Abstract
Background
Left ventricular thrombosis (LVT) is a well‐known complication of acute myocardial infarction, most commonly seen in anterior wall ST‐segment elevation myocardial infarction (STEMI). It is associated with systemic thromboembolism.
Hypothesis
Our aim was to evaluate the impact of LVT on in‐hospital mortality, thromboembolism, and bleeding in patients with anterior STEMI.
Methods
Data was collected from the Nationwide Inpatient Sample where patients with a primary diagnosis of “Anterior STEMI” [ICD9‐CM code 410.1] were included. Comparisons were made between patients with LVT [ICD9‐CM code 429.79] vs those without using propensity score matching (PSM).
Results
From 2002 to 2014, there were 157 891 cases of anterior STEMI. Among these, 649 (0.4%) had LVT. Post‐PSM, there was no difference in in‐hospital mortality between the groups with LVT and without (7.3% vs 8.6%). Thromboembolic event rate was higher with LVT compared to those without LVT (7.3% vs 2.1%). There was no difference in bleeding events between patients with LVT and those without (2.9% vs 3.2%). The baseline average length of stay in the group with LVT was longer than the group without LVT (7.9 ± 6.7 days vs 5.1 ± 6.0 days). The average hospitalization‐related costs were also significantly higher among patients with LVT compared to those without (95 598 USD vs 66 641 USD per stay) at baseline.
Conclusion
Among patients hospitalized with anterior STEMI, presence of LVT is associated with increased thromboembolic events, average length of hospital stay and average cost of hospitalization. However, it is not associated with increased in‐hospital mortality or bleeding events.
Keywords: anterior STEMI, cardiac thrombosis, left ventricular thrombus, thromboembolism
1. INTRODUCTION
Left ventricular thrombosis (LVT) is a known complication of acute myocardial infarction (AMI). LVT is most commonly seen in the setting of anterior ST‐segment elevation myocardial infarction (STEMI), which accounts for more than 90% of cases.1, 2, 3, 4 Occlusion of the left anterior descending artery results in a large infarcted area in the anterior wall of the left ventricle which contributes to stasis of blood flow, thus acting as a nidus for LVT formation.5 Several factors have been associated with increased risk of developing LVT, including but not limited to anterior wall infarct, left ventricular ejection fraction <30%, apical wall akinesis/dyskinesis, and LV aneurysm formation.1, 2, 3, 5, 6
The incidence of LVT in AMI in pre‐reperfusion therapy era was 7%‐46%.5, 7, 8 Following the discovery of percutaneous coronary intervention (PCI), the incidence of LVT in AMI decreased significantly to 4%‐6%.9, 10, 11
Systemic embolization is one of the most feared complications of LVT and occurs in 10%‐15% of patients not treated with anticoagulation.12, 13 Most thromboembolic events occur during the first 3 to 4 months after AMI.12, 13, 14, 15, 16, 17, 18 Several observational studies have shown that anticoagulation reduced the risk of embolization in patients with LVT after MI.7, 13, 14, 18
LVT is associated with significant morbidity however, there has been no study to date that evaluates early mortality related to LVT in AMI. The aim of this study was to evaluate in‐hospital mortality among STEMI patients with LVT. Additional endpoints of the study were rates of thromboembolic and bleeding events, length of hospital stay and total cost of hospitalization.
2. METHODS
2.1. Data source and study population
We conducted our analysis on unweighted hospital discharge data from the Healthcare Cost and Utilization Project—Nationwide Inpatient Sample (HCUP‐NIS) from 2002 through 2014. The Nationwide Inpatient Sample (NIS) is the largest, publicly available, all‐payer inpatient database in the United States. Annually, the NIS is composed of discharge‐level data from roughly 8 million hospitalizations and approximates a stratified sample of 20% of community hospitals in the United States. Each hospitalization within the database contains clinical and resource‐use information. Patients' diagnoses are documented in parallel, as both International Classification of Disease‐ninth edition‐Clinical Modification (ICD9‐CM) and clinically meaningful clusters of ICD9‐CMs, termed Clinical Classification Software (CCS) codes. Core hospital stay files contain details on patient demographics (eg, age, sex, race), International Classification of Diseases, ninth Revision, Clinical Modification (ICD9‐CM) diagnosis codes (15‐30, depending on the year), Elixhauser,19 comorbidities, length of hospital stay, discharge status, in‐hospital mortality and total charges among other variables. A comprehensive synopsis on NIS data is available at http://www.hcup-us.ahrq.gov. Patients aged more than 18 years who were admitted with a primary diagnosis of “Anterior STEMI” (ICD9‐CM codes 410.1x) were included in the study. Patients with missing mortality data and those transferred out of hospital were excluded from the analysis. Presence of cardiac thrombosis (CAT) was identified using the ICD9‐CM code 429.79. Death was defined within the NIS as in‐hospital mortality. Bleeding event was defined by the presence of at least one the following: hemorrhagic stroke/intracranial bleeding, gastrointestinal bleeding, hemoptysis, and unspecified hemorrhage (Table A1, Appendix). An arterial thromboembolic event was defined as the presence of either acute stroke or transient ischemic attack (TIA), acute mesenteric ischemia or atheroembolism. Comparisons were made between patients with CAT vs those without. In‐hospital mortality was the primary endpoint whereas arterial thromboembolism and bleeding event rates were analyzed as secondary endpoints. Different comorbidities were identified by using ICD9‐CM diagnoses and diagnosis‐related group (see Table A1). CAT has been used interchangeably with LVT throughout manuscript for simplicity.
