Summary
Introduction
Superficial siderosis is a rare, neurodegenerative disease caused by toxic accumulation of hemosiderin on the surface of the brain and the spinal cord, most commonly from chronic subarachnoid hemorrhage.
Aims
The aim of this study was to assess the clinical and radiological outcomes of superficial siderosis patients using deferiprone, a cell permeant iron chelator. Subjects obtained pre‐ and post‐treatment brain MRIs and weekly laboratory tests. Osirix software was used to develop a method of quantifying hemosiderin deposition. Three‐dimensional whole brain images of gradient echo images were rendered and compared by dividing the mean T2 hyperintensity to the maximal cerebrospinal fluid signal.
Results
A total of 38 subjects completed the study, of which clinical and radiological data were available for 30. The average age was 64 years (range 37‐86), 53% were male, 94% were white. Nineteen subjects (63%) reported either no progression of disease or an improvement in at least one neurological domain, with 40% of patients reporting a stabilization in hearing function and 30% reporting stable or improved coordination and walking. By MRI, there was an overall mean increase in T2 hyperintensity of the whole brain of 1%‐13% over the 2‐year time period in half of patients, indicating a reduction hemosiderosis. There were no cases of agranulocytosis, and declines of white blood cells counts and neutrophils averaged <10%. Fatigue was the most common side effect.
Conclusion
This is the first long‐term prospective study of superficial siderosis on the iron chelator, deferiprone. MRI quantification of hemosiderin appears to demonstrate a measurable reduction in half of patients and this correlated with a stabilized or improving disease course. A future placebo‐controlled trial is necessary to determine whether deferiprone is an effective therapy for superficial siderosis.
Keywords: deferiprone, hemosiderosis of the central nervous system, superficial siderosis
1. INTRODUCTION
Superficial siderosis (SS) is an iron overload condition caused by chronic, intermittent bleeding in the cerebrospinal fluid, that leads to a classic triad of progressive and typically irreversible hearing loss, ataxia, and myelopathy.1 The most common etiologies that lead to SS are trauma, previous surgical procedures, dural tears, and tumors of the central nervous system.2 The pathogenetic mechanism they all share is a buildup of hemosiderosis on the surface of the brain, brainstem, cerebellum, cranial nerve VIII, and spinal cord over years to decades with free‐iron toxicity to the underlying tissues.3 Although the estimated prevalence of SS is approximately 1 in 10 million, the natural history of the condition for those few patients is a progressive decline in neurological function with no known treatments.4
We investigated the potential for an iron chelator, deferiprone, for the treatment of SS.5, 6, 7, 8 Deferiprone is the only iron chelator that readily crosses the blood‐brain barrier to potentially chelate iron within the central nervous system.9 Previously, we demonstrated the safety of a 3‐month course of deferiprone in a 10‐subject open‐label pilot trial.10 Interestingly, four of the 10 subjects in that study demonstrated a slight reduction in hemosiderin deposition by MRI. We sought to investigate the potential benefit of deferiprone by MRI by conducting a 38‐subject real‐world observational study of SS patients on deferiprone for 2 years to see whether hemosiderosis could be reduced in a safe and tolerable manner.
2. METHODS
This was a 2‐year longitudinal observational study of 38 subjects who were seen at the Johns Hopkins Hospital between 2012 and 2016 with a diagnosis of SS based on typical MRI findings of hemosiderosis on the surface of the brain, brainstem, cerebellum, and/or spinal cord presenting with signs/symptoms of SS, including hearing loss, ataxia, dysphagia and dysarthria, and weakness, spasticity and bowel/bladder dysfunction that localizes to the MRI findings (Figure 1). In addition to a clinical and radiographic diagnosis of SS, inclusion criteria included a treatment plan with deferiprone at a starting dose of 1000 mg twice daily (approximately 30 mg/kg/day) based on our previous experience in an open‐label trial of deferiprone in SS.10 Subjects were treated 5 days per week with 2 days off to allow for partial iron repletion by diet to mitigate the risk of iatrogenic iron deficiency anemia. Monitoring involved weekly complete blood counts with differential with close attention to neutrophil counts given the reported 1%‐2% risk of agranulocytosis11; monthly liver function tests, zinc levels and ferritin levels to monitor toxicity, zinc deficiency due to mild zinc chelation ability of deferiprone, and compliance, respectively. MRI scans of the brains without contrast were requested yearly on a clinical basis and compared with predrug baseline scans. Neurological examinations were conducted at the start of the study and, when available, at the end of the 2‐year observational period. Patient‐reported neurological function and side effects were also collected by email and telephone correspondence to assess for improvements or declines in neurological function due to the deferprone.
