Summary
Aims
Depression is one of the most common nonmotor symptoms in Parkinson's disease (PD). But the pathogenesis is still unclear. Studies have shown that depression in PD is closely related to the white matter abnormalities, but the number of studies is still very small and lack of whole brain white matter lesions study.
Methods
In this study, we investigated whole brain white matter integrity in 31 depressed PD patients and 37 nondepressed PD patients by diffusion tensor imaging.
Results
There was no difference in age, gender, age of onset, disease duration, Hoehn‐Yahr scale, Unified Parkinson's Disease Rating Scale scores‐III, and Mini‐Mental State Examination scores between the two groups. The only difference was the Hamilton Depression Rating Scale. Depressed PD patients showed reduced fractional anisotropy values in the left anterior corona radiata, left posterior thalamic radiation, left cingulum, left superior longitudinal fasciculus, left sagittal stratum (including inferior longitudinal fasciculus and inferior fronto‐occipital fasciculus), and left uncinate fasciculus. In patients with depression, the Hamilton Depression Rating Scale (HDRS) was negatively correlated with the FA value in the left cingulum (r = −0.712, P = .032) and left superior longitudinal fasciculus (r = −0.699, P = .025).
Conclusions
This study suggested depression in PD was related to impaired white matter integrity especially the long contact fibers in the left hemisphere. These findings may be helpful for further understanding the potential mechanisms underlying depression in PD.
Keywords: depression, diffusion tensor imaging, Parkinson's disease, white matter fibers
1. INTRODUCTION
Parkinson's disease (PD) is one of the most common neurodegenerative disorders, which have typical motor symptoms including resting tremor, rigidity, bradykinesia, and postural reflex abnormalities.1, 2 Increasing studies have shown that most PD patients will experience a series of nonmotor symptoms such as paresthesia, sleep disorders, autonomic dysfunction, neuropsychiatric symptoms, and behavioral disorders.3, 4 Depression is the most common neuropsychiatric symptom, and up to 50% PD patients have suffered from this psychiatric disorder.5 Depressive symptoms can exist in the various stages of the course of PD, but the clinician recognition is still at a low level. Early recognition, diagnosis, and treatment of depression in PD patients will help to improve the quality of life and delay the progress of PD. But till now, the pathophysiological mechanism of depression in PD is still unclear.4
Pathological studies show that PD patients exhibit limbic system degeneration before the onset of dyskinesia, which suggest depressive symptoms can not only be secondary to motor disorders, but also be the initial symptom of PD4 , 6 With the development of neuroimaging, studies have shown that there are abnormalities of neurotransmitters, cortical and subcortical structures, network connections, and white matter fibers in PD with depression.7, 8 Positron emission tomography (PET) has shown a selective decrease in dopamine and noradrenaline innervation in the limbic system in PD with depression (PDD) patients. Magnetic resonance imaging (MRI) shows that there are changes in cortex and subcortical structures in PDD patients, mainly in the prefrontal lobe, temporal lobe, and limbic system. Resting‐state functional MRI (RS‐fMRI) shows that PDD patients have increased spontaneous neural activity in orbitofrontal cortex, while reduced network functional connectivity of the prefrontal‐limbic system. These results suggest the abnormalities of prefrontal‐limbic network may be involved in the complex pathophysiological mechanisms in PDD patients.7, 8
White matter degeneration is very common in PD, and the abnormal fiber connectivity can affect the network connections of the prefrontal‐limbic system and other brain regions.9, 10, 11, 12 Therefore, abnormal white matter fiber may be one of the important pathogenesis of depression in PD. The presence of disturbed emotional network in depression individuals in the general population further supports above assumption.13
Studies have attempted to identify abnormal white matter fibers connections in PDD patients. Using diffusion tensor imaging (DTI), PDD patients show decreased fractional anisotropy (FA) in the anterior cingulate bundle compared with PD without depression.12 The only one study which focuses on the whole brain white matter fibers connections using DTI has shown reduced FA in left uncinate fasciculus, superior longitudinal fasciculus, anterior thalamic radiation, forceps minor, and the inferior longitudinal fasciculus in PDD patients.10 But another study has shown no specific abnormalities in the uncinate fasciculus and corpus callosum in PDD patients,14 which are frequently damaged in major depressive disorder (MDD) patients.15 These inconsistencies are due to the less number of current related researches especially the whole brain white matter fibers study.
