Abstract
Background
In patients with atrial fibrillation, ablation decreases left atrial (LA) compliance, which may lead to left ventricular (LV) diastolic dysfunction. We aimed to examine serial changes in LV diastolic function after 2 ablation procedures and their related factors in patients with paroxysmal atrial fibrillation (PAF).
Hypothesis
LV diastolic function is different after 2 ablation procedures.
Methods
We enrolled 132 patients with PAF (76 males, mean age 67 years; cryoballoon [CB] ablation/radiofrequency [RF] ablation 60/72) who underwent a single ablation procedure. The transthoracic echocardiographic parameters were obtained before, 3 days after, and 6 months after ablation.
Results
The afterload‐related index of LV diastolic function, Ed/Ea = E/e' / (0.9 × systolic blood pressure), increased significantly at 3 days after ablation, especially after CB ablation (P <0.05), although no differences were observed in age, sex, LA size, LV size, and E/e' before ablation between CB ablation and RF ablation. Creatine kinase release after ablation was significantly higher in CB ablation than in RF ablation (P <0.001). The increment of Ed/Ea after CB ablation was positively correlated with LV ejection fraction (LVEF) before ablation (r =0.416; P <0.05). The elderly (age ≥ 75 years), females, and patients with hypertension were more likely to show impaired LV diastolic function transiently after 3 days of ablation, but the diastolic index was restored to baseline level after 6 months.
Conclusions
The increased Ed/Ea after CB ablation represented transient manifestation of underlying LV diastolic dysfunction in PAF patients with preserved LVEF with older age, female sex, and a history of hypertension.
Keywords: Cryoballoon Ablation, Diastolic Dysfunction, Paroxysmal Atrial Fibrillation, Radiofrequency Ablation, Transthoracic Echocardiography
1. INTRODUCTION
There is a potential risk that ablation procedures for patients with atrial fibrillation (AF) may decrease left atrial (LA) compliance, resulting in impaired LA diastolic function. Furthermore, the reduction in LA compliance after ablation may lead to relatively increased volume and elevation of left ventricular (LV) diastolic parameters.1 LA injury during the ablation procedure may be more severe in cryoballoon (CB) ablation than in radiofrequency (RF) ablation.2 However, the immediate difference in LV diastolic function due to acutely impaired LA compliance between the 2 ablation procedures, possibly resulting from the extent of the isolated area of the posterior LA wall during the acute phase, remains to be seen. We recently reported afterload‐related index for LV diastolic function is significantly higher in elderly (age ≥ 75 years) hypertensive females than in elderly hypertensive males.3, 4 In this study, we aimed to elucidate the differences in altered LV diastolic function shortly after ablation between patients with paroxysmal AF (PAF) treated with RF ablation and second‐generation CB ablation and to examine the factors that affect the manifestation of an underlying LV diastolic dysfunction in these patients.
2. METHODS
2.1. Patient selection
We enrolled 132 patients with PAF (76 males; mean age, 67 years) who consecutively underwent a single RF ablation (from June 2014 to May 2016; n = 72) or CB ablation (from June 2016 to June 2017; n = 60). These patients had symptomatic PAF that was refractory to drugs. We excluded the patients with significant mitral annular calcification, mitral stenosis, or moderate to severe mitral regurgitation. Medical histories were obtained by reviewing patients' medical records for electrocardiograms and Holter recordings of AF episodes. Oral anticoagulation therapy was necessary for ≥1 month before and 6 months after ablation. Blood samples were obtained before ablation and the day after ablation. The investigation conforms with the principles outlined in the Declaration of Helsinki and was approved by the institutional review board of our hospital. Written informed consent was obtained from all patients before catheter ablation.
2.2. Ablation procedure
After obtaining LA access, a bolus of heparin followed by additional boluses was administered to maintain an activated clotting time of 300 to 350 seconds. In case of RF ablation, we performed the extensive encircling pulmonary vein isolation (EEPVI) method using 2 circular mapping catheters (Optima; St. Jude Medical, Minneapolis, MN). Three‐dimensional (3D) mapping was performed with the NavX system (St. Jude Medical). Two Optima catheters were placed within the ipsilateral superior and inferior pulmonary veins (PVs). After constructing 3D electroanatomical maps with NavX, continuous circumferential ablation lines were created around the left‐ and right‐sided PVs by using a 3.5‐mm‐tip irrigated CoolFlex catheter (St. Jude Medical) at a maximum power of 30 W for 30 seconds at each site. The EEPVI endpoint was the absence or dissociation of local electrograms within the entire surrounded region together with the exit block through pacing within the PV ostia.
