Abstract
Background
We sought to evaluate the incremental prognostic benefit of carotid artery disease and subclinical coronary artery disease (CAD) features in addition to clinical evaluation in an asymptomatic population.
Methods
Over a 6‐year period, 10‐year Framingham risk score together with carotid ultrasound and coronary computed tomography angiography were evaluated for prediction of major adverse cardiac events (MACE).
Results
We enrolled 517 consecutive asymptomatic patients (63% male, mean age 64 ±10 years; 17.6% with diabetes). Median (interquartile range) coronary artery calcium score (CACS) was 34 (0–100). Over a median follow‐up of 4.4 (3.4–5.1) years, there were 53 MACE (10%). Patients experiencing MACE had higher CACS, incidence of carotid disease, presence of CAD ≥50%, and remodeled plaque as compared with patients without MACE. At multivariable analyses, presence of CAD ≥50% (HR: 5.14, 95% CI: 2.1–12.4) and percentage of segments with remodeled plaque (HR: 1.04, 95% CI: 1.03–1.06) independently predicted MACE (P < 0.001). Models adding CAD ≥50% or percentage of segments with remodeled plaque resulted in higher discrimination and reclassification ability compared with a model based on 10‐year FRS, carotid disease, and CACS. Specifically, the C‐statistic improved to 0.75 with addition of CAD and 0.84 when adding percentage of segments with remodeled plaque, whereas net reclassification improvement indices were 0.86 and 0.92, respectively.
Conclusions
In an asymptomatic population, CAD and plaque positive remodeling increase MACE prediction compared with a model based on 10‐year FRS, carotid disease, and CACS estimation. In the diabetes subgroup, percentage of segments with remodeled plaque was the only predictor of MACE.
Keywords: Calcium Scoring, Cardiovascular Prevention, Carotid Disease, Computed Tomography, Coronary Artery Disease, Diabetes Mellitus, Subclinical Atherosclerosis
1. INTRODUCTION
Prevention of cardiovascular (CV) disease is mandatory for ethical and economic reasons.1, 2 Studies have evaluated carotid and coronary atherosclerotic burden assessment in predicting CV events in healthy individuals.3, 4, 5, 6 Despite promising results, to date the clinical usefulness of noninvasive subclinical atherosclerosis evaluation in asymptomatic at‐risk individuals is still debated. The role of coronary computed tomography angiography (CCTA) in symptomatic patients is delineated, but it is still unknown in asymptomatic individuals with significant atherosclerosis because of a paucity of data.7, 8, 9 Accordingly, the aim of the study was to assess the prognostic benefit of carotid artery disease by ultrasound technique and subclinical coronary artery disease (CAD) using CCTA in an asymptomatic at‐risk adult population. Moreover, we analyzed the impact of each technique on improving net reclassification improvement (NRI) and integrated discrimination improvement (IDI) as compared with Framingham risk score (FRS). In addition, we sought to evaluate the prognostic impact of the comprehensive diagnostic approach in the subset of subjects with diabetes mellitus (DM).
2. METHODS
2.1. Study population and enrollment
We enrolled a total of 691 consecutive asymptomatic patients referred to Centro Cardiologico Monzino for carotid ultrasound (CUS) assessment and CCTA for screening between February 2004 and June 2010. Exclusion criteria included previous history of CV disease (n = 51), contraindication to CCTA (n = 102), and inadequate heart rate (>75 bpm; n = 21). Accordingly, 517 patients were included in the study and underwent a structured clinical interview, CV risk factors collection, 10‐year CV risk calculation according to FRS,10 CUS, and CCTA within 2 weeks of the enrollment. Written informed consent was obtained from all patients, and the institutional ethics committee approved the study protocol.
2.2. Carotid ultrasound
All patients underwent CUS evaluation using Hawk 2002 grayscale ultrasound with a special multifrequency linear probe (V‐K Medical Co., Chennai, India). Two experienced operators (D.C. and M.D.L.), blinded to the clinical, laboratory, and radiological findings, performed the measurements independently. Longitudinal and transverse views of the left and right carotid arteries were obtained. Carotid artery disease was defined as the presence of increased carotid intima‐media thickness (CIMT >0.9 mm) and/or any carotid plaque. Measurements were obtained on the far wall of the distal common carotid artery according to the Mannheim consensus.11 Carotid plaque was defined as a focal structure encroaching on the arterial lumen of ≥0.5 mm, 50% of the surrounding CIMT value, or demonstrating a thickness of ≥1.5 mm, as measured from the media‐adventitia interface to the intima‐lumen interface.
2.3. CCTA protocol and images analysis
CCTA was performed with a 64‐slice scanner (Discovery HD 750 Medical System; GE Healthcare, Milwaukee, WI). First, images were acquired for the calculation of coronary artery calcium score (CACS) and then contrast‐enhanced CCTA was performed. Retrospective and prospective electrocardiographic triggering (SnapShot Pulse; GE Healthcare) was used with variable padding, tube voltage, and tube current, as previously described.12, 13, 14 The dose‐length product was recorded.15 The CCTA images were transferred to a remote dedicated workstation (GE Healthcare). Two experienced operators evaluated the datasets (A.I.G. and G.P., both with ≥8 years of clinical experience in CCTA performance and analysis), blinded to all patient information. Consensus agreement was achieved by a third reader (D.A., with equal years of experience) in case of any disagreement. The American Heart Association (AHA) 16‐segment model was used to segment coronary arteries.16 CACS and atherosclerotic plaque showing noncalcified, mixed, calcified, and remodeled features were identified and evaluated as previously described.14, 17 Positive remodeling was defined whenever vessel diameter at the plaque site was ≥10% larger than that of the reference segment. Finally, the percentage of stenosis was derived according to the formula (diameter ref. – diameter min.) / diameter ref. × 100,14 where “ref.” is reference and “min.” is minimum. A stenosis ≥50% was considered obstructive CAD.
