Abstract
Background
Obesity can lead to increased oxidative stress which is one of the proposed mechanisms in the etiopathogenesis of takotsubo cardiomyopathy (TCM).
Hypothesis
The presence of obesity adversely impacts clinical outcomes in TCM patients.
Methods
We queried the Nationwide Inpatient Sample database (2010‐2014) to identify adult patients admitted with a principal diagnosis of TCM with and without obesity. We compared the categorical and continuous variables by Pearson χ2 and Student t test, respectively, in propensity‐score matched cohorts.
Results
The study cohort comprised 612 obese TCM (weighted n = 3034) and 5696 nonobese TCM (weighted n = 28 186) patients. Obese TCM patients were more often younger and private‐insurance enrollees. Cardiac complications including acute myocardial infarction (9.0% vs 7.4%; P = 0.04), cardiac arrest (2.3% vs. 0.4%; P < 0.001), cardiogenic shock (4.3% vs. 3.2%; P = 0.03), congestive heart failure (5.0% vs. 3.8%; P = 0.02), respiratory failure (12.9% vs. 11.0%; P = 0.021) and use of mechanical hemodynamic support (Impella; 0.2% vs. 0.0%, P = 0.02) were significantly higher among obese TCM patients. There were no significant differences between the 2 groups in all‐cause mortality (1.0% vs. 0.8%; P = 0.35), arrhythmia (24.5% vs. 22.7%, P = 0.123), length of stay (3.7 ±3.5 vs. 3.7 ±3.6 days; P = 0.68), and total hospital charges ($40 780.16 vs. $42 575.14; P = 0.08).
Conclusions
Obese TCM patients were more susceptible to developing TCM‐related cardiac complications than were nonobese TCM patients, without any impact on all‐cause in‐hospital mortality, LOS, and hospital charges.
Keywords: Apical Ballooning Syndrome, Body Mass Index, Cardiovascular Complications, Mortality, Obesity, Outcome, Stress Cardiomyopathy, Takotsubo Cardiomyopathy
1. INTRODUCTION
Takotsubo cardiomyopathy (TCM), also known as acute stress cardiomyopathy, broken‐heart syndrome, or apical ballooning syndrome, is characterized by a reversible apical left ventricular dysfunction in the absence of coronary artery disease. This condition has gained worldwide recognition after it was first described in Japan in 1990.1 There was a 3‐fold increase in hospitalizations for TCM in the United States from 2007 through 2012.2 A recent nationwide study reported a nearly 10% 30‐day readmission rate and an alarming $112 million cost of healthcare among TCM patients.3 Although the main etiology of TCM is not recognized yet, it has been shown to have a temporal relationship with an extremely emotional or physically stressful event. Once considered a benign condition,4 TCM has recently been linked to short‐ and long‐term comorbidities similar to acute coronary syndrome,5, 6, 7, 8 such as coronary artery disease, cardiogenic shock, atrial fibrillation, left bundle branch block, hypertension, hypercholesterolemia, diabetes mellitus, and others. In the light of these findings, a better understanding of the predictors of the outcomes in TCM is warranted.
Existing data suggest that obesity leads to the oxidative stress, which is significantly associated with the increased risk of development of cardiovascular (CV) diseases. There is limited data available on the impact of obesity on the TCM outcomes.9 The objective of this study was to compare the outcomes of TCM in obese vs nonobese patients by using the Nationwide Inpatient Sample database (NIS), which is the largest publicly accessible database in the United States (US).
2. METHODS
2.1. Study design and data source
This was a retrospective cohort study in the US by using the NIS database for the years 2010 to 2014. The NIS is maintained by the Agency for Healthcare Research and Quality (AHRQ) as a part of Healthcare Cost and Utilization Project (HCUP) and comprised an estimated 7 million to 8 million unweighted discharges each year.10 The NIS is a stratified 20% section of discharges from the community hospitals drawn from 44 states participating in HCUP, representing >95% of the US population. The NIS database includes 1 primary discharge diagnosis and up to 24 secondary discharge diagnoses, along with comorbidities already identified on admission, procedures performed, and complications occurring after admission. Discharge weights provided in the database were applied to achieve the national estimates. Detailed information on the self‐weight design of NIS is available at https://www.hcup-us.ahrq.gov/db/nation/nis/nisdbdocumentation.jsp.
