Summary
Background
Anhedonia, as a dysregulation of the reward circuit, is present in both Major Depressive Disorder (MDD) and schizophrenia (SZ).
Aims
To elucidate the clinical and neurobiological differences between schizophrenia (SZ) and depression (MDD) in regard to anhedonia, while reconciling the challenges and benefits of assessing anhedonia as a transdiagnostic feature under the Research Domain Criteria (RDoC) framework.
Methods
In this review, we summarize data from publications examining anhedonia or its underlying reward deficits in SZ and MDD. A literature search was conducted in OVID Medline, PsycINFO and EMBASE databases between 2000 and 2017.
Results
While certain subgroups share commonalities, there are also important differences. SZ may be characterized by a disorganization, rather than a deficiency, in reward processing and cognitive function, including inappropriate energy expenditure and focus on irrelevant cues. In contrast, MDD has been characterized by deficits in anticipatory pleasure, development of reward associations, and integration of information from past experience. Understanding the roles of neurotransmitters and aberrant brain circuitry is necessary to appreciate differences in reward function in SZ and MDD.
Conclusion
Anhedonia as a clinical presentation of reward circuit dysregulation is an important and relatively undertreated symptom of both SZ and MDD. In order to improve patient outcomes and quality of life, it is important to consider how anhedonia fits into both diagnoses.
Keywords: anhedonia, depression, reward circuit, schizophrenia
1. INTRODUCTION
Informed by advances in the fields of psychiatry and neuroscience, the National Institute of Mental Health (NIMH) Research Domain Criteria (RDoC) initiated a collective effort to map transdiagnostic dimensions of psychopathology onto underlying biological system abnormalities.1, 2 Among the 5 RDoC domains, the positive valence system is most implicated in various aspects of reward learning and behavior, including the clinical symptom of anhedonia. Importantly, anhedonia is a feature across psychiatric disorders including major depressive disorder (MDD) and schizophrenia (SZ). While it has traditionally been defined as an inability to experience pleasure,2, 3 neuroscientific findings suggest that anhedonia can also be conceptualized as a multifaceted clinical symptom resulting from an underlying deficit in reward circuitry.4, 5
Historically, the nucleus accumbens (NAc), ventral tegmental area (VTA), and associated mesolimbic dopamine pathways have been considered the key components of reward and pleasure.6, 7 However, recent neuroimaging findings suggest a more complex model of reward which includes the NAc, VTA, amygdala, prefrontal cortex, caudate, putamen, and orbitofrontal cortex.3, 8, 9 Neuroimaging studies further elucidate the complexity of reward processing by utilizing behavioral tasks designed to assess specific aspects of reward, including anticipation, motivation, and expectation.9 The neural pathway and task performance probed by various reward paradigms suggests there are a series of possible deficits that could lead to the clinical presentation of anhedonia.5, 10 A simple schematic adapted from Kring and Barch11 outlines the various aspects of the reward process (Figure 1), where a deficit in each of the areas could lead to overall dysregulation of the reward circuit.5, 11
Figure 1.
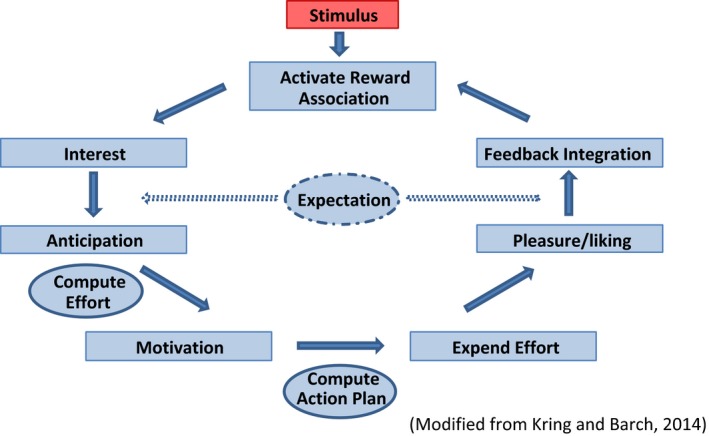
A simple schematic highlighting the key aspects of reward processing. Impairments in any part of the circuit can result in the presentation of anhedonia
Anhedonia is one of the core symptoms of MDD.12 Although traditionally considered a core symptom in SZ, it was subsequently reconceptualized to distinguish between consummatory and anticipatory components of hedonic experience. However, the transdiagnostic expression of anhedonia across MDD and SZ has been underexplored from a clinical perspective, despite being a predictor of poor clinical outcome and cognitive impairment.13 Zhang et al14 provide a convincing argument for the transdiagnostic examination of anhedonia at a substrate level using functional neuroimaging, finding similar patterns of brain activation across MDD and SZ in both anticipatory and consummatory anhedonia. As the field of psychiatry progresses toward increased incorporation of the transdiagnostic RDoC approach, it will become increasingly important to deepen our understanding of how aberrant changes in common neurobiological networks can lead to similar clinical presentations across different diagnoses. Our aim is to compare anhedonia across SZ and MDD, highlighting the potential contributions of neurobiology, clinical/behavioral presentation, and treatment strategies from a transdiagnostic perspective.
2. ANHEDONIA AS A CONSTRUCT
Reward processing includes interest in something that is deemed pleasurable by the individual. Anticipation of this pleasure is typically followed by an increase in motivation to formulate an appropriate plan to obtain the reward, followed by the expenditure of adequate effort to achieve the desired outcome. Past consummatory pleasure and reward learning serve to modulate future reward decision‐making and pleasure with respect to a specific stimulus. Reward learning, feedback integration, and the correct prediction of reward magnitude also play an important role in governing goal‐directed behavior.5, 11 Traditional definitions have emphasized the consummatory aspect of anhedonia; however, a deficit in any aspect of the reward system (see Table 1) could potentially preclude an individual from reaching the consummatory pleasure stage and thus present as an inability to feel pleasure.15 Importantly, reward processing is not necessarily linear where one facet follows the other, and instead, facets may occur in parallel (eg, interest itself may result in consummatory pleasure).5 In any case, this suggests that there may be differential neurobiological mechanisms underlying the clinical presentation of anhedonia.5, 16 In their neuroimaging meta‐analysis, Zhang and colleagues14 focused on consummatory and anticipatory pleasure—a common theme in anhedonia research since the development of the Temporal Experience of Pleasure Scale (TEPS) in 2006.17 However, the inclusion of other facets of reward such as feedback integration, reward learning, and motivation may be as important when delineating the neurobiological deficits associated with anhedonia.5 Generating a fuller understanding of the specific reward system impairments underlying anhedonia may serve to inform more targeted and personalized treatment interventions.