2.4. Follow‐up
Patient follow‐up was performed by checking medical records, by phone interview, and by communication with personal physicians by researchers unaware of the patients' CUS and CCTA results. All suspected new events were reviewed by 2 experienced investigators who evaluated all pertinent records. We defined MACE as a combined endpoint of coronary death, nonfatal myocardial infarction, and non–ST‐segment myocardial infarction.10, 18, 19 In case of multiple events in a given patient, the first event was included in the analysis.
2.5. Statistical analysis
Descriptive analyses were performed to characterize patients with and without MACE, and the distribution of variables within the group was evaluated using the independent t test or Mann–Whitney test for continuous variables and the χ2 or Fisher exact test for categorical data. Univariable and multivariable Cox proportional hazard models were used to estimate the incidence of MACE. Covariate selection in multivariable models was done using clinical and statistical criteria, particularly to avoid overfitting in the development of multivariable models. Multicollinearity was examined in all models and proportional hazard assumption was verified by use of the Schoenfeld test. To evaluate the utility of CUS parameters, first a clinical model including the FRS was built and then CIMT >0.9 plus any carotid plaque and CACS were added incrementally to the model; the additional value of the addition of CTA data were evaluated considering 2 separated models in which CAD >50% or information on segments with remodeled plaque were included in the model with clinical and CUS data. The clinical utility of the addition of CUS and CCTA data on the clinical model was evaluated in terms of discrimination and risk reclassification ability. The Harrell C‐statistic was used to evaluate discrimination ability, and risk reclassification was evaluated using continuous and categorical NRI estimated considering the survival nature of the data. Moreover, the IDI was also used to assess the incremental value of the addition of variable at each step (Figure 1).20 Categorical NRI was evaluated adapting standard thresholds of the FRS commonly used to define low, intermediate, and high risk over 10 years (<10%, 10%–20%, and > 20%, respectively) to the time frame of the study, thus considering 4.5% and 9% as cutoffs to discriminate risk categories.
Figure 1.
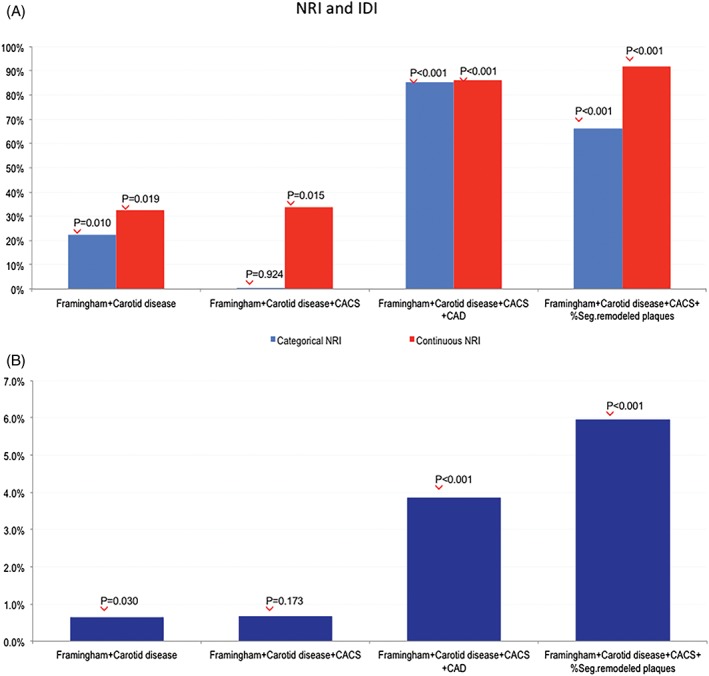
NRI and IDI. Incremental value of the incremental addition of carotid disease, CACS, CAD, and percentage of segments with remodeled plaque to the clinical model assessed by (A) categorical and continuous NRI and (B) IDI. P values are referred to the utility of the addition of each variable as compared with the previous step. Abbreviations: CACS, coronary artery calcium score; CAD, coronary artery disease; IDI, integrated discrimination improvement; NRI, net reclassification index
All tests were 2‐tailed, and a P value <0.05 was considered significant. Statistical analyses were performed using Stata software release 10 (StataCorp LP, College Station, TX) and R 2.11 (R Foundation, Vienna, Austria).
3. RESULTS
Five‐hundred‐seventy consecutive patients were enrolled in the study, including 328 (63%) males, with a mean age of 64 ±10 years. Subjects with DM numbered 91 (17.6%), and those without DM numbered 426 (82.4%). The baseline clinical characteristics are listed in Table 1. The overall evaluability of coronary segments imaged by CCTA was 89%. Patients with carotid disease, including both CIMT >0.9 mm and carotid plaque, showed a higher rate of CAD ≥50% when compared with those without carotid disease (58% and 28%, respectively, P < 0.001; see Supporting Information, Table S1, in the online version of this article).
Table 1.
