Abstract
Background
Thyroid dysfunction and myocardial fibrosis are both associated with cardiovascular events in patients with dilated cardiomyopathy (DCM).
Hypothesis
The combination of thyroid hormone (TH) and myocardial fibrosis (detected by late gadolinium enhancement [LGE]) is an independent and incremental predictor of adverse events in DCM.
Methods
We consecutively enrolled 220 idiopathic DCM patients with thyroid function and LGE assessment at Fuwai Hospital (China) from January 2010 to October 2011 and followed up through December 2015. Patients were divided into 4 groups according to the presence or absence of LGE and FT3 value (median level of 2.79 pg/mL): LGE‐positive + FT3 < 2.79 pg/mL, LGE‐positive + FT3 ≥ 2.79 pg/mL, LGE‐negative + FT3 < 2.79 pg/mL, and LGE‐negative + FT3 ≥ 2.79 pg/mL.
Results
During a median follow‐up of 61 months, 56 patients (25.5%) died, with 27/56 (48.2%), 8/45 (17.8%), 12/54 (22.2%), and 9/65 (13.8%) among 4 groups (P = 0.009), respectively. Multivariable Cox regression analysis identified LGE‐positive and FT3 < 2.79 pg/mL as a significant independent predictor of all‐cause mortality (hazard ratio: 2.893, 95% confidence interval: 1.323‐6.326, P = 0.008). Combining the predictive value of FT3 and LGE status significantly improved risk reclassification for all‐cause mortality, as indicated by the net reclassification improvement (0.28; P = 0.005) and integrated discrimination improvement (0.058; P = 0.001).
Conclusions
The findings suggest that the combination of FT3 and LGE yielded a more accurate predictive value for long‐term prognosis in patients with DCM, which may improve patient selection for intensive interventions.
Keywords: Dilated Cardiomyopathy, Free Triiodothyronine, Late Gadolinium Enhancement, Myocardial Fibrosis, Risk Stratification
1. INTRODUCTION
Nonischemic dilated cardiomyopathy (DCM) is one of the most common cardiac diseases, occurring in approximately one‐third of heart failure (HF) patients, and is associated with significant morbidity and mortality.1 The disease is characterized by left ventricular (LV) chamber enlargement with a malignant prognosis including sudden cardiac death (SCD) and progressive HF. A considerable number of patients with HF caused by DCM could progress into terminal stage rapidly, even under adequate drug therapy.2 Although numerous traditional and classical risk‐prediction models have been established, which are mainly based on cardiac‐function parameters, their clinical application and precise risk stratification remain limited.3, 4 Thus, an accurate risk classification of DCM patients is of paramount importance for intensive intervention selection, such as implantable cardioverter‐defibrillator, cardiac resynchronization therapy, and even heart transplantation.
A growing body of evidence suggests that thyroid dysfunction, even subtle changes of thyroid hormone (TH) level, could act as an important risk factor in the progression of cardiovascular (CV) diseases.5, 6, 7 It has been shown in our previous study that low free triiodothyronine (FT3) levels correlated with deterioration of cardiac function,8 and hypothyroid status was a strong predictor of poor prognosis in patients with DCM.9 Low FT3 level might indicate a determinant factor directly implicated in the evolution and prognosis of cardiac diseases.10 Thyroid dysfunction has been recognized by current guidelines as a modifiable risk factor that might accelerate HF.4, 11 Based on this knowledge, we hypothesized that TH could be added into the traditional risk‐prediction model and provide more accurate risk classification. In the past decades, cardiac magnetic resonance imaging (cMRI) has been increasingly utilized as a noninvasive imaging method in DCM patients for etiological diagnosis and risk stratification.12 cMRI with late gadolinium enhancement (LGE) sequences has further extended our ability to accurately identify and quantify myocardial fibrosis13 and provide prognostic value in HF patients,14, 15, 16 especially for arrhythmic outcomes.17 Interestingly, accumulating evidence has shown that TH plays an important role in the process of myocardial fibrosis, with increased collagen in hypothyroid animals that was not seen in hyperthyroid animals.18, 19
As both thyroid dysfunction and LGE have been proven to be independent prognostic factors in DCM, together with the potential interaction between them, we hypothesized that the combination of LGE and TH levels may provide more accurate information for risk stratification in patients with DCM. We tested the hypothesis with complete information on thyroid function and LGE profile from a well‐characterized population of 220 consecutive patients with DCM.
2. METHODS
2.1. Study population
We conducted a prospective observational study of 220 DCM patients who were admitted to Fuwai Hospital (National Center of Cardiovascular Diseases, China) from January 2010 to October 2011. This subgroup with complete information on thyroid function and LGE‐cMRI belongs to a cohort study of 458 DCM patients previously reported.9 Nonischemic DCM was diagnosed according to World Health Organization criteria.20 Exclusion criteria were as follows: intake of thyroxine, amiodarone, corticosteroids or antithyroid drugs; history of severe valvular disease, hypertensive heart disease, or tachycardia‐induced cardiomyopathy; and alcohol abuse, metal fragments in their bodies, implanted ferromagnetic devices, or unsuitable to undergo cMRI. The study was conducted according to the principles of the Declaration of Helsinki and was approved by the ethics committee of Fuwai Hospital, China. Informed consent was obtained from all patients enrolled in this study.
2.2. Thyroid function testing
Thyroid function profiles were assessed in all enrolled patients using radioimmunoassay (Immulite 2000; Siemens, Erlangen, Germany) in the Department of Nuclear Medicine of Fuwai Hospital when patients were stable under optimal pharmacologic therapy. The reference intervals of TH profiles in our hospital are as follows: TSH, 0.55 to 4.78 mIU/L; FT3, 1.79 to 4.09 pg/mL; FT4, 0.8 to 1.88 ng/dL; TT3, 0.65 to 1.91 ng/mL; and TT4, 4.29 to 12.47 μg/dL. The assay received regulatory approval based on measured functional sensitivity of 0.2 pg/mL for FT3, 0.1 ng/dL for FT4, 0.1 ng/mL for TT3, 0.3 μg/dL for TT4, and 0.008 mIU/L for TSH. The intra‐ and interassay coefficients of variation for all assays were ≤10.0%.