In the case of CB ablation, after gaining LA access, an inner lumen mapping catheter (Achieve; Medtronic, Minneapolis, MN) was advanced into each PV ostium. Then, a 28‐mm cryoballoon (Arctic Front Advance; Medtronic) was advanced, inflated, and positioned sequentially in the PV ostium of each vein. Optimal vessel occlusion was considered achieved upon selective contrast injection. Cryothermal applications lasted 4 minutes, and a bolus freeze following isolation was systematically performed in the initiation procedures. Subsequently, all procedures were performed with a single 3‐minute application for each vein. The freeze duration was reduced to 3 minutes because of the very short time to isolate numerous PVs.5 A second freeze was delivered if PV isolation failed after the first cycle in the case of nadir temperature > −40 °C and in early PV reconnection. To monitor right phrenic nerve activity during CB ablation in the right‐sided veins, a circular mapping catheter was placed in the superior vena cava at a high output of 1200‐ms cycle length to capture the phrenic nerve. PV isolation was confirmed at the end of the procedure using a circular mapping catheter. In both ablation groups, PV isolation was achieved in 100%. Patients were treated according to the physician's discretion and current guidelines.
2.3. Echocardiographic examination
Transthoracic echocardiography (Aplio 400; Toshiba Medical, Tokyo, Japan) was performed before, 3 days after, and 6 months after ablation under sinus rhythm in all patients. Measurements of echocardiographic parameters such as chamber size (LA dimension [LAD]), LA volume index [LAVI], and LV dimensions), LV ejection fraction (LVEF), stroke volume index (SVI), tricuspid regurgitation pressure gradient (TRPG), transmitral flow velocity (E/A), and tissue Doppler images of the mitral annular septal and lateral area (mean e') were obtained according to the American or European Society of Echocardiography criteria.4, 6, 7 All patients exhibited LVEF ≥50%. As an index for afterload, we examined effective arterial elastance (Ea) calculated by end‐systolic blood pressure (SBP)/SVI = (0.9 × SBP)/SVI.8, 9 We also examined 3 E/e'‐related indices of LV diastolic function, namely E/e', operant diastolic elastance = Ed = E/e'/SVI,10 and Ed/Ea = E/e'/(0.9 × SBP).3, 4 The index was calculated by dividing it by the body surface area. Echocardiographic measurements were compared between the procedures with RF ablation and CB ablation. Transesophageal echocardiography was performed in all patients before ablation to exclude the presence of LA thrombus.11
2.4. Statistical analysis
Continuous variables were expressed as mean ±SD, and categorical variables were presented as frequency and percentage. Differences in categorical variables between the groups were compared by using the χ2 test, and differences in continuous variables between the groups were compared with Student t test or Welch t test, appropriately. Between‐group differences were assessed by 1‐way analysis of variance or Kruskal‐Wallis analysis for 3 comparisons, and differences between pairs of each group were assessed by a post‐hoc Bonferroni test (t test or Mann–Whitney test). Correlations were tested using the Pearson or Spearman coefficient, and the P value was examined by regression analysis. A P value of <0.05 was considered significant.
3. RESULTS
3.1. Clinical and laboratory data before ablation
The clinical and laboratory data before ablation in all patients, including the CB ablation and RF ablation groups, are shown in Table 1. No significant differences were found in age, sex, and the incidence of comorbidities such as hypertension (HTN) and diabetes mellitus between the 2 ablation groups. No differences were also observed in the levels of estimated glomerular filtration rate and brain natriuretic peptide between both procedures. In echocardiographic data before ablation, no differences were observed in LAD, LAVI, LVEF, SVI, E/e', and TRPG between the 2 ablation groups (Table 2). In addition, there was no difference in SBP before the procedure between both ablation groups (CB ablation, 128 ±16 mm Hg, vs RF ablation, 126 ±16 mm Hg; P = 0.560).
