Abstract
The use of drug‐eluting stents (DES) vs bare‐metal stents (BMS) in saphenous vein graft (SVG) lesions remains controversial. We conducted a meta‐analysis of all randomized clinical trials comparing the outcomes of DES with BMS in SVG percutaneous coronary interventions. A search of PubMed, Embase, the Cochrane Register of Controlled Trials, and Clinicaltrials.gov was performed for all randomized clinical trials. We evaluated the short‐ and long‐term clinical outcomes of the following: all‐cause mortality, major adverse cardiovascular events (MACE), definite/probable stent thrombosis, target lesion revascularization (TLR), and target‐vessel revascularization (TVR). From a total of 1582 patients in 6 randomized clinical trials, 797 had DES and 785 had BMS. Patients with DES had lower short‐term MACE, TLR, and TVR in comparison with BMS (odds ratio [OR]: 0.56, 95% confidence interval [CI]: 0.35–0.91, P = 0.02; OR: 0.43, 95% CI: 0.19–0.99, P = 0.05; and OR: 0.45, 95% CI: 0.22–0.95, P = 0.04, respectively). However, there were no different outcomes for all‐cause mortality (P = 0.63) or stent thrombosis (P = 0.21). With long‐term follow‐up, there were no significant reductions of MACE (P = 0.20), TLR (P = 0.57), TVR (P = 0.07), all‐cause mortality (P = 0.29), and stent thrombosis (P = 0.76). The use of DES in SVG lesions was associated with lower short‐term MACE, TLR, and TVR in comparison with BMS. However, there were no significant differences with long‐term follow‐up.
Keywords: Bare‐Metal Stent, Drug‐Eluting Stent, Meta‐Analysis, Saphenous Vein Graft Lesion
1. INTRODUCTION
Although saphenous vein grafts (SVGs) are commonly used for coronary artery bypass graft (CABG) surgery, graft failure rates are estimated to be 10% to 25% within 1 year and almost 50% at 10 years after surgery.1, 2, 3 Therefore, secondary preventive measures after CABG are paramount to improving the long‐term clinical outcomes after surgery.4 The management of SVG failure can include medical treatment, repeat CABG, and percutaneous coronary intervention (PCI).2, 5 In comparison with SVG PCI, repeat CABG is the less preferred method for revascularization due to increased morbidity and mortality.6, 7, 8, 9
Analysis of the National Cardiovascular Data Registry (NCDR) from 2004 to 2009 showed that SVG PCIs represent about 5.7% of the total PCI volume.10 However, previous evidence has shown higher incidence of short‐ and long‐term major adverse cardiac events (MACE) in patients who underwent bypass‐graft PCI in comparison with native coronary PCI, including double the rate of in‐hospital mortality.11, 12 In contrast, evidence of the clinical outcomes of DES and BMS in SVG PCIs are conflicting and definite answers for long‐term safety are lacking.13
In a recent meta‐analysis of 4 randomized clinical trials (RCTs) and 36 observational studies, PCI with DES had lower rates of MACE, all‐cause mortality, and target‐vessel revascularization (TVR) in comparison with BMS.14 Another meta‐analysis of 5 RCTs showed a significant reduction of TVR in patients who received first‐generation DES in comparison with BMS.15 The result of the Drug‐Eluting Stents vs. Bare Metal Stents in Saphenous Vein Graft Angioplasty (DIVA) trial, which used a large percentage of second‐generation DES in comparison with BMS, was reported recently with both short‐ and long‐term clinical outcomes.16 Within this context, we conducted an updated meta‐analysis to compare the clinical outcomes of DES vs BMS in SVG lesions in both short‐term (≤12 months) and long‐term (>12 months) follow‐up.
2. METHODS
We conducted and reported our meta‐analysis according to the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses Protocols (PRISMA‐P) Statement 2015.17 We searched the databases of PubMed, Embase, the Cochrane Collaboration Central Register of Controlled Trials, Scopus, www.Clinicaltrials.gov, and conference proceedings of various international and national scientific meetings, from inception to September 2017. In addition, all reference reports on non‐RCTs were searched manually to avoid missing additional eligible RCTs. The keywords used for the systematic literature search were “saphenous vein graft,” “percutaneous intervention,” “drug‐eluting stent,” “bare‐metal stent,” “clinical trials,” and “randomized controlled trials.” We used Boolean operators to search heading connections. We included only RCTs and subgroup analyses from RCTs focused on the PCIs of SVGs and comparing DES vs BMS, regardless of the study language. We restricted our meta‐analysis to RCTs, as they carry less confounding biases in comparison with observational studies. The quality of the included studies was assessed to minimize biases. The comprehensive literature search was conducted by 2 authors independently (BK and MO) and all discrepancies were resolved by a consensus. The following inclusion criteria were used: (1) the study was an RCT, (2) the trial was randomized to DES vs BMS for SVG PCI, (3) the duration of follow‐up was ≥6 months, and (4) the trial reported ≥1 of these clinical outcomes: all‐cause mortality, MACEs, definite/probable stent thrombosis, target lesion revascularization (TLR), and TVR (Figure 1).