Table 1.
Reward system deficits implicated in anhedonia
| Deficit | MDD | SZ | Research |
|---|---|---|---|
| Motivation | Diminished | Diminished | Sherdell et al26 |
| Treadway et al118 | |||
| Fervaha et al59 | |||
| Attention allocation | Not evaluated | Toward nonreward cues | McCarthy et al38 |
| Barch et al39 | |||
| Anticipatory pleasure | Diminished | Diminished | Gard et al48 |
| Sherdell et al26 | |||
| Chase et al119 | |||
| Arrondo et al120 | |||
| Loas et al49 | |||
| Liu et al37 | |||
| Reward learning and integration | Diminished | Diminished pleasure recall | Marder and Galderesi30 |
| Steele et al121 | |||
| Kumar et al122 | |||
| Strauss et al117 | |||
|
Waltz et al44, 56
Gold et al57 | |||
|
Pizzagalli et al83
Vrieze et al36 | |||
| Prediction error | Reduced signaling in NAc, caudate, and midbrain | Reduced signaling in hippocampal areas | Gradin et al95 |
| Consummatory pleasure | Mildly diminished | Maintained | Sherdell et al26 |
| Strauss and Gold34 | |||
| Treadway and Zald16 | |||
| Da Silva et al53 |
MDD, major depressive disorder; SZ, schizophrenia.
The first challenge in exploring differences in reward circuitry across diagnostic groups is the current inability to assess different aspects of the reward system concurrently. For example, in the context of MDD, many self‐report or clinician‐rated scales still focus heavily on consummatory pleasure (eg, Snaith‐Hamilton Pleasure Scale)18 and many behavioral tasks only assess one or two aspects of the reward system.5 The only scale so far validated in an MDD sample that evaluates different aspects of anhedonia is the Dimensional Anhedonia Rating Scale (DARS).19 Traditional scales used to assess affective experience and reward system deficits in SZ, such as the Positive and Negative Syndrome Scale (PANSS)20 or the Scale for the Assessment of Negative Symptoms (SANS),21 lack targeted investigations of anticipation, interest, effort allocation, and motivation. Recently developed rating scales in SZ such as the Brief Negative Symptom Scale (BNSS)22 and the Clinical Assessment Interview for Negative Symptoms (CAINS)23 address some of the previous limitations, with more focused evaluations of anticipation, interest, and motivation, although their broader uptake remains limited. Drawing conclusions from individual studies assessing isolated aspects of the reward system can lead to an incomplete characterization of hedonic deficits and how the various facets of reward are correlated. Moving forward, we advocate for the use of multiple behavioral tasks and more refined clinical scales, which will serve to increase the transdiagnostic comparability across research studies.
3. THE PRESENTATION OF ANHEDONIA IN MDD AND SZ
An MDD diagnosis is characterized by the presence of five of a possible nine symptoms, of which anhedonia is a core diagnostic symptom.12 As a result, there are more than 200 potential symptom combinations that may fulfill a diagnosis.24 Patients with MDD presenting with anhedonic symptoms may demonstrate dissociation between consummatory pleasure and motivation, expectation of negative outcomes regardless of past positive outcomes, and increased risk avoidance.4, 25, 26, 27, 28 Anhedonia has also been found to be a prominent residual symptom following treatment.29
SZ is also a heterogeneous disorder, marked by the variable presentation of positive symptoms (ie, hallucinations and delusions), negative symptoms (ie, amotivation and diminished expression), and cognitive deficits. Historically, the core negative symptoms consisted of anhedonia, asociality, alogia, avolition, and affective flattening.21, 30 However, the current conceptualization of negative symptoms outlines two separate but interrelated subdomains: amotivation (within which anhedonia is subsumed) and diminished expression (including alogia and affective flattening).12, 31, 32, 33 Foussias et al33 suggest that amotivation is a more accurate description of what has previously been described as anhedonia in SZ.
Anhedonia has traditionally been considered a core symptom of both SZ and MDD; however, recent studies suggest that many of these individuals can indeed experience pleasure “in the moment”.16, 34 The difference between SZ and MDD may lie in the underlying facets of the reward system that are impaired and consequently lead to the clinical presentation of apparent anhedonia. Specifically, in MDD, impairments in effort expenditure, reward learning, prediction error, and/or motivation may preclude individuals from reaching the “reward stage” of experiencing pleasure at the time.25, 35, 36 Additionally, patients with MDD express difficulty in integrating information about past reward outcomes to inform future reward‐based decision‐making and fail to make reward‐maximizing choices when under stress.37 On the other hand, patients with SZ demonstrate difficulty in their ability to correctly allocate attention to relevant stimuli, resulting in an inability to make choices that optimize gains.38, 39 While patients with MDD share the inability to optimize gains, their underlying deficits differ from SZ. Patients with MDD are unable to adequately utilize rewarding or positive feedback to optimize reward outcomes; however, they do not differ from controls in their ability to utilize punishment information to avoid loss.40 Further, patients with SZ often experience memory deficits which may impede normal reward learning and pleasure recall, contributing to inaccurate appraisals of rewards and impaired decision‐making in pursuit of such rewards.30, 41
Similarly, in studies examining isolated components of the reward system, patients with SZ display impaired reward anticipation and expectancy, such that they fail to modulate their behavior in response to reward cues and have diminished procedural learning.42, 43, 44 In line with these findings, there is also evidence of reduced neural responses to reward‐predicting cues in both medicated and unmedicated patients with SZ.45, 46, 47 Some,48, 49, 50, 51 but not all,52, 53, 54, 55 studies utilizing the TEPS, developed to assess physical anhedonia (including sensory experiences) in SZ, report a specific reduction in anticipatory pleasure. Interestingly, these impairments have been linked to the overall severity of negative symptoms, including anhedonia48, 51 and amotivation.53 Other reports suggest a specific impairment in the speed of reward learning in SZ, rather than an inability to learn associations: this is especially prominent in response to positive feedback.44, 56, 57 Additionally, behavioral studies examining effort‐based decision‐making in SZ have consistently shown impaired effort allocation, such that patients are less willing to exert effort for high‐reward‐high‐probability conditions.39, 58, 59 Lastly, some studies have shown that patients with SZ demonstrate impairments in action selection decision‐making, or the ability to maintain, update, and integrate reward‐driven feedback to guide goal‐directed behavior,60, 61, 62, 63 although others have not.64, 65, 66, 67 Interestingly, impaired decision‐making has also been observed in people with SZ during real‐world executive functioning using goal planning and action tasks.68, 69 In addition, intact reward responsiveness70, 71 suggests that these deficits are not a function of reward insensitivity.