Figure 1.
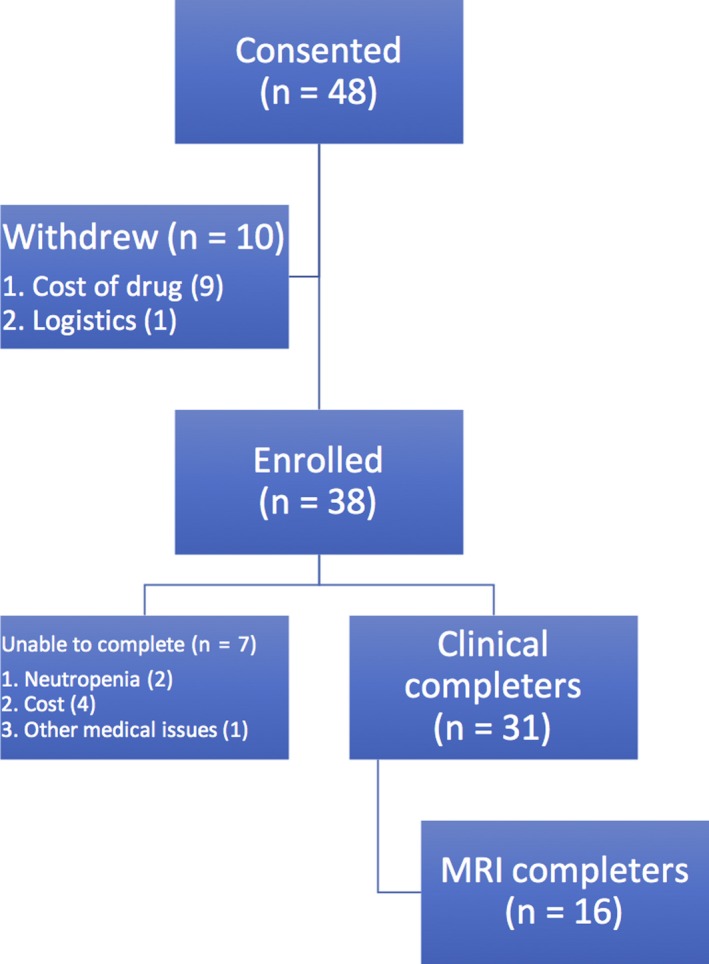
Consort diagram of the participants. Forty‐eight participants enrolled in the trial but 10 withdrew, most commonly because of inability to access/afford the deferiprone. A total of 38 participants completed the study
The susceptibility weighted (SWI) MRI images were compared over the course of the study by comparing the iron susceptibility signal in the 2‐year postdrug scan with the baseline MRI scan. Three‐dimensional images of the whole brain were rendered using Orisix software (version 8.0; Pixmeo SARL, Geneva, Switzerland), and the total T2 signal of the SWI images was averaged and standardized to the maximum T2 signal of the cerebrospinal fluid in the lateral ventricles (video S1). Lower average T2 signals indicate increased hemosiderosis while higher T2 signals indicate less hemosiderosis. Paired t‐tests were calculated to determine whether the changes in the whole brain iron content over the 2‐year observational period were significant defined by P values < 0.05.
Informed consent was obtained from each subject participating in the study, which was approved by the Johns Hopkins University Institutional Review Board.
3. RESULTS
Forty‐eight subjects were initially recruited to the study, of which 38 enrolled. The primary reason for exclusion was inability to acquire the drug due to declined coverage by insurance for off‐label use. The average age of the group was 64 years old (range 37‐86), 53% were male and 94% were white; the full demographics are listed in Table 1. Among the known etiologies of SS, dural tear was the most common cause but a large number of subjects with unknown bleeding sources despite imaging workup. Eight subjects underwent procedures to correct the bleed although it can be difficult to ascertain whether the bleeding continued, slowed or was stopped in these subjects.
Table 1.