2.2. Statistics
Demographics and baseline characteristics were summarized using descriptive statistics. Continuous data was expressed as mean ± one SD and analyzed using the student's t‐test or analysis of variance. Fischer's exact test or Pearson's χ 2 test was used for analysis of categorical variables. Trend analyses were performed using the Mantel‐Haenszel test of trend. Propensity score matching was used to identify a cohort of patient with similar baseline characteristics to those with LVT. Similar methods have been used in previously published data.20, 21 Propensity score matching was performed using 1:1 matching protocol without replacement and caliper width 10−3 of SD of the logit of propensity score using LVT as treatment variable. Matching variables included patient demographics, admission details, hospital characteristics, year of admission, comorbidities, and associated risk score as shown in Table 1. All study measures were compared between cases and non‐cases before and after matching. Results were considered statistically significant for P values < 0.05. IBM SPSS statistics version 23.0 (Armonk, New York) was used to perform data analysis.
Table 1.
Characteristics of patients with anterior STEMI, before and after propensity matching
| Before propensity‐score matching | After propensity‐score matching¥ | |||||
|---|---|---|---|---|---|---|
| Characteristics | Acute anterior STEMI without cardiac thrombosis (n = 157 242) | Acute anterior STEMI with cardiac thrombosis (n = 649) | P value | Acute anterior STEMI without cardiac thrombosis (n = 618) | Acute anterior STEMI with cardiac thrombosis (n = 618) | P value |
| Age in years (mean ± SD) | 63.9 ± 14.5 | 62.1 ± 13.7 | 0.002 | 61.6 ± 14.0 | 62.3 ± 13.73 | 0.40 |
| Sex‐female (%) | 34.0 | 26.5 | <0.001 | 26.1 | 27.0 | 0.74 |
|
Race
Caucasian (%) |
60.6 |
59.9 |
0.54 |
59.9 |
60.0 |
0.76 |
| African American (%) | 5.8 | 6.9 | 6.6 | 6.8 | ||
| Hispanic (%) | 6.0 | 6.6 | 5.3 | 6.6 | ||
| Other or missing (%) | 27.5 | 26.5 | 28.2 | 26.5 | ||
| Weekend admission (%) | 26.8 | 23.1 | 0.03 | 24.3 | 23.5 | 0.73 |
| Elective admission (%) | 7.7 | 8.2 | 0.63 | 7.8 | 8.4 | 0.69 |
| Payer information | 0.002 | 0.52 | ||||
| Medicare | 45.4 | 41.9 | 41.9 | 42.4 | ||
| Medicaid | 6.4 | 8.9 | 8.7 | 8.6 | ||
| Private | 36.4 | 35.7 | 35.9 | 35.8 | ||
| Self‐pay | 7.8 | 7.7 | 9.1 | 7.4 | ||
| No charge | 0.7 | 1.7 | 1.9 | 1.6 | ||
| Other | 3.4 | 4.0 | 2.4 | 4.2 | ||
| Hospital region | <0.001 | 0.81 | ||||
| Northwest | 17.9 | 23.3 | 22.7 | 22.7 | ||
| Midwest | 22.1 | 24.0 | 24.6 | 23.9 | ||
| South | 39.7 | 30.7 | 29.6 | 31.9 | ||
| West | 20.2 | 22.0 | 23.1 | 21.5 | ||
| Hospital bedsize | 0.09 | 0.28 | ||||
| Small | 9.1 | 6.6 | 4.9 | 7.0 | ||
| Medium | 23.1 | 22.9 | 23.9 | 23.1 | ||
| Large | 67.8 | 70.5 | 71.2 | 70.0 | ||
| Location/teaching status of hospital | <0.001 | 0.75 | ||||
| Rural | 8.9 | 6.0 | 5.3 | 6.3 | ||
| Urban non‐teaching | 42.5 | 34.0 | 35.8 | 35.4 | ||
| Urban teaching | 48.5 | 60.0 | 58.9 | 58.3 | ||
| Year | <0.001 | 0.96 | ||||
| 2002 | 12.3 | 8.0 | 9.4 | 8.4 | ||
| 2003 | 11.3 | 8.3 | 10.5 | 8.7 | ||
| 2004 | 9.5 | 7.1 | 6.8 | 7.4 | ||
| 2005 | 8.6 | 6.5 | 6.5 | 6.8 | ||
| 2006 | 9.0 | 9.9 | 10.7 | 10.0 | ||