International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM) codes were utilized to identify the disease, comorbidities, and outcomes. The requisite for informed consent and institutional review board approval was waived due to the anonymous and de‐identified nature of the data.
2.2. Patient selection and study setting
Patients age ≥ 18 years admitted with a principal diagnosis of TCM between January 2010 and December 2014 using ICD‐9 CM code 429.83 were included in the study.2, 11 The patients were excluded if they developed TCM after admission, if TCM was noted as one of the secondary discharge diagnoses, or if they had missing information such as age and weight. Eligible patients were further stratified into two study cohorts: obese vs nonobese. Obese patients were identified using the following ICD‐9‐CM codes defined in Elixhauser comorbidity classification software: 278.0, 278.00, 278.01, 278.03, 649.10‐649.14, and 793.91, V85.30‐V85.39, V85.41‐V85.45, and V85.54, which are inclusive of body mass index (BMI) >30 kg/m2 (Figure 1).12
Figure 1.
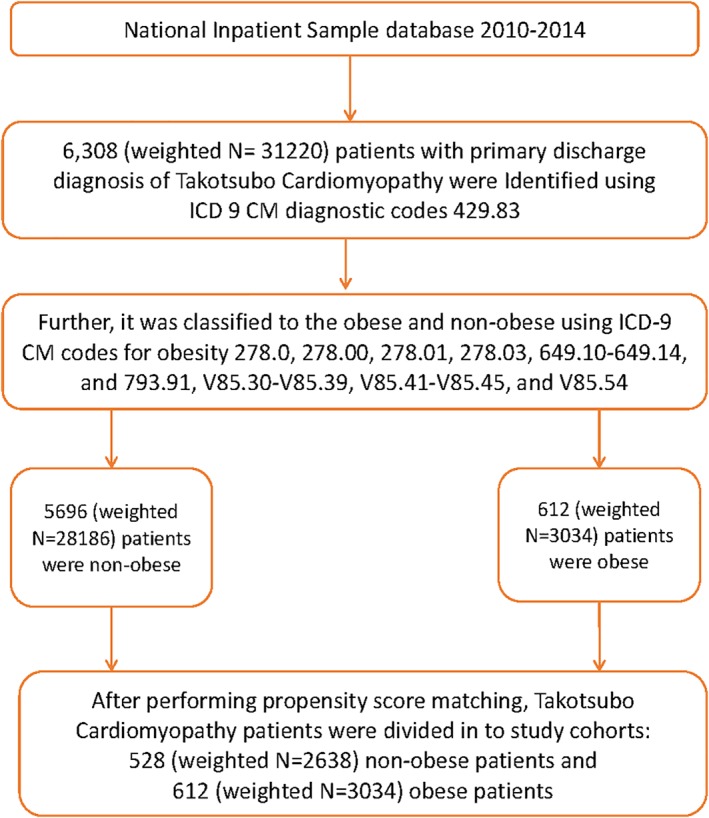
The study population selection algorithm. Abbreviations: ICD‐9‐CM, International Classification of Diseases, Ninth Revision, Clinical Modification
2.3. Variables
We acquired and compared the data between the obese vs nonobese groups on the baseline demographics, hospital characteristics, comorbidities provided in the database, and development of complications. Most of the patient and hospital‐level characteristics were directly extracted as provided in the NIS, whereas the AHRQ comorbidity measurements were used to identify the comorbid disorders. We queried the TCM cases for complications using ICD‐9‐CM codes in any of the secondary diagnoses fields (see Supporting Information, Table S1, in the online version of this article).
2.4. Outcomes
The primary outcomes were defined as the development of the all‐cause in‐hospital mortality, and other complications including acute myocardial infarction (AMI), arrhythmia, cardiogenic shock, cardiac arrest, mechanical circulatory support, venous thromboembolism, and respiratory failure. The secondary outcomes were the length of hospital stay (LOS) in days and total hospital charges.
2.5. Statistical analyses
Categorical variables were presented as the numbers and proportions using the χ2 test. The continuous variables were presented as the mean and SD and were matched using the Student t test. Discharge weights provided by the HCUP were applied to get the national estimates.