In summary, anhedonia may be best understood as a multifaceted symptom that manifests as a result of one or more deficits in the reward system, rather than the more traditional and narrow interpretation as a deficit solely in consummatory pleasure.34 Understanding these differential mechanisms of apparent anhedonia across individuals with MDD and SZ may present opportunities to refine assessment of the true nature of impairment, enabling more targeted and effective interventions to address this clinically important domain of symptomatology.
4. THE NEUROBIOLOGY OF REWARD SYSTEM DEFICITS IN MDD AND SZ
Findings from neuroimaging studies in MDD link anhedonia to dysfunction in the striatum, NAc, frontal cortices, and caudate during reward‐related tasks.3, 28, 72, 73 Further, using full‐brain connectivity analysis, Sharma et al74 identified key areas of hypoconnectivity between the NAc and various other brain regions, such as those within the default mode network (DMN) and the cingulo‐opercular network, across multiple disorders with reward system deficits including MDD and SZ. Hypoconnectivity between the NAc and DMN may be a potential mechanism through which cognition, such as memory capacity, may influence aspects of reward processing.
There is also evidence that the ventral striatum (VS), particularly the NAc, is important for anticipatory and expectation aspects of reward processing,5 and findings from preclinical mammalian models support the role of dopamine in these processes.6, 75, 76 Among the other monoamines, serotonin dysfunction is considered a key neurotransmitter deficiency in MDD.77 However, a significant number of patients do not respond to selective serotonin reuptake inhibitors (SSRIs),78 suggesting serotonin does not modulate all MDD symptoms. Furthering this idea, anhedonia has been found to be a predictor of SSRI nonresponse.79, 80 A link among anhedonia, treatment nonresponse, and dopamine is likely present, although the majority of research linking dopamine dysregulation to reward processing is derived from preclinical models.81, 82 However, Rizvi19 demonstrated that anhedonia was correlated with high D2/D3 binding (ie, poor dopaminergic tone) in the anterior cingulate cortex in humans. Furthermore, Pizzagalli et al83 reported that pramipexole (a D2/D3 agonist) impaired normal reward processing. There is also evidence that OROS methylphenidate, as an adjunctive treatment in MDD, demonstrated significant improvement in anhedonia, but not in depression symptoms overall.84 As such, the dopamine system may be an important therapeutic target for anhedonia.85 However, these findings must be interpreted with caution as there are clearly other networks at play. For example, Lally et al86 found that anhedonia in patients with MDD could be significantly reduced with a single infusion of ketamine, which is thought to work mainly at NMDA receptors. The efficacy of different treatment options in MDD may be partially due to the high degree of heterogeneity that exists between patients.87
In SZ, a disturbance in dopamine circuitry has been associated with both positive and negative symptoms and is often referred to as the “dopamine hypothesis”. The first iteration of this theory suggested excess dopamine to be the main cause of SZ—a theory which explained the efficacy of antipsychotic drugs with predominant effects in blocking dopamine transmission.88, 89 A subsequent reconceptualization of this theory by Davis and Kahn90 proposed that hyperdopaminergia is present in subcortical regions and is linked to the positive symptoms of SZ, whereas hypodopaminergia in the frontal cortex is associated with the negative symptoms of the illness. Building on this work, Howes and Kapur91 revised the theory to account for emerging evidence suggesting that dopamine dysregulation may be linked to an incorrect appraisal of stimuli.91 Such “aberrant attribution of salience”, or the transfer of focus from rewards or goals onto seemingly irrelevant stimuli, may be responsible for both disordered thoughts and hallucinations in SZ.92, 93 In this way, SZ differs from MDD in that individuals seem to be willing to expend energy, but due to misallocation of effort and attention, they are not able to integrate environmental cues and make choices that optimize reward gain.39
Prediction error tasks have been traditionally used to measure phasic dopamine bursts during reward expectation.5 The outcomes of prediction error studies, however, have been mixed for both SZ and MDD, likely due to the high degree of neurobiological heterogeneity within each diagnosis.73, 94 Interestingly, Gradin et al95 found that patients with SZ and MDD exhibited decreased encoding during prediction error tasks, although the underlying brain areas demonstrating diminished dopamine activity differed. Specifically, depressed patients showed decreased activity in the striatum and midbrain, whereas patients with SZ had reduced activity in the caudate, thalamus, insula, amygdala, and hippocampus. Interestingly, there was a correlation between decreased prediction error encoding activity and anhedonia severity in patients with MDD, whereas decreased level of encoding in patients with SZ was correlated with psychotic symptoms. The areas of dysregulation uncovered in SZ align more closely with memory, learning, and emotional processing. Furthermore, patients with SZ have demonstrated enhanced prediction error—but only in response to irrelevant cues.96 Interestingly, Pelizza and Ferrari97 found that individuals with SZ who were experiencing severe anhedonia also presented with a high degree of disorganized cognition. Overall, these findings suggest that the anhedonic phenotype in SZ reflects a degree of “disorganization” within the reward system due to disrupted cognition and aberrant stimulus processing.