Figure 1.
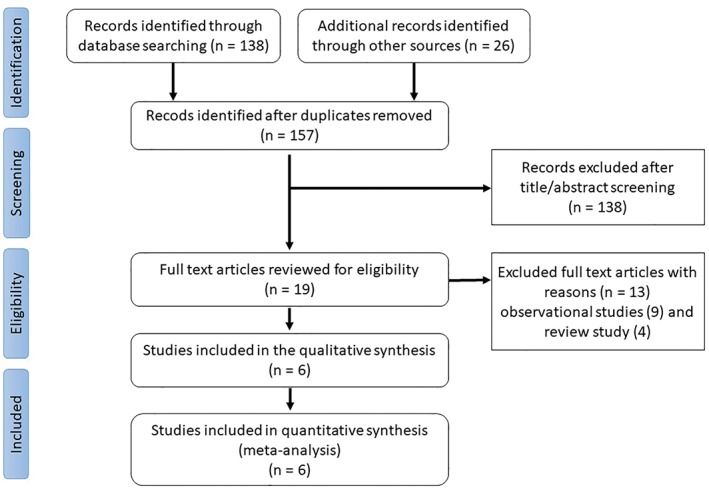
PRISMA flow diagram. Abbreviations: PRISMA, Preferred Reporting Items for Systematic Reviews and Meta‐Analyses
Data extraction was carried out by 2 independent authors (BK, MO) and discrepancies were resolved by a third author (AA). From each RCT we extracted the study characteristics and baseline demographics (Tables 1 and 2). We used Jadad scoring to measure the quality of each RCT.18 We evaluated the following clinical outcomes in both short‐ and long‐term follow‐up: all‐cause mortality, MACE, definite/probable stent thrombosis, TLR, and TVR. We should note that MACE has been defined as device‐ and/or patient‐oriented composite across the trials.19 We assumed that MACE was equivalent for device‐oriented composite in the DIVA trial. In long‐term follow‐up, we did not include the Stenting of Saphenous Vein Grafts (SOS) trial's extended post hoc analysis, as there were fewer patients included and the analysis was restricted to those from the highest‐enrolling site.20
Table 1.
Details of the included RCTs
| Study | Country (No. of Sites) | Year of Publication | Total Number of Patients | DES Type | Primary Endpoint | Events Rate, DES | Events Rate, BMS | P Value |
|---|---|---|---|---|---|---|---|---|
| RRISC | Belgium | 2006 | 75 | First‐generation SES | 6‐mo angiographic in‐stent late lumen loss | 0.38 ± 0.51 mm | 0.79 ± 0.66 mm | 0.001 |
| BASKET | Switzerland | 2009 | 47 | First‐generation SES and PES | MACE (cardiac death, nonfatal MI, and non–MI‐related TVR) | 21% | 62% | 0.007 |
| SOS | United States (5) | 2009 | 80 | First‐generation PES | Binary angiographic restenosis/lesion at 12 mo | 9% | 51% | 0.001 |
| ISAR‐CABG | Germany (4) | 2011 | 610 | First‐generation SES and PES | Combined incidence of death, MI, and TLR at 12 mo | 15% | 22% | 0.02 |
| BASKET‐SAVAGE | Europe (3)* | 2016 | 173 | First‐generation PES | MACE (cardiac death, nonfatal MI, and TVR) at 12 mo | 2.3% | 17.9% | <0.001 |
| DIVA | United States (25) | 2017 | 597 | First and second generation | TVF (composite of cardiac death, target‐vessel MI, and TVR) at 12 mo | 17% | 19% | 0.67 |
Abbreviations, BASKET, Basel Stent Kosten Effektivitäts (Cost‐Effectiveness) Trial; BASKET‐SAVAGE, Basel Stent Kosten Effektivitäts Trial–Saphenous Venous Graft Angioplasty Using Glycoprotein IIb/IIIa Receptor Inhibitors and Drug‐Eluting Stents; BMS, bare‐metal Stent; DES, drug‐eluting stent; DIVA, Drug‐Eluting Stents vs Bare‐Metal Stents in Saphenous Vein Graft Angioplasty Trial; ISAR‐CABG, Is Drug‐Eluting Stenting Associated With Improved Results in Coronary Artery Bypass Grafts Trial; MACE, major adverse cardiac events; MI, myocardial infarction; PES, paclitaxel‐eluting stent; RCT, randomized clinical trial; RRISC, Reduction of Restenosis In Saphenous Vein Grafts With Cypher Sirolimus‐Eluting Stent Trial; SES, sirolimus‐eluting stent; SOS, Stenting of Saphenous Vein Grafts Trial; TLR, target‐lesion revascularization; TVF, target‐vessel failure; TVR, target‐vessel revascularization.