To reduce the selection bias with the unmatched cohort, we performed a propensity score‐matched analysis of the obese vs. non‐obese TCM patients. We built multivariate logistic regression model after adjusting for the age, sex, race, median household income, hospital region, relevant CV comorbidities to measure a propensity score. The subsequent individually matched propensity score was used for nearest neighbor matching of both the groups (obese vs. nonobese) with caliper match tolerance of 0.01. Later, we compared and tabulated the TCM outcomes between both obese and nonobese groups in the propensity score‐matched cohort. More than 5% post‐matching difference between the two groups was considered the clinically significant difference. SPSS Statistics software, version 22.0 (IBM Corp., Armonk, NY) was utilized to perform all statistical analyses, and P ≤ 0.05 was considered significant.
3. RESULTS
3.1. Baseline characteristics
A total of 6308 (weighted n = 31 220) patients were discharged with a primary diagnosis of TCM during the study period from January 2010 to December 2014. Out of these, nearly 9.7% patients were obese. The study cohorts comprised 612 (weighted 3034) obese and 5696 nonobese (weighted 28 186) patients. Interestingly, the obese TCM cohort was younger (62.1 ±12.2 vs. 66.7 ±13.0 years; P < 0.001). The majority of the patients with TCM were white (81.4%), and a large number of them were female (93.9%). Compared with the nonobese cohort, the unmatched obese cohort consisted of a higher frequency of females (93.9% vs 91.2%; P < 0.001), African Americans (8.1% vs5.9%; P < 0.001), Hispanics (7.4% vs 6.0%; P ≤ 0.001), private payer (35.2% vs 28.9%; P < 0.001), lower income quartile (25.5% vs 23.3%; P < 0.001), and admitted to small and medium‐size hospitals (Table 1). Although in both the groups, the frequency of weekend (77.1% vs 76.8%; adjusted P = 0.68) and nonelective admissions (94.5% vs 94.2%; adjusted P = 0.44) was high, it was not statistically significant. After propensity score matching, matched cohorts of 612 (weighted 3034) obese vs. 528 (weighted 2638) nonobese patients were comparable in regard to sex, race, and hospital size. The groups also varied in terms of the patients' age, admission day/type, primary payer status, household income, hospital owner type, and region, with the standardized difference of <10% between both groups.
Table 1.
Baseline characteristics of obese vs nonobese TCM patients
| Variables | Unmatched Cohort (n = 6308, Weighted n = 31 220) | Propensity‐Matched Cohort (n = 1140, Weighted n = 5672) | ||||
|---|---|---|---|---|---|---|
| Nonobese | Obese | P Value | Nonobese | Obese | P Value | |
| Unweighted admission | 5696 | 612 | 528 | 612 | ||
| Weighted admission | 28 186 | 3034 | 2638 | 3034 | ||
| Mean age, y | 66.7 ± 13.0 | 62.1 ± 12.2 | <0.001 | 64.5 ± 12.9 | 62.1 ±12.2 | <0.001 |
| Age group, y | ||||||
| 18‐44 | 4.7 | 8.7 | 1.1 | 0.4 | ||
| 45‐64 | 37.1 | 46.8 | 4.0 | 6.3 | ||
| 65‐84 | 50.8 | 42.7 | 30.0 | 28.5 | ||
| ≥ 85 | 7.4 | 1.8 | 64.9 | 64.8 | ||