5. IMPLICATION OF ANHEDONIA IN MDD AND SZ FOR TREATMENT SELECTION AND OUTCOME
There is currently no treatment approved by the Food and Drug Administration (FDA) or other international agencies aimed at improving anhedonia in MDD,12 despite its association with poor clinical outcomes and quality of life.80, 98 Typically, SSRIs are first‐line treatments for a MDE; however, some studies have shown they can adversely affect dopamine transmission and induce “emotional blunting”.99, 100, 101, 102, 103 Current clinical guidelines recommend atypical antipsychotic agents, such as the dopamine D2 partial agonist aripiprazole, as first‐line adjunctive treatments for those patients who do not respond to SSRI monotherapy.104, 105, 106 Adjunctive aripiprazole has been shown to greatly improve overall depression severity in MDD and anhedonia in bipolar depression.107, 108
Antipsychotic medications represent the cornerstone of psychopharmacological treatment for SZ.109 Anhedonia (which falls under the avolition‐apathy subdomain of negative symptoms) is recognized as an associated feature of SZ in the DSM‐5,15 yet treatments targeting anhedonia and related negative symptoms in SZ are nonexistent. The FDA has highlighted the need to identify treatments for negative symptoms in SZ as a therapeutic priority.110 While most antipsychotic medications (with the exception of clozapine in treatment‐resistant populations) seem to be equally effective in treating positive symptoms, they have been less successful in treating the negative and cognitive symptoms of the illness.111, 112 In fact, there are very few pharmacological interventions that demonstrate improvement in persistent negative symptoms in SZ.113, 114 Cariprazine, an atypical agent with preferential D3 over D2 blocking effects, has been shown to have greater efficacy in treating negative symptoms in SZ compared to risperidone and may be a promising option for patients who have significant and persistent negative symptoms including amotivation.115 In a recent meta‐analysis,114 atypical antipsychotics were superior to typical antipsychotics in reducing anhedonia, likely based on the reduced propensity for these atypical agents to cause worsening of negative symptoms. There is also limited support for the psychostimulant lisdexamfetamine as an adjunctive therapy for predominant negative symptoms of SZ.116 At this time, cognitive behavioral therapy may be the best adjunctive intervention in SZ.117
Anhedonia across SZ and MDD may be due to different underlying neurobiological impairments, and going forward, the ultimate challenge lies in generating a more precise definition of reward system dysfunction as it is experienced by the individual, regardless of traditional diagnostic categories. Additionally, tailoring treatments to specific impairments, rather than the apparent clinical phenomenon or diagnostic label, may lead to more preferable clinical outcomes.
6. CONCLUSION
Anhedonia and underlying reward system deficits are common to both MDD and SZ. While it is important to investigate commonalities across these two disorders, one must also keep in mind that both conditions are highly heterogeneous. The current literature would suggest that, on the whole, the underlying dysregulation associated with anhedonia in MDD may not be similarly present in SZ, which may be due to the differences in the way anhedonia clinically presents across the two disorders. That said, what remains to be established is whether there are subgroups of individuals across these disorders for whom there are shared behavioral and neurobiological impairments that present clinically as anhedonia. It is also possible that part of the apparent reward system abnormalities evident in SZ may be more related to overall cognitive impairment as opposed to a reward processing specific deficit. Although MDD and SZ on the surface may appear to share a common hedonic impairment, there are many relevant clinical and neurobiological differences between the two disorders that require further investigation.
Moving forward, a more complete understanding of the neurotransmitter profile in individuals with MDD who experience anhedonic symptoms will be necessary to delineate the interplay between dopamine, norepinephrine, and serotonin. Additionally, a better understanding of how SZ diverges from MDD in terms of reward circuitry may provide novel targets for drug development aimed at alleviating persistent negative symptoms. While tailoring treatment and improving clinical outcomes are the ultimate goal, in the short term, it is important that the field develops more precise terminology for anhedonia that is more inclusive of its multiple facets. Understanding that anhedonia, as a symptom, is actually a manifestation of multiple reward circuit deficits arising from aberrant reward cue processing (ie, motivation, effort allocation, interest, consummatory pleasure, feedback integration, and reward learning) will be increasingly important as the field of psychiatry moves toward a transdiagnostic method of clinical assessment and treatment.
DISCLOSURES
SHK has received research funding or honoraria from the following sources: Abbott, Allergan, AstraZeneca, BMS, Brain Cells Inc., Brain Canada, Clera, CIHR, Eli Lilly, Janssen, Lundbeck, Lundbeck Institute, Ontario Brain Institute, Otsuka, Pfizer, Servier, St. Jude Medical, Sunovion, and Xian‐Janssen. GF has served on advisory boards for Hoffman‐La Roche and Takeda and received speakers’ fees from Hoffman‐La Roche, Lundbeck, and Novartis. SR has received research funding from Pfizer Canada, Canadian Biomarker Integration Network for Depression, CIHR, Ontario Brain Institute, and the Brain and Behavior Foundation.
ACKNOWLEDGMENTS
We would like to thank all members of the Arthur Sommer Rotenberg Suicide and Depression Studies Program and Centre for Addiction and Mental Health who supported us in the preparation of this manuscript, as well as the University of Toronto Institute of Medical Science Summer Undergraduate Research Program for their continued support of undergraduate research.
Lambert C, Da Silva S, Ceniti AK, Rizvi SJ, Foussias G, Kennedy SH. Anhedonia in depression and schizophrenia: A transdiagnostic challenge. CNS Neurosci Ther. 2018;24:615–623. 10.1111/cns.12854
Funding information
This research was conducted as part of the Canadian Biomarker Integration Network in Depression (CAN‐BIND) program. CAN‐BIND is an Integrated Discovery Program carried out in partnership with, and financial support from, the Ontario Brain Institute, an independent nonprofit corporation, funded partially by the Ontario Government. The opinions, results, and conclusions are those of the authors, and no endorsement by the Ontario Brain Institute is intended or should be inferred.
REFERENCES
- 1. Cuthbert BN, Insel TR. Toward the future of psychiatric diagnosis: the seven pillars of RDoC. BMC Med. 2013;126:1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nimh.nih.gov (2017). NIMH: Anhedonia (RDoC Element). [online] https://www.nimh.nih.gov/research-priorities/rdoc/units/behaviors/150689.shtml. Accessed Oct 1, 2017.