2.3. cMRI and LGE analysis
The procedure and acquisition of cMRI is the same as our previous study.21 All data were transferred to a separate workstation for analysis and were assessed by 2 independent readers. A third blinded reader adjudicated in cases of disagreement. Briefly, the LV was divided into 6 basal segments, 6 midventricular segments, 4 distal segments, and the apex based on a 17‐segment model. LGE was defined as area of signal enhancement ≥2 SD of the signal of nonenhanced myocardium.
2.4. Follow‐up and endpoints
Follow‐up for the patients started at the inception with thyroid function evaluation. We contacted patients by phone or periodically examined the patients every 6 months according to our follow‐up schedule. Patients' medical records would be reviewed if they were re‐admitted into our hospital. Median duration of follow‐up was 61 months (range, 1–72 months). Primary endpoint of the study was all‐cause mortality. The principal secondary endpoint was a composite of cardiac mortality (SCD, HF, stroke, or thromboembolic event) or cardiac transplantation. Two additional major secondary endpoints were also prespecified: an arrhythmic composite endpoint including SCD or aborted SCD, and an HF composite endpoint including HF death, HF hospitalization, or cardiac transplantation. Patients lost to follow‐up were censored upon last contact with them.
2.5. Statistical analysis
Continuous variables are presented as mean ± SD and were compared across multiple groups using 1‐way analysis of variance (ANOVA). Noncontinuous and categorical variables are presented as frequencies (percentages) and were compared using the χ2 test or Fisher exact test as appropriate. Kaplan–Meier analysis was used to generate survival curves, and the log‐rank test was used to assess the differences among the 4 different groups. Univariate and multivariate Cox proportional hazards models were used to estimate the hazard ratio (HR) with 95% confidence intervals (CI). The covariables in multivariate analysis were mainly selected due to the following reasons: the variable is known to be associated with thyroid function and CV events, and is therefore clinically important; and the variable which has significance in univariable analysis is also included in the multivariable analysis. Net reclassification improvement (NRI) and integrated discrimination improvement (IDI) analysis were used to assess the reclassification of patient risk of primary endpoints and each second endpoint. For each patient, the predicted overall risk of an adverse event was determined by a prediction model based on LGE and left ventricular ejection fraction (LVEF), and the relative improvement in patient reclassification was assessed by combing FT3, LGE, and LVEF. For all the endpoints, reclassification was examined using the thresholds of 0% to 10%, 10% to 20%, 20% to 30%, and ≥30% to stratify level of risk. All statistical analyses were performed using SAS version 9.2 (SAS Institute, Inc., Cary, NC). A 2‐tailed P value <0.05 was considered statistically significant.
3. RESULTS
3.1. Study population and baseline clinical characteristics
From January 2010 to October 2011, 458 consecutive DCM patients were admitted to Fuwai Hospital (National Center of Cardiovascular Diseases, China). Among the entire cohort, 220 DCM patients underwent cMRI and LGE evaluation and completed follow‐up through December 2015. For the study flowchart and subjects' disposition, see Supporting Information, Figure 1, in the online version of this article.
Figure 1.
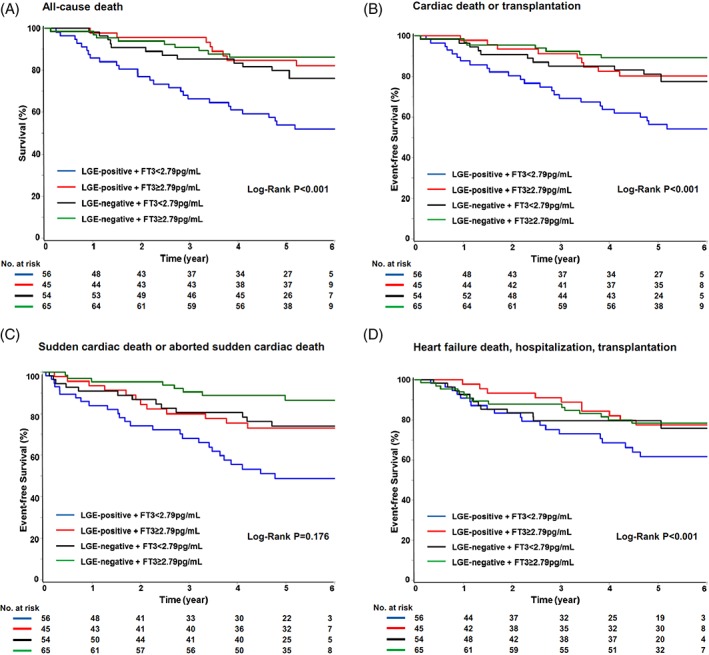
Cumulative Kaplan–Meier curves during follow‐up for (A) all‐cause death, (B) cardiac death or transplantation, (C) SCD or aborted SCD, and (D) HF, hospitalization, or transplantation. Abbreviations: FT3, free triiodothyronine; HF, heart failure; LGE, late gadolinium enhancement; SCD, sudden cardiac death
Table 1 shows the baseline clinical characteristics and FT3 levels of the study population. Patients were divided into 4 groups based on the presence or absence of LGE and the FT3 median value of 2.79 pg/mL: LGE‐positive + FT3 < 2.79 pg/mL (n = 56), LGE‐positive + FT3 ≥ 2.79 pg/mL (n = 45), LGE‐negative + FT3 < 2.79 pg/mL (n = 54), and LGE‐negative + FT3 ≥ 2.79 pg/mL (n = 65). Patients in the LGE‐positive + FT3 < 2.79 pg/mL group had the highest percentage of females and the lowest percentage of smokers. Significant differences were also detected for age, LVEF, systolic and diastolic blood pressure, FT4, and TT3. A significantly decreasing trend could be detected with respect to N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP) across the groups. No significant difference was found with regard to comorbidities and medications.