| Sex | <0.001 | 0.380 | ||||
| M | 8.8 | 6.1 | 6.6 | 6.1 | ||
| F | 91.2 | 93.9 | 93.4 | 93.9 | ||
| Admission day | 0.684 | 0.017 | ||||
| Weekend | 76.8 | 77.1 | 79.7 | 77.1 | ||
| Weekday | 23.2 | 22.9 | 20.3 | 22.9 | ||
| Type of admission | 0.446 | 0.001 | ||||
| Nonelective | 94.2 | 94.5 | 96.4 | 94.5 | ||
| Elective | 5.8 | 5.5 | 3.6 | 5.5 | ||
| Race | <0.001 | 0.068 | ||||
| White | 83.0 | 81.4 | 81.8 | 81.4 | ||
| American African | 5.9 | 8.1 | 9.1 | 8.1 | ||
| Hispanic | 6.0 | 7.4 | 5.9 | 7.4 | ||
| Asian and Pacific Islander | 1.8 | 0.4 | 0.4 | 0.4 | ||
| Native American | 0.5 | 0.7 | 0.4 | 0.7 | ||
| Others | 2.9 | 2.0 | 2.5 | 2.0 | ||
| Primary payer | <0.001 | <0.001 | ||||
| Medicare | 58.0 | 50.2 | 55.3 | 50.2 | ||
| Medicaid | 6.2 | 7.9 | 8.7 | 7.9 | ||
| Private including HMO | 28.9 | 35.2 | 29.3 | 35.2 | ||
| Self‐pay/no charge/other | 6.9 | 6.7 | 6.7 | 6.7 | ||
| Hospital characteristics | ||||||
| Median HHI categorya (percentiles) | <0.001 | <0.001 | ||||
| 0–25th | 23.3 | 25.5 | 21.0 | 25.5 | ||
| 26–50th | 25.6 | 29.7 | 25.3 | 29.7 | ||
| 51–75th | 25.9 | 24.3 | 26.5 | 24.3 | ||
| 76–100th | 25.2 | 20.6 | 27.1 | 20.6 | ||
| Hospital bed sizeb | <0.001 | 0.153 | ||||
| Small | 9.6 | 10.4 | 11.7 | 10.4 | ||
| Medium | 23.4 | 28.3 | 29.2 | 28.3 | ||
| Large | 67.0 | 61.3 | 59.1 | 61.3 | ||
| Control/ownership of hospital | <0.001 | <0.001 | ||||
| Government, nonfederal | 8.3 | 6.3 | 4.4 | 6.3 | ||
| Private, nonprofit | 80.9 | 83.7 | 89.8 | 83.7 | ||
| Private, investor‐owned | 10.8 | 10.0 | 5.8 | 10.0 | ||
| Hospital region | <0.001 | <0.001 | ||||
| Northeast | 19.6 | 15.3 | 47.2 | 15.3 | ||
| Midwest | 25.3 | 31.0 | 29.4 | 31.0 | ||
| South | 32.0 | 31.7 | 17.0 | 31.7 | ||
| West | 23.1 | 22.1 | 6.4 | 22.1 | ||
Abbreviations: F, female; HHI, household income; M, male; SD, standard deviation; TCM, takotsubo cardiomyopathy.
Data are presented as % or mean ± SD.
Represents a quartile classification of the estimated median HHI of residents in the patient's ZIP code. The quartiles are identified by values of 1 to 4, indicating the poorest to wealthiest populations. Derived from https://www.hcup‐us.ahrq.gov/db/vars/zipinc_qrtl/nisnote.jsp.
The bed size cutoff points divided into small, medium, and large have been derived from https://www.hcup‐us.ahrq.gov/db/vars/hosp_bedsize/nisnote.jsp.
3.2. Comorbidities comparison
After propensity‐matched analysis, the significantly high proportion of comorbidities in the obese TCM group in the unmatched cohort disappeared and remained significantly high only for congestive heart failure (5.0% vs. 3.8%, adjusted P = 0.02; Table 2). Also, the frequency of certain comorbidities significantly decreased among the obese TCM group such as the alcohol abuse (1.0% vs. 1.7%; P = 0.02), valvular heart disease (1.1% vs. 1.7%; P = 0.04), history of sudden cardiac arrest (0.0% vs. 0.4%; P < 0.001), and chronic pulmonary disease (27.9% vs. 32.4%; P < 0.001). In propensity‐matched cohorts, major relevant CV comorbidities were comparable in both groups.
Table 2.