Demographics of study participants
| Characteristic | Number of Participants |
|---|---|
| Participation | |
| Enrolled | 48 |
| Withdrew | 10 |
| Completed | 38 |
| Sex | |
| Male | 20 (53%) |
| Female | 18 (47%) |
| Race | |
| White | 36 (94%) |
| Black | 1 (3%) |
| Other | 1 (3%) |
| Age at Enrollment | |
| Average (median, range) | 64 (64, 37‐86 years) |
| Duration of disease | |
| Average (median, range) | 8.5 (7, 1‐33 years) |
| Cause of Disease | |
| Dural tear | 11 |
| Prior neurosurgical procedure | 8 |
| CNS tumor/cyst | 4 |
| Undetermined | 15 |
| Surgical correction of bleed | 8 (21%) |
3.1. Clinical
The most common clinical complaints on presentation were hearing loss and imbalance, followed by bowel/bladder dysfunction and symptomatic nystagmus. On examination, hearing impairment was present in 100% of patients and ataxia was found in 95% of patients. Post‐treatment clinical data were obtained from 31 of 38 subjects. After 2 years of deferiprone chelation therapy, 19 of 30 (63%) reported no progression or an improvement in at least one neurological domain. The other 11 (37%) reported a slowly progressive worsening in all clinical domains similar to the progression experienced prior to starting the therapy; however, most patients reported mixed results with worsening in some neurological domains and stabilization in others (Table 2). Overall, 40% of patients reported a stabilization in hearing function, 30% reported stable or improved coordination and walking.
Table 2.
Clinical observations
| Clinical (out of 31 participants) | Better | Same | Worse |
|---|---|---|---|
| Hearing | 0 | 13 (42%) | 18 (58%) |
| Coordination | 2 (6%) | 8 (26%) | 21 (68%) |
| Walking | 1 (3%) | 9 (29%) | 21 (68%) |
| Fine Motor Function | 0 | 9 (29%) | 22 (71%) |
| Bowel/Bladder | 1 (3%) | 12 (39%) | 18 (58%) |
There was no indication that the eight subjects who underwent a procedure to stop the bleeding had better outcomes. Of those eight, only one reported stabilization or improvement in clinical function. The most common side effect was fatigue reported by 27% of subjects. The fatigue was dose dependent with a reduction in the morning dose leading to the greatest reduction of the side effect experienced during the day.
3.2. Serology and Hematology
Given the anticipated 1%‐2% risk of agranulocytosis associated with deferiprone, all subjects were asked to obtain weekly complete blood counts with differentials to monitor neutrophil counts. While there were no episodes of agranulocytosis among the 38 subjects in this study, two subjects (5%) had episodes of neutropenia to a minimum absolute neutrophil count of 900 cells/μl and were asked to stop taking the deferiprone out of abundance of caution (Table 3). In both cases, neutrophil counts rebounded within 4 weeks and subjects resumed using deferiprone. There was a slight cumulative decline in total WBC count and neutrophil count over the course of the trial that was statistically significantly but not clinically meaningful (Figure S1A‐B).
Table 3.
Safety profile and side effects
| Lab | Change in value(%) |
|---|---|
| Total white blood cell count | −5 |
| Neutrophil (%) | −3 |
| Absolute neutrophil count | −3 |
| Side effect | Frequency(%) |
| Fatigue | 25 |
| Joint pain | 4 |
| Mouth sores | 4 |
Liver function tests remained within 4 × normal limits in all subjects (Figure S1C‐D). Ferritin levels consistently declined over time in all subjects as expected in compliant patients (Figure S1E). As deferiprone is also a weak zinc chelator, zinc levels were monitored monthly. In five subjects, mild zinc deficiency was noted by blood testing but none of these subjects endorsed symptoms of zinc deficiency (Figure S1F). For zinc deficiency, subjects stopped using deferiprone for 2 weeks while taking 20‐40 mg of daily zinc tablets. In all cases, zinc levels returned to baseline levels and deferiprone was resumed.