Table 1.
Clinical and laboratory characteristics before ablation
| All, N = 132 | CB, n = 60 | RF, n = 72 | P Valuea | |
|---|---|---|---|---|
| Mean age, y | 67 ± 10 | 67 ±12 | 66 ±9 | 0.652 |
| Male sex | 76 (57) | 36 (60) | 40 (55) | 0.610 |
| HTN | 101 (76) | 54 (77) | 47 (65) | 0.155 |
| DM | 27 (20) | 16 (26) | 11 (15) | 0.081 |
| Dyslipidemia | 35 (26) | 19 (31) | 16 (22) | 0.224 |
| Laboratory data | ||||
| FBG, mg/dL | 106 ±23 | 105 ±28 | 106 ±18 | 0.735 |
| eGFR, mL/min/1.73 m2 | 66.2 ±16.6 | 66.1 ±16.1 | 66.4 ±17.1 | 0.930 |
| BNP, pg/mL | 110.3 ±160.1 | 100.8 ±168.6 | 118.5 ±153.1 | 0.533 |
Abbreviations: BNP, brain natriuretic peptide; CB, cryoballoon; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; FBG, fasting blood glucose; HTN, hypertension; RF, radiofrequency; SD, standard deviation.
Data are expressed as n (%) or mean ±SD.
P values represent the comparison of data between the CB and RF groups.
Table 2.
Changes in echocardiographic data before and 3 days after ablation
| All | P Valuea | CB | P Valuea | RF | P Valuea | ||||
|---|---|---|---|---|---|---|---|---|---|
| Before | 3 Days After | Before | 3 Days After | Before | 3 Days After | ||||
| LVDd, mm | 46 ± 4 | 45 ±4 | 0.201 | 45 ±4 | 45 ±3 | 0.919 | 47 ±4 | 45 ±4 | 0.103 |
| LVDs, mm | 29 ±4 | 28 ±3 | 0.167 | 28 ±3 | 28 ±3 | 0.978 | 30 ±4 | 28 ±4 | 0.080 |
| LVEF, % | 66.0 ±7.3 | 66.8 ±7.3 | 0.428 | 67.1 ±7.7 | 66.9 ±7.5 | 0.897 | 65.1 ±6.9 | 66.7 ±7.3 | 0.234 |
| SVI, mL/m2 | 39.2 ±8.0 | 38.3 ±8.0 | 0.413 | 38.2 ±7.7 | 38.1 ±8.7 | 0.929 | 40.1 ±8.3 | 38.5 ±7.3 | 0.310 |
| LAD, mm | 39 ±6 | 39 ±6 | 0.962 | 39 ±6 | 39 ±5 | 0.715 | 39 ±6 | 39 ±7 | 0.799 |
| LAVI, mL/m2 | 33 ±11 | 32 ±14 | 0.611 | 33 ±8 | 31 ±10 | 0.388 | 33 ±13 | 33 ±16 | 0.972 |
| TRPG, mm Hg | 22 ±5 | 22 ±5 | 0.808 | 23 ±6 | 22 ±5 | 0.588 | 21 ±5 | 22 ±5 | 0.437 |
| Ea, mm Hg × m2/mL | 3.05 ±0.71 | 3.20 ±0.72 | 0.166 | 3.13 ±0.72 | 3.16 ±0.76 | 0.870 | 2.97 ±0.69 | 3.24 ±0.69 | 0.074 |
Abbreviations: CB, cryoballoon; Ea, effective arterial elastance; LAD, left atrial dimension; LAVI, left atrial volume index; LVDd, left ventricular end‐diastolic dimension; LVDs, left ventricular end‐systolic dimension; LVEF, left ventricular ejection fraction; RF, radiofrequency; SD, standard deviation; SVI, stroke volume index; TRPG, tricuspid regurgitation pressure gradient.
Data are expressed as mean ±SD.
P values represent the comparison of the data before and 3 days after ablation.