Table 1.
Baseline clinical characteristics, cardiac function, and thyroid hormone levels
| LGE‐positive + FT3 < 2.79 pg/mL, n = 56 | LGE‐positive + FT3 ≥ 2.79 pg/mL, n = 45 | LGE‐negative + FT3 < 2.79 pg/mL, n = 54 | LGE‐negative + FT3 ≥ 2.79 pg/mL, n = 65 | P Value | |
|---|---|---|---|---|---|
| Age, y | 49.95 ± 13.50 | 45.40 ± 12.75 | 54.43 ± 12.40 | 48.23 ± 14.81 | 0.009 |
| Female sex | 20 (35.7) | 7 (15.6) | 19 (35.2) | 13 (20.0) | 0.032 |
| BMI, kg/m2 | 23.83 ± 3.59 | 25.43 ± 4.56 | 24.92 ± 4.77 | 25.27 ± 4.06 | 0.220 |
| Smoking | 17 (30.4) | 26 (57.8) | 24 (44.4) | 37 (56.9) | 0.011 |
| DM | 12 (21.4) | 7 (15.6) | 15 (27.8) | 6 (9.2) | 0.053 |
| Dyslipidemia | 24 (42.9) | 19 (42.2) | 20 (37.0) | 22 (33.8) | 0.716 |
| Anemia | 9 (16.1) | 4 (8.9) | 6 (11.1) | 3 (4.6) | 0.197 |
| AF | 44 (78.6) | 33 (73.3) | 33 (61.1) | 40 (61.5) | 0.110 |
| Renal dysfunction | 9 (16.1) | 6 (13.3) | 15 (27.8) | 12 (18.5) | 0.283 |
| SBP, mm Hg | 106.29 ± 15.20 | 115.36 ± 14.13 | 110.56 ± 23.11 | 117.54 ± 16.55 | 0.003 |
| DBP, mm Hg | 69.05 ± 12.38 | 73.36 ± 10.94 | 68.46 ± 14.33 | 74.37 ± 10.39 | 0.017 |
| NT‐proBNP, pg/mL | 2639.4 ± 1831.4 | 1921.9 ± 1468.3 | 2069.3 ± 1762.8 | 1577.1 ± 1661.0 | 0.013 |
| LVEDD, mma | 71.54 ± 12.72 | 70.98 ± 13.99 | 70.05 ± 12.28 | 67.02 ± 11.03 | 0.193 |
| LVEDV, mLa | 215.30 ± 57.56 | 237.55 ± 95.47 | 250.01 ± 77.98 | 244.68 ± 116.16 | 0.723 |
| LVEDV index, mL/m2a | 155.01 ± 56.39 | 134.38 ± 52.47 | 146.13 ± 50.43 | 131.30 ± 57.43 | 0.109 |
| CO, L/mina | 4.16 ± 1.66 | 4.63 ± 1.10 | 4.26 ± 1.58 | 4.47 ± 1.77 | 0.578 |
| LVEF, %a | 22.46 ± 8.54 | 24.87 ± 10.12 | 24.73 ± 9.59 | 29.39 ± 13.17 | 0.004 |
| NYHA functional class | 0.102 | ||||
| I–II | 10 (17.9) | 11 (24.4) | 12 (22.2) | 23 (35.4) | |
| III–IV | 46 (82.1) | 34 (75.6) | 42 (77.8) | 42 (64.6) | |
| TSH, mIU/L | 2.34 ± 1.78 | 2.56 ± 1.67 | 5.64 ± 17.29 | 2.22 ± 1.55 | 0.121 |
| FT4, ng/dL | 1.26 ± 0.28 | 1.42 ± 0.37 | 1.26 ± 0.23 | 1.37 ± 0.30 | 0.012 |
| TT3, ng/mL | 0.91 ± 0.33 | 1.13 ± 0.28 | 0.91 ± 0.30 | 1.09 ± 0.29 | <0.001 |
| TT4, μg/dL | 7.82 ± 1.65 | 8.50 ± 1.70 | 7.57 ± 2.25 | 8.12 ± 1.99 | 0.092 |
| Medications at baseline | |||||
| β‐Blocker | 47 (83.9) | 39 (86.7) | 43 (79.6) | 59 (90.8) | 0.375 |
| ACEI/ARB | 32 (57.1) | 30 (66.7) | 37 (68.5) | 49 (75.4) | 0.204 |
| Loop diuretic | 49 (87.5) | 40 (88.9) | 48 (88.9) | 55 (84.6) | 0.885 |
| Aldosterone antagonist | 44 (78.6) | 41 (91.1) | 38 (70.4) | 52 (80.0) | 0.088 |
| Digoxin | 38 (67.9) | 29 (64.4) | 32 (59.3) | 37 (56.9) | 0.614 |
Abbreviations: ACEI, angiotensin‐converting enzyme inhibitor; AF, atrial fibrillation; ARB, angiotensin II receptor blocker; BMI, body mass index; cMRI, cardiac magnetic resonance imaging; CO, cardiac output; DBP, diastolic blood pressure; DM, diabetes mellitus; FT3, free triiodothyronine; FT4, free thyroxine; LGE, late gadolinium enhancement; LVEDD, left ventricular end‐diastolic diameter; LVEDV, left ventricular end‐diastolic volume; LVEF, left ventricular ejection fraction; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; NYHA, New York Heart Association; SBP, systolic blood pressure; SD, standard deviation; TSH, thyroid stimulating hormone; TT3, total triiodothyronine; TT4, total thyroxine.
Data are presented as n (%) or mean ± SD.
Detected by cMRI.
3.2. Prognostic value of the combination of LGE and FT3 level
All study outcomes and composite endpoints are shown in Table 2, and Kaplan–Meier estimates of time to event are shown in the Figure.