Baseline characteristics
| All Patients | CHD Events | No CHD Events | P Value | |
|---|---|---|---|---|
| N (%) | 517 (100) | 53 (10.3) | 464 (89.8) | NS |
| Age, y | 64 ± 10 | 65 ±10 | 63 ±10 | NS |
| Male sex | 328 (63.4) | 36 (67.9) | 292 (62.9) | NS |
| Risk factors | ||||
| HTN | 368 (71.2) | 43 (81.1) | 325 (70) | NS |
| Smoking | 155 (30) | 17 (32) | 138 (30) | NS |
| Hyperlipidemia | 324 (63) | 40 (75) | 284 (61) | 0.042 |
| DM | 91 (18) | 16 (30) | 75 (16) | 0.011 |
| Family history of CAD | 153 (30) | 16 (30) | 137 (30) | NS |
| Medical therapy | ||||
| β‐Blockers | 144 (28) | 16 (30) | 128 (28) | NS |
| ACEIs | 238 (46) | 29 (55) | 209 (45) | NS |
| ASA | 229 (64) | 34 (64) | 195 (42) | 0.002 |
| Nitrates | 77 (15) | 17 (32) | 60 (13) | <0.001 |
| Statins | 181 (35) | 25 (47) | 156 (34) | NS |
| Ca antagonists | 116 (22) | 16 (30) | 100 (22) | NS |
| Antihypertensives | 349 (68) | 41 (77) | 308 (66) | NS |
| FRS | 12 (7–21) | 16 (8–23) | 12 (6–20) | NS |
| FRS categories | ||||
| Low, <10% | 226 (44) | 19 (36) | 207 (45) | NS |
| Intermediate, 10%–20% | 168 (33) | 19 (36) | 149 (32) | |
| High, >20% | 123 (24) | 15 (28) | 108 (23) | |
| CUS | ||||
| CIMT >0.9 mm | 389 (75) | 45 (85) | 344 (74) | NS |
| Carotid plaque | 295 (57) | 39 (74) | 256 (55) | 0.011 |
| CCTA characteristics | ||||
| CACS | 34 (0–100) | 87 (52–157) | 15 (0–91) | <0.001 |
| CAD ≥50% | 226 (44) | 46 (87) | 180 (39) | <0.001 |
| 1 vessel ≥50% | 112 (22) | 22 (42) | 90 (19) | <0.001 |
| 2 vessels ≥50% | 72 (14) | 14 (26) | 58 (13) | |
| 3 vessels ≥50% | 41 (8) | 10 (19) | 31 (7) | |
| LM ≥50% | 2 (0.3) | 1 (2) | 1 (0.2) | NS |
| Segments with stenosis | 0 (0–8) | 1 (0–7) | 0 (0–8) | <0.001 |
| ≥1 segment with noncalcific plaque | 191 (37) | 28 (53) | 163 (35) | 0.016 |
| ≥1 segment with calcific plaque | 172 (33) | 22 (42) | 150 (32) | NS |
| ≥1 segment with mixed plaque | 220 (43) | 46 (87) | 174 (37) | <0.001 |
| ≥1 segment with remodeled plaque | 223 (43) | 49 (92) | 174 (37) | <0.001 |
Abbreviations: ACEI, angiotensin‐converting enzyme inhibitor; ASA, acetylsalicylic acid (aspirin); Ca, calcium; CACS, coronary artery calcium score; CAD, coronary artery disease; CCTA, coronary computed tomography angiography; CHD, coronary heart disease; CIMT, carotid intima‐media thickness; CUS, carotid ultrasound; DM, diabetes mellitus; FRS, Framingham risk score; HTN, hypertension; IQR, interquartile range; LM, left main artery; NS, not significant; SD, standard deviation.
Data are presented as n (%), mean ± SD, or median (IQR).
Patients in whom CCTA showed ≥1 segment with noncalcified plaque, calcified plaque, mixed plaque, and remodeled plaque were more represented in the carotid disease group when compared with those without carotid disease (42% vs 32%, P = 0.018; 43% vs 22%, P < 0.001; 54% vs 30%, P < 0.001; and 54% vs 31%, P < 0.001, respectively). Moreover, CACS was significantly higher in patients with carotid disease (59 [0 min–132 max] vs 0 [0 min–65 max]; P < 0.001) as compared with patients without carotid disease.
Over a median follow‐up of 4.4 years (interquartile range [IQR], 3.4–5.1 years), there were a total of 53 MACE (10%) including 6 cardiac deaths (1%), 13 nonfatal myocardial infarction (4%), and 34 non–ST‐segment myocardial infarction (7%). No patients were lost to follow‐up. The mean 10‐year FRS for CVD events was intermediate (12%) without statistically significant differences between the 2 groups (Table 1).
At carotid ultrasound evaluation, patients with carotid plaque were 74% in the MACE group and 55% in the no‐MACE group, respectively (P = 0.011). Mean CACS was 87 (IQR, 52–157) in the MACE group and 15 (IQR, 0–91) in the no‐MACE group (P < 0.001). At CCTA analysis, the presence of CAD ≥50% was 46 (87%) in the MACE group and 180 (39%) in the no‐MACE group (P < 0.001). The number of patients with noncalcific, mixed, and remodeled plaque was significantly higher in the MACE group as compared with the no‐MACE group (P = 0.016, P < 0.001, and P < 0.001, respectively). The mean radiation dose during CCTA was 4.3 ±1.0 mSv, with no difference between the 2 groups. Interobserver agreement for the detection of carotid disease, CACS, coronary artery stenosis, and plaque features was good (κ value of 0.81, 0.92, 0.78, and 0.85, respectively), whereas intraobserver agreement was very good in all situations (κ value of 0.92, 0.96, 0.90, and 0.93, respectively).
