Abstract
Background
Apixaban is a non–vitamin K oral anticoagulant approved for prevention of stroke and systemic embolism in patients with nonvalvular atrial fibrillation (AF). Current labeling recommends dose reduction based on patient age, weight, and renal function.
Hypothesis
The aim of this study was to analyze adherence to current labeling instructions concerning initial apixaban dosing in clinical practice and identify factors associated with inappropriate dose reduction.
Methods
Patients with AF initiated on apixaban in 2016 were identified in the Heart Center Leipzig database. Records were screened to identify patient characteristics, prescribed apixaban dose, renal function, and further dosing‐relevant secondary diagnoses and co‐medication.
Results
We identified 569 consecutive patients with AF initiated on apixaban. In 301 (52.9%) patients, apixaban was prescribed in standard dose (5 mg b.i.d.) and in 268 (47.1%) in a reduced dose (2.5 mg b.i.d.). Of 268 patients receiving a reduced dose, 163 (60.8%) did not meet labeling criteria for dose reduction. In univariate and multivariate regression analysis, age (OR: 0.736, 95% CI: 0.664–0.816, P < 0.0001), patient weight (OR: 1.120, 95% CI: 1.076–1.166, P < 0.0001), and serum creatinine level (OR: 0.910, 95% CI: 0.881–0.940, P < 0.0001) were independent predictors for apixaban underdosage.
Conclusions
In clinical practice, apixaban dosing is frequently inconsistent with labeling. Factors associated with inappropriate dose reduction are age, patient weight, and serum creatinine level, the same factors used as criteria for dose adjustment. However, in underdosed patients, the 3 factors did not meet the criteria for dose reduction.
Keywords: Anticoagulation, Apixaban, Atrial Fibrillation, Dose Reduction
1. INTRODUCTION
Atrial fibrillation (AF) is the most common cardiac arrhythmia encountered in clinical practice and is associated with an increased risk for stroke and systemic thromboembolism. To prevent patients with AF from experiencing a stroke, antithrombotic therapy is crucial. Compared with vitamin K antagonists (VKAs), non–vitamin K antagonist oral anticoagulants (NOACs) have more predictable pharmacodynamics, have fewer drug and food interactions, and do not require therapeutic‐drug‐level monitoring.1 NOACs are used in fixed doses, with recommended adjustments based on specific patient factors or concomitant medications. These dose adjustments were approved in large clinical trials, but adherence to the recommended doses in clinical practice is crucial to achieve maximal benefit and requires detailed evaluation.2, 3, 4, 5 This study focuses on apixaban, a direct inhibitor of factor Xa. In the Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE) trial, only 428 patients received reduced‐dose apixaban. Therefore, the evidence for reduced‐dose apixaban is very limited.
The standard dose of apixaban is 5 mg b.i.d. for prevention of strokes or systemic embolism in patients with nonvalvular AF. A reduced dose (2.5 mg b.i.d.) is required if ≥2 of the 3 following clinical criteria are present: actual body weight ≤60 kg, age ≥80 years, and serum creatinine (sCr) ≥1.5 mg/dL (133 μmol/L). Furthermore, a dose reduction is recommended for patients with severe renal dysfunction (estimated glomerular filtration rate [eGFR] 15–29 mL/min/1.73 m2) according to European Medicines Agency (EMA)‐approved labeling.6 This dose recommendation is based on extrapolation of pharmacokinetic data, because the ARISTOTLE trial excluded patients with eGFR <25 mL/min/1.73 m2 from the trial.
In this study, we aim to analyze adherence to current labeling concerning initial apixaban dosing in clinical practice and to identify factors associated with inappropriate dose reduction.
2. METHODS
2.1. Patient population
We performed a retrospective study, using the hospital's electronic health records to identify consecutive patients with AF initiated on apixaban in 2016. Patients were excluded for the following reasons: indication for oral anticoagulant treatment other than AF (venous thromboembolism or secondary prevention of recurrent venous thromboembolism), lack of documentation of patient weight and renal function, or previous diagnosis indicating valvular AF (mitral stenosis or mechanical heart valves; Figure 1). Interventions within the last 6 months having an effect on anticoagulant therapy, such as transfemoral aortic valve replacement (TAVR) or coronary stenting, were included in the analysis.
Figure 1.