Comparison of comorbidities in obese vs nonobese TCM patients
| Comorbidities | Unmatched Cohort | Propensity‐Matched Cohort | ||||
|---|---|---|---|---|---|---|
| Nonobese | Obese | P Value | Nonobese | Obese | P Value | |
| Alcohol abuse | 3.8 | 1.0 | <0.001a | 1.7 | 1.0 | 0.025 |
| HTN | 63.0 | 77.4 | <0.001a | 75.3 | 77.4 | 0.072 |
| DM, uncomplicated | 15.3 | 37.2 | <0.001a | 38.4 | 37.2 | 0.336 |
| DM with chronic complications | 1.9 | 4.5 | <0.001a | 5.1 | 4.5 | 0.290 |
| Smoking | 33.9 | 32.5 | 0.135 | 33.7 | 32.5 | 0.322 |
| Dyslipidemia | 48.1 | 58.7 | <0.001a | 57.0 | 58.7 | 0.189 |
| Coronary atherosclerosis | 4.9 | 3.1 | <0.001a | 4.0 | 3.1 | 0.084 |
| Coagulopathy | 3.2 | 2.2 | 0.002a | 2.3 | 2.2 | 0.735 |
| CHF | 3.9 | 5.0 | 0.003a | 3.8 | 5.0 | 0.023a |
| Valvular disease | 1.7 | 1.1 | 0.01a | 1.7 | 1.1 | 0.046a |
| PVD | 6.5 | 4.5 | <0.001a | 4.3 | 4.5 | 0.776 |
| Previous MI | 6.5 | 6.9 | 0.380 | 7.8 | 6.9 | 0.203 |
| Previous PCI | 4.6 | 4.8 | 0639 | 5.7 | 4.8 | 0.111 |
| Previous CABG | 1.4 | 1.0 | 0.068 | 1.3 | 1.0 | 0.233 |
| History of sudden cardiac arrest | 0.4 | 0.0 | 0.001a | 0.4 | 0.0 | 0.001a |
| Chronic pulmonary disease | 25.6 | 27.9 | <0.001a | 32.4 | 27.9 | <0.001a |
| Renal failure | 5.9 | 10.1 | <0.001a | 10.2 | 10.1 | 0.922 |
| RA/collagen vascular diseases | 4.4 | 4.9 | 0.266 | 5.5 | 4.9 | 0.294 |
| Hypothyroidism | 18.1 | 18.7 | 0.387 | 19.1 | 18.7 | 0.662 |
| Liver disease | 1.6 | 2.1 | 0.03a | 1.9 | 2.1 | 0.567 |
| Fluid electrolyte disorders | 23.1 | 21.1 | 0.01a | 22.7 | 21.1 | 0.128 |
Abbreviations: CABG, coronary artery bypass grafting; CHF, congestive heart failure; CI, confidence interval; DM, diabetes mellitus; HTN, hypertension; MI, myocardial infarction; PCI, percutaneous coronary intervention; PVD, peripheral vascular disease; RA, rheumatoid arthritis; TCM, takotsubo cardiomyopathy.
Data are presented as %.
P values significant <0.05 at 95% CI.
3.3. Outcomes among the obese vs nonobese TCM patients
There were no significant differences in all‐cause in‐hospital mortality, development of arrhythmia, venous thromboembolism, and mechanical circulatory support among the obese and nonobese TCM patients in the unmatched and matched cohorts (Table 3). However, AMI, cardiac arrest, and respiratory failure were significantly higher among obese TCM patients in both the unmatched and matched cohorts. Nevertheless, in propensity score‐matched cohorts, cardiogenic shock (4.3% vs 3.2%; P = 0.03) and the need for mechanical circulatory support (Impella placement; 0.2% vs 0.0%, P = 0.02) turned out to be significantly higher in the obese TCM group compared with the nonobese TCM group, respectively. There were no significant differences in the LOS and total charges for the hospitalization of both propensity‐matched cohorts.
Table 3.
Complications in obese vs nonobese TCM patients
| Complications | Unmatched Cohort | Propensity‐Matched Cohort | ||||
|---|---|---|---|---|---|---|
| Nonobese | Obese | P Value | Nonobese | Obese | P Value | |
| All‐cause in‐hospital mortality | 1.3 | 1.0 | 0.143 | 0.8 | 1.0 | 0.354 |
| AMI | 8.0 | 9.0 | 0.041a | 7.4 | 9.0 | 0.025a |
| Arrhythmia | 24.2 | 24.5 | 0.683 | 22.7 | 24.5 | 0.123 |
| Cardiogenic shock | 4.6 | 4.3 | 0.435 | 3.2 | 4.3 | 0.032a |
| Cardiac arrest | 1.2 | 2.3 | <0.001a | 0.4 | 2.3 | <0.001a |
| Mechanical circulatory support | 2.5 | 2.5 | 0.993 | 2.5 | 2.5 | 0.985 |
| IABP | 2.3 | 2.3 | 0.997 | 2.5 | 2.3 | 0.699 |
| PVAD (Impella) | 0.1 | 0.2 | 0.792 | 0.0 | 0.2 | 0.037a |
| VTE | 4.2 | 4.5 | 0.376 | 4.5 | 4.5 | 0.952 |
| Respiratory failure | 11.0 | 12.9 | 0.002a | 11.0 | 12.9 | 0.021a |
| LOS, d | 3.7 ±3.7 | 3.7 ±3.5 | 0.987 | 3.7 ±3.6 | 3.7 ±3.5 | 0.685 |
| Mean total hospital charges | $43 268.32 | $40 780.16 | <0.001a | $42 575.14 | $40 780.16 | 0.081 |
Abbreviations: AMI, acute myocardial infarction; CI, confidence interval; IABP, intra‐aortic balloon pump; LOS, length of stay; PVAD, percutaneous ventricular assist device; SD, standard deviation; TCM, takotsubo cardiomyopathy; VTE, venous thromboembolism.