The univariate analysis (Table 2) showed that carotid plaque, but not only CIMT, was a significant predictor of MACE (P = 0.007). Notably, CIMT alone was not significantly associated with the risk of cardiac events. The presence of significant CAD; the number of vessels with stenosis ≥50%; the number of segments with noncalcific, mixed, and remodeled plaque; and CACS were significant predictors of MACE. The number of segments with calcified plaque was not a significant predictor of MACE.
Table 2.
Univariate and multivariate Cox models
| HR | 95% CI | P Value | |
|---|---|---|---|
| Univariate models | |||
| Male sex | 1.252 | (0.703–2.230) | NS |
| Age | 1.018 | (0.989–1.047) | NS |
| Smoker | 1.111 | (0.624–1.978) | NS |
| DM | 2.152 | (1.197–3.869) | 0.010 |
| HTN | 1.824 | (0.916–3.629) | NS |
| Hyperlipidemia | 1.899 | (1.016–3.550) | 0.045 |
| Family history of CAD | 1.044 | (0.581–1.877) | NS |
| Antihypertensives | 1.697 | (0.892–3.228) | NS |
| ACEIs | 1.468 | (0.854–2.521) | NS |
| Nitrates | 2.990 | (1.679–5.325) | <0.001 |
| β‐Blockers | 1.112 | (0.619–1.999) | NS |
| Ca antagonists | 1.554 | (0.864–2.793) | NS |
| ASA | 2.349 | (1.340–4.119) | 0.003 |
| Statins | 1.705 | (0.994–2.925) | NS |
| FRS | 6.878 | (0.810–58.434) | NS |
| CIMT >0.9 mm | 1.964 | (0.926–4.168) | NS |
| Carotid plaque | 2.180 | (1.184–4.016) | 0.012 |
| Carotid diseasea | 2.231 | (1.241–4.010) | 0.007 |
| CACS | 1.001 | (1.0–1.002) | 0.001 |
| CAD ≥50% | 9.702 | (4.378–21.502) | <0.001 |
| 1 vessel ≥50% | 6.314 | (3.061–13.028) | <0.001 |
| 2 vessels ≥50% | 5.277 | (2.286–12.181) | <0.001 |
| 3 vessels ≥50% | 10.649 | (4.409–25.718) | <0.001 |
| LM ≥50% | 7.166 | (0.988–51.96) | NS |
| Segment with stenosis | 1.278 | (1.136–1.437) | <0.001 |
| % Segments with noncalcific plaque | 1.038 | (1.015–1.062) | 0.001 |
| % Segments with calcific plaque | 1.010 | (0.991–1.029) | NS |
| % Segments with mixed plaque | 1.047 | (1.033–1.062) | <0.001 |
| % Segments with remodeled plaque | 1.063 | (1.049–1.077) | <0.001 |
| Multivariate models | |||
| FRS | 3.444 | (0.373–31.769) | NS |
| Carotid diseasea | 2.082 | (1.141–3.801) | 0.017 |
| FRS | 2.809 | (0.294–26.830) | NS |
| Carotid diseasea | 1.908 | (1.036–3.516) | 0.038 |
| CACS | 1.001 | (1.001–1.002) | 0.018 |
| FRS | 0.614 | (0.051–7.429) | NS |
| Carotid diseasea | 1.293 | (0.706–2.367) | NS |
| CACS | 1.000 | (0.999–1.001) | NS |
| CAD | 9.079 | (3.916–21.047) | <0.001 |
| FRS | 0.626 | (0.053–7.253) | NS |
| Carotid diseasea | 1.267 | (0.671–2.396) | NS |
| CACS | 1.000 | (0.999–1.001) | NS |
| % Segments with remodeled plaque | 1.061 | (1.044–1.077) | <0.001 |
Abbreviations: ACEI, angiotensin‐converting enzyme inhibitor; ASA, acetylsalicylic acid (aspirin); Ca, calcium; CACS, coronary artery calcium score; CAD, coronary artery disease; CI, confidence interval; CIMT, carotid intima‐media thickness; DM, diabetes mellitus; FRS, Framingham risk score; HR, hazard ratio; HTN, hypertension; LM, left main artery; NS, not significant.
Carotid disease refers to CIMT >0.9 mm + carotid plaque.
On multivariate analysis (Table 2), carotid disease was a significant independent predictor of MACE when added to FRS, and both carotid disease and CACS were independent predictors when added to FRS. CUS data were no more significant when CCTA parameters were included in the model, with the latter being the only significant independent predictors.
The C‐statistic for the FRS model was 0.57 (95% CI: 0.50–0.65). Although the addition of carotid disease did not improve discrimination ability of the model (C‐statistic: 0.62, 95% CI: 0.56–0.69, P = 0.126), the further addition of CACS (C‐statistic: 0.66, 95% CI: 0.59–0.72, P = 0.026), and CCTA data (C‐statistic: 0.75, 95% CI: 0.69–0.80, P = 0.003) for CAD (C‐statistic: 0.84, 95% CI: 0.80–0.88, P < 0.001 for the percentage of segments with remodeled plaque) significantly improved model performance in terms of discriminatory power.
In terms of risk reclassification (Figure 2), the addition of CUS and CCTA data progressively improved model performance at each step according to both continuous and categorical NRI. According to categorical NRI and IDI, the addition of CACS to the model including FRS and carotid disease did not significantly improve risk reclassification, whereas the inclusion of the presence of CAD ≥50% to the model including FRS, carotid disease, and CACS correctly reclassified 85.4% of subjects with an IDI of 3.9%. Finally, the model adding the percentage of segments with remodeled plaque allowed for a correct reclassification 66.1% of subjects with an IDI of 6.0% as compared with the model including FRS, CUS, and CACS data.