3.3. Radiography
All subjects obtained noncontrast MRI scans of the brain, upon which the diagnosis of SS was made, within 6 months of starting deferiprone. Repeat scans after the completion of the 2‐year observational period were obtained in all subjects, but as this was only an observational study, all scans were obtained for clinical use. A comparison of the prestudy and poststudy MRI parameters were performed to identify pairs of scans with the same echo time and the same captured brain volume and found that 16 subjects met these criteria (Table S1). Among these 16 subjects, eight (50%) showed a decrease in the total iron content of the brain by MRI after 2 years of deferiprone use (Table 4). In this group of “responders,” the average decrease in total iron content was 5% with a range of 1%‐13%. In the 50% of subjects who showed an increased total iron content, the increase was 36% with a range of 2%‐116%. A sub‐analysis of the MRI “responders” shows that 63% were male with an average age of 66 years. We did not have enough participants to assess for an increased chance of responding to deferiprone among those with a history of intervention to stop the bleeding. Clinically, all but one (88%) of the subjects who responded by MRI reported improvement in symptoms in at least neurological domain. The one exception was the subject who improved the least by MRI. Even among the subjects with increasing iron deposits by MRI despite deferiprone, only 38% reported no improvement in any neurological domain. An example MRI for a responder is shown in Figure 2.
Table 4.
MRI changes and clinical correlation
| Participant | Mean T2/Max CSF | MRI Changea | Clinical Changeb | |
|---|---|---|---|---|
| Predrug | Postdrug | |||
| 1c | 0.362 | 0.374 | +0.03 | Same |
| 2c | 0.370 | 0.375 | +0.01 | Worse |
| 3 | 0.395 | 0.316 | −0.25 | Worse |
| 4 | 0.559 | 0.473 | −0.18 | Improved |
| 5 | 1.024 | 0.474 | −1.16 | Improved |
| 6 | 0.419 | 0.420 | +0.01 | Same |
| 7 | 0.367 | 0.418 | +0.13 | Same |
| 8 | 0.355 | 0.288 | −0.23 | Same |
| 9 | 0.512 | 0.364 | −0.41 | Same |
| 10 | 0.323 | 0.338 | +0.04 | Same |
| 11 | 0.330 | 0.359 | +0.08 | Same |
| 12 | 0.390 | 0.423 | +0.08 | Same |
| 13 | 0.407 | 0.399 | −0.02 | Worse |
| 14c | 0.430 | 0.376 | −0.14 | Same |
| 15 | 0.304 | 0.312 | +0.02 | Same |
| 16 | 0.388 | 0.292 | −0.33 | Worse |
A positive value indicates reduced hemosiderosis.
Same or improved in any neurological function. Worse indicates decline in all neurological functions.
Underwent procedure to stop the bleeding, such as surgery or blood patching.
Figure 2.
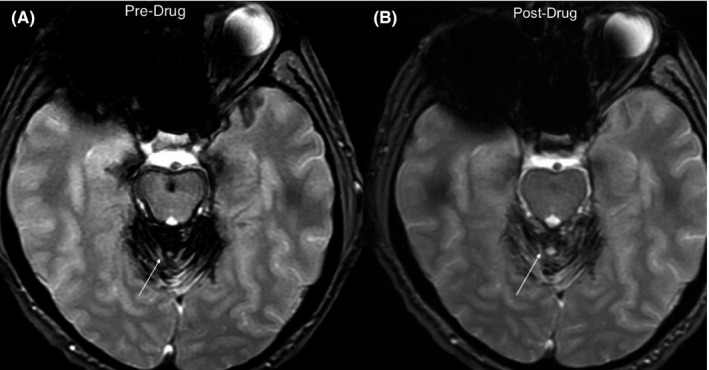
Example of MRI susceptibility weighted images from subject 7 (Table 4) before starting on deferiprone (A) compared to the same axial cut after 2 years of deferiprone (B). The hypointense dark areas identified by the arrows represent artifact from hemosiderosis, which are grossly reduced after deferiprone use
4. DISCUSSION
In this longitudinal observational study of a relatively large cohort of patients with superficial siderosis, we report on the potential benefit of long‐term iron chelation with the cell‐permeable chelator, deferiprone. Despite the significant biases and limits inherent in an observational study, this one provides two important conclusions. First, iron chelation in superficial siderosis appears to lead to reduction in iron deposits over time in more than half of subjects. In our previous open label with deferiprone in superficial siderosis, minor MRI were visible in four of 10 subjects in as little as 3 months,10 with more obvious MRI changes over longer periods of time.7, 8 The reduction in iron deposits is an objective calculation of the entire brain as measured by an index of total brain T2 signal to the max CSF signal. Second, there may be a clinical correlation to reduced brain MRI manifested as a stabilization in disease progression in at least one neurological domain, most commonly hearing and balance.