Therefore, the aim of this study was to investigate the whole brain white matter fibers integrity in PD patients with or without depression using DTI. The FA value of every region of interest (ROI) was analyzed by an automated approach based on an International Consortium of Brain Mapping (ICBM) template. We hoped to further identify and confirm the white matter fibers defects in PDD patients by a simple and automated method, which may help us to further understand the pathogenesis and diagnosis the depression in PD patients.
2. METHODS
2.1. Participants
A total of 68 PD patients were recruited from the Department of Neurology, Affiliated Drum Tower Hospital of Nanjing University Medical School, from January 2013 to December 2015. Diagnosis of PD was performed according to UK PD Brain Bank criteria by two neurologists experienced in movement disorders. Patients over 75 years old, course of disease greater than 10 years, and Hoehn‐Yahr (H‐Y) scale greater than 4 were excluded. Unipolar depression was diagnosed according to the Diagnostic and Statistical Manual of Mental Disorders fourth edition (DSM‐IV) criteria by an experienced psychiatrist. Patients who had used antidepressants or had significant cognitive impairment or mental disorders were excluded too.
68 PD patients were divided into two groups: PD with depression (PDD, n = 31) and PD with no depression (PDnD, n = 37). All the subjects had signed an informed consent, and this study was approved by ethics committee of the Affiliated Drum Tower Hospital, Nanjing University Medical School. Motor disability was evaluated using the motor part of the Unified Parkinson's Disease Rating Scale (UPDRS‐III) and H‐Y scale, depression was measured by the Hamilton Depression Rating Scale (HDRS), and cognitive impairment was assessed with Mini‐Mental State Examination (MMSE). The clinical assessments and MRI scans were proceeded in the same period, and anti‐parkinsonian medicine was suspended at least 12 hours prior to the measurements. Detailed features of the two groups were showed in Table 1.
Table 1.
Demographical and clinical data
| Index | PDD | PDnD | P value |
|---|---|---|---|
| N | 31 | 37 | ‐ |
| Age (years±SD) | 58.8 ± 8.67 | 59.1 ± 11.4 | 0.902 |
| Age of onset (years±SD) | 55.3 ± 8.26 | 56.7 ± 10.7 | 0.547 |
| Duration (years±SD) | 3.23 ± 3.04 | 2.40 ± 2.53 | 0.229 |
| Male/Female | 18/13 | 23/14 | 0.731 |
| UPDRS‐III | 20.6 ± 11.8 | 17.8 ± 12.8 | 0.355 |
| H‐Y scale | 2.16 ± 1.02 | 1.84 ± 1.00 | 0.216 |
| MMSE | 27.6 ± 1.87 | 27.7 ± 1.80 | 0.937 |
| HDRS | 25.2 ± 3.96 | 5.43 ± 3.99 | <0.001a |
Indicates statistical significant difference (P < 0.01).
PDD, PD with depression; PDnD, PD with no depression; UPDRS‐III, Unified Parkinson's Disease Rating Scale scores‐III; H‐Y scale, Hoehn‐Yahr scale; MMSE, Mini‐Mental State Examination scores; HDRS, Hamilton Depression Rating Scale.
2.2. Diffusion tensor imaging acquisition and data processing
All DTI scans were performed on a 3.0 Tesla MR scanner (Achieva 3.0T TX, Philips Medical Systems, Netherlands), using an 8‐channel head coil. DTI was performed with an echo planar imaging (EPI) sequence with echo time (TE)/repetition time (TR)=55/8400 msec, 32 diffusion‐sensitive gradient directions (b = 1000 msec/mm2), in‐plane resolution of 2.0 mm, and a slice thickness of 2.5 mm. All participants were scanned on the same MR scanner.