3.2. Alterations of echocardiographic data after ablation
No changes were noted in LA size, LV size, LVEF, SVI, and TRPG 3 days after ablation in all patients and in both ablation groups (Table 2). Furthermore, Ea did not differ significantly before and 3 days after ablation in all patients and in both ablation groups (Table 2). SBP did not differ significantly between the 2 ablation groups 3 days after ablation (129 ±14 mm Hg vs 136 ±22 mm Hg; P = 0.084). However, the 3 indices for LV diastolic function increased significantly at 3 days after ablation in all patients (Figure 1A). Furthermore, these indices increased significantly only in the CB ablation group (Figure 1B). The 3 indices for LV diastolic function were restored to baseline levels at 6 months after ablation in the CB ablation group. The increment of Ed/Ea, but not E/e' or Ed, shortly after CB ablation was positively and significantly correlated with LVEF before ablation (ΔE/e' vs LVEF, r = 0.317, P = 0.086; ΔEd vs LVEF, r = 0.286, P = 0.125; ΔEd/Ea vs LVEF, r = 0.416, P = 0.021). Other indices for LV diastolic function such as E/A and deceleration time were not altered after ablation in both treatment groups (data not shown).
Figure 1.
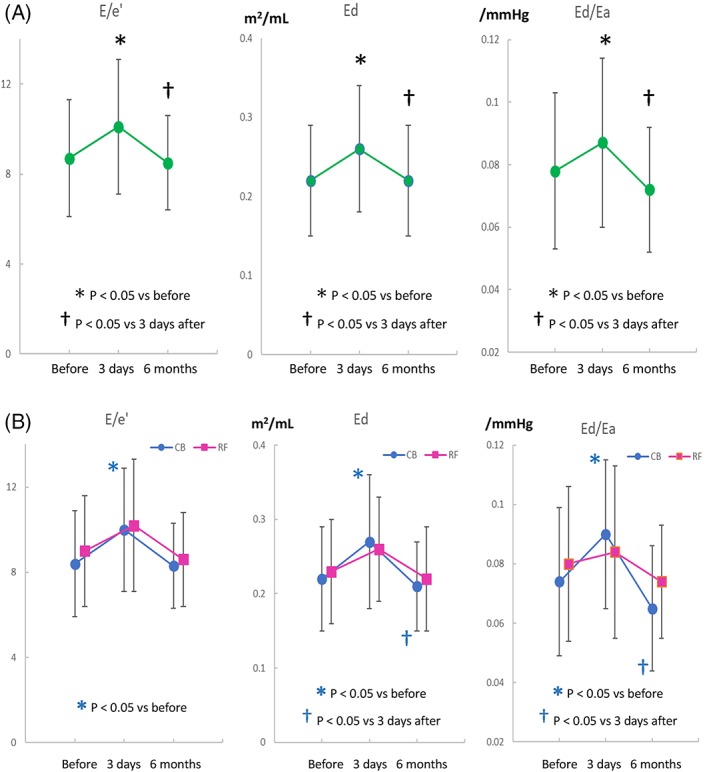
(A) Serial changes in E/e'‐related indices for LV diastolic function such as E/e', operant diastolic elastance = Ed = E/e'/SVI, and Ed/effective arterial elastance (Ea) = E/e'/(0.9 × SBP) before and after ablation in all patients with PAF and (B) the differences in the changes of E/e'‐related indices between patients treated with CB ablation (blue closed circles) and RF ablation (red closed squares). No significant differences were observed in these indices of RF ablation group. Abbreviations: CB, cryoballoon; LV, left ventricular; PAF, paroxysmal atrial fibrillation; RF, radiofrequency; SBP, systolic blood pressure; SVI, stroke volume index
3.3. Modulating factors for altered diastolic function
Among all patients with PAF, sex difference was observed in the alterations of the indices for LV diastolic function after ablation. These indices did not differ significantly between sexes before ablation. However, there were significant differences in E/e' (P < 0.001), Ed (P = 0.038), and Ed/Ea (P = 0.001; Table 3) 3 days after ablation between sexes, although these parameters similarly increased after ablation in both sexes. Ea did not differ significantly between sexes before ablation (P = 0.416) and 3 days after ablation (P = 0.715). Similar changes in LV diastolic function after ablation were observed between all patients age ≥ 75 years and < 75 years or between all patients with and without HTN (Table 3). Changes in these parameters after ablation did not differ significantly between the patients with and without diabetes mellitus (Table 3). Changes in Ed/Ea observed in all patients were essentially similar in patients with PAF treated with CB ablation (Figure 2). In patients treated with RF ablation, the alterations were less prominent than those in patients treated with CB ablation (Figure 2). In multivariate logistic regression analysis for the increment of Ed/Ea, the P value for CB ablation was smaller (P = 0.075) compared with that for age (P = 0.875), sex (P = 0.261), or the incidence of HTN (P = 0.592).