| 2007 | 7.7 | 7.9 | 7.3 | 8.3 | ||
| 2008 | 7.7 | 10.2 | 9.7 | 10.2 | ||
| 2009 | 6.9 | 7.9 | 8.6 | 7.9 | ||
| 2010 | 5.7 | 8.0 | 7.8 | 7.6 | ||
| 2011 | 5.8 | 5.9 | 5.3 | 5.8 | ||
| 2012 | 5.4 | 6.3 | 4.2 | 6.1 | ||
| 2013 | 5.1 | 7.2 | 6.5 | 6.0 | ||
| 2014 | 5.0 | 6.9 | 6.8 | 6.6 | ||
| Hypertension with and without complications (%) | 54.7 | 51.9 | 0.14 | 51.3 | 52.0 | 0.80 |
| Diabetes without complications (%) | 22.9 | 24.0 | 0.50 | 26.2 | 23.2 | 0.22 |
| Diabetes with complications (%) | 3.0 | 4.4 | 0.04 | 3.8 | 3.4 | 0.73 |
| Dyslipidemia (%) | 48.0 | 48.4 | 0.86 | 48.1 | 48.2 | 0.95 |
| Atrial fibrillation or flutter (%) | 12.4 | 16.9 | <0.001 | 15.2 | 16.0 | 0.69 |
| Chronic pulmonary disease (%) | 14.9 | 11.1 | 0.006 | 12.4 | 11.6 | 0.68 |
| Current or past smoker (%) | 36.5 | 35.1 | 0.46 | 41.1 | 36.7 | 0.11 |
| History of stroke or TIA (%) | 2.7 | 1.8 | 0.18 | 1.1 | 1.9 | 0.35 |
| History of myocardial infarction (%) | 6.2 | 5.2 | 0.32 | 5.5 | 4.2 | 0.29 |
| History of DVT or PE (%) | 1.1 | 1.7 | 0.18 | 1.6 | 1.6 | 1.00 |
| History of GI disorders (%) | 0.9 | 0.3 | 0.10 | 0.2 | 0.3 | 0.56 |
| Drug abuse (%) | 2.1 | 2.5 | 0.43 | 2.8 | 2.6 | 0.84 |
| Alcohol abuse (%) | 2.9 | 3.9 | 0.13 | 5.9 | 3.8 | 0.07 |
| Peripheral vascular disorders (%) | 6.1 | 7.6 | 0.09 | 8.1 | 7.5 | 0.71 |
| Aortic atherosclerosis (%) | 1.6 | 3.7 | <0.001 | 3.9 | 3.4 | 0.64 |
| Deficiency anemias (%) | 8.9 | 7.9 | 0.41 | 8.1 | 7.5 | 0.71 |
| Chronic blood loss anemia | 0.8 | 0.6 | 0.64 | 0.5 | 0.7 | 0.71 |
| Collagen vascular disease or rheumatoid arthritis (%) | 1.7 | 1.9 | 0.81 | 1.5 | 2.0 | 0.52 |
| Hypothyroidism (%) | 6.6 | 4.8 | 0.06 | 6.1 | 4.7 | 0.29 |
| Liver disease (%) | 0.8 | 0.8 | 0.95 | 0.8 | 0.8 | 0.99 |
| Renal failure (%) | 7.0 | 7.9 | 0.33 | 6.8 | 7.4 | 0.68 |
| Obesity (%) | 9.2 | 6.5 | 0.02 | 6.9 | 6.4 | 0.70 |
| Depression (%) | 4.4 | 4.7 | 0.73 | 4.6 | 4.7 | 0.91 |
| Coagulopathy (%) | 3.6 | 4.0 | 0.57 | 4.5 | 3.9 | 0.64 |
| Hypercoagulable disorder (%) | 0.2 | 0.6 | 0.05 | 0.2 | 0.3 | 0.56 |
| Obstructive sleep apnea (%) | 1.5 | 2.0 | 0.28 | 1.1 | 1.9 | 0.24 |
| Long‐term anticoagulant use (%) | 1.7 | 4.6 | <0.001 | 3.2 | 3.9 | 0.54 |
| Long‐term antiplatelet use (%) | 4.8 | 4.6 | 0.83 | 5.2 | 4.7 | 0.69 |
| Swan‐Ganz catheter insertion (%) | 5.7 | 9.4 | <0.001 | 8.7 | 8.7 | 1.00 |
| Coronary artery bypass grafting (%) | 9.1 | 7.2 | 0.10 | 9.2 | 7.3 | 0.21 |
| Percutaneous coronary intervention (%) | 64.4 | 64.9 | 0.80 | 64.4 | 65.2 | 0.76 |
| Intra‐aortic balloon pump use (%) | 11.6 | 18.0 | <0.001 | 15.9 | 16.7 | 0.70 |
| Shock (%) | 10.4 | 17.9 | <0.001 | 15.0 | 16.0 | 0.63 |
| Acute cardiorespiratory failure (%) | 13.7 | 23.0 | <0.001 | 17.6 | 20.6 | 0.19 |
| Vasopressor use (%) | 0.8 | 1.2 | 0.26 | 0.6 | 1.1 | 0.36 |
| Ventilator use (%) | 9.3 | 10.2 | 0.45 | 10.2 | 8.9 | 0.43 |
| Lymphoma (%) | 0.4 | 0.6 | 0.26 | 0.7 | 0.5 | 0.69 |
| Metastatic cancer (%) | 0.6 | 0.7 | 0.86 | 1.2 | 0.7 | 0.35 |
| Solid tumor without metastasis (%) | 1.6 | 1.7 | 0.80 | 1.3 | 1.8 | 0.49 |
Abbreviations: DVT, deep vein thrombosis; PE, pulmonary embolism; SD: SD, APRDRG, all patient refined diagnosis‐related groups; TIA, transient ischemic attack. ¥ Standardized differences for all variables <0.1.