Diffusion tensor imaging data analysis was performed by a pipeline toolbox for analyzing brain diffusion images (PANDA).16 The main procedure includes: (i) correcting for the eddy current effect and calculating diffusion tensor (DT) metrics using the flirt and dtifit command of FSL, respectively; (ii) normalizing: registrations of all the individual FA images to the FMRIB58_FA template by calling the fnirt command of FSL; (iii) output for atlas‐based analysis: calculating the regional diffusion metrics by averaging the FA values within each region of the International Consortium of Brain Mapping template (ICBM‐DTI‐81).17 Visualization of the results was performed by the BrainNet Viewer.18
2.3. Statistical analysis
Statistical analysis was performed using SPSS version 17. Descriptive data were presented as mean ± standard deviations. Categorical data were analyzed using chi‐square test. Comparison of continuous data between two groups was performed using Student's t test. Analysis of variance was used to compare the FA difference between depression and nondepression groups of PD patients with UPDRS‐III and MMSE as covariate. Correlations between the FA values and HRDS in PD with depression patients were examined using Pearson correlation. P < 0.05 was considered as statistically significant.
3. RESULTS
3.1. Demographical and clinical data
As shown in Table 1, 68 PD patients were divided into PD with depression (PDD, n = 31) and PD with no depression (PDnD, n = 37). There were no significant differences in age, age of onset, disease duration, or gender between the two groups. Clinical assessments including UPDRS‐III, H‐Y scale, and MMSE had also shown no difference between the two groups. The only difference between the two groups was the HDRS (P < 0.001). All patients were right‐handed and with right onset.
3.2. FA changes in different brain regions
In this study, we defined the ROIs based on the Johns Hopkins University (JHU) White Matter (WM) tractography atlas.17 The FA values of every ROI were measured by PANDA.16 Compared to the PDnD group, PDD patients showed lower FA values in several brain regions including left anterior corona radiata, left posterior thalamic radiation, left cingulum, left superior longitudinal fasciculus, left sagittal stratum (including inferior longitudinal fasciculus and inferior fronto‐occipital fasciculus), and left uncinate fasciculus (Table 2 and Figures 1 and 2). In these six regions of the right hemisphere, there was no significant difference between the two groups (Table 2).
Table 2.
FA values between groups of different regions
| Regions | Hemisphere | PDD (n = 31) | PDnD (n = 37) | F | P value |
|---|---|---|---|---|---|
| Anterior corona radiata | L | 0.670 ± 0.203 | 0.780 ± 0.121 | 4.68 | 0.033a |
| R | 0.725 ± 0.210 | 0.777 ± 0.119 | 4.35 | 0.264 | |
| Posterior thalamic radiation | L | 0.720 ± 0.135 | 0.806 ± 0.096 | 5.15 | 0.01a |
| R | 0.773 ± 0.151 | 0.799 ± 0.106 | 5.11 | 0.473 | |
| Cingulum | L | 0.686 ± 0.157 | 0.785 ± 0.115 | 4.74 | 0.025a |
| R | 0.716 ± 0.168 | 0.742 ± 0.120 | 3.71 | 0.25 | |
| Superior longitudinal fasciculus | L | 0.694 ± 0.151 | 0.776 ± 0.107 | 5.01 | 0.027a |
| R | 0.728 ± 0.161 | 0.757 ± 0.115 | 4.67 | 0.462 | |
| Sagittal stratum | L | 0.717 ± 0.147 | 0.797 ± 0.105 | 4. 68 | 0.027a |
| R | 0.771 ± 0.155 | 0.802 ± 0.109 | 4.50 | 0.407 | |
| Uncinate fasciculus | L | 0.598 ± 0.150 | 0.695 ± 0.115 | 4.34 | 0.045a |
| R | 0.634 ± 0.168 | 0.647 ± 0.115 | 5.54 | 0.728 |
Indicates statistical significant difference (P < .05).
PDD, PD with depression; PDnD, PD with no depression; L, left; R, Right.
Figure 1.