The results of this study suggest that deferiprone may be clinically effective in this disease population. Long‐term natural history studies in this population are sparse. In 2007, we published the most extensive analysis of superficial siderosis to date with a review of 270 case reports published over the previous 100 years.1 In this analysis, we reported that the natural history of superficial siderosis is a progressive neurological decline with no exceptions. Prior to our study with deferiprone, there have been no successful treatment options that could stop the progression, much less improve outcomes. Only one case report described a patient who underwent surgical correction of the bleed with subsequent improvement in neurological function but long‐term follow‐up beyond 15 months was not yet available in that case.11 Therefore, this study is particular meaningful in superficial siderosis because for the first time there appears to be a clinical benefit in stopping progression of a disease that previously could not be stopped.
There was no long‐standing toxicity or development of agranulocytosis in this 2‐year study. In previous studies, a 1%‐2% risk of agranulocytosis has been reported.12 Although none of the subjects in this study developed agranulocytosis, two subjects were found to have a decline in neutrophils counts that prompted discontinuation of deferiprone until the counts returned to baseline. In both cases, the neutrophil counts rebounded on discontinuation. Deferiprone was not rechallenged upon resolution of those events out of abundance of caution. In previously reported experience with deferiprone, 10 patients with neutropenia were rechallenged upon recovery of the neutrophil count whereupon only one patient experienced agranulocytosis and no neutropenia or agranulocytosis were observed in the other nine.12 One trial evaluated if mild neutropenia would necessarily proceed to agranulocytosis with continued deferiprone exposure. Deferiprone was maintained in seven consecutive patients who experienced mild neutropenia and all seven patients normalized their neutrophil counts despite continued deferiprone use.13
Fatigue was a common complaint among patients on deferiprone in this study and is similar to reported rates of fatigue in other patient populations.14 Fatigue appeared to be dose dependent in this patient population and was mitigated by dose reductions especially in the morning dose. In most cases, reducing the dose from 1000 mg in the morning to 500 mg in the morning greatly improved the fatigue. In some cases, the morning dose was added to the evening dose or eliminated altogether.
The potential benefit of deferiprone in superficial siderosis patients is based on the rationale that removing the iron deposits from the brain may lead to healing and improvement in neurological function. Interestingly, among subjects who had identifiable sources of bleeding and underwent procedures to correct the bleeding did not have better outcomes: only one of eight had improvement in clinical function associated with reduced hemosiderosis by MRI. This may be due to the fact that subjects with identifiable bleeds had more bleeding—making it easier to detect on imaging. It is possible that chelation of iron may lead only to stabilizing the disease progression, as healing in the central nervous system is limited. In the worst case scenario, iron chelation might have no sustained clinical benefit over time, if an irreversible neurodegenerative cascade is triggered by long‐standing hemosiderosis. In other words, perhaps patients who did not respond to deferiprone passed a “point of no return” where the brain's support mechanisms could not be repaired further.
Another possibility for why some patients responded well to chelation and others did not is that perhaps the responders chelate their iron less effectively naturally than the nonresponders. Ferritin and haptoglobins are very good at binding free iron and they have to release the iron in order for chelators to bind them. So, perhaps the nonresponders are not letting their iron go. Further studies on the specific isoforms of ferritin and haptoglobins in superficial siderosis patients may shed light on this idea.
The major limitations of this study relate to the observational design. The rarity of the disease and limited resources available to study the potential benefit of deferiprone in this patient population were the driving factors behind the design. Because this study was observational only, it biased subjects who had access to our clinical center and access to the drug which costs approximately $48,000/patient/year in the United States. It was also biased to those who could remain in close contact for laboratory monitoring and MRI acquisition. Despite our best attempts to standardize MRI protocols, the observational design left us with more than half of clinical MRIs of unsuitable quality for accurate comparison over the 2‐year period. Nevertheless, despite these limitations, this study provides a launching point for a randomized, placebo controlled trial of deferiprone in superficial siderosis. A randomized, placebo‐controlled trial is required to determine whether deferiprone has any true clinical benefit. This study suggests that a clinical outcome measure that includes measures of hearing and balance have the best chance of showing a response to deferiprone. Given the slow progression of superficial siderosis in natural history studies,1, 15 a future trial must be of sufficient duration to separate the experimental and placebo arms. This study suggests that 2 years may be of sufficient duration to detect clinical benefit.