- 3. Der‐Avakian A, Markou A. The neurobiology of anhedonia and other reward‐related deficits. Trends Neurosci. 2012;35:68‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Whitton AE, Treadway MT, Pizzagalli DA. Reward processing dysfunction in major depression, bipolar disorder and schizophrenia. Curr Opin Psychiatry. 2015;28:7‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rizvi SJ, Pizzagalli DA, Sproule BA, Kennedy SH. Assessing anhedonia in depression: potentials and pitfalls. Neurosci Biobehav Rev. 2016;65:21‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nestler EJ, Carlezon WA. The mesolimbic dopamine reward circuit in depression. Biol Psychiat. 2006;59:1151‐1159. [DOI] [PubMed] [Google Scholar]
- 7. Ikemoto S. Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens–olfactory tubercle complex. Brain Res Rev. 2007;56:27‐78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gorwood P. Neurobiological mechanisms of anhedonia. Dialogues Clin Neurosci. 2008;10:291‐299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Russo SJ, Nestler EJ. The brain reward circuitry in mood disorders. Nature Rev Neurosci. 2013;14:609‐625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Misaki M, Suzuki H, Savitz J, Drevets WC, Bodurka J. Individual variations in nucleus accumbens responses associated with major depressive disorder symptoms. Sci Rep. 2016;6:1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kring AM, Barch DM. The motivation and pleasure dimension of negative symptoms: neural substrates and behavioral outputs. Eur Neuropsychopharmacol. 2014;24:725‐736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Edition F. Diagnostic and Statistical Manual of Mental Disorders (DSM‐5), 5th edn Washington, DC: America Psychiatric Association; 2013. [Google Scholar]
- 13. McIntyre RS, Woldeyohannes HO, Soczynska JK, et al. Anhedonia and cognitive function in adults with MDD: results from the International Mood Disorders Collaborative Project. CNS Spectr. 2016;21:362‐366. [DOI] [PubMed] [Google Scholar]
- 14. Zhang B, Lin P, Shi H, et al. Mapping anhedonia‐specific dysfunction in a transdiagnostic approach: an ALE meta‐analysis. Brain Imaging Behav. 2016;10:920‐939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Treadway MT, Zald DH. Parsing anhedonia: translational models of reward‐processing deficits in psychopathology. Curr Dir Psychol Sci. 2013;22:244‐249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Treadway MT, Zald DH. Reconsidering anhedonia in depression: lessons from translational neuroscience. Neurosci Biobehav Rev. 2011;35:537‐555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gard DE, Gard MG, Kring AM, John OP. Anticipatory and consummatory components of the experience of pleasure: a scale development study. J Res Pers. 2006;40:1086‐1102. [Google Scholar]
- 18. Snaith RP, Hamilton M, Morley S, Humayan A, Hargreaves D, Trigwell P. A scale for the assessment of hedonic tone the Snaith‐Hamilton Pleasure Scale. Br J Psychiatry. 1995;167:99‐103. [DOI] [PubMed] [Google Scholar]
- 19. Rizvi S. Anhedonia in Major Depressive Disorder: Exploration of a Predictive Clinical Phenotype [Doctoral Dissertation]. Toronto, ON: University of Toronto; 2015. [Google Scholar]
- 20. Kay SR, Fiszbein A, Opfer LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261. [DOI] [PubMed] [Google Scholar]
- 21. Andreasen N. Negative symptoms in schizophrenia: definition and reality. Arch Gen Psychiatry. 1982;39:784‐788. [DOI] [PubMed] [Google Scholar]
- 22. Kirkpatrick B, Strauss GP, Nguyen L, et al. The brief negative symptom scale: psychometric properties. Schizophr Bull. 2010;37:300‐305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Horan WP, Kring AM, Gur RE, Reise SP, Blanchard JJ. Development and psychometric validation of the clinical assessment interview for negative symptoms (CAINS). Schizophr Res. 2011;132:140‐145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Østergaard SD, Jensen SO, Bech P. The heterogeneity of the depressive syndrome: when numbers get serious. Acta Psychiatr Scand. 2011;124:495‐496. [DOI] [PubMed] [Google Scholar]
- 25. Ubl B, Kuehner C, Kirsch P, Ruttorf M, Diener C, Flor H. Altered neural reward and loss processing and prediction error signalling in depression. Soc Cogn Affect Neurosci. 2015;10:1102‐1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sherdell L, Waugh CE, Gotlib IH. Anticipatory pleasure predicts motivation for reward in major depression. J Abnorm Psychol. 2012;121:51‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Smoski MJ, Lynch TR, Rosenthal MZ, Cheavens JS, Chapman AL, Krishnan RR. Decision‐making and risk aversion among depressive adults. J Behav Ther Exp Psychiatry. 2008;39:567‐576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dichter GS, Kozink RV, McClernon FJ, Smoski MJ. Remitted major depression is characterized by reward network hyperactivation during reward anticipation and hypoactivation during reward outcomes. J Affect Disord. 2012;136:1126‐1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nierenberg AA. Residual symptoms in depression: prevalence and impact. J Clin Psychiatry. 2015;76:e1480. [DOI] [PubMed] [Google Scholar]
- 30. Marder SR, Galderisi S. The current conceptualization of negative symptoms in schizophrenia. World Psychiatry. 2017;16:14‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Messinger JW, Trémeau F, Antonius D, et al. Avolition and expressive deficits capture negative symptom phenomenology: implications for DSM‐5 and schizophrenia research. Clin Psyc Rev. 2011;31:161‐168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Millan MJ, Fone K, Steckler T, Horan WP. Negative symptoms of schizophrenia: clinical characteristics, pathophysiological substrates, experimental models and prospects for improved treatment. Eur Neuropsychopharmacol. 2014;24:645‐692. [DOI] [PubMed] [Google Scholar]
- 33. Foussias G, Remington G. Negative symptoms in schizophrenia: avolition and Occam's razor. Schizophr Bull. 2008;36:359‐369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Strauss GP, Gold JM. A new perspective on anhedonia in schizophrenia. Am J Psychiatry. 2012;169:364‐373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hershenberg R, Satterthwaite TD, Daldal A, et al. Diminished effort on a progressive ratio task in both unipolar and bipolar depression. J Affect Disord. 2016;196:97‐100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Vrieze E, Pizzagalli DA, Demyttenaere K, et al. Reduced reward learning predicts outcome in major depressive disorder. Biol Psychiat. 2013;73:639‐645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Liu WH, Chan RC, Wang LZ, et al. Deficits in sustaining reward responses in subsyndromal and syndromal major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:1045‐1052. [DOI] [PubMed] [Google Scholar]