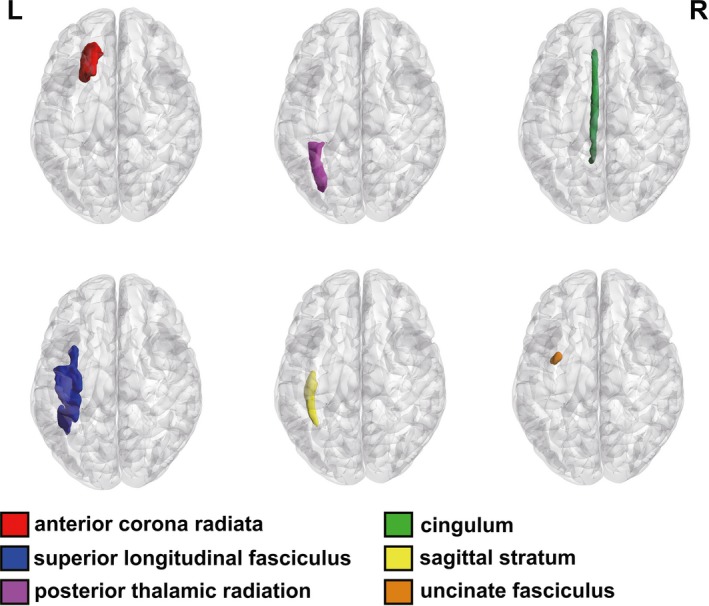
Regional patterns of FA changes in PD with depression. (Left anterior corona radiata, left posterior thalamic radiation, left cingulum, left superior longitudinal fasciculus, left sagittal stratum, and left uncinate fasciculus. L, Left; R, Right)
Figure 2.
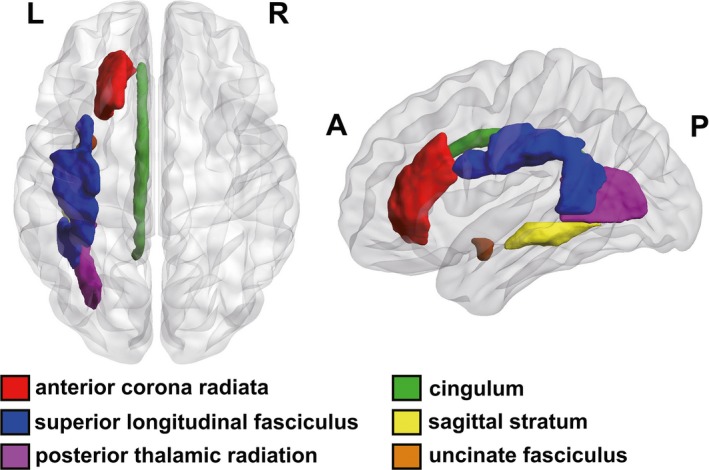
Localization of FA change regions in depressed PD patients. (Left anterior corona radiata, left posterior thalamic radiation, left cingulum, left superior longitudinal fasciculus, left sagittal stratum, and left uncinate fasciculus. L, Left; R, Right; A, Anterior; P, Posterior)
3.3. Correlation between FA values and disease characteristics
Correlation analysis showed that the FA values in the left cingulum (r = −0.712, P = .032) and left superior longitudinal fasciculus (r = −0.699, P = .025) of the PDD group were negatively correlated with HDRS scores (Table 3), but no correlation was found with other disease characteristics including age, duration, UPDRS‐III, H‐Y scale, and MMSE. After controlling for age, duration, UPDRS‐III, H‐Y scale, and MMSE, the negative correlation mentioned above was still significant.
Table 3.
Correlation analysis between FA values and HDRS scores
| Regions | r | P value |
|---|---|---|
| Left anterior corona radiata | −0.309 | 0.203 |
| Left posterior thalamic radiation | −0.234 | 0.563 |
| Left cingulum | −0.712 | 0.032a |
| Left superior longitudinal fasciculus | −0.699 | 0.025a |
| Left sagittal stratum | −0.464 | 0.309 |
| Left uncinate fasciculus | −0.541 | 0.207 |
Indicates statistical significant difference (P < .05).
FA, fractional anisotropy; HDRS, Hamilton Depression Rating Scale.
4. DISCUSSION
In this study, we explored the association between depressive symptoms and whole brain white matter lesions in PD patients using DTI technology. Results showed depressed PD patients exhibited degeneration of several fibers including left anterior corona radiata, left posterior thalamic radiation, left cingulum, left superior longitudinal fasciculus, left sagittal stratum (including inferior longitudinal fasciculus and inferior fronto‐occipital fasciculus), and left uncinate fasciculus. And the degree of degeneration in left cingulum and left superior longitudinal fasciculus was positively associated with the severity of depression.