Places include: Switzerland (2), Germany (2), and Denmark (2).
Table 2.
Baseline demographic characteristics included in the meta‐analysis
| Characteristic | RRISC | BASKET | SOS | ISAR‐CABG | BASKET‐SAVAGE | DIVA | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DES, n = 38 | BMS, n = 37 | DES, n = 34 | BMS, n = 13 | DES, n = 41 | BMS, n = 39 | DES, n = 303 | BMS, n = 307 | DES, n = 89 | BMS, n = 84 | DES, n = 292 | BMS, n = 305 | |
| Age, y | 73 ± 7 | 72 ± 8 | 71 ± 8 | 71 ± 8 | 66 ± 9 | 67 ± 9 | 71.4 ± 9 | 71.5 ± 9.3 | 71 ± 8 | 71 ± 9 | 69 ± 7.4 | 68.2 ± 7.7 |
| Male sex | 31 (82) | 33 (89) | 27 (79) | 13 (100) | 41 (100) | 39 (100) | 263 (87) | 259 (84) | 80 (90) | 75 (89) | 290 (99) | 305 (100) |
| DM | 6 (16) | 5 (14) | 10 (29) | 2 (17) | 18 (44) | 17 (44) | 111 (37) | 107 (35) | 41 (46) | 34 (41) | 173 (59) | 187 (61) |
| Current smoking | 2 (5) | 4 (11) | 6 (18) | 0 | 12 (29) | 9 (23) | 25 (8) | 18 (6) | — | — | 61 (21) | 72 (24) |
| Prior MI | 17 (45) | 15 (40) | 20 (59) | 6 (46) | 23 (66) | 23 (59) | 170 (56) | 168 (55) | 59 (66) | 50 (60) | 163 (56) | 153 (50) |
| HTN | 22 (58) | 21 (57) | 30 (88) | 11 (83) | 38 (93) | 37 (95) | 216 (71) | 223 (73) | 81 (91) | 75 (89) | 278 (95) | 296 (97) |
| Hyperlipidemia | 33 (87) | 31 (84) | 27 (79) | 12 (92) | 40 (98) | 37 (95) | 268 (88) | 264 (86) | 76 (85) | 73 (87) | 287 (98) | 294 (96) |
| BMI, kg/m2 | 26.4 ± 3.1 | 26.4 ± 3.9 | — | — | 29.9 ± 4.6 | 28.9 ± 3.9 | 27.5 ± 4.2 | 27.4 ± 3.7 | — | — | 30.6 ± 5.6 | 30.4 ± 5.3 |
| LVEF, % | 68 ± 18 | 72 ± 12 | — | — | 20 (EF 51%) | 22 (EF 61%) | 49.2 ± 12.2 | 49.5 ± 13.8 | — | — | 52.5 ± 47 | 49.4 ± 13.3 |
| Graft age, y | 12.4 ± 4.6 | 12.6 ± 5.9 | — | — | 11 ± 6 | 12 ± 6 | 13.4 ± 5.6 | 13.7 ± 5.2 | 12 ± 5 | 14 ± 6 | 13.9 ± 6.7 | 12.8 ± 6.8 |
| Indication for PCI | ||||||||||||
| Stable angina | — | — | 22 (65) | 4 (31) | 12 (29) | 13 (33) | 188 (62) | 183 (60) | 45 (51) | 46 (55) | 117 (40) | 105 (34) |
| ACS | — | — | 9 (27%) | 7 (54%) | — | — | — | — | 33 (37) | 33 (39) | — | — |
| UA | 23 (60) | 19 (51) | 16 (39) | 14 (36) | 115 (38) | 124 (40) | — | — | 89 (30) | 95 (31) | ||
| NSTEMI | — | — | — | — | 10 (24) | 8 (21) | — | — | — | — | 66 (23) | 74 (24) |
| STEMI | — | — | 3 (9) | 2 (15) | — | — | — | — | — | — | — | — |
| Other | — | — | — | — | 3 (7) | 4 (10) | — | — | 18 (20) | 16 (19) | 17 (6) | 30 (10) |
| Embolic protective device | 37 (79) | 41 (84) | — | — | 29 (51) | 31 (56) | <5% | <5% | — | — | 199 (69) | 211 (69) |
| Stent length, mm | 23.4 ± 7 | 22.9 ± 8 | 41 ± 25 | 46 ± 30 | 18 ± 6 | 18 ± 6 | 26.8 ± 15.4 | 27.5 ± 13.4 | 31 ± 19 | 30 ± 20 | 27 ± 18.9 | 26.6 ± 18.3 |
Abbreviations: ACS, acute coronary syndrome; BASKET, Basel Stent Kosten Effektivitäts (Cost‐Effectiveness) Trial; BASKET‐SAVAGE, Basel Stent Kosten Effektivitäts Trial–Saphenous Venous Graft Angioplasty Using Glycoprotein IIb/IIIa Receptor Inhibitors and Drug‐Eluting Stents; BMI, body mass index; BMS, bare‐metal stent; DES, drug‐eluting stent; DIVA, Drug‐Eluting Stents vs Bare‐Metal Stents in Saphenous Vein Graft Angioplasty Trial; DM, diabetes mellitus; HTN, hypertension; ISAR‐CABG, Is Drug‐Eluting Stenting Associated With Improved Results in Coronary Artery Bypass Grafts Trial; LVEF, left ventricular ejection fraction; MI, myocardial infarction; NSTEMI, non–ST‐segment elevation myocardial infarction; PCI, percutaneous coronary intervention; RRISC, Reduction of Restenosis In Saphenous Vein Grafts With Cypher Sirolimus‐Eluting Stent Trial; SD, standard deviation; SOS, Stenting of Saphenous Vein Grafts Trial; STEMI, ST‐segment elevation myocardial infarction; UA, unstable angina.