Data are presented as % or mean ±SD.
P values <0.05 significant at 95% CI.
4. DISCUSSION
To our knowledge, this is the first study analyzing a large national cohort about the association of obesity with TCM outcomes. With an established association between the obesity and CV disorders, our hypothesis was to look at the disparities in the outcomes for TCM in relation to the obesity by using the NIS. We found that approximately 10% of the hospitalized TCM patients were obese. The obese cohort consisted mainly of female patients (93.9%) who predominantly had nonelective (94.5%) and weekend (77.1%) admissions. The entire study population consisted of patients age ≥ 45 years in both the obese and the nonobese groups. However, unmatched and propensity‐matched obese cohort consisted of comparatively more younger patients. Propensity‐matched cohorts showed no difference in the all‐cause in‐hospital mortality in both groups. However, cardiac complications such as AMI, cardiogenic shock, cardiac arrest, Impella placement, and respiratory failure were higher in the obese TCM group. The LOS and the total hospital charges were comparable in the propensity‐matched obese and nonobese groups.
Patients with TCM have a higher prevalence of multiple CV risk factors and associated comorbidities. In a systematic review of 1109 TCM patients that focused on the clinical characteristics and comorbidities, it was found that obesity was present in 17%, hypertension in 54%, dyslipidemia in 32%, diabetes in 17%, and smoking in 22% of the patients. In another study from NIS in 2008, smoking, alcohol abuse, hyperlipidemia, and anxiety were found to significantly associate with TCM.13
All of the above‐mentioned comorbid conditions are often associated with endothelial dysfunction, which is considered a predisposing factor in the development of TCM.14 Nonetheless, information concerning with the effects of obesity and other CV risk factors on the outcomes in TCM is exceptionally constrained. In our study, the obese TCM group was comparatively younger, belonged to the lower income group, and most were admitted to hospitals in the southern and western US regions.
Although there were no racial or sex differences among the obese and nonobese TCM groups, we found that the overall study population was overrepresented by the female sex. This finding is similar to other previously published studies.13, 15 Deshmukh et al. reported similar findings with almost 90.4% of patients being females13 in the NIS 2008 database study. They also found that women age > 55 years had 4.8× higher odds for developing TCM when compared with women age < 55 years. The higher occurrence of TCM in the females and older age groups has been hypothesized with various possible mechanisms. Historically, it has been observed that males are biologically more protected against the stress‐induced catecholamine cardiotoxicity than females. It has also been proposed that higher cardiac adrenergic receptor density in males, especially at the apex, provides an improved protection against catecholamine levels by delaying the complete saturation.16 Interestingly, Krishnamoorthy et al. reported higher mortality among male TCM patients, likely due to a higher proportion of CV complexities and cardiac arrest.17
There were no significant differences in the both groups in terms of the cardiovascular morbidities and related procedures. However, the obese patients had a significantly higher prevalence of congestive heart failure while the significantly low frequency of alcohol abuse, valvular heart disease, previous sudden cardiac arrest, and chronic pulmonary disorder. Obesity is known to cause left ventricular (LV) dilation, increased LV wall stress, and LV diastolic dysfunction.18 Diastolic dysfunction due to cardiac remodeling owing to the intravascular volume expansion among the obese could be a possible explanation for the higher prevalence of congestive heart failure among the obese TCM group.19 Obesity is also often associated with obstructive sleep apnea, and obstructive sleep apnea has been associated with high catecholamine levels.20 Increase in catecholamine levels is one of the most accepted pathogenic mechanisms in TCM.21 Overall, an excellent long‐term prognosis of the disease with the recovery of the LV dysfunction within the few weeks could be a potential reason for the lower and nonsignificant difference in the in‐hospital all‐cause mortality, LOS, and hospitalization charges, despite higher complications among the obese TCM.15, 22, 23