Depression is one of the most common mood disturbances in PD, but its pathogenesis is unclear.4 Studies using advanced neuroimaging generally suggest increased neural activity in the prefrontal regions and decreased functional connectivity between the prefrontal‐limbic networks in depressed PD patients.7 Most of these studies have adopted position emission tomography, single‐photon emission computed tomography, structural MR, or RS‐fMRI. Given the dysfunction of the prefrontal‐limbic network connectivity may be one of the most important pathogenesis involved in PD with depression, DTI, the only noninvasive method to detect, track, and display the white matter tracts in vivo, might help us better to explore the pathogenesis. But to the best of our knowledge, there are only four relevant research using DTI and only one report on the study of whole brain white matter integrity was reported.
So, our study investigated the whole brain white matter fibers lesions in depressed PD patients using DTI and the DTI data were postprocessed by PANDA based on the JHU WM tractography atlas. PANDA is a pipeline toolbox for fully automated analyzing brain diffusion images which can help us analyze DTI data faster and better.16 The JHU WM tractography atlas is well established for parcellation of the entire WM into multiple ROIs automatically. This WM atlas in the standard space may provide better statistical sensitivity and accuracy.17 Therefore, this was a new study which focused on whole brain WM fibers in depressed PD patients by a more convenient and reliable DTI analyzation method.
Previous DTI studies have shown WM fibers lesions in depressed PD patients, such as the bilateral anterior cingulate cortex, bilateral mediodorsal thalamic areas, and multiple tracts connecting to the left frontal and deep temporal lobes including left uncinate fasciculus, left superior and inferior longitudinal fasciculus, left anterior thalamic radiation, and left forceps minor.10, 11, 12 In this study, we also found WM fibers degeneration in left cingulum, left superior and inferior longitudinal fasciculus, and left uncinate fasciculus in PD with depression patients. All of the above regions belong to the long contact WM fibers in the brain. These findings further confirmed that the dysfunction of the prefrontal‐limbic networks may be the vital pathological basis in PD with depression patients.
Studies using T1‐weighted imaging have shown the depressed PD patients had decreased gray matter (GM) volumes in the prefrontal, parietal, and insular regions as well as the limbic system (anterior cingulate cortices and amygdala).19, 20, 21, 22, 23 These structural abnormalities were found to further support our DTI findings. As we know, the prefrontal cortex and limbic systems are the main brain regions for emotion regulation. The prefrontal cortex connects with various sensory areas, such as the visual and the somatic sensory cortex. Neurons in the prefrontal network are responsible for responding to multimodal sensory stimuli and assessing these stimuli.24 Malfunction of this area may cause disturbance in decision making and evaluation of emotional stimuli, which may give rise to various psychotic disorders such as depression.25, 26 Similarly, abnormalities of the WM fibers that connect the prefrontal cortex to each region of the brain especially the limbic systems can also cause abnormal assessment of mood. In primary depression, DTI studies also have found significant correlations between depression and altered integrity of white matter tracts that contribute to emotional regulation,27, 28 such as the superior longitudinal fasciculus, corpus callosum, uncinate fasciculus, internal and external capsule, cingulum and anterior corona radiata, thalamic projection fibers, and other association fibers in the limbic system.29, 30 Compared to our findings in depressed PD patients, white matter damage is more extensive in patients with primary depression, but all these impaired WM regions or fibers mainly located in the prefrontal cortex, limbic system, and the contact fibers between them. Studies using RS‐fMRI have also shown decreased functional connectivity between the prefrontal‐limbic networks31, 32, 33 and impaired interhemispheric synchrony34 in PD with depression patients. Therefore, the abnormal sensory integration and evaluation caused by white matter deficits which we found might be an important reason for depression in PD.