3. RESULTS
3.1. Baseline characteristics
A total of 157 891 patients with the primary diagnosis of anterior STEMI were included, of which 649 (0.4%) cases had LVT. Table 1 shows the baseline characteristics of the study population in patients with anterior STEMI depending on the presence of LVT. A great majority of the admissions occurred at large, urban teaching hospitals. Before propensity matching, patients with LV thrombus were younger (62.1 ± 13.7 vs 63.9 ± 14.5 years; P < 0.001) and a majority of them were males (73.5% vs 66%; P < 0.001) compared to those without LVT. Patients with LVT had a higher incidence of atrial fibrillation (16.9% vs 12.4%; P < 0.001), aortic atherosclerosis (3.7% vs 1.6%; P < 0.001), use of long‐term anticoagulation (4.6% vs 1.7%; P < 0.001), use of Swan‐Ganz catheter (9.4% vs 5.7%; P < 0.001), intra‐aortic balloon pump use (18.0% vs 11.6%; P < 0.001), cardiogenic shock (17.9% vs 10.4%; P < 0.001), and acute respiratory failure (23% vs 13.7%; P < 0.001) compared to patients without LVT. Conversely; patients without LVT were more likely to have COPD (14.9% vs 11.1%; P = 0.006) and obesity (9.2% vs 6.5%; P = 0.02). There were similarities in the comorbid conditions such as hypertension, diabetes, dyslipidemia, current or prior smoking, alcohol or drug abuse; prior stroke or myocardial infarction; peripheral vascular disease, deficiency anemias, collagen vascular diseases, liver diseases, hypothyroidism, chronic kidney disease, coagulopathy, cancer, use of PCI or coronary artery bypass grafting, vasopressor use and ventilator use, between the two groups as noted in Table 1.
3.2. Study endpoints
The mean length of stay in the group with LVT was longer than the group without LVT (7.9 ± 6.7 days vs 5.1 ± 6.0 days; P < 0.01) at baseline. The baseline average cost of healthcare per patient in the group with LVT was also higher compared to the group without LVT ($95 598 vs $66 641; P < 0.01).
Table 2 shows the outcome comparisons for primary and secondary endpoints. Annual trends in outcomes at baseline can be seen in Figure 1. At baseline, in‐hospital mortality occurred in 8.5% of acute anterior STEMI without LVT (n = 157 242) and 7.7% of acute anterior STEMI with LVT (n = 649). There was no significant difference in the in‐hospital mortality between the two groups (P = 0.462). After propensity matching using the 1:1 matching protocol, we matched 618 patients with anterior STEMI with LVT to 618 patients with anterior STEMI without LVT. There was no difference in in‐hospital mortality between the two groups (8.6% vs 7.3%; P = 0.4) after propensity matching.
Table 2.
Primary and secondary outcomes among propensity matched groups
| Before propensity‐score matching | After propensity‐score matching | |||||||
|---|---|---|---|---|---|---|---|---|
| Endpoint | Acute anterior STEMI without cardiac thrombosis (n = 157 242) | Acute anterior STEMI with cardiac thrombosis (n = 649) | Odds ratio (95% confidence intervals) | P value | Acute anterior STEMI without cardiac thrombosis (n = 618) | Acute anterior STEMI with cardiac thrombosis (n = 618) | Odds ratio (95% confidence intervals) | P value |
| Primary endpoint | ||||||||
| Hospital mortality | 8.5 | 7.7 | 0.89 (0.67‐1.19) | 0.462 | 8.6 | 7.3 | 0.83 (0.55‐1.26) | 0.400 |
| Secondary endpoints | ||||||||
| Thromboembolic event a | 1.8 | 6.9 | 4.17 (3.07‐5.65) | <0.001 | 2.1 | 7.3 | 3.65 (1.95‐6.84) | <0.001 |
| Bleeding event b | 2.4 | 2.8 | 1.18 (0.74‐1.89) | 0.478 | 3.2 | 2.9 | 0.89 (0.47‐1.71) | 0.742 |
Thromboembolic event defined as presence of at least one of following: acute ischemia stroke, transient ischemic attack, acute mesenteric ischemia, or atheroembolic event.
Bleeding event defined as presence of at least one of following: hemorrhagic stroke, intracranial bleeding, gastrointestinal bleeding, hemoptysis, and other unspecified hemorrhage.
Figure 1.