Table 2.
Study outcome data during a median follow‐up of 61 months
| LGE‐positive + FT3 < 2.79 pg/mL, n = 56 | LGE‐positive + FT3 ≥ 2.79 pg/mL, n = 45 | LGE‐negative + FT3 < 2.79 pg/mL, n = 54 | LGE‐negative + FT3 ≥ 2.79 pg/mL, n = 65 | P Value | |
|---|---|---|---|---|---|
| All‐cause mortality | 27 (48.2) | 8 (17.8) | 12 (22.2) | 9 (13.8) | <0.001 |
| Cardiac deaths | 24 (42.9) | 8 (17.8) | 9 (16.7) | 7 (10.8) | <0.001 |
| Cardiac transplantation | 5 (8.9) | 3 (6.7) | 3 (5.6) | 0 (0.0) | 0.038 |
| SCD | 12 (21.4) | 4 (8.9) | 6 (11.1) | 6 (9.2) | 0.185 |
| Aborted SCD | 7 (12.5) | 8 (17.8) | 6 (11.1) | 9 (13.8) | 0.806 |
| HF death | 12 (21.4) | 4 (8.9) | 3 (5.6) | 1 (1.5) | 0.002 |
| HF hospitalization | 15 (26.8) | 6 (13.3) | 8 (14.8) | 7 (10.8) | 0.115 |
| ICD/CRT implantation | 5 (8.9) | 6 (13.3) | 3 (5.6) | 5 (7.7) | 0.591 |
| Cardiac deaths or transplantation | 25 (44.6) | 9 (20.0) | 11 (20.4) | 7 (10.8) | <0.001 |
| SCD or aborted SCD | 19 (33.9) | 10 (22.2) | 12 (22.2) | 14 (21.5) | 0.381 |
| HF death, HF hospitalization, cardiac transplantation | 26 (46.4) | 12 (26.7) | 13 (24.1) | 8 (12.3) | <0.001 |
Abbreviations: CRT, cardiac resynchronization therapy; FT3, free triiodothyronine; HF, heart failure; ICD, implantable cardioverter‐defibrillator; LGE, late gadolinium enhancement; SCD, sudden cardiac death.
Data are presented as n (%).
3.2.1. Primary endpoint: all‐cause mortality
After a median follow‐up of 61 months, 56 patients (25.5%) died in total, with rates of 27/56 (48.2%), 8/45 (17.8%), 12/54 (22.2%), and 9/65 (13.8%) in patients with LGE‐positive + FT3 < 2.79 pg/mL, LGE‐positive + FT3 ≥ 2.79 pg/mL, LGE‐negative + FT3 < 2.79 pg/mL, and LGE‐negative + FT3 ≥ 2.79 pg/mL status, respectively (Table 2). Among the 4 groups, LGE‐positive + FT3 < 2.79 pg/mL status was associated with a higher rate of all‐cause mortality endpoint (Figure 1A; log‐rank test P < 0.001). Atrial fibrillation, NT‐proBNP, LVEF, left ventricular end‐diastolic diameter, left ventricular end‐diastolic volume index assessed by cMRI, LGE status, and FT3 were found to be significantly associated with all‐cause mortality in the univariate Cox analysis (Table 3). In multivariate Cox survival analysis, LGE‐positive + FT3 < 2.79 pg/mL was significantly associated with all‐cause mortality (HR: 2.893, 95% CI: 1.323‐6.326, P = 0.008; Table 3). NT‐proBNP and LVEF were also found to be independently correlated with all‐cause mortality in the multivariable Cox analysis (Table 3). Interaction effect between LGE status and FT3 level in the multivariate Cox model of all‐cause mortality was not significant (interaction P = 0.214).
Table 3.
Univariate and multivariate Cox analysis for all‐cause mortality
| Variables | HR (95% CI) | P Value |
|---|---|---|
| Univariate regression | ||
| Age | 1.000 (0.982‐1.020) | 0.966 |
| Female sex | 1.183 (0.669‐2.092) | 0.564 |
| BMI | 0.955 (0.898‐1.017) | 0.152 |
| Smoking | 0.588 (0.340‐1.016) | 0.057 |
| DM | 0.709 (0.335‐1.498) | 0.367 |
| Anemia | 1.075 (0.461‐2.508) | 0.867 |
| Renal insufficiency | 1.325 (0.712‐2.466) | 0.374 |
| AF | 2.060 (1.065‐3.985) | 0.032 |
| NT‐proBNP (per 100 pg/mL) | 1.038 (1.025‐1.051) | <0.001 |
| LVEF | 0.961 (0.939‐0.983) | <0.001 |
| LVEDD | 1.054 (1.034‐1.074) | <0.001 |
| LVEDV index | 1.011 (1.006‐1.015) | <0.001 |
| LGE‐negative + FT3 ≥ 2.79 pg/mL | 1 (Ref) | |
| LGE‐negative + FT3 < 2.79 pg/mL | 1.653 (0.696‐3.923) | 0.254 |
| LGE‐positive + FT3 ≥ 2.79 pg/mL | 1.160 (0.445‐3.023) | 0.762 |
| LGE‐positive + FT3 < 2.79 pg/mL | 4.064 (1.903‐8.679) | <0.001 |
| Multivariate regression | ||
| NT‐proBNP (per 100 pg/mL) | 1.028 (1.014‐1.043) | <0.001 |
| LVEF | 0.964 (0.937‐0.991) | 0.009 |
| LGE‐negative + FT3 ≥ 2.79 pg/mL | 1 (Ref) | |
| LGE‐negative + FT3 < 2.79 pg/mL | 1.617 (0.666‐3.923) | 0.288 |
| LGE‐positive + FT3 ≥ 2.79 pg/mL | 1.095 (0.414‐2.896) | 0.855 |
| LGE‐positive + FT3 < 2.79 pg/mL | 2.893 (1.323‐6.326) | 0.008 |
Abbreviations: AF, atrial fibrillation; BMI, body mass index; CI, confidence interval; DM, diabetes mellitus; FT3, free triiodothyronine; HR, hazard ratio; LGE, late gadolinium enhancement; LVEDD, left ventricular end‐diastolic diameter; LVEDV, left ventricular end‐diastolic volume; LVEF, left ventricular ejection fraction; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; Ref, reference.