- 38. McCarthy JM, Treadway MT, Bennett ME, Blanchard JJ. Inefficient effort allocation and negative symptoms in individuals with schizophrenia. Schizophr Res. 2016;170:278‐284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Barch DM, Treadway MT, Schoen N. Effort, anhedonia, and function in schizophrenia: reduced effort allocation predicts amotivation and functional impairment. J Abnorm Psychol. 2014;123:387‐397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Henriques JB, Davidson RJ. Decreased responsiveness to reward in depression. Cogn Emot. 2000;14:711‐724. [Google Scholar]
- 41. Kring AM, Moran EK. Emotional response deficits in schizophrenia: insights from affective science. Schizophr Bull. 2008;34:819‐834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Murray GK, Corlett PR, Clark L, et al. Substantia nigra/ventral tegmental reward prediction error disruption in psychosis. Mol Psychiatry. 2008;13:267‐276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Waltz JA, Schweitzer JB, Ross TJ, et al. Abnormal responses to monetary outcomes in cortex, but not in the basal ganglia, in schizophrenia. Neuropsychopharmacology. 2010;35:2427‐2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Waltz JA, Frank MJ, Wiecki TV, Gold JM. Altered probabilistic learning and response biases in schizophrenia: behavioral evidence and neurocomputational modeling. Neuropsychol. 2011;25:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Schlagenhauf F, Juckel G, Koslowski M, et al. Reward system activation in schizophrenic patients switched from typical neuroleptics to olanzapine. Psychopharmacology. 2008;196:673‐684. [DOI] [PubMed] [Google Scholar]
- 46. Juckel G, Schlagenhauf F, Koslowski M, et al. Dysfunction of ventral striatal reward prediction in schizophrenic patients treated with typical, not atypical, neuroleptics. Psychopharmacology. 2006;187:222‐228. [DOI] [PubMed] [Google Scholar]
- 47. Simon JJ, Cordeiro SA, Weber MA, et al. Reward system dysfunction as a neural substrate of symptom expression across the general population and patients with schizophrenia. Schizophr Bull. 2015;41:1370‐1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gard DE, Kring AM, Gard MG, Horan WP, Green MF. Anhedonia in schizophrenia: distinctions between anticipatory and consummatory pleasure. Schizophr Res. 2007;93:253‐260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Loas G, Monestes JL, Yon V, Thomas P, Gard DE. Anticipatory anhedonia in schizophrenia subjects. L'Encéphale. 2010;36:85‐87. [DOI] [PubMed] [Google Scholar]
- 50. Favrod J, Ernst F, Giuliani F, Bonsack C. Validation of the temporal experience of pleasure scale (TEPS) in a French‐speaking environment. L'Encephale. 2009;35:241‐248. [DOI] [PubMed] [Google Scholar]
- 51. Chan RC, Wang Y, Huang J, et al. Anticipatory and consummatory components of the experience of pleasure in schizophrenia: cross‐cultural validation and extension. Psychiatry Res. 2010;175:181‐183. [DOI] [PubMed] [Google Scholar]
- 52. Strauss GP, Wilbur RC, Warren KR, August SM, Gold JM. Anticipatory vs. consummatory pleasure: what is the nature of hedonic deficits in schizophrenia? Psychiatry Res. 2011;187:36‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Da Silva S, Saperia S, Siddiqui I, et al. Investigating consummatory and anticipatory pleasure across motivation deficits in schizophrenia and healthy controls. Psychiatry Res. 2017;254:112‐117. [DOI] [PubMed] [Google Scholar]
- 54. Cassidy CM, Lepage M, Harvey PO, Malla A. Cannabis use and anticipatory pleasure as reported by subjects with early psychosis and community controls. Schizophr Res. 2012;137:39‐44. [DOI] [PubMed] [Google Scholar]
- 55. Edwards CJ, Cella M, Tarrier N, Wykes T. Predicting the future in schizophrenia: the discrepancy between anticipatory and consummatory pleasure. Psychiatry Res. 2015;229:462‐469. [DOI] [PubMed] [Google Scholar]
- 56. Waltz JA, Frank MJ, Robinson BM, Gold JM. Selective reinforcement learning deficits in schizophrenia support predictions from computational models of striatal‐cortical dysfunction. Biol Psychiatry. 2007;62:756‐764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Gold JM, Waltz JA, Matveeva TM, et al. Negative symptoms and the failure to represent the expected reward value of actions: behavioral and computational modeling evidence. Arch Gen Psychiatry. 2012;69:129‐138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Treadway MT, Peterman JS, Zald DH, Park S. Impaired effort allocation in patients with schizophrenia. Schizophr Res. 2015;161:382‐385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Fervaha G, Graff‐Guerrero A, Zakzanis KK, Foussias G, Agid O, Remington G. Incentive motivation deficits in schizophrenia reflect effort computation impairments during cost‐benefit decision‐making. J Psychiatr Res. 2013;47:1590‐1596. [DOI] [PubMed] [Google Scholar]
- 60. Beninger RJ, Wasserman J, Zanibbi K, Charbonneau D, Mangels J, Beninger BV. Typical and atypical antipsychotic medications differentially affect two nondeclarative memory tasks in schizophrenic patients: a double dissociation. Schizophr Res. 2003;61:281‐292. [DOI] [PubMed] [Google Scholar]
- 61. Ritter LM, Meador‐Woodruff JH, Dalack GW. Neurocognitive measures of prefrontal cortical dysfunction in schizophrenia. Schizophr Res. 2004;68:65‐73. [DOI] [PubMed] [Google Scholar]
- 62. Kester HM, Sevy S, Yechiam E, Burdick KE, Cervellione KL, Kumra S. Decision‐making impairments in adolescents with early‐onset schizophrenia. Schizophr Res. 2006;85:113‐123. [DOI] [PubMed] [Google Scholar]
- 63. Premkumar P, Fannon D, Kuipers E, Simmons A, Frangou S, Kumari V. Emotional decision‐making and its dissociable components in schizophrenia and schizoaffective disorder: a behavioural and MRI investigation. Neuropsychologia. 2008;46:2002‐2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Cavallaro R, Cavedini P, Mistretta P, et al. Basal‐corticofrontal circuits in schizophrenia and obsessive‐compulsive disorder: a controlled, double dissociation study. Biol Psychiatry. 2003;54:437‐443. [DOI] [PubMed] [Google Scholar]