Table 3.
Risk factors and changes in 3 indices of diastolic function before and 3 days after ablation in patients with PAF
| Males | Females | P Value (M vs F) | Age < 75 y | Age ≥ 75 y | P Value (<75 vs ≥75) | HTN– | HTN+ | P Value (HT– vs HT+) | DM– | DM+ | P Value (DM– vs DM+) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E/e' | ||||||||||||
| Before | 8.0 ±2.3 | 9.2 ±2.4 | 0.054 | 8.0 ±2.3 | 9.1 ±2.4 | 0.063 | 7.8 ±2.2 | 8.6 ±2.5 | 0.209 | 8.3 ±2.5 | 8.4 ±2.8 | 0.978 |
| 3 days after | 9.2 ±2.4 | 11.9 ±3.5 | <0.001 | 9.4 ±3.0 | 11.3 ±2.6 | 0.010 | 8.8 ±3.1 | 10.6 ±2.9 | 0.026 | 10.1 ±3.1 | 9.7 ±2.9 | 0.685 |
| P value (before vs after) | <0.001 | 0.003 | 0.001 | <0.001 | 0.118 | <0.001 | <0.001 | 0.184 | ||||
| Ed = E/e'/SVI, m2/mL | ||||||||||||
| Before | 0.21 ±0.07 | 0.22 ±0.08 | 0.434 | 0.20 ±0.06 | 0.25 ±0.07 | 0.002 | 0.20 ±0.05 | 0.22 ±0.08 | 0.169 | 0.21 ±0.07 | 0.21 ±0.07 | 0.951 |
| 3 days after | 0.25 ±0.07 | 0.29 ±0.09 | 0.038 | 0.24 ±0.07 | 0.30 ±0.09 | 0.009 | 0.23 ±0.06 | 0.28 ±0.08 | 0.016 | 0.26 ±0.08 | 0.26 ±0.09 | 0.950 |
| P value (before vs after) | <0.001 | 0.002 | <0.001 | <0.001 | 0.039 | <0.001 | <0.001 | 0.077 | ||||
| Ed/Ea, /mm Hg | ||||||||||||
| Before | 0.069 ± 0.021 | 0.078 ±0.021 | 0.104 | 0.069 ±0.019 | 0.079 ±0.023 | 0.053 | 0.069 ±0.022 | 0.074 ±0.021 | 0.374 | 0.071 ±0.020 | 0.076 ±0.028 | 0.439 |
| 3 days after | 0.079 ±0.021 | 0.102 ±0.033 | 0.001 | 0.081 ±0.028 | 0.098 ±0.019 | 0.014 | 0.077 ±0.030 | 0.091 ±0.024 | 0.048 | 0.086 ±0.028 | 0.086 ±0.022 | 0.961 |
| P value (before vs after) | 0.002 | 0.002 | 0.003 | 0.001 | 0.149 | <0.001 | <0.001 | 0.348 | ||||
Abbreviations: DM, diabetes mellitus; Ea, effective arterial elastance; Ed, operant diastolic elastance; F, female; HTN, hypertension; M, male; PAF, paroxysmal atrial fibrillation; SD, standard deviation; SVI, stroke volume index.
Data are expressed as mean ± SD.
Figure 2.