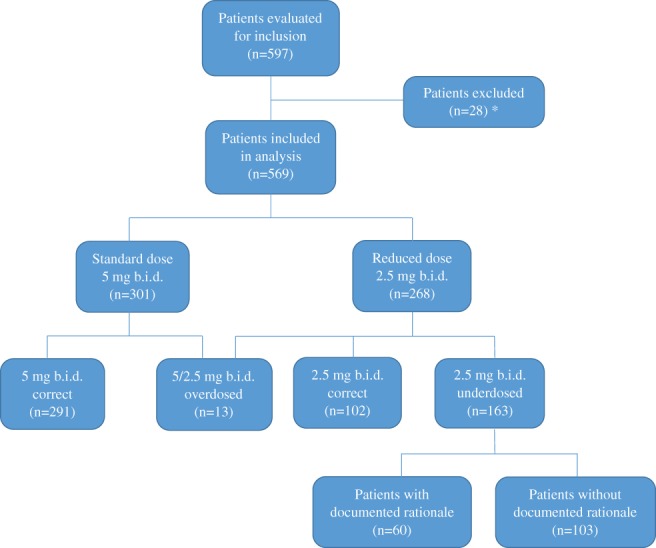
Patient selection. *Reasons for exclusion: 20 patients had indication for OAC treatment other than AF (VTE or secondary prevention of recurrent VTE) or previous diagnosis indicating valvular AF (mitral stenosis or mechanical heart valves); 5 patients had no documented weight; and 3 patients had no documented sCr level. Abbreviations: AF, atrial fibrillation; b.i.d., twice daily; OAC, oral anticoagulation; sCr, serum creatinine; VTE, venous thromboembolism
Patients were categorized as overdosed if they were administered the standard dose (5 mg b.i.d.) even though they met the criteria for a dose adjustment or if apixaban was contraindicated (ie, eGFR <15 mL/min). Patients who received a reduced dose (2.5 mg b.i.d.), despite being eligible for the higher dose, were defined as underdosed. We statistically compared patient characteristics among underdosed patients and patients receiving a reduced dose consistent with the package insert (PI) labeling. Furthermore, we scrutinized the physician letters of underdosed patients for documented reasons for the off‐label treatment.
2.2. Statistical analysis
Data analysis was performed with SPSS software, version 21 (IBM Corp., Armonk, NY). Continuous variables are reported as mean ± SD and categorical variables are presented as numbers with percentages. Comparison tests were performed using parametric (Student t test) and nonparametric (χ2 test) tests. Multivariable regression analysis that included variables with a P value <0.1 found in univariate analysis was performed to identify independent predictors of suboptimal apixaban therapy. A P value of <0.05 was considered statistically significant.
3. RESULTS
We included 569 consecutive patients with nonvalvular AF. The mean age was 72.6 ± 11.5 years, and 243 (42.7%) were women. The mean body mass index was 29.1 ± 9.2 kg/m2. The estimated risk for stroke, as summarized by the mean CHA2DS2‐VASc score, was 3.8 ± 1.8. The eGFR was widely distributed, with a mean of 59.5 ± 24.7 mL/min/1.73 m2. Forty‐six (8.1%) patients had a bleeding event prior to the start of apixaban therapy. The most common type of prior bleeding event was gastrointestinal. The majority of patients (432; 75.9%) had not taken any oral anticoagulant previously (naïve cohort). Overall, 167 (29.3%) were on concomitant single antiplatelet therapy (SAPT) and only 12 (2.1%) were on dual antiplatelet therapy (DAPT). Approximately 94 (16.5%) patients received a TAVR and 61 (10.7%) a coronary intervention/coronary stent within 6 months before apixaban therapy was started (Table 1).
Table 1.