3.2.2. Cardiac mortality or cardiac transplantation
Of the 56 all‐cause deaths, cardiac death occurred in 48 patients (85.7%). These included 28 SCDs and 20 HF deaths. In addition, orthotopic cardiac transplantation was performed in 11 patients (5%) for end‐stage HF. LGE‐positive + FT3 < 2.79 pg/mL status was associated with a markedly higher risk of the secondary composite endpoint of cardiac mortality or cardiac transplantation compared with LGE‐negative + FT3 ≥ 2.79 pg/mL status (44.6% vs 10.8%; HR: 4.961, 95% CI: 2.141‐11.50, P < 0.001; Figure [B] and Supporting Information, Table S1, in the online version of this article). This association remained significant following adjustment for other significant prognostic variables including age, sex, smoking, diabetes mellitus, atrial fibrillation, NT‐proBNP, and LVEF. Similarly, no significant interaction effect between LGE status and FT3 level in the multivariate Cox model of CV mortality or cardiac transplantation was observed (interaction P = 0.175).
3.2.3. Arrhythmic composite endpoint and HF composite endpoint
There was no significant difference among the 4 groups for the arrhythmic composite endpoint, including SCD and aborted SCD (Table 2 and Figure [C]). The HF composite endpoint occurred in 59 patients, with a significant decreasing rate among the different groups (Table 2 and Figure [D]). Univariate analysis showed that LGE‐positive + FT3 < 2.79 pg/mL status was markedly associated with greater risk of HF composite endpoint compared with LGE‐negative + FT3 ≥ 2.79 pg/mL status (HR: 5.010, 95% CI: 2.265‐11.08, P < 0.001; Figure [D] and Supporting Information, Table S3, in the online version of this article). Similar association was observed for NT‐proBNP, LVEDD, and LVEDV index in the univariate analysis. In multivariate analysis, LGE‐positive + FT3 < 2.79 pg/mL status was still an independent predictor of HF composite endpoint (HR: 4.675, 95% CI: 2.209‐10.77).
The interaction P value between LGE status and FT3 level was 0.3065 in the model of arrhythmic composite endpoint and 0.9590 in the model of HF composite endpoint.
3.3. Incremental prognostic value risk reclassification by the combination of FT3 and LGE level
We assessed reclassification of risk separately for all‐cause mortality and the secondary composite endpoints (cardiac death and cardiac transplantation, arrhythmic and HF composite endpoints) after addition of FT3 to a risk model (Cox survival analysis) based on traditional risk factors, including LVEF and LGE status (see Supporting Information, Table S4, in the online version of this article). For all‐cause mortality (Table 4), the addition of FT3 to LVEF and LGE–based risk‐prediction model resulted in 14 correct (up) reclassifications and 9 incorrect (down) reclassifications in the 56 patients who died. Additionally, 54 correct (down) reclassifications and 22 incorrect (up) reclassifications occurred in the 164 survivors. Overall, the combination of FT3 and LGE status significantly improved the reclassification (NRI, 0.28; P = 0.005) and the integrated discrimination (IDI, 0.058; P = 0.001). For the secondary composite endpoint (cardiac death and cardiac transplantation; see Supporting Information, Table S5, in the online version of this article), the addition of FT3 yielded 16 correct (up) reclassifications and 11 incorrect (down) reclassifications in the 48 patients who had cardiac death or transplantation. Similarly, 58 correct (down) reclassifications and 25 incorrect (up) reclassifications occurred in the 172 patients who did not experience cardiac death or transplantation. Overall, 29% of patients were correctly reclassified after adding FT3 status to the risk model (NRI, 0.29; P = 0.010). Risk reclassification for arrhythmic and HF composite endpoints are shown in the Supporting Information, Tables S6 and S7, in the online version of this article.
Table 4.
Risk reclassification with the addition of FT3 level to a risk model based on LGE and LVEF for all‐cause mortality
| Predicted Risk With LGE and LVEF, % | Predicted Risk With LGE Plus FT3 and LVEF, % | ||||
|---|---|---|---|---|---|
| 0–10 | 10–20 | 20–30 | ≥30 | Total | |
| Deaths | |||||
| 0–10 | 0 | 1a | 0a | 0a | 1 |
| 10–20 | 0b | 6 | 6a | 0a | 12 |
| 20–30 | 0b | 1b | 7 | 7a | 15 |
| ≥30 | 0b | 4b | 4b | 20 | 28 |
| Total | 0 | 12 | 17 | 27 | 56 |
| Survivors | |||||
| 0–10 | 20 | 1b | 0b | 0b | 22 |
| 10–20 | 14a | 33 | 11b | 1b | 81 |
| 20–30 | 0a | 18a | 13 | 9b | 45 |
| ≥30 | 0a | 10a | 12a | 22 | 42 |
| Total | 34 | 62 | 36 | 32 | 164 |
Abbreviations: FT3, free triiodothyronine; LGE, late gadolinium enhancement; LVEF, left ventricular ejection fraction.
Values are the number of patients in corresponding risk category (0%–10%, 10%–20%, 20%–30%, and ≥30%) according to the risk model based on LGE and LVEF and the risk model based on LGE plus FT3 and LVEF for all‐cause mortality.
Correct reclassifications.
Incorrect reclassifications.