Figure 2.
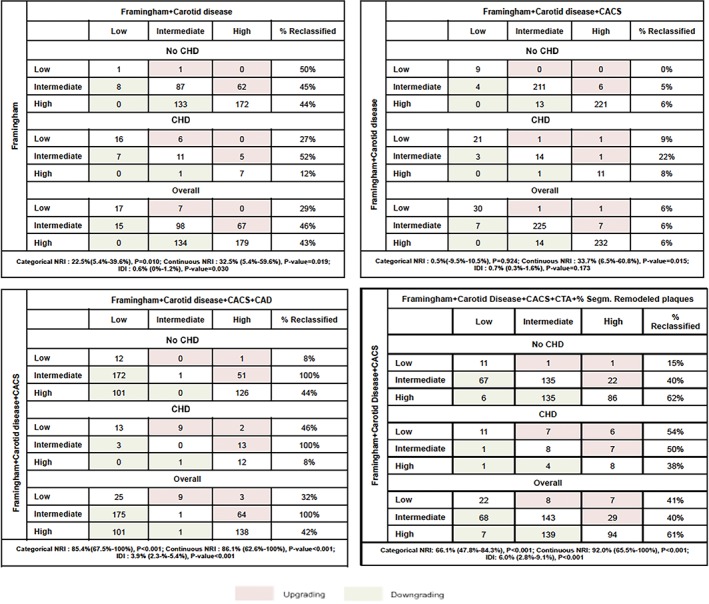
Risk reclassification showing number of subjects and percentage of subjects reclassified when the clinical model was updated, adding incrementally (A) carotid disease, (B), CACS, (C) CAD, and (D) percentage of segments with remodeled plaque. For each section, categorical and continuous NRI as well as IDI are reported with their 95% CI. Abbreviations: CACS, coronary artery calcium score; CAD, coronary artery disease; CHD, coronary heart disease; CI, confidence interval; CTA, computed tomography angiography; IDI, integrated discrimination improvement; NRI, net reclassification index
Considering the subset of the population with DM, the univariate analysis showed that statins and the percentage of segments with remodeled plaque were the only predictors of MACE (Table 3). The C‐statistic for the model including FRS and the percentage of segments with remodeled plaque was 0.83.
Table 3.
Univariate and multivariate Cox models in DM population
| Multivariate Cox Model | |||||
|---|---|---|---|---|---|
| Univariate Cox Models | HR (95% CI) | P Value | HR (95% CI) | P Value | Harrell's C‐statistic |
| Male sex | 3.030 (0.862–10.651) | NS | |||
| Age | 1.001 (0.946–1.059) | NS | |||
| Smoker | 1.282 (0.445–3.694) | NS | |||
| HTN | 1.454 (0.330–6.404) | NS | |||
| Hyperlipidemia | 1.098 (0.381–3.163) | NS | |||
| Family history | 0.966 (0.336–2.781) | NS | |||
| Antihypertensives | 0.690 (0.222–2.140) | NS | |||
| ACEIs | 0.699 (0.262–1.865) | NS | |||
| β‐Blockers | 1.294 (0.450–3.725) | NS | |||
| Ca antagonists | 1.793 (0.665–4.832) | NS | |||
| ASA | 1.558 (0.502–4.834) | NS | |||
| Statins | 3.454 (1.143–10.997) | 0.028 | |||
| FRS | 4.275 (0.119–153.302) | NS | 1.512 (1.028–1.078) | NS | 0.827 (0.777–0.878) |
| CIMT >0.9 mm | 4.653 (0.614–35.259) | NS | |||
| Carotid plaque | 1.980 (0.638–6.150) | NS | |||
| Carotid diseasea | 2.188 (0.705–6.796) | NS | |||
| CAD ≥50% | 1.230 (0.458–3.308) | NS | |||
| % Segments with noncalcific plaque | 1.026 (0.992–1.061) | NS | |||
| % Segments with calcific plaque | 1.013 (0.983–1.044) | NS | |||
| % Segments with mixed plaque | 1.026 (1.000–1.053) | 0.050 | |||
| % Segments with remodeled plaque | 1.053 (1.029–1.078) | <0.001 | 1.053 (1.028–1.078) | <0.001 | |
| CACS | 1.001 (0.999–1.003) | NS | |||
Abbreviations: ACEI, angiotensin‐converting enzyme inhibitor; ASA, acetylsalicylic acid (aspirin); Ca, calcium; CACS, coronary artery calcium score; CAD, coronary artery disease; CI, confidence interval; CIMT, carotid intima‐media thickness; DM, diabetes mellitus; FRS, Framingham risk score; HR, hazard ratio; HTN, hypertension; NS, not significant.
Carotid disease refers to CIMT >0.9 mm + carotid plaque.
4. DISCUSSION
The main findings of this study in an asymptomatic adult population are the following: (1) carotid disease assessment is able to predict MACE occurrence more accurately than traditional 10‐year FRS; (2) CACS allows slightly better event prediction as compared with clinical plus CUS evaluation and loses its signal when coronary stenosis information or plaque features by CCTA are considered; (3) significant coronary stenosis and positive plaque remodeling represent the most powerful tool of reclassification of patient risk who experienced a MACE, which was most pronounced when a starting model including clinical risk and carotid and coronary stenosis placed them in an intermediate‐risk category; and (4) in the subgroup of subjects with DM, the percentage of segments with remodeled plaque is the only predictor of MACE.