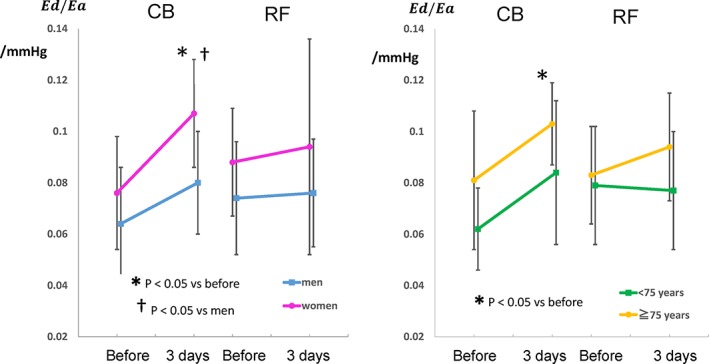
Sex‐ and age‐related changes in diastolic elastance (Ed)/arterial elastance (Ea) = Ed/Ea, before and 3 days after CB and RF ablation. Abbreviations: CB, cryoballoon; RF, radiofrequency
Significant differences were observed in serum levels of creatine kinase (CB ablation: 287 ±100 IU/L vs RF ablation: 128 ±60 IU/L, P < 0.001) and creatine kinase‐MB isoenzyme (CB ablation: 29 ±10 IU/L/37 °C vs RF ablation: 18 ±8 IU/L/37 °C, P = 0.008) 24 hours after ablation between the 2 ablation groups, although there were no differences in these levels before ablation.
4. DISCUSSION
4.1. Manifestation of underlying LV diastolic dysfunction after ablation
Ablation could be useful to determine patients with PAF who have an underlying LV diastolic dysfunction. No changes in LA size and LVEF were observed, but increases in E/e'‐related indices showing transient manifestation of LV diastolic dysfunction shortly after ablation were observed in patients with PAF, especially in the CB ablation group in our study. The impaired LA compliance leading to relatively increased volume may transiently progress LV diastolic dysfunction that is not evident before ablation.1, 12 As significantly positive correlation was found between LVEF before ablation and increment of Ed/Ea, even in the patients with PAF with preserved LV systolic function, the acute reduction of LA compliance by CB ablation procedure may provoke the acute impairment of LV diastolic function.
During the chronic phase, although there is no difference in the recurrence rate between CB ablation and RF ablation procedures in general,13, 14 intact areas of the posterior LA wall after PV isolation with CB ablation are larger (48%) than the areas encircled by RF ablation (37%).15 However, Kenigsberg et al. reported that the intact areas of the posterior LA wall immediately after PV isolation with CB ablation were only 27%.16 Myocardial damage during the procedure is reported to be larger in CB ablation than in RF ablation,2 which was also observed in our study. The edematous change during the acute phase might be greater in CB ablation than in RF ablation. The difference in the manifestation of LV diastolic dysfunction may be due to the different isolation areas of each PV antrum between CB ablation and RF ablation.
We reported the alterations of LV diastolic function after ablation using the indices exhibiting different aspects for LV diastolic function, such as E/e', Ed = E/e'/SVI, and Ed/Ea = E/e'/(0.9 × systolic blood pressure). These parameters showed almost similar changes after ablation, confirming the altered LV diastolic function after ablation. However, E/e' values showed heterogeneity of variance (Kruskal‐Wallis analysis) than the other 2 indices (1‐way analysis of variance). It is well known that Ed and Ea are higher in females than in males, under stable conditions.17, 18 Given that LV diastolic function is obviously affected by the extent of the afterload, the elevated Ed could be an epiphenomenon because of an associated increase in Ea. Therefore, Ed must be corrected by indexing to Ea as Ed/Ea. Ed/Ea may be a more suitable index for LV diastolic function among the 3 E/e'‐related indices, considering the P values and correlation coefficient in our study.
An ablation procedure for AF in patients with LV diastolic dysfunction is associated with an increased short‐term or long‐term recurrence risk.19, 20 Furthermore, the presence of LV hypertrophy in patients with PAF, possibly having underlying LV diastolic dysfunction, is a strong and independent predictor of recurrence after ablation.21 Because the elderly, females, or patients with HTN exhibited impaired LV diastolic function after ablation, a large‐scale prospective study to elucidate the difference in the future incidence of heart failure with preserved ejection fraction (HFpEF) and/or AF and their related factors among patients with PAF with and without LV diastolic dysfunction shortly after ablation is warranted.
4.2. Clinical implications
Not all patients with PAF with ablation procedures show LV diastolic dysfunction shortly after ablation, although AF per se is a well‐known risk factor for HFpEF.22 In our study, the elderly, females, or patients with HTN with PAF and preserved LV systolic function were at risk for impaired LV diastolic function after ablation, in whom the incidence of HFpEF may be high in the near future when LA compliance is acutely and/or persistently impaired by any reasons. Acutely altered LV diastolic function brought by ablation procedures in patients with PAF may represent a clue to clarify one of the various mechanisms for the occurrence of HFpEF.