Baseline patient characteristics
| Characteristics | N = 569 |
|---|---|
| Mean age, y | 72.6 ± 11.5 |
| Female sex | 243 (42.7) |
| Weight, kg | 83.6 ± 18.7 |
| BMI, kg/m2 | 29.1 ± 9.2 |
| sCr, μmol/l | 113.1 ± 55.7 |
| eGFR, mL/min/1.73 m2 | 59.5 ± 24.7 |
| Apixaban dose | |
| 2.5 mg b.i.d. | 268 (47.1) |
| 5 mg b.i.d. | 301 (52.9) |
| Prior OAC | |
| None | 432 (75.9) |
| Rivaroxaban | 41 (7.2) |
| Dabigatran | 8 (1.4) |
| Phenprocoumon | 82 (14.4) |
| Edoxaban | 6 (1.1) |
| Antiplatelet drugs | |
| None | 390 (68.5) |
| ASA | 16 (2.8) |
| Clopidogrel | 148 (26.0) |
| ASA + clopidogrel | 12 (2.1) |
| Ticagrelor | 3 (0.6) |
| Interventions | |
| Ablation | 78 (13.7) |
| TAVR | 94 (16.5) |
| Cardioversion | 110 (19.3) |
| Stent/PTCA | 61 (10.7) |
| Pacemaker | 75 (13.2) |
| Prior bleeding episodes | 46 (8.1) |
| Localization | |
| GI | 10 (21.7) |
| Epistaxis | 7 (15.2) |
| Rectal | 3 (6.5) |
| Intracranial | 6 (13.0) |
| Hemorrhagic stroke | 1 (2.2) |
| Bleeding hemorrhoids | 2 (4.4) |
| Congenital coagulation disorders | 2 (4.4) |
| Acquired coagulation disorders | 5 (10.9) |
| Other | 10 (21.7) |
| CHA2DS2‐VASc score | 3.8 ± 1.8 |
| CHF | 136 (23.9) |
| HTN | 507 (89.1) |
| DM | 203 (35.2) |
| Stroke/TIA | 59 (10.3) |
| Vascular diseases | 208 (36.5) |
Abbreviations: ASA, acetylsalicylic acid (aspirin); BMI, body mass index; CHA2DS2‐VASc, CHF, HTN, age > 75 y, DM, stroke/TIA, vascular disease, age 65–74 y, sex category (female); CHF, chronic heart failure; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; GI, gastrointestinal; HTN, hypertension; OAC, oral anticoagulation; PTCA, percutaneous transluminal coronary angioplasty; sCr, serum creatinine; SD, standard deviation; TAVR, transcatheter aortic valve replacement; TIA, transient ischemic attack.
Data are presented as n (%) or mean ±SD.
Out of 569 patients, 301 (52.9%) were administered apixaban in the standard dose and 268 (47.1%) in a reduced dose. Of 268 patients receiving a reduced dose, 102 (38.1%) met the criteria for a dose reduction and 163 (60.8%) were underdosed. A group of only 13 patients (2.3%) was overdosed. Due to its small size, we did not include this group in the statistical analysis.
Underdosed patients were younger, had a higher weight, had a lower sCr level and lower CHA2DS2‐VASc‐score, more often had previous strokes, and were more often on concomitant DAPT compared with patients receiving a reduced dose according to the PI (Table 2). Of the 163 underdosed patients, 93 (57.1%) met only 1 dose‐reduction criterion and 70 (42.9%) met no dose‐reduction criteria. Among patients with only 1 dose‐reduction criterion, 54 (58.1%) were age ≥80 years, 8 (8.6%) had a weight of ≤60 kg, and 30 (32.3%) had a sCr level of ≥1.5 mg/dL.
Table 2.