Today, carotid artery disease is used as a surrogate marker of preclinical coronary atherosclerosis.21, 22, 23, 24 Studies showed that the combination of CIMT and carotid plaque assessment improves the ability to identify subclinical coronary disease,3, 25 and, hence, the prediction of ischemic CVD. Accordingly, the results of our study reinforce the concept that carotid artery disease information incrementally predicts MACE occurrence with respect to FRS. In fact, it reclassifies almost half of subjects from intermediate to high or low risk. Coronary calcium scoring is a powerful marker of coronary atherosclerosis26, 27, 28 and has also demonstrated the ability to reclassify a portion of patients in the intermediate‐risk group by traditional risk factors. The BioImage Study4 prospectively enrolled 5808 asymptomatic adults and showed that detection of subclinical carotid or coronary atherosclerosis improves 3‐year risk prediction of MACE occurrence and reclassification compared with conventional risk factors, with comparable results for either modality. In the present study, we demonstrate that CACS modestly improves prediction of clinical event occurrence and very weakly reclassifies individuals to a model including FRS and carotid disease. On the other hand, CCTA allows for the direct and noninvasive assessment of CAD and has proven to be an accurate test to evaluate the degree of stenosis as compared with invasive coronary angiography.29, 30, 31 Compared with the case of symptomatic patients where the indication of CCTA has been established,32, 33 few studies have aimed to evaluate the appropriateness of CCTA in asymptomatic patients to substantiate the clinical value and prognostic usefulness over traditional strategies.6, 34 In our study the presence of significant CAD independently predicted MACE in an at‐risk asymptomatic population. Importantly, adding CAD to a model including FRS, carotid diseases, and CACS produced a wide reclassification of patient risk. In particular, 81% of patients who experienced MACE initially included in the intermediate‐risk group were reclassified as high risk and, on the contrary, 76% who did not experience cardiac events and were initially included in the intermediate‐risk group were reclassified as lower risk.
The unique ability of CCTA to identify adverse plaque features such as low‐density noncalcified plaque and positive remodeling of the vessel is of great clinical value.35, 36, 37, 38, 39 Despite the power of coronary stenosis information in predicting event occurrence and correctly reclassifying most patients, the present analysis shows that the characterization of plaque and, specifically, the presence of positive remodeling, are able to further improve risk stratification, particularly lowering the risk of patients who do not show this feature.
Interestingly, the separate analysis of the subjects with DM in this population has shown that the assessment of carotid disease and CACS as risk‐prediction markers is not useful. The only significant parameter is represented by the percentage of segments with remodeled plaque (the presence of mixed plaque reaches the limit of statistical significance). These findings could find a conceptual explanation based on the fact that patients with DM have a condition of high coronary risk per se,40, 41 such that standard risk stratification does not generate any additional prognostic information. Recently, the American Diabetes Association (ADA) and AHA issued a joint statement urging the identification of asymptomatic patients with subclinical CAD in whom more aggressive lifestyle or treatment changes would prevent progression of the disease and the reduction of future clinical events.42 Because it is currently difficult to hypothesize a stratification with the use of CCTA of all DM patients for economic reasons, a useful strategy could be to screen patients when DM has been present for >10 years.43
4.1. Study limitations
Some limitations are present in this study. First, the study enrolled a relatively low number of patients, and its results need to be confirmed in a larger population. Second, our population is highly selected, mainly due to the limitations of the use of CCTA. Moreover, we decided to include common IMT in the analysis, as the existing literature demonstrated the added predictive value of its measurement to carotid plaque burden. However, it should be mentioned that this assumption cannot hold true for carotid bifurcation and internal IMT, whose data have not been analyzed to date. Further studies are warranted to confirm the preliminary evidence emerging from this investigation. It is highly probable that other features, such as the napkin‐ring sign, local and systemic inflammation, and plaque attenuation values will be able to add important information for the purpose of MACE prediction.
5. CONCLUSION
In an asymptomatic at‐risk population, carotid disease assessment is able to predict MACE occurrence more accurately than traditional clinical scores and with comparable accuracy to the coronary calcium score. Coronary artery stenosis and plaque positive remodeling represent the most powerful tools of risk reclassification in this wide subset of patients. In the subgroup of subjects with DM, the percentage of segments with remodeled plaque was the only predictor of MACE.
Conflicts of interest
G. Pontone has served on the speakers bureau and as a consultant for General Electric and has served as a consultant for HeartFlow, Medtronic, and Bayer. D. andreini has served on the speakers bureau for General Electric. F. Cademartiri has served as a consultant for Guerbet and Siemens. The authors declare no other potential conflicts of interest.