Data are presented as n (%) or mean ± SD.
We performed an aggregate data meta‐analysis of clinical outcomes (short‐ and long‐term follow‐up) for the 6 RCTs comparing DES with BMS. We used RevMan, version 5.3 for Windows (Cochrane Collaboration, Oxford, UK) for our meta‐analysis. We calculated the odds ratios (ORs) and 95% confidence intervals (CIs) to summarize the effect size for each short‐ and long‐term clinical outcome. We used a P value cutoff of <0.05 as a significant statistical result. Heterogeneity between studies was explored by Cochran's Q statistic (P < 0.05) and I 2 statistic. The effect measures were pooled together using a random‐effect model to account for between‐study variation. Short‐ and long‐term clinical outcomes were assessed at ≤12 months and > 12 months, respectively.
3. RESULTS
We identified 6 RCTs16, 21, 22, 23, 24, 25 with a pooled sample of 1582 patients; 1456 (92%) were male. There were 797 patients in the DES group and 785 in the BMS group. The mean age was 70.1 ± 8.4 years. In terms of short‐term clinical outcomes, the median follow‐up was 12 months. Our pooled sample showed a statistically significant reduction in MACE (OR: 0.56, 95% CI: 0.35–0.91, P = 0.02), TLR (OR: 0.43; 95% CI: 0.19–0.99; P = 0.05), and TVR (OR: 0.45, 95% CI: 0.22–0.95, P = 0.04) with DES in comparison with BMS. However, no statistically significant results were obtained from analyzing all‐cause mortality (OR: 1.14, 95% CI: 0.66–1.97, P = 0.63) and stent thrombosis (OR: 0.53, 95% CI: 0.20–1.43, P = 0.21; Figure 2).
Figure 2.
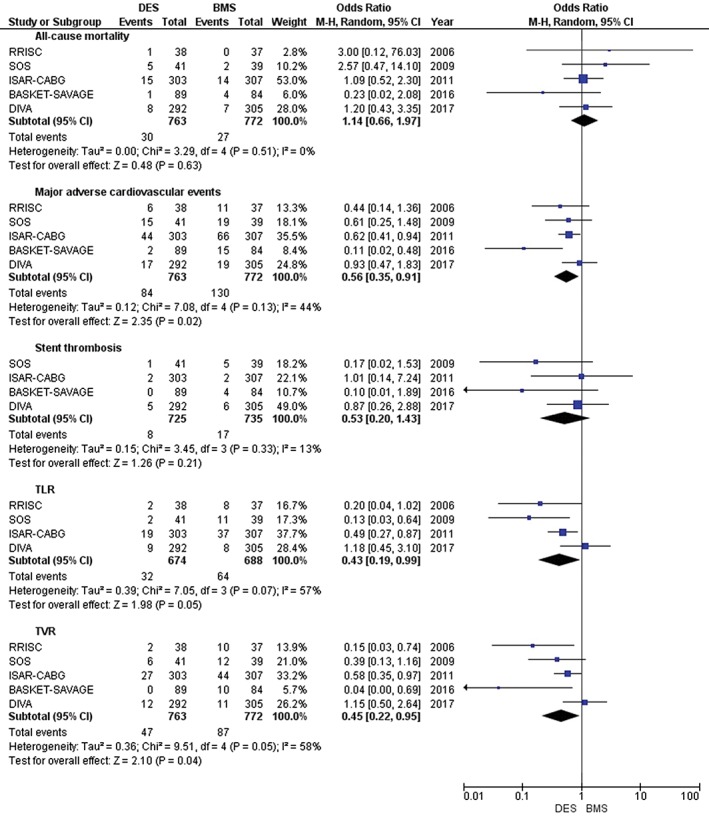
Forest plot showing short‐term clinical outcomes. Abbreviations: BASKET‐SAVAGE, Basel Stent Kosten Effektivitäts (Cost‐Effectiveness) Trial–Saphenous Venous Graft Angioplasty Using Glycoprotein IIb/IIIa Receptor Inhibitors and Drug‐Eluting Stents; BMS, bare‐metal stent; CI, confidence interval; DES, drug‐eluting stent; df, degrees of freedom; DIVA, Drug‐Eluting Stents vs. Bare Metal Stents In Saphenous Vein Graft Angioplasty; ISAR‐CABG, Is Drug‐Eluting Stenting Associated With improved Results in Coronary Artery Bypass Grafts?; M‐H, Mantel–Haenszel; RRISC, Reduction of Restenosis In Saphenous Vein Grafts With Cypher Sirolimus‐Eluting Stent Trial; SOS, Stenting of Saphenous Vein Grafts