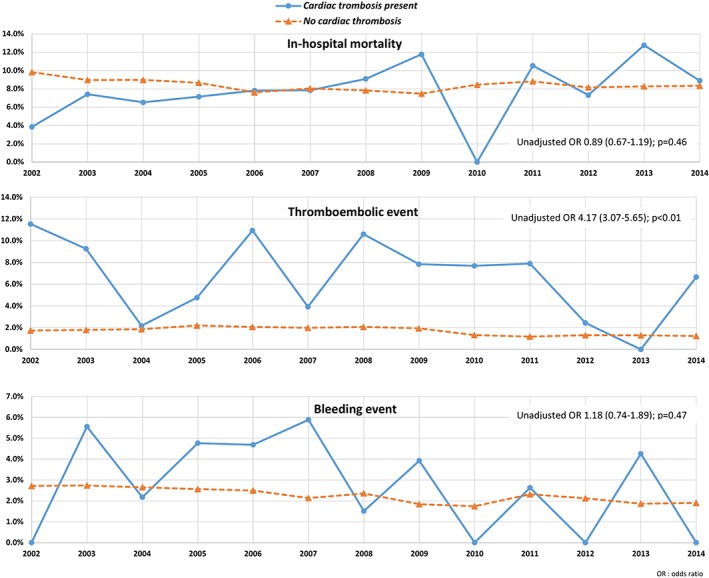
Annual trends in primary and secondary endpoints among patients with an anterior ST‐segment elevation myocardial infarction (STEMI) depending on the presence of left ventricular thrombosis (LVT)
Within the pre‐matched sample, thromboembolic event rates were higher in the group with LVT compared to the group without LVT (6.9% vs 1.8%; P < 0.01). This difference was maintained after propensity matching (7.3% vs 2.1%; P < 0.01). On the other hand, bleeding events did not differ significantly among those with LVT and those with LVT at baseline (2.8% vs 2.4% respectively; P = 0.47). Propensity matching revealed a similar lack of difference in bleeding event rates among those with and without LVT (2.9 vs 3.2%; P = 0.74).
Patients with LVT had higher rates of ischemic stroke (4.2% vs 1.2%; P < 0.01), transient ischemic attack (1.7% vs 0.2%; P < 0.01) and atheroembolism (1.1% vs 0.4%; P < 0.01). There was no difference in rates of hemorrhagic stroke, GI bleeding and acute mesenteric ischemia among patients with LVT compared to those without.
4. DISCUSSION
The major finding of this study is that patients with LVT post anterior STEMI had significantly increased thromboembolic event rates compared to patients without LVT. However, there was no significant difference in the inpatient mortality rates and bleeding rates between the two groups.
Using a large cohort of real‐world anterior STEMI patients across the United States, we found that the rate of cardiac thrombosis in the percutaneous coronary intervention era has decreased considerably. While the incidence of LV thrombus in pre‐perfusion era was as high as 40%,7, 8 there are few studies on LV thrombus post‐PCI. In a study by Weinsaft et al,3 using delayed cardiac magnetic resonance (D‐CMR), LV thrombus was present in 8% of post‐STEMI patients treated with PCI. Compared to D‐CMR, echocardiography diagnosed LV thrombus in only 1/third of patients with LV thrombus detected by D‐CMR. This means that although PCI has substantially decreased the rates of LV thrombus, a great percentage of LV thrombus are potentially missed depending on the imaging modality performed.
Our study showed that there was no significant difference in hospital mortality among anterior STEMI patients with and without LVT. To the best of our knowledge, this study is the first and largest to compare the rates of in‐hospital mortality between STEMI patients with and without LVT. The overall mortality rates in STEMI (7.7% w/LVT vs 8.5% w/o LVT) in our study are comparable to reported 30‐day mortality rate, of 2.5%‐10% in patients admitted with STEMI.22, 23, 24, 25, 26
In our study, the rate of thromboembolic events was 4‐fold higher among patients with anterior STEMI with LVT compared to those without LVT. In a prospective observational study by Stratton and Resnick, systemic thromboembolism occurred in 13% patients with LV thrombus after a mean follow‐up of 22 months.12, 13 The actual incidence of early thromboembolism in real practice is expected to be higher than that observed in our study (6.9%), as most embolic events occur within first 3 to 4 months after discharge. The majority of evidence evaluating anticoagulation for LV thrombus is based on data in the pre‐PCI era. The European guidelines recommend vitamin‐K antagonist for at least 3 to 6 months, while the American guidelines recommend indefinite treatment in patients without increased risk of bleeding.27 Both guidelines suggest reevaluation of LV thrombus with routine imaging and considering stopping anticoagulation with continuation of antiplatelet therapy. Prophylactic warfarin without LVT on echocardiography has been studied in high‐risk STEMI patients (anterior wall infarct and LVEF ≤ 40%).28 It was not associated with reduced thromboembolic events but was associated with increased risk of bleeding. Surprisingly, lower all‐cause mortality was noted at 1‐year. In the future, studies are needed to establish use of contrast imaging and anticoagulation in the post‐PCI era.