4. DISCUSSION
The present study explored the prognostic value of LGE and FT3 levels in DCM and identified LGE‐positive and FT3 < 2.79 pg/mL as a significant independent predictor of all‐cause mortality. LGE‐positive and FT3 < 2.79 pg/mL was also independently associated with cardiac mortality or transplantation and the HF composite endpoints. Combination of FT3 and LGE status significantly improved risk reclassification for all‐cause mortality and the composite of cardiac mortality or cardiac transplantation. To the best of our knowledge, this is the first clinical study demonstrating that a combination of fibrosis status (LGE) and important physiological (thyroid function) indices can provide more clinically relevant information for assessing the risk of long‐term mortality in patients with DCM than LGE status alone.
Human myocardial biopsy has indicated that the histological basis for LGE in DCM is focal myocardial fibrosis, which is a very common histological feature of the failing heart.22, 23 Although the interaction between LGE and FT3 level in Cox model was not significant in the present study, the influence of thyroid hormone (TH) on myocardial fibrosis has attracted much attention. The underlying pathophysiological mechanisms of myocardial fibrosis are various, but low thyroid function has been consistently shown to contribute to myocardial fibrosis. Chen et al. provided a comprehensive insight into the underlying mechanisms that hypothyroidism promoted myocardial fibrosis through the TH receptor (TR) β1.24 THs could also accelerate the breakdown of collagen types I and III by increasing matrix metalloproteinase‐1 activity.18 Consistent with animal experiments, results obtained from our previous clinical observation study revealed that the presence of LGE was more frequent in relatively low T3 conditions.21
LGE detected by cMRI has grown to be a powerful predictor for cardiac events in DCM patients. Two recently published systematic reviews16, 25 reported that the presence of LGE in DCM was a high‐risk status with poor prognosis. Additionally, a UK cohort enrolling 472 nonischemic DCM patients demonstrated that LGE was associated with the extent of LV dilatation and systolic impairment, and DCM patents could be reclassified into more accurate risk stratification based on LGE status.15 Corrado et al. studied a consecutive series of DCM patients with arrhythmic events as the primary endpoints. Their findings strongly suggested that clinical application of LGE has obvious advantage over LVEF in predicting arrhythmic events and SCD.17 More recently, the study by Pontone et al further confirmed LGE was a better independent predictor of CV events and could be used to refine risk classification.26 To avoid missing patients at high risk for cardiac events who cannot be identified with traditional risk factors and LGE, we added FT3 to create a simple, convenient model for predicting outcomes in patients with DCM. In the present cohort, the combination of FT3 level and LGE status provides more information for assessing the prognosis of DCM.
In the NRI analysis, our findings demonstrated that the addition of thyroid function significantly improved the risk stratification for all‐cause mortality, CV death, and HF composite outcomes. Current clinical decision‐making and prognostic assessment in DCM patients are predominantly based on LVEF27; however, it is acknowledged that LVEF has some limitations regarding its sensitivity and specificity.17, 28 Our study suggests that the combined use of FT3 and LGE has the potential to generate a more accurate and reliable risk stratification for patients with nonischemic DCM. It may have a clinical implication not only to accurately identify low‐risk patients with severe impairment in systolic function, thus avoiding excess costs and potential complications of aggressive interventions, but also to facilitate identification of high‐risk patients with more preserved LVEF who may benefit from intensive therapies.
Although presence of LGE seems to be better than traditional LVEF in predicting arrhythmic events and SCD,17 the present study showed that FT3 level, on the basis of LGE and LVEF predicting model, did not provide more predictive value for the composite endpoint of SCD or aborted SCD and the composite endpoint of HF death, HF hospitalization, or cardiac transplantation. As for SCD, increasing data suggest that myocardial fibrosis might constitute the pathophysiological basis for ventricular arrhythmias due to scar‐related reentry.29, 30 Results obtained from our previous clinical observation indicated that the presence of LGE was significantly associated with FT3 level. In addition, a number of animal studies have indicated that TH treatment may inhibit or even reverse myocardial fibrosis in heart diseases.31, 32 The low event rate in our study participants may also limit the power to detect the difference. The overlapped effects of LGE and FT3 on myocardial fibrosis might explain why FT3 did not improve the risk classification for SCD. It may also be responsible for the less significant improvement of predicting value of FT3 model in the HF composite endpoint.
4.1. Study limitations
Despite the encouraging findings, our study has some limitations. First, thyroid function profiles based on serum TH level rather than myocardial tissue level might not accurately reflect the focal myocardial TH in HF. As mentioned previously, cardiac‐tissue TH could be down‐regulated by the increased induction of type III iodothyronine deiodinase in cardiac diseases, which is independent from alterations of serum TH levels.33, 34 It would be desirable if myocardial TH levels were available and evaluated in the predictive value for HF. Further investigation to define the relationship of serum and myocardial tissue THs in human heart disease is certainly needed. Additionally, HF was shown to be associated with altered cardiac TH signaling, as evidenced by changes in myocardial expression of TH nuclear receptors in animal experiments, especially TH receptor α1.35, 36 The clinical implication of TH receptor α1 in guiding diagnosis and prognosis needs further investigation in clinical settings.
5. CONCLUSION
We identified LGE‐positive and FT3 < 2.79 pg/mL as a significant independent predictor of all‐cause mortality. LGE‐positive and FT3 < 2.79 pg/mL was also independently associated with cardiac mortality or transplantation, and the HF composite endpoint. Combination of FT3 and LGE status significantly improved risk reclassification and integrated discrimination for adverse events in DCM. The combination of fibrosis status (LGE) and important physiological (thyroid function) indices can provide more clinically relevant information for assessing the risk of long‐term mortality in patients with DCM. However, the potential clinical application of FT3 and LGE in the risk stratification of patients with nonischemic DCM deserves further investigation in prospective randomized trials.
Conflicts of interest
The authors declare no potential conflicts of interest.
Supporting information
Figure S1.