With longer follow‐up (median 32.4 years), the reduction of MACE, TLR, and TVR became statistically insignificant (OR: 0.58, 95% CI: 0.26–1.32, P = 0.20; OR: 0.66, 95% CI: 0.15–2.82, P = 0.57; and OR: 0.48, 95% CI: 0.22–1.07, P = 0.07, respectively). Additionally, other clinical outcomes remain statistically insignificant with long‐term follow‐up: specifically, all‐cause mortality (OR: 1.63, 95% CI: 0.66–4.00, P = 0.29) and stent thrombosis (OR: 0.84, 95% CI: 0.28–2.55, P = 0.76; Figure 3).
Figure 3.
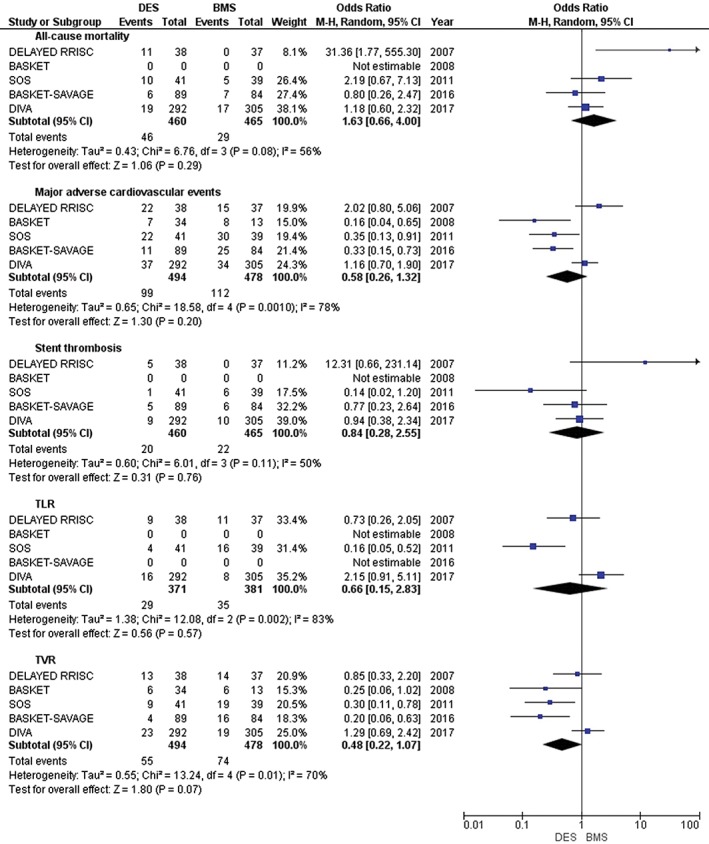
Forest plot showing long‐term clinical outcomes. Abbreviations: BASKET, Basel Stent Kosten Effektivitäts (Cost‐Effectiveness) Trial; BASKET‐SAVAGE, Basel Stent Kosten Effektivitäts Trial–Saphenous Venous Graft Angioplasty Using Glycoprotein IIb/IIIa Receptor Inhibitors and Drug‐Eluting Stents; BMS, bare‐metal stent; CI, confidence interval; DELAYED RRISC, Death and Events at Long‐Term Follow‐Up Analysis: Extended Duration of the Reduction of Restenosis In Saphenous Vein Grafts With Cypher Stent; DES, drug‐eluting stent; df, degrees of freedom; DIVA, Drug‐Eluting Stents vs. Bare Metal Stents In Saphenous Vein Graft Angioplasty; ISAR‐CABG, Is Drug‐Eluting Stenting Associated With improved Results in Coronary Artery Bypass Grafts?; M‐H, Mantel–Haenszel; SOS, Stenting of Saphenous Vein Grafts
4. DISCUSSION
The result of our meta‐analysis of 6 RCTs demonstrated a short‐term benefit of DES in comparison with BMS in MACE, TLR, and TVR. The reduction in MACE could be explained by the reduction in both TLR and TVR. However, the effect on MACE was ameliorated with longer follow‐up as the reduction of TLR and TVR became insignificant. In our study, we have not included the very long post hoc analysis of SOS,20 as it had only analyzed the highest enrolled patients from a single center and hence had a lower total sample size from the original study.