Baseline characteristics according to dose groups
| Characteristics | Group 1, 5 mg Correct, n = 294 | Group 2, 2.5 mg Correct, n = 102 | Group 3, 2.5 mg Incorrect, n = 166 | Group 2 vs Group 3, P Value |
|---|---|---|---|---|
| Female sex | 107 (36.4) | 55 (53.9) | 74 (44.6) | 0.137 |
| Male sex | 187 (63.6) | 47 (46.1) | 92 (55.4) | 0.137 |
| Mean age, y | 66.9 ± 11.4 | 82.0 ± 7.1 | 76.5 ± 7.9 | <0.0001 |
| ≤74 | 199 (67.7) | 13 (12.7) | 53 (31.9) | |
| 75–79 | 72 (24.5) | 10 (9.8) | 57 (34.4) | |
| ≥80 | 23 (7.8) | 79 (77.5) | 56 (33.7) | |
| Weight, kg | 87.6 ± 19.6 | 74.8 ± 17.3 | 82.2 ± 15.5 | 0.00038 |
| ≥66 | 262 (89.1) | 66 (64.7) | 142 (85.6) | |
| 61–65 | 18 (6.1) | 7 (6.9) | 14 (8.4) | |
| ≤60 | 14 (4.8) | 29 (28.4) | 10 (6.0) | |
| sCr, μmol/l | 89.8 ± 25.8 | 173.4 ± 70.1 | 107.6 ± 31.1 | <0.0001 |
| ≤119 | 278 (94.6) | 20 (19.6) | 119 (71.7) | |
| 120–132 | 11 (3.7) | 2 (2.0) | 17 (10.2) | |
| ≥133 | 5 (1.7) | 80 (78.4) | 30 (18.1) | |
| eGFR, mL/min/1.73 m2 | 73.7 ± 20.3 | 32.0 ± 15.5 | 55.3 ± 18.0 | <0.0001 |
| BMI, kg/m2 | 29.7 ± 11.6 | 27.5 ± 5.3 | 29.0 ± 5.2 | 0.019 |
| Interventions | ||||
| TAVR | 7 (2.4) | 33 (32.4) | 54 (32.5) | 0.976 |
| Ablation | 74 (25.2) | 0 (0.0) | 4 (2.4) | 0.114 |
| Cardioversion | 82 (27.9) | 9 (8.8) | 17 (10.2) | 0.703 |
| Stent/PTCA | 6 (2.0) | 17 (16.7) | 34 (20.5) | 0.44 |
| Pacemaker | 34 (11.6) | 18 (17.6) | 23 (13.9) | 0.402 |
| OAC naïve | 226 (76.9) | 73 (71.6) | 131 (78.9) | 0.171 |
| OAC previously received | 68 (23.1) | 29 (28.4) | 35 (21.1) | 0.171 |
| SAPT | 22 (7.5) | 54 (52.9) | 87 (52.4) | 0.615 |
| DAPT | 2 (0.7) | 0 (0.0) | 10 (6.0) | 0.012 |
| Prior bleeding episodes | 13 (4.4) | 12 (11.8) | 21 (12.7) | 0.83 |
| CHA2DS2‐VASc score | 2.9 ± 1.8 | 5.0 ± 1.3 | 4.4 ± 1.4 | 0.001 |
| CHF | 52 (17.7) | 34 (33.3) | 47 (28.3) | 0.385 |
| HTN | 241 (82.0) | 99 (97.1) | 157 (94.6) | 0.34 |
| DM | 75 (25.5) | 43 (42.2) | 77 (46.4) | 0.499 |
| Stroke/TIA | 22 (7.5) | 18 (17.6) | 15 (9.0) | 0.037 |
| Vascular disease | 63 (21.4) | 57 (55.9) | 80 (48.2) | 0.221 |
Abbreviations: BMI, body mass index; CHA2DS2‐VASc, CHF, HTN, age > 75 y, DM, stroke/TIA, vascular disease, age 65–74 y, sex category (female); CHF, congestive heart failure; DAPT, dual antiplatelet therapy; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; HTN, hypertension; OAC, oral anticoagulation; PTCA, percutaneous transluminal coronary angioplasty; SAPT, single antiplatelet therapy; sCr, serum creatinine; SD, standard deviation; TAVR, transcatheter aortic valve replacement; TIA, transient ischemic attack.
Data are presented as n (%) or mean ± SD.
Moreover, in underdosed patients, the values of age, weight, and sCr were more often close to the cutoff value compared with patients receiving doses consistent with the PI (Table 2).
3.1. Predictors of apixaban underdosage
In multivariate regression analysis, age (odds ratio [OR]: 0.736, 95% confidence interval [CI]: 0.664–0.816, P < 0.0001), weight (OR: 1.120, 95% CI: 1.076–1.166, P < 0.0001), and sCr (OR: 0.910, 95% CI: 0.881–0.940, P < 0.0001) were the only independent predictors of apixaban underdosage. Other factors that were included in the model (concomitant DAPT, previous stroke/transient ischemic attack, and coronary interventions within the last 6 months prior to the start of apixaban therapy) had no significant influence on off‐label dose reductions of apixaban.