Supporting information
Table S1 CCTA characteristics according to carotid disease
Guaricci AI, Lorenzoni V, Guglielmo M, et al. Prognostic relevance of subclinical coronary and carotid atherosclerosis in a diabetic and nondiabetic asymptomatic population. Clin Cardiol. 2018;41:769–777. 10.1002/clc.22952
REFERENCES
- 1. Mozaffarian D, Benjamin EJ, Go AS, et al. Heart Disease and Stroke Statistics—2016 Update: a report from the American Heart Association [published correction appears in Circulation. 2016;133:e599]. Circulation. 2016;133:e38–e360. [DOI] [PubMed] [Google Scholar]
- 2. Barton P, Andronis L, Briggs A, et al. Effectiveness and cost effectiveness of cardiovascular disease prevention in whole populations: modelling study. BMJ. 2011;343:d4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nambi V, Chambless L, Folsom AR, et al. Carotid intima‐media thickness and presence or absence of plaque improves prediction of coronary heart disease risk: the ARIC (Atherosclerosis Risk In Communities) study. J Am Coll Cardiol. 2010;55:1600–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Baber U, Mehran R, Sartori S, et al. Prevalence, impact, and predictive value of detecting subclinical coronary and carotid atherosclerosis in asymptomatic adults: the BioImage study. J Am Coll Cardiol. 2015;65:1065–1074. [DOI] [PubMed] [Google Scholar]
- 5. Guaricci AI, Brunetti ND, Di Biase M, et al. Cardiovascular clinical risk constrains to a powerful primary prevention: carotid atherosclerosis in toto and low dose computed tomography coronary angiography? Int J Cardiol. 2015;178:147–148. [DOI] [PubMed] [Google Scholar]
- 6. Maffei E, Seitun S, Nieman K, et al. Assessment of coronary artery disease and calcified coronary plaque burden by computed tomography in patients with and without diabetes mellitus. Eur Radiol. 2011;21:944–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cho I, Chang HJ, Hartaigh BO, et al. Incremental prognostic utility of coronary CT angiography for asymptomatic patients based upon extent and severity of coronary artery calcium: results from the Coronary CT Angiography Evaluation for Clinical Outcomes International Multicenter (CONFIRM) study [published correction appears in Eur Heart J. 2015;36:3287]. Eur Heart J. 2015;36:501–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Maffei E, Seitun S, Guaricci AI, et al. Chest pain: coronary CT in the ER. Br J Radiol. 2016;89:20150954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Min JK, Labounty TM, Gomez MJ, et al. Incremental prognostic value of coronary computed tomographic angiography over coronary artery calcium score for risk prediction of major adverse cardiac events in asymptomatic diabetic individuals. Atherosclerosis. 2014;232:298–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. D'Agostino RB Sr, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117:743–753. [DOI] [PubMed] [Google Scholar]
- 11. Touboul PJ, Hennerici MG, Meairs S, et al. Mannheim carotid intima‐media thickness and plaque consensus (2004–2006–2011): an update on behalf of the advisory board of the 3rd, 4th and 5th Watching the Risk symposia, at the 13th, 15th and 20th European Stroke Conferences, Mannheim, Germany, 2004; Brussels, Belgium, 2006; and Hamburg, Germany, 2011. Cerebrovasc Dis. 2012;34:290–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Guaricci AI, Schuijf JD, Cademartiri F, et al. Incremental value and safety of oral ivabradine for heart rate reduction in computed tomography coronary angiography. Int J Cardiol. 2012;156:28–33. [DOI] [PubMed] [Google Scholar]
- 13. Guaricci AI, Maffei E, Brunetti ND, et al. Heart rate control with oral ivabradine in computed tomography coronary angiography: a randomized comparison of 7.5 mg vs 5 mg regimen. Int J Cardiol. 2013;168:362–368. [DOI] [PubMed] [Google Scholar]
- 14. Pontone G, Bertella E, Mushtaq S, et al. Coronary artery disease: diagnostic accuracy of CT coronary angiography—a comparison of high and standard spatial resolution scanning. Radiology. 2014;271:688–694. [DOI] [PubMed] [Google Scholar]
- 15. Geleijns J, Golding S, Menzel HG. A workshop on quality criteria for computed tomography held in Arhus, Denmark, November 1998. Eur Radiol. 2000;10:544–545. [DOI] [PubMed] [Google Scholar]
- 16. Austen WG, Edwards JE, Frye RL, et al. A reporting system for patients evaluated for coronary artery disease: report of the Ad Hoc Committee for Grading of Coronary Artery Disease, Council on Cardiovascular Surgery, America Heart Association. Circulation. 1975;51(4 suppl):5–40. [DOI] [PubMed] [Google Scholar]
- 17. Hoffmann U, Moselewski F, Nieman K, et al. Noninvasive assessment of plaque morphology and composition in culprit and stable lesions in acute coronary syndrome and stable lesions in stable angina by multidetector computed tomography. J Am Coll Cardiol. 2006;47:1655–1662. [DOI] [PubMed] [Google Scholar]
- 18. Thygesen K, Alpert JS, White HD; Joint ESC/ACCF/AHA/WHF Task Force for the Redefinition of Myocardial Infarction. Universal definition of myocardial infarction. Circulation. 2007;116:2634–2653. [DOI] [PubMed] [Google Scholar]
- 19. Anderson JL, Adams CD, Antman EM, et al; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2012 ACCF/AHA focused update incorporated into the ACCF/AHA 2007 guidelines for the management of patients with unstable angina/non–ST‐elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines [published correction appears in Circulation. 2013;127:e863–e864]. Circulation. 2013;127:e663–e828. [DOI] [PubMed] [Google Scholar]
- 20. Pencina MJ, D'Agostino RB Sr, Steyerberg EW. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med. 2011;30:11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Amato M, Montorsi P, Ravani A, et al. Carotid intima‐media thickness by B‐mode ultrasound as surrogate of coronary atherosclerosis: correlation with quantitative coronary angiography and coronary intravascular ultrasound findings. Eur Heart J. 2007;28:2094–2101. [DOI] [PubMed] [Google Scholar]