The mechanism underpinning venous graft occlusion are multifactorial and largely attributed to the accelerated progression of atherosclerotic disease in the adjacent stented segment, restenosis, and/or stent thrombosis.2, 26 Although DES have been shown to reduce in‐stent restenosis risks, they also have been associated with late stent thrombosis.27 Although we noticed an insignificant lower trend for stent thrombosis in short‐term follow‐up with DES (OR: 0.53, 95% CI: 0.20–1.43, P = 0.21), the trend became higher with longer follow‐up (OR: 0.84, 95% CI: 0.28–2.55, P = 0.76). The increasing trend could be explained in part by the mechanism of late stent thrombosis with DES.
In a large study from the National Cardiovascular Data Registry (CathPCI) of patients age ≥ 65 years (n = 49 325) who underwent SVG stenting between 2005 and 2009, DES were associated with lower all‐cause mortality with no observed difference in MI or urgent revascularization at 3 years.28 However, our pooled results showed no clear benefit of DES over BMS with regard to all‐cause mortality. Therefore, large powered RCTs are warranted to detect these effects, if they truly exist.
In comparison with first‐generation DES, newer DES have shown better safety and efficacy in native coronary arteries.29 The comparison outcome of first‐generation vs second‐generation DES in SVG lesions has shown similar long‐term outcomes.30 In a cohort study of 2471 patients comparing DES vs BMS in SVG PCI at all Veterans Affairs hospitals, second‐generation DES had lower mortality rates in comparison with first‐generation DES, but the difference was not statistically significant.31 In the current RCTs, different types of stents, predominantly first generation, have been used across all trials, and only the DIVA trial used second‐generation DES in the majority of patients (>89%). Whether second‐generation DES are superior to first generation DES in SVG PCIs remains unclear, and further large RCTs are warranted.
4.1. Study limitations
In our study, we observed some limitations with the included RCTs. First, different types of stents were used across trials, which could lead to different results. Therefore, we suggest a larger study that can detect the differences between the different types of DES. Second, the lower‐than‐expected recruitment rate was common in the previous trials. Third, the samples of patients enrolled in the previous trials were small in size (3 trials had ≤80 patients), with only 2 trials enrolling relatively larger number of patients (DIVA enrolled 599 and analyzed 597; Is Drug‐Eluting Stenting Associated With improved Results in Coronary Artery Bypass Grafts? [ISAR‐CABG] enrolled 610).24, 32 Fourth, different trials reported variable embolic protective device utilization (ISAR‐CABG <5% and DIVA >65%).24, 32 These devices might have influenced the outcomes, as they provide additional protection for periprocedural MACE. Fifth, some studies performed routine angiographic follow‐up rather than clinically driven follow‐up, which could increase the rate for detecting restenosis and thus higher repeated revascularization. Finally, there were discrepancies in MACE definition among the trials, which could have affected the interpretation of our results. Trials have used device‐oriented composite (cardiac death, target‐vessel MI, and TLR) and/or patient‐oriented composite (all‐cause death, any MI, and any repeat revascularization) definitions.19 In the future, a more agreed‐upon definition of clinical outcomes is strongly suggested.
The major strength of our study is that we have included only RCTs, which, unlike observational studies, eliminates potential biases. In addition, we analyzed both short‐ and long‐term clinical outcomes of all available RCTs. Such separation could form a valid basis for future trials. One of the main limitations of our study is that some trials have not reported short‐ and long‐term clinical outcomes, which could have led to different results. In addition, individual data were not available in the included trials and, therefore, limited analysis was performed. Although mortality was similar between both groups despite the reduction of short‐term MACE, TLR, and TVR in DES group, it is possible that the longer duration of dual antiplatelet therapy in the DES group might increase the bleeding episodes associated with the increase of mortality. Additionally, the study is still underpowered for significant clinical outcomes. Furthermore, we were not able to generalize our results to all types of DES because only a few studies have used second‐generation DES, which might affect the clinical outcomes of our results. Also, mostly males (~92%) were involved in the included RCTs. Although sex differences are unlikely to affect the clinical outcomes of these RCTs, generalization of the results to females should be examined with caution.
5. CONCLUSION
In our meta‐analysis of patients with SVG lesions treated with PCI, DES were associated with short‐term lower MACE, TLR, and TVR in comparison with BMS. Other clinical outcomes, including all‐cause mortality and stent thrombosis, were not significant between DES and BMS.
ACKNOWLEDGMENTS
The authors thank Katherine Negele, editorial assistant, research department, Hurley Medical Center, for assistance with manuscript editing.