We did not find a significant difference in the in‐hospital bleeding events between anterior STEMI patients with and without LVT. Patients with an LVT are usually treated with vitamin‐K antagonist for at least 3 months29 in addition to DAPT, thus increasing the bleeding risk. A literature review revealed that patients who received vitamin‐K antagonist in addition to DAPT had 12% risk of bleeding per year compared to 3.7% in patients who received DAPT alone.5, 30 Our findings suggest that it may be reasonable to use an aggressive antithrombotic approach in the inpatient setting, considering a lack of increase in bleeding among cardiac thrombosis patients, however we cannot be certain given the lack of information on concurrent medication use and the future bleeding events the occurred beyond index hospitalization. Nonetheless, the marked increase in thromboembolic events does warrant attention from clinicians and a possible need for using timely and aggressive antithrombotic therapies while monitoring for bleeding.
We noted that development of LVT in patients with anterior STEMI during index hospitalization increases mean length of stay by 3 days and average cost of hospitalization by 30 000 USD. Development of post‐MI CAT requires prolonged monitoring and initiation of anticoagulation with bridging in these patients.
Our study has several limitations due to the administrative nature of the national databases and their reliance on accuracy of coding as noted in previously published studies evaluating myocardial infarction patients.31, 32 The nature of the codes available may not have been completely representative of LV thrombus and thus the incidence of thrombus in our study was far lower than previously reported, and can be attributed to the reliance on coding. In addition, the ICD9‐CM code is used for CAT though we used it interchangeably with LVT. Important factors that may contribute to LVT such infarct size, ejection fraction, door‐to‐balloon time, sudden cardiac arrest were not studied. Imaging findings such as thrombus mobility and thrombus protrusion which are shown to be associated with thromboembolism could not be studied. The lack of data on the antithrombotic regimen used is another major limitation. However, these limitations are counterbalanced by the considerable sample size and absence of selection bias. The findings of our study are limited to index stay and cannot be extrapolated to post‐discharge events. It is also likely that the actual rate of detection of LVT and occurrence of thromboembolic or bleeding events may be underestimated because of undercoding for less severe forms of disease such as low risk bleeding events. The temporal sequence of detection of thrombosis and occurrence of events cannot be ascertained, and information on the type or degree of anticoagulation is not available due to administrative nature of database.
5. CONCLUSION
Using a large national hospital database, we found that LVT in the presence of an anterior STEMI is associated with increased in‐hospital thromboembolic events, length of hospital stay and total cost of hospitalization. In contrast, anterior STEMI with LVT was not associated with higher in‐hospital mortality or bleeding risks. These data suggest that among patients found to have LVT following anterior STEMI, event rates remain high and judicious but early use of anticoagulant strategies may be warranted. Prospective data on the natural course of LVT on anticoagulation among patients with anterior STEMI, and the associated complication rates is needed.
ACKNOWLEDGMENTS
No study specific funding was used to support this work. The authors are solely responsible for the study design, conduct and analyses, drafting and editing of the manuscript and its final contents. All authors had access to the data and a role in writing the manuscript.
Conflict of interest
The authors declare no potential conflict of interests.
Table A1.
ICD‐9CM codes used for diagnoses and procedures
| Variable | ICD‐9CM codes |
|---|---|
| Hypertension with and without complications, diabetes with and without complications, chronic pulmonary disease, peripheral vascular disorders, renal failure, obesity, coagulopathy, liver disease, depression, valvular disease, drug abuse, alcohol abuse, deficiency anemia, chronic blood loss anemia, collagen vascular disease or rheumatoid arthritis, hypothyroidism, liver disease, lymphoma, metastatic cancer, solid tumor without metastasis, depression, coagulation disorder | Elixhauser comorbidity measures within database |
| Dyslipidemia | 272.0, 272.1, 272.2, 272.3, 272.4 |
| Current or past smoker | 305.1, V158.2 |
| History of stroke without residual defects or transient ischemic attack | V125.4, 438 |
| History of myocardial infarction | 412 |
| History of DVT or PE | V125.1, V125.5 |
| History of GI disease | V127.x |
| Long‐term antiplatelet use | V586.3, V586.6 |
| Long‐term anticoagulant use | V586.1 |