On stress testing, a cohort of >2000 systolic heart failure patients demonstrated the existence of strong obesity paradox with the best survival among obese patients.24 However, in our study, obese TCM patients were more likely to develop AMI, cardiac arrest, cardiogenic shock, respiratory failure, and requiring the use of Impella. Several possible mechanisms exist behind the higher incidence of CV complications among the obese TCM cohort. Recently, Riders et al. described that the obesity was associated with 22% lower peak filling pressure and continued diastolic dysfunction during catecholamine‐induced inotropic stress.19 They found that excessive catecholamine during the stress exacerbates the myocardial energetic deficits, worsening the cardiac contractility. Impaired blood flow during the state of acute stress owing to the ventricular hypertrophy and microvascular dysfunction in the obese cohort was also suspected to play a role.19 It is also suggested that the intravascular volume expansion may cause cardiac remodeling in obese subjects that leads to the diastolic dysfunction owing to the reduced myocardial energetic due to the loss of total creatine pool.25
Another mechanism for the worse outcomes in obese TCM patients could be the myocardial susceptibility to the oxidative stress. Preclinical studies suggest that obesity creates a state of oxidative stress, which is exacerbated by the sympathetic hyperactivity leading to transient myocardial dysfunction.26, 27, 28 Association between catecholamine and some inflammatory pathways has also been explored in the pathogenesis of TCM but their definite association with the obesity is yet to be established.23, 29, 30, 31 Inflammatory mediators such as IL‐6 are secreted from the adipocytes in the presence of stressful stimulus via catecholamine‐mediated pathway.32, 33, 34 However, there is a need for further research to clearly delineate the association of IL‐6 with obesity and resultant poor outcomes in the TCM patients.
4.1. Study limitations
We performed this study from the NIS database, which is a nationally representative database minimizing the single center associated selection biases and increase in the generalizability of the results. Use of nationwide sample with weighted data helps in generalizing the results for the entire population of the United States rather than a specific region and similar approach has been successfully utilized previously.35 Hales et al. reported obesity prevalence rate of approximately 39% in the United States, which was significantly higher than the current 10% obesity prevalence among the TCM cohort.36 Therefore, underreporting of obesity is one of the major limitations readers should consider while interpreting results as the NIS data is restricted to hospitalized obese patients (exclusive of outpatient TCM patients). However, data related to medication use, laboratory and other clinical data were not available which limited further evaluation of pathophysiology affecting the outcomes. Another potential limitation of the study is the inability to stratify the obese patients based on BMI, which could have given detailed insights. To minimize the bias, we have assessed the outcomes in propensity‐matched cohorts; however, it is not possible to control all the confounders and residual imbalances of all the variables in the retrospective analysis, which could have affected the outcomes to some extent.
5. CONCLUSION
In this nationally representative population‐based retrospective cohort study, we concluded that the obese patients hospitalized with TCM are more prone to develop cardiac complications such as AMI, cardiac arrest, cardiogenic shock, and advanced mechanical circulatory support during the hospitalization. However, there was no significant difference in the term of all‐cause in‐hospital mortality between the obese and nonobese TCM patients. Further prospective studies are warranted to endorse our study results.
Conflict of interest
The authors declare no potential conflicts of interest.
Supporting information
Supplementary Table 1 International Classification of Disease ninth Revision ‐Clinical Modification Codes (ICD‐9 CM) and Clinical Classifications Software (CCS) and procedure (PR) codes used for Comorbidities and Complications not provided by NIS database.