3.2. Rationales for apixaban underdosage as documented in medical records
For most underdosed patients (103; 63.2%), no rationale was documented in the discharge letter. In 60 underdosed patients, rationales were recorded as follows: 24 (40.0%) patients due to concomitant antiplatelet therapy, 15 (25.0%) due to impaired renal function, 11 (18.3%) due to prior bleeding events, 4 (6.7%) due to advanced age, 1 due to both advanced age and impaired renal function, 1 due to low body weight, 1 due to Mallory‐Weiss syndrome, 1 due to an esophageal stent, 1 due to immune thrombocytopenia, and 1 due to cirrhosis.
3.3. Sensitivity analysis
Patient characteristics between those with and without documented rationale for underdosing did not differ except for age, received TAVR, received SAPT, and prior bleeding events (Table 3). Furthermore, we repeated the multivariate regression analysis including only patients with no documented rationale this time. In this analysis, age (OR: 0.808, 95% CI: 0.744–0.878, P < 0.0001), weight (OR: 1.085, 95% CI: 1.049–1.123, P < 0.0001), and sCr (OR: 0.947, 95% CI: 0.933–0.962, P < 0.0001) were again the only independent predictors of apixaban underdosage.
Table 3.
Comparison of patients with and without documented rationale for apixaban underdosing
| Characteristics | No Documented Rationale, n = 103 | Documented Rationale, n = 60 | P Value |
|---|---|---|---|
| Female sex | 51 (49.5) | 22 (36.7) | 0.107 |
| Male sex | 52 (50.5) | 38 (63.3) | 0.107 |
| Mean age, y | 77.4 ± 6.9 | 75.0 ± 8.6 | 0.020 |
| ≤74 | 32 (31.1) | 20 (33.3) | |
| 75–79 | 31 (30.1) | 26 (43.3) | |
| ≥80 | 40 (38.8) | 14 (23.3) | |
| Weight, kg | 82.3 ± 16.0 | 82.1 ±15.1 | 0.738 |
| ≥66 | 89 (86.4) | 54 (90.0) | |
| 61–65 | 8 (7.8) | 4 (6.7) | |
| ≤60 | 6 (5.8) | 2 (3.3) | |
| sCr, μmol/L | 107.6 ± 29.2 | 108.7 ± 33.5 | 0.707 |
| ≤119 | 75 (72.8) | 42 (70.0) | |
| 120–132 | 12 (11.7) | 4 (6.7) | |
| ≥133 | 16 (15.5) | 14 (23.3) | |
| eGFR, mL/min/1.73 m2 | 53.9 ± 17.1 | 57.1 ±19.0 | 0.152 |
| BMI, kg/m2 | 29.4 ± 5.4 | 28.4 ± 4.8 | 0.311 |
| TAVR | 42 (40.8) | 12 (2.0) | 0.004 |
| Ablation | 3 (2.9) | 1 (1.7) | 0.593 |
| Cardioversion | 8 (7.8) | 9 (15.0) | 0.174 |
| Stent/PTCA | 20 (19.4) | 14 (23.3) | 0.487 |
| Pacemaker | 15 (14.6) | 6 (10.0) | 0.977 |
| OAC naïve | 83 (80.1) | 45 (75.0) | 0.763 |
| OAC received | 20 (19.4) | 15 (25.0) | 0.763 |
| SAPT | 63 (61.2) | 25 (41.7) | 0.008 |
| DAPT | 3 (2.9) | 7 (11.7) | 0.30 |
| Prior bleeding episodes | 7 (6.8) | 14 (23.3) | 0.002 |
| CHA2DS2‐VASc score | 4.5 ± 1.3 | 4.3 ± 1.6 | 0.398 |
| CHF | 27 (26.2) | 18 (30.0) | 0.564 |
| HTN | 100 (97.1) | 54 (90.0) | 0.66 |
| DM | 50 (48.5) | 24 (40.0) | 0.278 |
| Stroke/TIA | 7 (6.8) | 8 (13.3) | 0.290 |
| Vascular disease | 48 (46.6) | 31 (51.7) | 0.572 |
Abbreviations: BMI, body mass index; CHA2DS2‐VASc, CHF, HTN, age > 75 y, DM, stroke/TIA, vascular disease, age 65–74 y, sex category (female); CHF, congestive heart failure; DAPT, dual antiplatelet therapy; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; HTN, hypertension; OAC, oral anticoagulation; PTCA, percutaneous transluminal coronary angioplasty; SAPT, single antiplatelet therapy; sCr, serum creatinine; SD, standard deviation; TAVR, transcatheter aortic valve replacement; TIA, transient ischemic attack.