| Vasopressor use | Procedure code 00.17 |
| Ventilator use | Procedure codes 967.0, 967.1, 967.2, 960.4, 960.5 |
| Atrial fibrillation or flutter | 427.31, 427.32 |
| Ventricular tachycardia or fibrillation | 427.1, 427.41, 427.42 |
| Coronary artery disease | 414.Xx, V458.1, V458.2 |
| Non ST elevation MI | 410.70, 410.71, 410.72 |
| Hypercoagulable disease | 289.81, 289.82, 270.4 |
| Ischemic stroke | 433.x1 |
| Transient ischemic attack | 435.x |
| Shock | 785.5x |
| Percutaneous coronary intervention | Procedure codes 00.66, 17.55, 36.01, 36.02, 36.05, 36.06, 36.07 |
| Coronary artery bypass grafting | Procedure codes 361.x |
| Swan‐Ganz catheter insertion | 372.1, 372.3, 896.3, 896.4, 896.6, 896.7, 896.8 |
| Intra‐aortic balloon pump use | Procedure code 376.1 |
| Acute mesenteric ischemia | 557.0 |
| Atheroembolism | 445.0x, 445.8x, 444 |
| Hemorrhagic stroke or intracranial bleed | 430, 431, 432.x |
| Gastrointestinal bleeding | 578.0, 578.1, 578.9 |
| Hemoptysis | 786.30, 786.31, 786.39 |
| Unspecified hemorrhage | 459.0 |
| Transfusion of blood products | V58.2, procedure codes 99.0x |
Ram P, Shah M, Sirinvaravong N, et al. Left ventricular thrombosis in acute anterior myocardial infarction: Evaluation of hospital mortality, thromboembolism, and bleeding. Clin Cardiol. 2018;41:1289–1296. 10.1002/clc.23039
REFERENCES
- 1. Asinger RW, Mikell FL, Elsperger J, Hodges M. Incidence of left‐ventricular thrombosis after acute transmural myocardial infarction. Serial evaluation by two‐dimensional echocardiography. N Engl J Med. 1981;305(6):297‐302. 10.1056/NEJM198108063050601. [DOI] [PubMed] [Google Scholar]
- 2. Halperin JL, Fuster V. Left ventricular thrombus and stroke after myocardial infarction: toward prevention or perplexity? J Am Coll Cardiol. 1989;14(4):912‐914. [DOI] [PubMed] [Google Scholar]
- 3. Weinsaft JW, Kim J, Medicherla CB, et al. Echocardiographic algorithm for post‐myocardial infarction LV thrombus: a gatekeeper for thrombus evaluation by delayed enhancement CMR. JACC Cardiovasc Imaging. 2016;9(5):505‐515. 10.1016/j.jcmg.2015.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Abdelmoneim SS, Pellikka PA, Mulvagh SL. Contrast echocardiography for assessment of left ventricular thrombi. J Ultrasound Med. 2014;33(8):1337‐1344. 10.7863/ultra.33.8.1337. [DOI] [PubMed] [Google Scholar]
- 5. Delewi R, Zijlstra F, Piek JJ. Left ventricular thrombus formation after acute myocardial infarction. Heart. 2012;98(23):1743‐1749. 10.1136/heartjnl-2012-301962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chiarella F, Santoro E, Domenicucci S, Maggioni A, Vecchio C. Predischarge two‐dimensional echocardiographic evaluation of left ventricular thrombosis after acute myocardial infarction in the GISSI‐3 study. Am J Cardiol. 1998;81(7):822‐827. 10.1016/S0002-9149(98)00003-4. [DOI] [PubMed] [Google Scholar]
- 7. Weinreich DJ, Burke JF, Pauletto FJ. Left ventricular mural thrombi complicating acute myocardial infarction. Long‐term follow‐up with serial echocardiography. Ann Intern Med. 1984;100(6):789‐794. 10.7326/0003-4819-100-6-789. [DOI] [PubMed] [Google Scholar]
- 8. Nihoyannopoulos P, Smith GC, Maseri A, Foale RA. The natural history of left ventricular thrombus in myocardial infarction: a rationale in support of masterly inactivity. J Am Coll Cardiol. 1989;14(4):903‐911. [DOI] [PubMed] [Google Scholar]
- 9. Rehan A, Kanwar M, Rosman H, et al. Incidence of post myocardial infarction left ventricular thrombus formation in the era of primary percutaneous intervention and glycoprotein IIb/IIIa inhibitors. A prospective observational study. Cardiovasc Ultrasound. 2006;4:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Osherov AB, Borovik‐Raz M, Aronson D, et al. Incidence of early left ventricular thrombus after acute anterior wall myocardial infarction in the primary coronary intervention era. Am Heart J. 2009;157(6):1074‐1080. 10.1016/j.ahj.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 11. Gianstefani S, Douiri A, Delithanasis I, et al. Incidence and predictors of early left ventricular thrombus after ST‐elevation myocardial infarction in the contemporary era of primary percutaneous coronary intervention. Am J Cardiol. 2014;113(7):1111‐1116. 10.1016/j.amjcard.2013.12.015. [DOI] [PubMed] [Google Scholar]
- 12. Stratton JR, Resnick AD. Increased embolic risk in patients with left ventricular thrombi. Circulation. 1987;75(5):1004‐1011. 10.1161/01.CIR.75.5.1004. [DOI] [PubMed] [Google Scholar]
- 13. Vaitkus PT, Barnathan ES. Embolic potential, prevention and management of mural thrombus complicating anterior myocardial infarction: a meta‐analysis. J Am Coll Cardiol. 1993;22(4):1004‐1009. [DOI] [PubMed] [Google Scholar]
- 14. Keating EC, Gross SA, Schlamowitz RA, et al. Mural thrombi in myocardial infarctions. Prospective evaluation by two‐dimensional echocardiography. Am J Med. 1983;74(6):989‐995. 10.1016/0002-9343(83)90798-2. [DOI] [PubMed] [Google Scholar]