4.3. Study limitations
We performed echocardiographic examination 3 days after ablation because of the individual differences in the extent of volume overload during the procedures. In general, >2 weeks of maintained sinus rhythm after cardioversion might be necessary for a complete restoration of LA function in patients with AF. Although the restoration of sinus rhythm after ablation results in significant improvements of ventricular systolic function in AF patients with systolic dysfunction free from ventricular fibrosis,23 whether the diastolic properties can transiently worsen before LVEF improves remains undefined. Because our patients had preserved LV systolic function, our finding may not necessarily apply to patients with impaired LV systolic function. LA baroreceptors play an important role in cardiopulmonary baroreceptor reflex.24 Ablation procedures for AF may damage the LA baroreceptors located at the junction of LA and PVs and reduce the ability to regulate LA pressure changes. The relationship between the differences in the effects of the 2 ablation procedures on LA baroreceptors and on LV diastolic function is needed to be defined.
5. CONCLUSION
The increase in afterload‐related index of LV diastolic function after CB ablation represents a transient manifestation of underlying LV diastolic dysfunction in patients with PAF. Acutely impaired LA compliance may provoke the manifestation of LV diastolic dysfunction in patients with preserved LVEF, with older age, female sex, and a history of HTN.
Conflicts of interest
The authors declare no potential conflicts of interest.
Minamisaka T, Watanabe T, Shinoda Y, et al. Transient manifestation of left ventricular diastolic dysfunction following ablation in patients with paroxysmal atrial fibrillation. Clin Cardiol. 2018;41:978–984. 10.1002/clc.22990
REFERENCES
- 1. Witt CM, Fenstad ER, Cha YM, et al. Increase in pulmonary arterial pressure after atrial fibrillation ablation: incidence and associated findings. J Interv Card Electrophysiol. 2014;40:47–52. [DOI] [PubMed] [Google Scholar]
- 2. Miyazaki S, Kuroi A, Hachiya H, et al. Early recurrence after pulmonary vein isolation of paroxysmal atrial fibrillation with different ablation technologies: prospective comparison of radiofrequency vs. second‐generation cryoballoon ablation. Circ J. 2016;80:346–353. [DOI] [PubMed] [Google Scholar]
- 3. Hoshida S, Shinoda Y, Ikeoka K, et al. Age‐ and sex‐related differences in diastolic function and cardiac dimensions in a hypertensive population. ESC Heart Fail. 2016;3:270–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hoshida S, Shinoda Y, Ikeoka K, et al. Fluctuation of dynamic diastolic function relative to static cardiac structure: new insights into the underlying mechanism of heart failure with preserved ejection fraction in elderly patients. Circ J. 2017;81:755–758. [DOI] [PubMed] [Google Scholar]
- 5. Chierchia GB, Di Giovanni G, Sieira‐Moret J, et al. Initial experience of three‐minute freeze cycles using the second‐generation cryoballoon ablation: acute and short‐term procedural outcomes. J Interv Card Electrophysiol. 2014;39:145–151. [DOI] [PubMed] [Google Scholar]
- 6. Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. [DOI] [PubMed] [Google Scholar]
- 7. Nagueh SF, Appleton CP, Gillebert TC, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. Eur J Echocardiogr. 2009;10:165–193. [DOI] [PubMed] [Google Scholar]
- 8. Sunagawa K, Maughan WL, Burkhoff D, et al. Left ventricular interaction with arterial load studied in isolated canine ventricle. Am J Physiol. 1983;245(5 part 1):H773–H780. [DOI] [PubMed] [Google Scholar]
- 9. Kelly RP, Ting CT, Yang TM, et al. Effective arterial elastance as index of arterial vascular load in humans. Circulation. 1992;86:513–521. [DOI] [PubMed] [Google Scholar]