Table S1. Univariate and Multivariate Cox Analysis for Cardiac Death or Cardiac Transplantation
Table S2. Univariate and Multivariate Cox Analysis for Sudden Cardiac Death or Aborted Sudden Cardiac Death
Table S3. Univariate and Multivariate Cox Analysis for Heart Failure Death or Heart Failure hospitalization or Cardiac Transplantation
Table S4. Reclassification of Adverse Events by Combined LGE and FT3 Level.
Table S5. Risk Reclassification with the Addition of FT3 Level to a Risk Model Based on LGE for Cardiac Death or Cardiac Transplantation*.
Table S6. Risk Reclassification with the Addition of FT3 Level to a Risk Model Based on LGE and LVEF for Sudden Cardiac Death or Aborted Sudden Cardiac Death*.
Table S7. Risk Reclassification with the Addition of FT3 Level to a Risk Model Based on LGE and LVEF for Heart Failure Death or Heart Failure hospitalization or Cardiac Transplantation*.
Acknowledgments
The authors acknowledge Wei Li, Yang Wang, and Yanyan Zhao (Medical Research & Biometrics Center, Fuwai Hospital, National Center for Cardiovascular Disease, China) for their help with the statistical analyses.
Zhang K, Wang W, Zhao S, et al. Long‐term prognostic value of combined free triiodothyronine and late gadolinium enhancement in nonischemic dilated cardiomyopathy. Clin Cardiol. 2018;41:96–103. 10.1002/clc.22858
Funding information National Natural Science Foundation of China, Grant/Award number: 81470485; CAMS Innovation Fund for Medical Sciences, Grant/Award number: CIFMS 2016‐I2M‐1‐009; Capital Clinical Featured Application Research Project, Grant/Award number: z151100004015175; Natural Science Foundation of Beijing Municipality, Grant/Award number: 7152123.
REFERENCES
- 1. Jefferies JL, Towbin JA. Dilated cardiomyopathy. Lancet. 2010;375:752–762. [DOI] [PubMed] [Google Scholar]
- 2. Bozkurt B, Colvin M, Cook J, et al. Current Diagnostic and Treatment Strategies for Specific Dilated Cardiomyopathies: a Scientific Statement from the American Heart Association. Circulation. 2016;134:e579–e646. [DOI] [PubMed] [Google Scholar]
- 3. Echouffo‐Tcheugui JB, Greene SJ, Papadimitriou L, et al. Population risk prediction models for incident heart failure: a systematic review. Circ Heart Fail. 2015;8:438–447. [DOI] [PubMed] [Google Scholar]
- 4. Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC), developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–2200. [DOI] [PubMed] [Google Scholar]
- 5. Floriani C, Gencer B, Collet TH, et al. Subclinical thyroid dysfunction and cardiovascular diseases: 2016 update. Eur Heart J. 2017. doi: 10.1093/eurheartj/ehx050. [DOI] [PubMed] [Google Scholar]
- 6. Cappola AR, Fried LP, Arnold AM, et al. Thyroid status, cardiovascular risk, and mortality in older adults. JAMA. 2006;295:1033–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tseng FY, Lin WY, Lin CC, et al. Subclinical hypothyroidism is associated with increased risk for all‐cause and cardiovascular mortality in adults. J Am Coll Cardiol. 2012;60:730–737. [DOI] [PubMed] [Google Scholar]
- 8. Wang WY, Tang YD, Yang M, et al. Free triiodothyronine level indicates the degree of myocardial injury in patients with acute ST‐elevation myocardial infarction. Chin Med J (Engl). 2013;126:3926–3929. [PubMed] [Google Scholar]
- 9. Wang W, Guan H, Gerdes AM, et al. Thyroid status, cardiac function, and mortality in patients with idiopathic dilated cardiomyopathy. J Clin Endocrinol Metab. 2015;100:3210–3218. [DOI] [PubMed] [Google Scholar]
- 10. Pantos C, Mourouzis I. Translating thyroid hormone effects into clinical practice: the relevance of thyroid hormone receptor alpha1 in cardiac repair. Heart Fail Rev. 2015;20:273–282. [DOI] [PubMed] [Google Scholar]
- 11. Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:e240–e327. [DOI] [PubMed] [Google Scholar]
- 12. Pennell DJ. Cardiovascular magnetic resonance. Circulation. 2010;121:692–705. [DOI] [PubMed] [Google Scholar]
- 13. Mewton N, Liu CY, Croisille P, et al. Assessment of myocardial fibrosis with cardiovascular magnetic resonance. J Am Coll Cardiol. 2011;57:891–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pöyhönen P, Kivistö S, Holmström M, et al. Quantifying late gadolinium enhancement on CMR provides additional prognostic information in early risk‐stratification of nonischemic cardiomyopathy: a cohort study. BMC Cardiovasc Disord. 2014;14:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gulati A, Jabbour A, Ismail TF, et al. Association of fibrosis with mortality and sudden cardiac death in patients with nonischemic dilated cardiomyopathy [published correction appears in JAMA. 2013;310:99]. JAMA. 2013;309:896–908. [DOI] [PubMed] [Google Scholar]
- 16. Kuruvilla S, Adenaw N, Katwal AB, et al. Late gadolinium enhancement on cardiac magnetic resonance predicts adverse cardiovascular outcomes in nonischemic cardiomyopathy: a systematic review and meta‐analysis. Circ Cardiovasc Imaging. 2014;7:250–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Perazzolo Marra M, De Lazzari M, Zorzi A, et al. Impact of the presence and amount of myocardial fibrosis by cardiac magnetic resonance on arrhythmic outcome and sudden cardiac death in nonischemic dilated cardiomyopathy. Heart Rhythm. 2014;11:856–863. [DOI] [PubMed] [Google Scholar]
- 18. Ghose Roy S, Mishra S, Ghosh G, et al. Thyroid hormone induces myocardial matrix degradation by activating matrix metalloproteinase‐1. Matrix Biol. 2007;26:269–279. [DOI] [PubMed] [Google Scholar]