Conflicts of interest
Dr. Mustafa Hassan has received a research grant from Abbott.
The remaining authors report no relationships that could be construed as a conflict of interest.
Kheiri B, Osman M, Abdalla A, Ahmed S, Bachuwa G, Hassan M. The short‐ and long‐term outcomes of percutaneous intervention with drug‐eluting stent vs bare‐metal stent in saphenous vein graft disease: An updated meta‐analysis of all randomized clinical trials. Clin Cardiol. 2018;41:685–692. 10.1002/clc.22908
REFERENCES
- 1. Goldman S, Zadina K, Moritz T, et al; VA Cooperative Study Group #207/297/364 . Long‐term patency of saphenous vein and left internal mammary artery grafts after coronary artery bypass surgery: results from a Department of Veterans Affairs Cooperative Study. J Am Coll Cardiol. 2004;44:2149–2156. [DOI] [PubMed] [Google Scholar]
- 2. Harskamp RE, Lopes RD, Baisden CE, et al. Saphenous vein graft failure after coronary artery bypass surgery: pathophysiology, management, and future directions. Ann Surg. 2013;257:824–833. [DOI] [PubMed] [Google Scholar]
- 3. Hess CN, Lopes RD, Gibson CM, et al. Saphenous vein graft failure after coronary artery bypass surgery: insights from PREVENT IV. Circulation. 2015;132:1445–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Goyal A, Alexander JH, Hafley GE, et al; PREVENT IV Investigators . Outcomes associated with the use of secondary prevention medications after coronary artery bypass graft surgery. Ann Thorac Surg. 2007;83:993–1001. [DOI] [PubMed] [Google Scholar]
- 5. Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Catheter Cardiovasc Interv. 2012;79:453–495. [DOI] [PubMed] [Google Scholar]
- 6. Lee MS, Park SJ, Kandzari DE, et al. Saphenous vein graft intervention. JACC Cardiovasc Interv. 2011;4:831–843. [DOI] [PubMed] [Google Scholar]
- 7. Fitzgibbon GM, Kafka HP, Leach AJ, et al. Coronary bypass graft fate and patient outcome: angiographic follow‐up of 5065 grafts related to survival and reoperation in 1388 patients during 25 years. J Am Coll Cardiol. 1996;28:616–626. [DOI] [PubMed] [Google Scholar]
- 8. Yap C, Sposato L, Akowuah E, et al. Contemporary results show repeat coronary artery bypass grafting remains a risk factor for operative mortality. Ann Thorac Surg. 2009;87:1386–1391. [DOI] [PubMed] [Google Scholar]
- 9. Christenson JT, Schmuziger M, Simonet F. Reoperative coronary artery bypass procedures: risk factors for early mortality and late survival. Eur J Cardiothorac Surg. 1997;11:129–133. [DOI] [PubMed] [Google Scholar]
- 10. Brilakis ES, Wang TY, Rao SV, et al. Frequency and predictors of drug‐eluting stent use in saphenous vein bypass graft percutaneous coronary interventions: a report from the American College of Cardiology National Cardiovascular Data CathPCI registry. JACC Cardiovasc Interv. 2010;3:1068–1073. [DOI] [PubMed] [Google Scholar]
- 11. Roffi M, Mukherjee D, Chew DP, et al. Lack of benefit from intravenous platelet glycoprotein IIb/IIIa receptor inhibition as adjunctive treatment for percutaneous interventions of aortocoronary bypass grafts: a pooled analysis of five randomized clinical trials. Circulation. 2002;106:3063–3067. [DOI] [PubMed] [Google Scholar]
- 12. Laham RJ, Carrozza JP, Berger C, et al. Long‐term (4‐ to 6‐year) outcome of Palmaz‐Schatz stenting: paucity of late clinical stent‐related problems. J Am Coll Cardiol. 1996;28:820–826. [DOI] [PubMed] [Google Scholar]
- 13. Stefanadis C. Are drug‐eluting stents safe in the long term after saphenous vein graft intervention?: lessons learned from real‐world practice. J Am Coll Cardiol. 2014;64:1837–1839. [DOI] [PubMed] [Google Scholar]
- 14. Mosleh W, Gandhi S, Elsiddig M, et al. Comparison of drug‐eluting stents with bare‐metal stents for pci of saphenous vein graft lesions: systematic review and meta‐analysis. J Invasive Cardiol. 2016;28:E139–E169. [PubMed] [Google Scholar]
- 15. Bavishi C, Chatterjee S, Stone GW. Does current evidence favor drug‐eluting stents over bare‐metal stents for saphenous venous graft interventions? Insights from an updated meta‐analysis of randomized controlled trials. JACC Cardiovasc Interv. 2016;9:2456–2458. [DOI] [PubMed] [Google Scholar]
- 16. Brilakis ES. Drug‐Eluting Stents vs. Bare Metal Stents In Saphenous Vein Graft Angioplasty (DIVA). ESC17 presentation slides at: http://www.acc.org/education‐and‐meetings/image‐and‐slide‐gallery/media‐detail?id=2a3e25652ed949e58c5bd415d3ee37a8. Published 2017. Accessed: September 29th, 2017.