- 10. Redfield MM, Jacobsen SJ, Borlaug BA, et al. Age‐ and gender‐related ventricular‐vascular stiffening: a community‐based study. Circulation. 2005;112:2254–2262. [DOI] [PubMed] [Google Scholar]
- 11. Watanabe T, Shinoda Y, Ikeoka K, et al. Dabigatran exhibits low intensity of left atrial spontaneous echo contrast in patients with nonvalvular atrial fibrillation as compared with warfarin. Heart Vessels. 2017;32:326–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shoemaker MB, Hemnes AR, Robbins IM, et al. Left atrial hypertension after repeated catheter ablations for atrial fibrillation. J Am Coll Cardiol. 2011;57:1918–1919. [DOI] [PubMed] [Google Scholar]
- 13. Chierchia GB, Di Giovanni G, Ciconte G, et al. Second‐generation cryoballoon ablation for paroxysmal atrial fibrillation: 1‐year follow‐up. Europace. 2014;16:639–644. [DOI] [PubMed] [Google Scholar]
- 14. Ciconte G, Baltogiannis G, de Asmundis C, et al. Circumferential pulmonary vein isolation as index procedure for persistent atrial fibrillation: a comparison between radiofrequency catheter ablation and second‐generation cryoballoon ablation. Europace. 2015;17:559–565. [DOI] [PubMed] [Google Scholar]
- 15. Miyazaki S, Taniguchi H, Hachiya H, et al. Quantitative analysis of the isolation area during the chronic phase after a 28‐mm second‐generation cryoballoon ablation demarcated by high‐resolution electroanatomic mapping. Circ Arrhythm Electrophysiol. 2016;9:e003879. [DOI] [PubMed] [Google Scholar]
- 16. Kenigsberg DN, Martin N, Lim HW, et al. Quantification of the cryoablation zone demarcated by pre‐ and postprocedural electroanatomic mapping in patients with atrial fibrillation using the 28‐mm second‐generation cryoballoon. Heart Rhythm. 2015;12:283–290. [DOI] [PubMed] [Google Scholar]
- 17. Goto T, Ohte N, Fukuta H, et al. Relationship between effective arterial elastance, total vascular resistance, and augmentation index at the ascending aorta and left ventricular diastolic function in older women. Circ J. 2013;77:123–129. [DOI] [PubMed] [Google Scholar]
- 18. Gori M, Lam CSP, Gupta DK, et al; PARAMOUNT Investigators . Sex‐specific cardiovascular structure and function in heart failure with preserved ejection fraction. Eur J Heart Fail. 2014;16:535–542. [DOI] [PubMed] [Google Scholar]
- 19. Cha YM, Wokhlu A, Asirvatham SJ, et al. Success of ablation for atrial fibrillation in isolated left ventricular diastolic dysfunction: a comparison to systolic dysfunction and normal ventricular function. Circ Arrhythm Electrophysiol. 2011;4:724–732. [DOI] [PubMed] [Google Scholar]
- 20. Kosiuk J, Breithardt OA, Bode K, et al. The predictive value of echocardiographic parameters associated with left ventricular diastolic dysfunction on short‐ and long‐term outcomes of catheter ablation of atrial fibrillation. Europace. 2014;16:1168–1174. [DOI] [PubMed] [Google Scholar]
- 21. Li SN, Wang L, Dong JZ, et al. Electrocardiographic left ventricular hypertrophy predicts recurrence of atrial arrhythmias after catheter ablation of paroxysmal atrial fibrillation. Clin Cardiol. 2018;41:797–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rusinaru D, Leborgne L, Peltier M, et al. Effect of atrial fibrillation on long‐term survival in patients hospitalised for heart failure with preserved ejection fraction. Eur J Heart Fail. 2008;10:566–572. [DOI] [PubMed] [Google Scholar]
- 23. Prabhu S, Kistler PM. Atrial fibrillation, an underappreciated reversible cause of cardiomyopathy: Implications for clinical practice from the CAMERA‐MRI study. Heart Lung Circ. 2018;27:652–655. [DOI] [PubMed] [Google Scholar]
- 24. Oren RM, Schobel HP, Weiss RM, et al. Importance of left atrial baroreceptors in the cardiopulmonary baroreflex of normal humans. J Appl Physiol (1985). 1993;74:2672–2680. [DOI] [PubMed] [Google Scholar]