- 22. Gaibazzi N, Rigo F, Facchetti R, et al. Differential incremental value of ultrasound carotid intima‐media thickness, carotid plaque, and cardiac calcium to predict angiographic coronary artery disease across Framingham risk score strata in the APRES multicentre study. Eur Heart J Cardiovasc Imaging. 2015;17:991–1000. [DOI] [PubMed] [Google Scholar]
- 23. Cohen GI, Aboufakher R, Bess R, et al. Relationship between carotid disease on ultrasound and coronary disease on CT angiography. JACC Cardiovasc Imaging. 2013;6:1160–1167. [DOI] [PubMed] [Google Scholar]
- 24. Guaricci AI, Arcadi T, Brunetti ND, et al. Carotid intima‐media thickness and coronary atherosclerosis linkage in symptomatic intermediate‐risk patients evaluated by coronary computed tomography angiography. Int J Cardiol. 2014;176:988–993. [DOI] [PubMed] [Google Scholar]
- 25. Xie W, Liang L, Zhao L et al. Combination of carotid intima‐media thickness and plaque for better predicting risk of ischaemic cardiovascular events in Chinese subjects. Heart. 2011;97:1326–1331. [DOI] [PubMed] [Google Scholar]
- 26. Rumberger JA, Simons DB, Fitzpatrick LA, et al. Coronary artery calcium area by electron beam computed tomography and coronary atherosclerotic plaque area: a histopathologic correlative study. Circulation. 1995;92:2157–2162. [DOI] [PubMed] [Google Scholar]
- 27. Arad Y, Goodman KJ, Roth M, et al. Coronary calcification, coronary disease risk factors, C‐reactive protein, and atherosclerotic cardiovascular disease events: the St. Francis Heart study. J Am Coll Cardiol. 2005;46:158–165. [DOI] [PubMed] [Google Scholar]
- 28. Detrano R, Guerci AD, Carr JJ, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358:1336–1345. [DOI] [PubMed] [Google Scholar]
- 29. Achenbach S, Goroll T, Seltmann M, et al. Detection of coronary artery stenoses by low‐dose, prospectively ECG‐triggered, high‐pitch spiral coronary CT angiography. JACC Cardiovasc Imaging. 2011;4:328–337. [DOI] [PubMed] [Google Scholar]
- 30. Achenbach S, Narula J. Coronary CT angiography: from sensitivity to specificity. JACC Cardiovasc Imaging. 2011;4:1227–1229. [DOI] [PubMed] [Google Scholar]
- 31. Neglia D, Rovai D, Caselli C, et al; EVINCI Study Investigators . Detection of significant coronary artery disease by noninvasive anatomical and functional imaging. Circ Cardiovasc Imaging. 2015;8 10.1161/CIRCIMAGING.114.002179. [DOI] [PubMed] [Google Scholar]
- 32. Maffei E, Seitun S, Martini C, et al. Prognostic value of computed tomography coronary angiography in patients with chest pain of suspected cardiac origin [article in English and Polish]. Radiol Med. 2011;116:690–705. [DOI] [PubMed] [Google Scholar]
- 33. Shaw LJ, Hausleiter J, Achenbach S, et al; CONFIRM Registry Investigators . Coronary computed tomographic angiography as a gatekeeper to invasive diagnostic and surgical procedures: results from the multicenter CONFIRM (Coronary CT Angiography Evaluation for Clinical Outcomes: an International Multicenter) registry. J Am Coll Cardiol. 2012;60:2103–2114. [DOI] [PubMed] [Google Scholar]
- 34. Nucifora G, Schuijf JD, van Werkhoven JM, et al. Prevalence of coronary artery disease across the Framingham risk categories: coronary artery calcium scoring and MSCT coronary angiography. J Nucl Cardiol. 2009;16:368–375. [DOI] [PubMed] [Google Scholar]
- 35. Motoyama S, Kondo T, Sarai M, et al. Multislice computed tomographic characteristics of coronary lesions in acute coronary syndromes. J Am Coll Cardiol. 2007;50:319–326. [DOI] [PubMed] [Google Scholar]
- 36. Maurovich‐Horvat P, Hoffmann U, Vorpahl M, et al. The napkin‐ring sign: CT signature of high‐risk coronary plaques? JACC Cardiovasc Imaging. 2010;3:440–444. [DOI] [PubMed] [Google Scholar]
- 37. Motoyama S, Ito H, Sarai M, et al. Plaque characterization by coronary computed tomography angiography and the likelihood of acute coronary events in mid‐term follow‐up. J Am Coll Cardiol. 2015;66:337–346. [DOI] [PubMed] [Google Scholar]
- 38. Thomsen C, Abdulla J. Characteristics of high‐risk coronary plaques identified by computed tomographic angiography and associated prognosis: a systematic review and meta‐analysis. Eur Heart J Cardiovasc Imaging. 2016;17:120–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Guaricci AI, Pontone G, Fusini L, et al. Additional value of inflammatory biomarkers and carotid artery disease in prediction of significant coronary artery disease as assessed by coronary computed tomography angiography. Eur Heart J Cardiovasc Imaging. 2017;18:1049–1056. [DOI] [PubMed] [Google Scholar]
- 40. Haffner SM, Lehto S, Rönnemaa T, et al. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339:229–234. [DOI] [PubMed] [Google Scholar]
- 41. Maffei E, Seitun S, Nieman K, et al. Assessment of coronary artery disease and calcified coronary plaque burden by computed tomography in patients with and without diabetes mellitus. Eur Radiol. 2011;21:944–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Fox CS, Golden SH, Anderson C, et al. Update on prevention of cardiovascular disease in adults with type 2 diabetes mellitus in light of recent evidence: a Scientific Statement from the American Heart Association and the American Diabetes Association. Diabetes Care. 2015;38:1777–1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rana JS, Liu JY, Moffet HH, et al. Diabetes and prior coronary heart disease are not necessarily risk equivalent for future coronary heart disease events. J Gen Intern Med. 2016;31:387–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 CCTA characteristics according to carotid disease