- 15. Visser CA, Kan G, Meltzer RS, Dunning AJ, Roelandt J. Embolic potential of left ventricular thrombus after myocardial infarction: a two‐dimensional echocardiographic study of 119 patients. J Am Coll Cardiol. 1985;5(6):1276‐1280. [DOI] [PubMed] [Google Scholar]
- 16. Kupper AJ, Verheugt FW, Peels CH, Galema TW, Roos JP. Left ventricular thrombus incidence and behavior studied by serial two‐dimensional echocardiography in acute anterior myocardial infarction: left ventricular wall motion, systemic embolism and oral anticoagulation. J Am Coll Cardiol. 1989;13(7):1514‐1520. [DOI] [PubMed] [Google Scholar]
- 17. Keren A, Goldberg S, Gottlieb S, et al. Natural history of left ventricular thrombi: their appearance and resolution in the posthospitalization period of acute myocardial infarction. J Am Coll Cardiol. 1990;15(4):790‐800. [DOI] [PubMed] [Google Scholar]
- 18. Cregler LL. Antithrombotic therapy in left ventricular thrombosis and systemic embolism. Am Heart J. 1992;123(4 Pt 2):1110‐1114. 10.1016/0002-8703(92)91069-D. [DOI] [PubMed] [Google Scholar]
- 19. Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8‐27. 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 20. Shah M, Patnaik S, Maludum O, et al. Mortality in sepsis: comparison of outcomes between patients with demand ischemia, acute myocardial infarction and neither demand ischemia nor acute myocardial infarction. Clin Cardiol. 2018;41:936‐944. 10.1002/clc.22978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shah M, Parikh K, Patel B, et al. Use of therapeutic hypothermia among patients with coagulation disorders–a Nationwide analysis. Resuscitation. 2018;124:35‐42. 10.1016/j.resuscitation.2018.01.005. [DOI] [PubMed] [Google Scholar]
- 22. Roe MT, Messenger JC, Weintraub WS, et al. Treatments, trends, and outcomes of acute myocardial infarction and percutaneous coronary intervention. J Am Coll Cardiol. 2010;56(4):254‐263. [DOI] [PubMed] [Google Scholar]
- 23. Jernberg T, Johanson P, Held C, Svennblad B, Lindback J, Wallentin L. Association between adoption of evidence‐based treatment and survival for patients with ST‐elevation myocardial infarction. JAMA. 2011;305(16):1677‐1684. 10.1001/jama.2011.522. [DOI] [PubMed] [Google Scholar]
- 24. McManus DD, Gore J, Yarzebski J, Spencer F, Lessard D, Goldberg RJ. Recent trends in the incidence, treatment, and outcomes of patients with STEMI and NSTEMI. Am J Med. 2011;124(1):40‐47. 10.1016/j.amjmed.2010.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rosamond WD, Chambless LE, Heiss G, et al. Twenty‐two‐year trends in incidence of myocardial infarction, coronary heart disease mortality, and case fatality in 4 US communities, 1987‐2008. Circulation. 2012;125(15):1848‐1857. 10.1161/CIRCULATIONAHA.111.047480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tobbia P, Brodie BR, Witzenbichler B, et al. Adverse event rates following primary PCI for STEMI at US and non‐US hospitals: three‐year analysis from the HORIZONS‐AMI trial. EuroIntervention. 2013;8(10):1134‐1142. 10.4244/EIJV8I10A176. [DOI] [PubMed] [Google Scholar]
- 27. O'Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST‐elevation myocardial infarction: executive summary: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines. Circulation. 2013;127(4):529‐555. 10.1161/CIR.0b013e3182742c84. [DOI] [PubMed] [Google Scholar]
- 28. Shavadia JS, Youngson E, Bainey KR, Bakal J, Welsh RC. Outcomes and prognostic impact of prophylactic oral anticoagulation in anterior ST‐segment elevation myocardial infarction patients with left ventricular dysfunction. J Am Heart Assoc. 2017;6(7):e006054 10.1161/JAHA.117.006054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vandvik PO, Lincoff AM, Gore JM, et al. Primary and secondary prevention of cardiovascular disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e637S‐e668S. 10.1378/chest.11-2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nayak D, Aronow WS, Sukhija R, McClung JA, Monsen CE, Belkin RN. Comparison of frequency of left ventricular thrombi in patients with anterior wall versus non‐anterior wall acute myocardial infarction treated with antithrombotic and antiplatelet therapy with or without coronary revascularization. Am J Cardiol. 2004;93(12):1529‐1530. 10.1016/j.amjcard.2004.02.066. [DOI] [PubMed] [Google Scholar]
- 31. Shah M, Patnaik S, Patel B, et al. Trends in mechanical circulatory support use and hospital mortality among patients with acute myocardial infarction and non‐infarction related cardiogenic shock in the United States. Clin Res Cardiol. 2018;107(4):287‐303. 10.1007/s00392-017-1182-2. [DOI] [PubMed] [Google Scholar]
- 32. Agrawal S, Garg L, Shah M, et al. Thirty‐day readmissions after left ventricular assist device implantation in the United States: insights from the Nationwide readmissions database. Circ Heart Fail. 2018;11(3):e004628‐e004628. 10.1161/CIRCHEARTFAILURE.117.004628. [DOI] [PubMed] [Google Scholar]