- 19. Zhang Y, Dedkov EI, Teplitsky D, et al. Both hypothyroidism and hyperthyroidism increase atrial fibrillation inducibility in rats. Circ Arrhythm Electrophysiol. 2013;6:952–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Richardson P, McKenna W, Bristow M, et al. Report of the 1995 World Health Organization/International Society and Federation of Cardiology Task Force on the definition and classification of cardiomyopathies. Circulation. 1996;93:841–842. [DOI] [PubMed] [Google Scholar]
- 21. Wang W, Guan H, Fang W, et al. Free triiodothyronine level correlates with myocardial injury and prognosis in idiopathic dilated cardiomyopathy: evidence from cardiac MRI and SPECT/PET imaging. Sci Rep. 2016;6:39811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. McCrohon JA, Moon JC, Prasad SK, et al. Differentiation of heart failure related to dilated cardiomyopathy and coronary artery disease using gadolinium‐enhanced cardiovascular magnetic resonance. Circulation. 2003;108:54–59. [DOI] [PubMed] [Google Scholar]
- 23. de Leeuw N, Ruiter DJ, Balk AH, et al. Histopathologic findings in explanted heart tissue from patients with end‐stage idiopathic dilated cardiomyopathy. Transpl Int. 2001;14:299–306. [DOI] [PubMed] [Google Scholar]
- 24. Chen WJ, Lin KH, Lee YS. Molecular characterization of myocardial fibrosis during hypothyroidism: evidence for negative regulation of the pro‐alpha1(I) collagen gene expression by thyroid hormone receptor. Mol Cell Endocrinol. 2000;162:45–55. [DOI] [PubMed] [Google Scholar]
- 25. Duan X, Li J, Zhang Q, et al. Prognostic value of late gadolinium enhancement in dilated cardiomyopathy patients: a meta‐analysis. Clin Radiol. 2015;70:999–1008. [DOI] [PubMed] [Google Scholar]
- 26. Pontone G, Guaricci AI, Andreini D, et al. Prognostic benefit of cardiac magnetic resonance over transthoracic echocardiography for the assessment of ischemic and nonischemic dilated cardiomyopathy patients referred for the evaluation of primary prevention implantable cardioverter‐defibrillator therapy. Circ Cardiovasc Imaging. 2016;9:pii:e004956. [DOI] [PubMed] [Google Scholar]
- 27. Epstein AE, DiMarco JP, Ellenbogen KA, et al. 2012 ACCF/AHA/HRS focused update incorporated into the ACCF/AHA/HRS 2008 guidelines for device‐based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Circulation. 2013;127:e283–e352. [DOI] [PubMed] [Google Scholar]
- 28. Halliday BP, Cleland JGF, Goldberger JJ, et al. Personalizing risk stratification for sudden death in dilated cardiomyopathy: the past, present, and future. Circulation. 2017;136:215–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Iles L, Pfluger H, Lefkovits L, et al. Myocardial fibrosis predicts appropriate device therapy in patients with implantable cardioverter‐defibrillators for primary prevention of sudden cardiac death. J Am Coll Cardiol. 2011;57:821–828. [DOI] [PubMed] [Google Scholar]
- 30. Tschabrunn CM, Roujol S, Nezafat R, et al. A swine model of infarct‐related reentrant ventricular tachycardia: electroanatomic, magnetic resonance, and histopathological characterization. Heart Rhythm. 2016;13:262–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chen YF, Weltman NY, Li X, et al. Improvement of left ventricular remodeling after myocardial infarction with eight weeks L‐thyroxine treatment in rats. J Transl Med. 2013;11:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Khalife WI, Tang YD, Kuzman JA, et al. Treatment of subclinical hypothyroidism reverses ischemia and prevents myocyte loss and progressive LV dysfunction in hamsters with dilated cardiomyopathy. Am J Physiol Heart Circ Physiol. 2005;289:H2409–H2415. [DOI] [PubMed] [Google Scholar]
- 33. Liu Y, Redetzke RA, Said S, et al. Serum thyroid hormone levels may not accurately reflect thyroid tissue levels and cardiac function in mild hypothyroidism. Am J Physiol Heart Circ Physiol. 2008;294:H2137–H2143. [DOI] [PubMed] [Google Scholar]
- 34. Wassner AJ, Jugo RH, Dorfman DM, et al. Myocardial induction of type 3 deiodinase in dilated cardiomyopathy. Thyroid. 2017;27:732–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pantos C, Mourouzis I, Galanopoulos G, et al. Thyroid hormone receptor alpha1 downregulation in postischemic heart failure progression: the potential role of tissue hypothyroidism. Horm Metab Res. 2010;42:718–724. [DOI] [PubMed] [Google Scholar]
- 36. Mourouzis I, Kostakou E, Galanopoulos G, et al. Inhibition of thyroid hormone receptor α1 impairs post‐ischemic cardiac performance after myocardial infarction in mice. Mol Cell Biochem. 2013;379:97–105. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1.
Table S1. Univariate and Multivariate Cox Analysis for Cardiac Death or Cardiac Transplantation
Table S2. Univariate and Multivariate Cox Analysis for Sudden Cardiac Death or Aborted Sudden Cardiac Death
Table S3. Univariate and Multivariate Cox Analysis for Heart Failure Death or Heart Failure hospitalization or Cardiac Transplantation
Table S4. Reclassification of Adverse Events by Combined LGE and FT3 Level.
Table S5. Risk Reclassification with the Addition of FT3 Level to a Risk Model Based on LGE for Cardiac Death or Cardiac Transplantation*.
Table S6. Risk Reclassification with the Addition of FT3 Level to a Risk Model Based on LGE and LVEF for Sudden Cardiac Death or Aborted Sudden Cardiac Death*.
Table S7. Risk Reclassification with the Addition of FT3 Level to a Risk Model Based on LGE and LVEF for Heart Failure Death or Heart Failure hospitalization or Cardiac Transplantation*.