- 65. Evans CE, Bowman CH, Turnbull OH. Subjective awareness on the Iowa Gambling Task: the key role of emotional experience in schizophrenia. J Clin Exp Neuropsychol. 2005;27:656‐664. [DOI] [PubMed] [Google Scholar]
- 66. Rodríguez‐Sánchez JM, Crespo‐Facorro B, Iglesias RP, et al. Prefrontal cognitive functions in stabilized first‐episode patients with schizophrenia spectrum disorders: a dissociation between dorsolateral and orbitofrontal functioning. Schizophr Res. 2005;77:279‐288. [DOI] [PubMed] [Google Scholar]
- 67. Wilder KE, Weinberger DR, Goldberg TE. Operant conditioning and the orbitofrontal cortex in schizophrenic patients: unexpected evidence for intact functioning. Schizophr Res. 1998;30:169‐174. [DOI] [PubMed] [Google Scholar]
- 68. Semkovska M, Bédard MA, Godbout L, Limoge F, Stip E. Assessment of executive dysfunction during activities of daily living in schizophrenia. Schizophr Res. 2004;69:289‐300. [DOI] [PubMed] [Google Scholar]
- 69. Jovanovski D, Zakzanis KK, Young DA, Campbell Z. Assessing the relationship between insight and everyday executive deficits in schizophrenia: a pilot study. Psychiatry Res. 2007;151:47‐54. [DOI] [PubMed] [Google Scholar]
- 70. Cohen AS, Minor KS, Najolia GM. A framework for understanding experiential deficits in schizophrenia. Psychiatry Res. 2010;178:10‐16. [DOI] [PubMed] [Google Scholar]
- 71. Llerena K, Strauss GP, Cohen AS. Looking at the other side of the coin: a meta‐analysis of self‐reported emotional arousal in people with schizophrenia. Schizophr Res. 2012;142:65‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Pizzagalli DA, Holmes AJ, Dillon DG, et al. Reduced caudate and nucleus accumbens response to rewards in unmedicated individuals with major depressive disorder. Am J Psych. 2009;166:702‐710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Stoy M, Schlagenhauf F, Sterzer P, et al. Hyporeactivity of ventral striatum towards incentive stimuli in unmedicated depressed patients normalizes after treatment with escitalopram. J Psychopharmacol. 2012;26:677‐688. [DOI] [PubMed] [Google Scholar]
- 74. Sharma A, Wolf DH, Ciric R, et al. Common dimensional reward deficits across mood and psychotic disorders: a connectome‐wide association study. Am J Psychiatry. 2017;174:657‐666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Howes OD, Williams M, Ibrahim K, et al. Midbrain dopamine function in schizophrenia and depression: a post‐mortem and positron emission tomographic imaging study. Brain. 2013;136:3242‐3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Björklund A, Dunnett SB. Dopamine neuron systems in the brain: an update. Trends Neurosci. 2007;30:194‐202. [DOI] [PubMed] [Google Scholar]
- 77. Owens MJ, Nemeroff CB. Role of serotonin in the pathophysiology of depression: focus on the serotonin transporter. Clin Chem. 1994;40:288‐295. [PubMed] [Google Scholar]
- 78. Trivedi MH, Rush AJ, Wisniewski SR, et al. Evaluation of outcomes with citalopram for depression using measurement‐based care in STAR* D: implications for clinical practice. Am J Psychiatry. 2006;163:28‐40. [DOI] [PubMed] [Google Scholar]
- 79. McMakin DL, Olino TM, Porta G, et al. Anhedonia predicts poorer recovery among youth with selective serotonin reuptake inhibitor treatment–resistant depression. J Am Acad Child Psychiatry. 2012;51:404‐411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Uher R, Perlis RH, Henigsberg N, et al. Depression symptom dimensions as predictors of antidepressant treatment outcome: replicable evidence for interest‐activity symptoms. Psychol Med. 2012;42:967‐980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Salamone JD, Correa M, Mingote S, Weber SM. Nucleus accumbens dopamine and the regulation of effort in food‐seeking behavior: implications for studies of natural motivation, psychiatry, and drug abuse. J Pharmacol Exp Ther. 2003;305:1‐8. [DOI] [PubMed] [Google Scholar]
- 82. Nakajima S, Liu X, Lau CL. Synergistic interaction of D1 and D2 dopamine receptors in the modulation of the reinforcing effect of brain stimulation. Behav Neurosci. 1993;107:161. [DOI] [PubMed] [Google Scholar]
- 83. Pizzagalli DA, Evins AE, Schetter EC, et al. Single dose of a dopamine agonist impairs reinforcement learning in humans: behavioral evidence from a laboratory‐based measure of reward responsiveness. Psychopharmacol. 2008;196:221‐232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Rizvi SJ, Geraci J, Ravindran A, Kennedy SH. Predictors of response to adjunctive osmotic‐release methylphenidate or placebo in patients with major depressive disorder: effects of apathy/anhedonia and fatigue. J Clin Psychopharmacol. 2014;34:755‐759. [DOI] [PubMed] [Google Scholar]
- 85. Mawlawi O, Martinez D, Slifstein M, et al. Imaging human mesolimbic dopamine transmission with positron emission tomography: I. Accuracy and precision of D2 receptor parameter measurements in ventral striatum. J Cereb Blood Flow Metab. 2001. Sep;21:1034‐1057. [DOI] [PubMed] [Google Scholar]
- 86. Lally N, Nugent AC, Luckenbaugh DA, Niciu MJ, Roiser JP, Zarate CA Jr. Neural correlates of change in major depressive disorder anhedonia following open‐label ketamine. J Psychopharmacol. 2015;29:596‐607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Goldberg D. The heterogeneity of “major depression”. World Psychiatry. 2011;10:226‐228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Matthysse ST. Antipsychotic drug actions: a clue to the neuropathology of schizophrenia? Fed Proc. 1973;32:200‐205. [PubMed] [Google Scholar]
- 89. Snyder SH. The dopamine hypothesis of schizophrenia: focus on the dopamine receptor. Am J Psychiatry. 1976;133:197‐202. [DOI] [PubMed] [Google Scholar]
- 90. Davis KL, Kahn RS. Dopamine in schizophrenia: a review and reconceptualization. Am J Psychiatry. 1991;148:1474. [DOI] [PubMed] [Google Scholar]
- 91. Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III—the final common pathway. Schizophr Bull. 2009;35:549‐562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Kapur S. Psychosis as a state of aberrant salience: a framework linking biology, phenomenology, and pharmacology in schizophrenia. Am J Psychiatry. 2003;160:13‐23. [DOI] [PubMed] [Google Scholar]
- 93. Winton‐Brown TT, Fusar‐Poli P, Ungless MA, Howes OD. Dopaminergic basis of salience dysregulation in psychosis. Trends Neurosci. 2014;37:85‐94. [DOI] [PubMed] [Google Scholar]