In addition to the above findings of long contact WM fibers abnormalities, we also found degeneration of left anterior corona radiata and left posterior thalamic radiation in depressed PD patients. These two degenerated fibers have been found in primary depression29, 30 but were not reported in previous DTI studies in PD. Corona refers to the radial white matter between the internal capsule and the cerebral cortex. The posterior thalamic radiation is a bundle of fibers projecting from the thalamus to the occipital lobe. They connect with multiple sensory areas, including somatosensory cortex and visual cortex. Degeneration of these two fibers can also cause disturbance of sensory integration and evaluation, which might give rise to depression.25, 26
However, one study reported no difference of the FA in the corpus callosum and uncinate fasciculus in PD with depression patients.14 This inconsistency may be due to the small sample size (6 PDD and 6 PDnD patients) and mild depressive symptoms. More importantly, this study adopted ROI strategy rather than the whole brain WM evaluation and this may add to selection bias of the brain regions. In our study, a larger sample size and better whole brain WM evaluation method enabled us to reveal the WM degenerations and the results were basically consistent with other DTI studies.
Furthermore, we found the degree of degeneration in left cingulum and left superior longitudinal fasciculus was positively associated with the severity of depression. This result was different to the previous DTI studies which have shown the inverse correlation between depression and FA values mainly in left deep temporal cortex10 or mediodorsal thalamic areas.11 Studies also found inverse correlation between depression and WM or GM volume mainly in orbitofrontal, temporal, and limbic regions.19, 20, 23 But cingulum and superior longitudinal fasciculus are important long contact fibers connecting prefrontal cortex and limbic system. Based on the critical role of communication in prefrontal‐limbic networks for assessing stimuli and regulating emotions, the more severe degeneration in these fibers might cause more serious depression. The similar results have been reported in major depressive disorder.35 In PD, RS‐fMRI studies have shown depression was inversely correlated with functional connectivity between the amygdala and prefrontal and posterior cingulate cortices.32, 33 These findings further support our results.
In this study, we also found degenerated fibers mainly in the left hemisphere. These results were consistent with previous reports.10 Previous studies have shown that depression and anxiety are associated with prefrontal dysfunction, especially in the left prefrontal lobe.36 Epidemiological studies have also shown that PD patients with right onset are more likely to suffer from depression.37 In this study, all PD patients were right‐handed and with right onset. The above left hemisphere WM fiber abnormalities may be related to the right onset and may also be the inherent characteristics of PD with depression. These uncertainties need our further studies to confirm.
5. CONCLUSION
In summary, our study showed impaired long contact fiber integrity in white matter was related to depression in PD by a more convenient and reliable whole brain WM DTI analyzation method. But the pathogenesis of depression in PD is very complicated and the mechanisms are still need to be explored. Imaging study is noninvasive, and it provides us an objective idea of the possible connectivity between symptoms and the brain structures. However, the imaging features are not unique and studies using DTI on depression in PD are still scarce. We are in urgent need of more larger samples and more comprehensive researches to reveal the unique features or imaging markers in depressed PD patients. These findings may underlie the neural mechanisms of depression in PD and contribute to the diagnosis and treatment of depression in PD.
CONFLICT OF INTEREST
The authors declared no potential conflict of interests with respect to the authorship and/or publication of this article.
ACKNOWLEDGMENT
This work was supported by the National Natural Science Foundation of China (Grant No. 81301074, 81230026, 81630028), the Natural Science Foundation (BE2016610) of Jiangsu Province of China, and Jiangsu Provincial Key Medical Discipline. The National Key Research and Development Program of China (2016YFC1300500).
Wu J‐Y, Zhang Y, Wu W‐B, Hu G, Xu Y . Impaired long contact white matter fibers integrity is related to depression in Parkinson's disease. CNS Neurosci Ther. 2018;24:108–114. 10.1111/cns.12778
The first two authors contributed equally to this work.
Contributor Information
Gang Hu, Email: ghu@njmu.edu.cn.
Yun Xu, Email: xuyun20042001@aliyun.com.
