients with type 2 diabetes mellitus and acute coronary syndrome (EXAMINE): a cardiovascular safety study of the dipeptidyl peptidase‐4 inhibitor alogliptin in patients with type 2 diabetes with acute coronary syndrome. Am Heart J. 2011;162:620.e1–626.e1. [DOI] [PubMed] [Google Scholar]
- 3. Zannad F, Cannon CP, Cushman WC, et al; EXAMINE Investigators . Heart failure and mortality outcomes in patients with type 2 diabetes taking alogliptin versus placebo in EXAMINE: a multicentre, randomised, double‐blind trial. Lancet. 2015;385:2067–2076. [DOI] [PubMed] [Google Scholar]
- 4. Hicks KA, Tcheng JE, Bozkurt B, et al. 2014 ACC/AHA Key Data Elements and Definitions for Cardiovascular Endpoint Events in Clinical Trials: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Data Standards (Writing Committee to Develop Cardiovascular Endpoints Data Standards) [published correction appears in J Am Coll Cardiol 2015;66:982]. J Am Coll Cardiol. 2015;66:403–469. [DOI] [PubMed] [Google Scholar]
- 5. Giorda CB, Avogaro A, Maggini M, et al; Diabetes and Informatics Study Group . Recurrence of cardiovascular events in patients with type 2 diabetes: epidemiology and risk factors. Diabetes Care. 2008;31:2154–2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hess CN, Clare RM, Neely ML, et al. Differential occurrence, profile, and impact of first recurrent cardiovascular events after an acute coronary syndrome. Am Heart J. 2017;187:194–203. [DOI] [PubMed] [Google Scholar]
- 7. White WB, Kupfer S, Zannad F, et al; EXAMINE Investigators . Cardiovascular mortality in patients with type 2 diabetes and recent acute coronary syndromes from the EXAMINE trial. Diabetes Care. 2016;39:1267–1273. [DOI] [PubMed] [Google Scholar]
- 8. Zhuo X, Zhang P, Hoerger TJ. Lifetime direct medical costs of treating type 2 diabetes and diabetic complications. Am J Prev Med. 2013;45:253–261. [DOI] [PubMed] [Google Scholar]
- 9. Brandle M, Zhou H, Smith BR, et al. The direct medical cost of type 2 diabetes. Diabetes Care. 2003;26:2300–2304. [DOI] [PubMed] [Google Scholar]
- 10. Kohli P, Wallentin L, Reyes E, et al. Reduction in first and recurrent cardiovascular events with ticagrelor compared with clopidogrel in the PLATO Study. Circulation. 2013;127:673–680. [DOI] [PubMed] [Google Scholar]
- 11. Murphy SA, Cannon CP, Wiviott SD, et al. Reduction in recurrent cardiovascular events with intensive lipid‐lowering statin therapy compared with moderate lipid‐lowering statin therapy after acute coronary syndromes from the PROVE IT‐TIMI 22 (Pravastatin or Atorvastatin Evaluation and Infection Therapy–Thrombolysis In Myocardial Infarction 22) trial. J Am Coll Cardiol. 2009;54:2358–2362. [DOI] [PubMed] [Google Scholar]
- 12. Gaglia MA Jr, Waksman R. Overview of the 2010 Food and Drug Administration Cardiovascular and Renal Drugs Advisory Committee meeting regarding ticagrelor. Circulation. 2011;123:451–456. [DOI] [PubMed] [Google Scholar]
- 13. US Department of Health and Human Services, Agency for Healthcare Research and Quality , Stanton MW, Rutherford M. The High Concentration of U.S. Health Care Expenditures. Rockville, MD: Agency for Healthcare Research and Quality; 2005. Research in Action, Issue 19. AHRQ publication 06–0060. [Google Scholar]
- 14. Feltner C, Jones CD, Cené CW, et al. Transitional care interventions to prevent readmissions for persons with heart failure: a systematic review and meta‐analysis. Ann Intern Med. 2014;160:774–784. [DOI] [PubMed] [Google Scholar]