- 94. Rothkirch M, Tonn J, Köhler S, Sterzer P. Neural mechanisms of reinforcement learning in unmedicated patients with major depressive disorder. Brain. 2017;140:1147‐1157. [DOI] [PubMed] [Google Scholar]
- 95. Gradin VB, Kumar P, Waiter G, et al. Expected value and prediction error abnormalities in depression and schizophrenia. Brain. 2011;134:1751‐1764. [DOI] [PubMed] [Google Scholar]
- 96. Morris RW, Vercammen A, Lenroot R, et al. Disambiguating ventral striatum fMRI‐related bold signal during reward prediction in schizophrenia. Mol Psychiatry 2012;17:280‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Pelizza L, Ferrari A. Anhedonia in schizophrenia and major depression: state or trait? Ann Gen Psychiatry. 2009;8:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Lally N, Nugent AC, Luckenbaugh DA, Ameli R, Roiser JP, Zarate CA. Anti‐anhedonic effect of ketamine and its neural correlates in treatment‐resistant bipolar depression. Transl Psychiat. 2014;4:e469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Kennedy SH, Lam RW, McIntyre RS, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 clinical guidelines for the management of adults with major depressive disorder: section 3. pharmacological treatments. The. Can J Psychiat. 2016;61:540‐560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Argyropoulos SV, Nutt DJ. Anhedonia revisited: is there a role for dopamine‐targeting drugs for depression? J Psychopharmacol. 2013;27:869‐877. [DOI] [PubMed] [Google Scholar]
- 101. Gargoloff PD, Corral R, Herbst L, Marquez M, Martinotti G, Gargoloff PR. Effectiveness of agomelatine on anhedonia in depressed patients: an outpatient, open‐label, real‐world study. Hum Psychopharmacol. 2016;31:412‐418. [DOI] [PubMed] [Google Scholar]
- 102. McCabe C, Mishor Z, Cowen PJ, Harmer CJ. Diminished neural processing of aversive and rewarding stimuli during selective serotonin reuptake inhibitor treatment. Biol Psychiat. 2010;67:439‐445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Dunlop BW, Nemeroff CB. The role of dopamine in the pathophysiology of depression. Arch Gen Psychiatry. 2007;64:327‐337. [DOI] [PubMed] [Google Scholar]
- 104. Gelenberg AJ, Freeman MP, Markowitz JC, et al. Practice guideline for the treatment of patients with major depressive disorder third edition. Am J Psychiatry 2010;167:1.20068118 [Google Scholar]
- 105. Nelson JC, Papakostas GI. Atypical antipsychotic augmentation in major depressive disorder: a meta‐analysis of placebo‐controlled randomized trials. Focus. 2010;8:570‐582. [DOI] [PubMed] [Google Scholar]
- 106. Marcus RN, McQuade RD, Carson WH, et al. The efficacy and safety of aripiprazole as adjunctive therapy in major depressive disorder: a second multicenter, randomized, double‐blind, placebo‐controlled study. J Clin Psychopharmacol. 2008;28:156‐165. [DOI] [PubMed] [Google Scholar]
- 107. Berman RM, Fava M, Thase ME, et al. Aripiprazole augmentation in major depressive disorder: a double‐blind, placebo‐controlled study in patients with inadequate response to antidepressants. CNS Spectr. 2009;14:197‐206. [DOI] [PubMed] [Google Scholar]
- 108. Mazza M, Squillacioti MR, Pecora RD, Janiri L, Bria P. Effect of aripiprazole on self‐reported anhedonia in bipolar depressed patients. Psychiatry Res. 2009;165:193‐196. [DOI] [PubMed] [Google Scholar]
- 109. Lehman AF, Lieberman JA, Dixon LB, et al. Practice guideline for the treatment of patients with schizophrenia. Am J Psychiatry. 2004;161(2 suppl.):1‐56. [PubMed] [Google Scholar]
- 110. Laughren T, Levin R. Food and Drug Administration commentary on methodological issues in negative symptom trials. Schizophr Bull. 2011;37:255‐256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Keefe RS, Bilder RM, Davis SM, et al. Neurocognitive effects of antipsychotic medications in patients with chronic schizophrenia in the CATIE Trial. Arch Gen Psychiatry. 2007;64:633‐647. [DOI] [PubMed] [Google Scholar]
- 112. Lieberman JA, Stroup TS, McEvoy JP, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353:1209‐1223. [DOI] [PubMed] [Google Scholar]
- 113. Leucht S, Corves C, Arbter D, Engel RR, Li C, Davis JM. Second‐generation versus first‐generation antipsychotic drugs for schizophrenia: a meta‐analysis. Lancet. 2009;373:31‐41. [DOI] [PubMed] [Google Scholar]
- 114. Fusar‐Poli P, Papanastasiou E, Stahl D, et al. Treatments of negative symptoms in schizophrenia: meta‐analysis of 168 randomized placebo‐controlled trials. Schizophr Bull. 2014;41:892‐899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Németh G, Laszlovszky I, Czobor P, et al. Cariprazine versus risperidone monotherapy for treatment of predominant negative symptoms in patients with schizophrenia: a randomised, double‐blind, controlled trial. Lancet. 2017;389:1103‐1113. [DOI] [PubMed] [Google Scholar]
- 116. Lasser RA, Dirks B, Nasrallah H, et al. Adjunctive lisdexamfetamine dimesylate therapy in adult outpatients with predominant negative symptoms of schizophrenia: open‐label and randomized‐withdrawal phases. Neuropsychopharmacol. 2013;38:2140‐2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Strauss GP. The emotion paradox of anhedonia in schizophrenia: or is it? Schizophr Bull. 2013;39:247‐250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Treadway MT, Buckholtz JW, Schwartzman AN, Lambert WE, Zald DH. Worth the ‘EEfRT’? The effort expenditure for rewards task as an objective measure of motivation and anhedonia. PLoS One. 2009;4:e6598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Chase HW, Nusslock R, Almeida JR, Forbes EE, LaBarbara EJ, Phillips ML. Dissociable patterns of abnormal frontal cortical activation during anticipation of an uncertain reward or loss in bipolar versus major depression. Bipolar Disord. 2013;15:839‐854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Arrondo G, Segarra N, Metastasio A, et al. Reduction in ventral striatal activity when anticipating a reward in depression and schizophrenia: a replicated cross‐diagnostic finding. Front Psychol. 2015;6:1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Steele JD, Kumar P, Ebmeier KP. Blunted response to feedback information in depressive illness. Brain. 2007;130:2367‐2374. [DOI] [PubMed] [Google Scholar]
- 122. Kumar P, Waiter G, Ahearn T, Milders M, Reid I, Steele JD. Abnormal temporal difference reward‐learning signals in major depression. Brain. 2008;131:2084‐2093. [DOI] [PubMed] [Google Scholar]