Data are presented as n (%) or mean ±SD.
4. DISCUSSION
Our study shows that apixaban in clinical practice is frequently prescribed at lower than the recommended dose. Off‐label dosing has been observed with other NOACs, too. In a recent large US clinical study, Steinberg et al. found that 9.4% of 5738 AF patients treated with a NOAC (dabigatran, rivaroxaban, or apixaban) for prevention of stroke were underdosed and 3.4% were overdosed.7 Furthermore, Steinberg et al. assigned the frequency of under‐ and overdosing to each NOAC. Compared with our results, in this study the frequency of overdosed apixaban was almost equal (2.3% vs 2.1%, respectively), whereas the frequency of underdosed apixaban was clearly rarer (28.6% vs 11.8%, respectively). This difference could be explained by the different models of the study. We used data from a specialized heart center, but Steinberg et al. used data from a nationally representative sample of sites, including not only cardiologists as prescribers, but also primary‐care providers and neurologists. Moreover, Steinberg et al. complied with US Food and Drug Administration (FDA) labeling for apixaban, which, in contrast to the EMA labeling, does not include an explicit dose‐reduction recommendation for patients with severe renal dysfunction (eGFR 15–29 mL/min/1.73 m2). Steinberg et al. did not consider the prescribing practices of physicians.
In another US clinical study, though a much smaller one, Megan et al found that out of 24 patients receiving apixaban in a reduced dose, only 3 (12.5%) were dosed according to the PI and 21 (87.5%) were underdosed.8 Compared with that, our proportion between patients with appropriate reduced doses and underdosed patients is more balanced (38.1% vs 60.8%, respectively). The substantial difference between both results might be caused by the different sizes of the patient populations and the fact that Megan et al. also complied with FDA labeling for apixaban.
To our knowledge, this is the first analysis of the association between off‐label dose reductions of apixaban only and patient characteristics. In multivariate regression analysis, we could demonstrate that the decision for a lower‐than‐recommended dose of apixaban is significantly associated with the following 3 patient factors: age, weight, and sCr level. The same factors are used as dose‐reduction criteria in the EMA‐approved PI of apixaban. According to PI labeling, ≥2 of the 3 following clinical criteria must be present for a 2.5‐mg b.i.d. dose of apixaban: actual body weight ≤ 60 kg, age ≥ 80 years, and sCr ≥1.5 mg/dL (133 μmol/L). These dose‐reduction criteria used in the ARISTOTLE trial were chosen because, particularly in combination, they predict increased apixaban exposure based on pharmacokinetic modeling.9, 10, 11
We found that physicians often decided for a reduced dose if only 1 criterion exceeded the cutoff value or if criteria almost reached the cutoff value (Table 2). Hence, we assume that the main cause for off‐label dose reduction of apixaban is unawareness of the 2‐of‐3 criteria rule for dosage reduction or the location close to the cutoff limits of the values of age, weight, and sCr.
An important question is the impact of these clinical practices on the risk of stroke in patients with AF. In people with normal renal function and lower age, apixaban 2.5 mg is associated with about 50% lower plasma concentrations than is treatment with 5.0 mg.12 This raises concerns about the safety of the 2.5‐mg dose in patients who have only 1 or 0 dose‐reduction criteria to maintain effective stroke prevention. Currently, few data exist on the effect of the 2.5‐mg b.i.d. dose of apixaban on clinical outcomes in patients with AF with <2 dose‐reduction criteria. Analyzing a large US administrative database, Yao et al. recently showed that underdosing is associated with a higher risk of stroke but with no statistically significant difference in major bleeding in apixaban‐treated patients.13 On the other hand, Steinberg et al, who analyzed all 3 NOACS together in the above‐mentioned study, did not find this association. They found that underdosing of NOACs is significantly associated with increased cardiovascular hospitalization.7 Therefore, more analyses are necessary to confirm an association between apixaban underdosing and higher risk of stroke.