- 17. Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta‐analysis protocols (PRISMA‐P) 2015 statement. Syst Rev. 2015;4:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. [DOI] [PubMed] [Google Scholar]
- 19. Cutlip DE, Windecker S, Mehran R, et al; Academic Research Consortium . Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007;115:2344–2351. [DOI] [PubMed] [Google Scholar]
- 20. Sosa A, Chao H, Guerra A, et al. Paclitaxel‐eluting vs. bare metal stent implantation in saphenous vein graft lesions: very long‐term follow‐up of the SOS (Stenting of Saphenous Vein Grafts) trial. Int J Cardiol. 2015;186:261–263. [DOI] [PubMed] [Google Scholar]
- 21. Vermeersch P, Agostoni P, Verheye S, et al; DELAYED RRISC Investigators . Increased late mortality after sirolimus‐eluting stents versus bare‐metal stents in diseased saphenous vein grafts: results from the randomized DELAYED RRISC trial. J Am Coll Cardiol. 2007;50:261–267. [DOI] [PubMed] [Google Scholar]
- 22. Jeger RV, Schneiter S, Kaiser C, et al. Drug‐eluting stents compared with bare metal stents improve late outcome after saphenous vein graft but not after large native vessel interventions. Cardiology. 2009;112:49–55. [DOI] [PubMed] [Google Scholar]
- 23. Brilakis ES, Lichtenwalter C, Abdel‐karim, et al. Continued benefit from paclitaxel‐eluting compared with bare‐metal stent implantation in saphenous vein graft lesions during long‐term follow‐up of the SOS (Stenting of Saphenous Vein Grafts) trial. JACC Cardiovasc Interv. 2011;4:176–182. [DOI] [PubMed] [Google Scholar]
- 24. Mehilli J, Pache J, Abdel‐Wahab M, et al; ISAR‐CABG Investigators . Drug‐eluting versus bare‐metal stents in saphenous vein graft lesions (ISAR‐CABG): a randomised controlled superiority trial [published correction appears in Lancet 2012;379:122]. Lancet. 2011;378:1071–1078. [DOI] [PubMed] [Google Scholar]
- 25. Jeger RV, Farah A, Engstrøm T, et al. Drug‐eluting vs. bare metal stents in saphenous vein grafts: the prospective randomized BASKET‐SAVAGE Trial. Presented at: European Society of Cardiology Congress 2016; August 27–31, 2016; Rome, Italy.
- 26. Bryan AJ, Angelini GD. The biology of saphenous vein graft occlusion: etiology and strategies for prevention. Curr Opin Cardiol. 1994;9:641–649. [DOI] [PubMed] [Google Scholar]
- 27. Camenzind E, Steg PG, Wijns W. Stent thrombosis late after implantation of first‐generation drug‐eluting stents: a cause for concern. Circulation. 2007;116:1440–1455. [DOI] [PubMed] [Google Scholar]
- 28. Brennan JM, Sketch MH Jr, Dai D, et al. Safety and clinical effectiveness of drug‐eluting stents for saphenous vein graft intervention in older individuals: results from the Medicare‐linked National Cardiovascular Data Registry CathPCI Registry (2005–2009). Catheter Cardiovasc Interv. 2016;87:43–49. [DOI] [PubMed] [Google Scholar]
- 29. Bangalore S, Toklu B, Amoroso N, et al. Bare metal stents, durable polymer drug‐eluting stents, and biodegradable polymer drug‐eluting stents for coronary artery disease: mixed treatment comparison meta‐analysis. BMJ. 2013;347:f6625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pokala NR, Menon RV, Patel SM, et al. Long‐term outcomes with first‐ vs. second‐generation drug‐eluting stents in saphenous vein graft lesions. Catheter Cardiovasc Interv. 2016;87:34–40. [DOI] [PubMed] [Google Scholar]
- 31. Aggarwal V, Stanislawski MA, Maddox TM, et al. Safety and effectiveness of drug‐eluting versus bare‐metal stents in saphenous vein bypass graft percutaneous coronary interventions: insights from the Veterans Affairs CART program. J Am Coll Cardiol. 2014;64:1825–1836. [DOI] [PubMed] [Google Scholar]
- 32. Brilakis ES, Banerjee S, Edson R, et al. Rationale and design of the Drug‐Eluting Stents vs Bare‐Metal Stents in Saphenous Vein Graft Angioplasty (DIVA) Trial. Clin Cardiol. 2017;40:946–954. [DOI] [PMC free article] [PubMed] [Google Scholar]