DISCLOSURES
RAK, KS, XL, and MAM have no disclosures to report. ML receives research funding from Alexion; Genzyme, Acorda; Sanofi; TG Therapeutics; TerumoBCT; Alnylam. Consulted for Chugai/Roche, Sanofi, Alexion, Acorda. Serves on the Scientific Advisory Board for Alexion and Acorda.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supporting information
Kessler RA, Li X, Schwartz K, Huang H, Mealy MA, Levy M. Two‐year observational study of deferiprone in superficial siderosis. CNS Neurosci Ther. 2018;24:187–192. 10.1111/cns.12792
Funding information
An unrestricted educational grant of $60,000 from Apopharma was provided for this study. Apopharma is the manufacturer of deferiprone. Role of Funding Source: The funding source provided solicited advice on the design of this study and the dosing of the deferiprone, but had no role during its execution, analyses, interpretation of the data, or decision to publish results. Feedback on the manuscript was solicited, and some of the recommended changes to the language were accepted at the discretion of the authors.
REFERENCES
- 1. Levy M, Turtzo C, Llinas RH. Superficial siderosis: a case report and review of the literature. Nat Clin Pract Neurol. 2007;3:54‐58; quiz 9. [DOI] [PubMed] [Google Scholar]
- 2. Kumar N, Cohen‐Gadol AA, Wright RA, Miller GM, Piepgras DG, Ahlskog JE. Superficial siderosis. Neurology. 2006;66:1144‐1152. [DOI] [PubMed] [Google Scholar]
- 3. Koeppen AH, Dickson AC, Chu RC, Thach RE. The pathogenesis of superficial siderosis of the central nervous system. Ann Neurol. 1993;34:646‐653. [DOI] [PubMed] [Google Scholar]
- 4. Wilson D, Chatterjee F, Farmer SF, et al. Infratentorial superficial siderosis: classification, diagnostic criteria, and rational investigation pathway. Ann Neurol. 2017;81:333‐343. [DOI] [PubMed] [Google Scholar]
- 5. Cummins G, Crundwell G, Baguley D, Lennox G. Treatment of superficial siderosis with iron chelation therapy. BMJ Case Rep. 2013;2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kuo PH, Kuo SH, Lo RY. Deferiprone reduces hemosiderin deposition in superficial siderosis. Can J Neurol Sci. 2017;44:219‐220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Levy M, Llinas RH. Deferiprone reduces hemosiderin deposits in the brain of a patient with superficial siderosis. AJNR Am J Neuroradiol. 2011;32:E1‐E2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Levy M, Llinas RH. Update on a patient with superficial siderosis on deferiprone. AJNR Am J Neuroradiol. 2012;33:E99‐E100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Arthur AS, Fergus AH, Lanzino G, Mathys J, Kassell NF, Lee KS. Systemic administration of the iron chelator deferiprone attenuates subarachnoid hemorrhage‐induced cerebral vasospasm in the rabbit. Neurosurgery. 1997;41:1385‐1391; discussion 91‐2. [DOI] [PubMed] [Google Scholar]
- 10. Levy M, Llinas R. Pilot safety trial of deferiprone in 10 subjects with superficial siderosis. Stroke. 2012;43:120‐124. [DOI] [PubMed] [Google Scholar]
- 11. Shih P, Yang BP, Batjer HH, Liu JC. Surgical management of superficial siderosis. Spine J. 2009;9:e16‐e19. [DOI] [PubMed] [Google Scholar]
- 12. Tricta F, Uetrecht J, Galanello R, et al. Deferiprone‐induced agranulocytosis: 20 years of clinical observations. Am J Hematol. 2016;91:1026‐1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. El‐Beshlawy AM, El‐Alfy MS, Sari TT, Chan LL, Tricta F. Continuation of deferiprone therapy in patients with mild neutropenia may not lead to a more severe drop in neutrophil count. Eur J Haematol. 2014;92:337‐340. [DOI] [PubMed] [Google Scholar]
- 14. Cohen A, Galanello R, Piga A, Vullo C, Tricta F. A multi‐center safety trial of the oral iron chelator deferiprone. Ann N Y Acad Sci. 1998;850:223‐226. [DOI] [PubMed] [Google Scholar]
- 15. Rodriguez FR, Srinivasan A. Superficial siderosis of the CNS. AJR Am J Roentgenol. 2011;197:W149‐W152. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