Furthermore, we found that physicians mostly do not justify their decision for an inappropriate reduced dose in physician letters. If recorded, the most frequent reasons were concomitant antiplatelet therapy, impaired renal function, and prior bleeding events. In our opinion, documentation of reasons for inappropriate dose reduction is important to increase the awareness of dose‐reduction rules of apixaban and subsequently to reduce the frequency of inappropriate dose reductions.
4.1. Study limitations
This study has several limitations. First, it was a retrospective, single‐center study. Furthermore, not all concomitant medications that might impact apixaban treatment were collected. Association with clinical outcomes (strokes, systemic embolism) was not reported.
5. CONCLUSION
In this retrospective study, apixaban was often administered underdosed (28.6%) in patients with nonvalvular AF. The factors associated significantly with apixaban doses lower than recommended were age, weight, and sCr level. These are the same factors that are used as criteria for dose adjustment. However, in underdosed patients, the 3 factors did not meet the criteria for dose reduction. Factors associated with increased bleeding risk, such as prior bleeding events or concomitant SAPT or DAPT, had no significant influence on off‐label dose reductions of apixaban.
Conflicts of interest
The authors declare no potential conflicts of interest.
Buchholz A, Ueberham L, Gorczynska K, et al. Initial apixaban dosing in patients with atrial fibrillation. Clin Cardiol. 2018;41:671–676. 10.1002/clc.22949
REFERENCES
- 1. Mega JL, Simon T. Pharmacology of antithrombotic drugs: an assessment of oral antiplatelet and anticoagulant treatments. Lancet. 2015;386:281–291. [DOI] [PubMed] [Google Scholar]
- 2. Connolly SJ, Ezekowitz MD, Yusuf S, et al; RE‐LY Committee and Investigators . Dabigatran versus warfarin in patients with atrial fibrillation [published correction appears in N Engl J Med. 2010;363:1877]. N Engl J Med. 2009;361:1139–1151. [DOI] [PubMed] [Google Scholar]
- 3. Granger C, Alexander J, McMurray J, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–992. [DOI] [PubMed] [Google Scholar]
- 4. Patel MR, Mahaffey KW, Garg J, et al; ROCKET‐AF Investigators . Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–891. [DOI] [PubMed] [Google Scholar]
- 5. Giugliano RP, Ruff CT, Braunwald E, et al; ENGAGE AF‐TIMI 48 Investigators . Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369:2093–2104. [DOI] [PubMed] [Google Scholar]
- 6. European Medicines Agency . Eliquis (apixaban): summary of product characteristics. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_‐_Product_Information/human/002148/WC500107728.pdf. Accessed December 6, 2017.
- 7. Steinberg A, Shrader P, Thomas L, et al; ORBIT‐AF Investigators and Patients . Off‐label dosing of non–vitamin K antagonist oral anticoagulants and adverse outcomes: the ORBIT‐AF II Registry. J Am Coll Cardiol. 2016;68:2597–2604. [DOI] [PubMed] [Google Scholar]
- 8. Barra ME, Fanikos J, Connors JM, et al. Evaluation of dose‐reduced direct oral anticoagulant therapy. Am J Med. 2016;129:1198–1204. [DOI] [PubMed] [Google Scholar]
- 9. Frost CE, Song Y, Shenker A, et al. Effects of age and sex on the single‐dose pharmacokinetics and pharmacodynamics of apixaban. Clin Pharmacokinet. 2015;54:651–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Upreti VV, Wang J, Barrett YC, et al. Effect of extremes of body weight on the dose pharmacokinetics, pharmacodynamics, safety and tolerability of apixaban in healthy subjects. Br J Clin Pharmacol. 2013;76:908–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chang M, Yu Z, Shenker A, et al. Effect of renal impairment on the pharmacokinetics, pharmacodynamics, and safety of apixaban. J Clin Pharmacol. 2016;56:637–645. [DOI] [PubMed] [Google Scholar]
- 12. Frost C, Nepal S, Wang J, et al. Safety, pharmacokinetics and pharmacodynamics of multiple oral doses of apixaban, a factor Xa inhibitor, in healthy subjects. Br J Clin Pharmacol. 2013;76:776–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yao X, Shah ND, Sangaralingham LR, et al. Non–vitamin K antagonist oral anticoagulant dosing in patients with atrial fibrillation and renal dysfunction. J Am Coll Cardiol. 2017;69:2779–2790. [DOI] [PubMed] [Google Scholar]