REFERENCES
- 1. Marti MJ, Tolosa E. Parkinson disease: new guidelines for diagnosis of Parkinson disease. Nat Rev Neurol. 2013;9:190‐191. [DOI] [PubMed] [Google Scholar]
- 2. Chaudhuri KR, Healy DG, Schapira AH. National institute for clinical E. non‐motor symptoms of Parkinson's disease: diagnosis and management. Lancet Neurol. 2006;5:235‐245. [DOI] [PubMed] [Google Scholar]
- 3. Gallagher DA, Schrag A. Psychosis, apathy, depression and anxiety in Parkinson's disease. Neurobiol Dis. 2012;46:581‐589. [DOI] [PubMed] [Google Scholar]
- 4. Rana AQ, Ahmed US, Chaudry ZM, Vasan S. Parkinson's disease: a review of non‐motor symptoms. Expert Rev Neurother. 2015;15:549‐562. [DOI] [PubMed] [Google Scholar]
- 5. Reijnders JS, Ehrt U, Weber WE, Aarsland D, Leentjens AF. A systematic review of prevalence studies of depression in Parkinson's disease. Mov Disord. 2008;23:183‐189; quiz 313. [DOI] [PubMed] [Google Scholar]
- 6. Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging. 2003;24:197‐211. [DOI] [PubMed] [Google Scholar]
- 7. Wen MC, Chan LL, Tan LC, Tan EK. Depression, anxiety, and apathy in Parkinson's disease: insights from neuroimaging studies. Eur J Neurol. 2016;23:1001‐1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chagas MH, Linares IM, Garcia GJ, Hallak JE, Tumas V, Crippa JA. Neuroimaging of depression in Parkinson's disease: a review. Int Psychogeriatr. 2013;25:1953‐1961. [DOI] [PubMed] [Google Scholar]
- 9. Gattellaro G, Minati L, Grisoli M, et al. White matter involvement in idiopathic Parkinson disease: a diffusion tensor imaging study. AJNR Am J Neuroradiol. 2009;30:1222‐1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Huang P, Xu X, Gu Q, et al. Disrupted white matter integrity in depressed versus non‐depressed Parkinson's disease patients: a tract‐based spatial statistics study. J Neurol Sci. 2014;346:145‐148. [DOI] [PubMed] [Google Scholar]
- 11. Li W, Liu J, Skidmore F, Liu Y, Tian J, Li K. White matter microstructure changes in the thalamus in Parkinson disease with depression: a diffusion tensor MR imaging study. Am J Neuroradiol. 2010;31:1861‐1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Matsui H, Nishinaka K, Oda M, et al. Depression in Parkinson's disease. Diffusion tensor imaging study. J Neurol. 2007;254:1170‐1173. [DOI] [PubMed] [Google Scholar]
- 13. Liao Y, Huang X, Wu Q, et al. Is depression a disconnection syndrome? Meta‐analysis of diffusion tensor imaging studies in patients with MDD. J Psychiatry Neurosci. 2013;38:49‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Surdhar I, Gee M, Bouchard T, Coupland N, Malykhin N, Camicioli R. Intact limbic‐prefrontal connections and reduced amygdala volumes in Parkinson's disease with mild depressive symptoms. Parkinsonism Relat Disord. 2012;18:809‐813. [DOI] [PubMed] [Google Scholar]
- 15. Zhang A, Leow A, Ajilore O, et al. Quantitative tract‐specific measures of uncinate and cingulum in major depression using diffusion tensor imaging. Neuropsychopharmacology. 2012;37:959‐967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cui Z, Zhong S, Xu P, He Y, Gong G. PANDA: a pipeline toolbox for analyzing brain diffusion images. Front Hum Neurosci. 2013;7:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mori S, Oishi K, Jiang H, et al. Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. NeuroImage. 2008;40:570‐582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Xia M, Wang J, He Y. BrainNet Viewer: a network visualization tool for human brain connectomics. PLoS ONE. 2013;8:e68910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Feldmann A, Illes Z, Kosztolanyi P, et al. Morphometric changes of gray matter in Parkinson's disease with depression: a voxel‐based morphometry study. Mov Disord. 2008;23:42‐46. [DOI] [PubMed] [Google Scholar]
- 20. Kostic VS, Agosta F, Petrovic I, et al. Regional patterns of brain tissue loss associated with depression in Parkinson disease. Neurology. 2010;75:857‐863. [DOI] [PubMed] [Google Scholar]
- 21. Cardoso EF, Maia FM, Fregni F, et al. Depression in Parkinson's disease: convergence from voxel‐based morphometry and functional magnetic resonance imaging in the limbic thalamus. NeuroImage. 2009;47:467‐472. [DOI] [PubMed] [Google Scholar]
- 22. Gyorfi O, Nagy H, Bokor M, et al. Reduced CA2‐CA3 Hippocampal subfield volume is related to depression and normalized by l‐DOPA in newly diagnosed Parkinson's disease. Front Neurol. 2017;8:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. van Mierlo TJ, Chung C, Foncke EM, Berendse HW, van den Heuvel OA. Depressive symptoms in Parkinson's disease are related to decreased hippocampus and amygdala volume. Mov Disord. 2015;30:245‐252. [DOI] [PubMed] [Google Scholar]
- 24. Rolls ET. Convergence of sensory systems in the orbitofrontal cortex in primates and brain design for emotion. Anat Rec A Discov Mol Cell Evol Biol. 2004;281:1212‐1225. [DOI] [PubMed] [Google Scholar]
- 25. Bechara A, Damasio H, Damasio AR. Emotion, decision making and the orbitofrontal cortex. Cereb Cortex. 2000;10:295‐307. [DOI] [PubMed] [Google Scholar]
- 26. Berlin HA, Rolls ET, Iversen SD. Borderline personality disorder, impulsivity, and the orbitofrontal cortex. Am J Psychiatry. 2005;162:2360‐2373. [DOI] [PubMed] [Google Scholar]
- 27. Murphy ML, Frodl T. Meta‐analysis of diffusion tensor imaging studies shows altered fractional anisotropy occurring in distinct brain areas in association with depression. Biol Mood Anxiety Disord. 2011;1:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wang L, Leonards CO, Sterzer P, Ebinger M. White matter lesions and depression: a systematic review and meta‐analysis. J Psychiatr Res. 2014;56:56‐64. [DOI] [PubMed] [Google Scholar]
- 29. Kieseppä T, Eerola M, Mäntylä R, et al. Major depressive disorder and white matter abnormalities: a diffusion tensor imaging study with tract‐based spatial statistics. J Affect Disord. 2010;120:240‐244. [DOI] [PubMed] [Google Scholar]
- 30. Korgaonkar MS, Grieve SM, Koslow SH, Gabrieli JD, Gordon E, Williams LM. Loss of white matter integrity in major depressive disorder: evidence using tract‐based spatial statistical analysis of diffusion tensor imagin. Hum Brain Mapp. 2011;32:2161‐2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hu X, Song X, Yuan Y, et al. Abnormal functional connectivity of the amygdala is associated with depression in Parkinson's disease. Mov Disord. 2015;30:238‐244. [DOI] [PubMed] [Google Scholar]
- 32. Lou Y, Huang P, Li D, et al. Altered brain network centrality in depressed Parkinson's disease patients. Mov Disord. 2015;30:1777‐1784. [DOI] [PubMed] [Google Scholar]
- 33. Huang P, Xuan M, Gu Q, et al. Abnormal amygdala function in Parkinson's disease patients and its relationship to depression. J Affect Disord. 2015;183:263‐268. [DOI] [PubMed] [Google Scholar]
- 34. Zhu Y, Song X, Xu M, et al. Impaired interhemispheric synchrony in Parkinson's disease with depression. Sci Rep. 2016;6:27477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Won E, Choi S, Kang J, et al. Association between reduced white matter integrity in the corpus callosum and serotonin transporter gene DNA methylation in medication‐naive patients with major depressive disorder. Transl Psychiatry. 2016;6:e866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hama S, Yamashita H, Shigenobu M, et al. Post‐stroke affective or apathetic depression and lesion location: left frontal lobe and bilateral basal ganglia. Eur Arch Psychiatry Clin Neurosci. 2007;257:149‐152. [DOI] [PubMed] [Google Scholar]
- 37. Foster PS, Drago V, Crucian GP, et al. Anxiety and depression severity are related to right but not left onset Parkinson's disease duration. J Neurol Sci. 2011;305:131‐135. [DOI] [PubMed] [Google Scholar]