Desai R, Singh S, Baikpour M, et al. Does obesity affect the outcomes in takotsubo cardiomyopathy? Analysis of the Nationwide Inpatient Sample database, 2010‐2014. Clin Cardiol. 2018;41:1028–1034. 10.1002/clc.22999
REFERENCES
- 1. Sato H, Tateishi H, Uchida T, et al. Tako‐tsubo‐like left ventricular dysfunction due to multivessel coronary spasm In: Kodama K, Haze K, Hori M, eds. Clinical Aspects of Myocardial Injury: From Ischemia to Heart Failure [in Japanese]. Tokyo, Japan: Kagakuhyoronsha Publishing Co; 1990:56‐64. [Google Scholar]
- 2. Khera R, Light‐McGroary K, Zahr F, et al. Trends in hospitalization for takotsubo cardiomyopathy in the United States. Am Heart J. 2016;172:53‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shah M, Ram P, Lo KB, et al. Etiologies, predictors and economic impact of readmission within one month among patients with takotsubo cardiomyopathy. Clin Cardiol. 2018;1–8. 10.1002/clc.22974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gianni M, Dentali F, Grandi AM, et al. Apical ballooning syndrome or takotsubo cardiomyopathy: a systematic review. Eur Heart J. 2006;27:1523‐1529. [DOI] [PubMed] [Google Scholar]
- 5. Elesber AA, Prasad A, Lennon RJ, et al. Four‐year recurrence rate and prognosis of the apical ballooning syndrome. J Am Coll Cardiol. 2007;50:448‐452. [DOI] [PubMed] [Google Scholar]
- 6. Sharkey SW, Windenburg DC, Lesser JR, et al. Natural history and expansive clinical profile of stress (tako‐tsubo) cardiomyopathy. J Am Coll Cardiol. 2010;55:333‐341. [DOI] [PubMed] [Google Scholar]
- 7. Previtali M, Repetto A, Camporotondo R, et al. Clinical characteristics and outcome of left ventricular ballooning syndrome in a European population. Am J Cardiol. 2011;107:120‐125. [DOI] [PubMed] [Google Scholar]
- 8. Templin C, Ghadri JR, Diekmann J, et al. Clinical features and outcomes of Takotsubo (stress) cardiomyopathy. N Engl J Med. 2015;373:929‐938. [DOI] [PubMed] [Google Scholar]
- 9. Brunelli E, La Russa D, Pellegrino D. Impaired oxidative status is strongly associated with cardiovascular risk factors. Oxid Med Cell Longev. 2017;2017:6480145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Healthcare Cost and Utilization Project (HCUP) . HCUP databases. Rockville, MD: Agency for Healthcare Research and Quality; 2018. https://www.hcup-us.ahrq.gov/nisoverview.jsp. Accessed February 2018. [Google Scholar]
- 11. Minhas AS, Hughey AB, Kolias TJ. Nationwide trends in reported incidence of takotsubo cardiomyopathy from 2006 to 2012. Am J Cardiol. 2015;116:1128‐1131. [DOI] [PubMed] [Google Scholar]
- 12. Healthcare Cost and Utilization Project (HCUP) . HCUP Elixhauser Comorbidity Software. Rockville, MD: Agency for Healthcare Research and Quality; June 2017. https://www.hcup-us.ahrq.gov/toolssoftware/comorbidity/comorbidity.jsp. Accessed February 10, 2018. [Google Scholar]
- 13. Deshmukh A, Kumar G, Pant S, et al. Prevalence of Takotsubo cardiomyopathy in the United States. Am Heart J. 2012;164:66.e1‐71.e1. [DOI] [PubMed] [Google Scholar]
- 14. Pelliccia F, Parodi G, Greco C, et al. Comorbidities frequency in Takotsubo syndrome: an international collaborative systematic review including 1109 patients. Am J Med. 2015;128:654.e11‐659.e11. [DOI] [PubMed] [Google Scholar]
- 15. Akashi YJ, Goldstein DS, Barbaro G, et al. Takotsubo cardiomyopathy: a new form of acute, reversible heart failure. Circulation. 2008;118:2754‐2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Stöllberger C, Finsterer J. Why does takotsubo (“broken heart syndrome”) affect more females than males? Int J Cardiol. 2011;147:175‐176. [DOI] [PubMed] [Google Scholar]
- 17. Krishnamoorthy P, Garg J, Sharma A, et al. Gender differences and predictors of mortality in takotsubo cardiomyopathy: analysis from the National Inpatient Sample 2009–2010 database. Cardiology. 2015;132:131‐136. [DOI] [PubMed] [Google Scholar]
- 18. Alpert MA. Obesity cardiomyopathy: pathophysiology and evolution of the clinical syndrome. Am J Med Sci. 2001;321:225‐236. [DOI] [PubMed] [Google Scholar]
- 19. Rider OJ, Francis JM, Ali MK, et al. Effects of catecholamine stress on diastolic function and myocardial energetics in obesity. Circulation. 2012;125:1511‐1519. [DOI] [PubMed] [Google Scholar]
- 20. Vardhan V, Shanmuganandan K. Hypertension and catecholamine levels in sleep apnoea. Med J Armed Forces India. 2012;68:33‐38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Goyal H, Singla U, Lawrence JD, et al. Tale of a tube and a pot—a case of Takotsubo cardiomyopathy occurring after MRI. Int J Cardiol. 2016;225:140‐141. [DOI] [PubMed] [Google Scholar]
- 22. Akashi YJ, Musha H, Kida K, et al. Reversible ventricular dysfunction takotsubo cardiomyopathy. Eur J Heart Fail. 2005;7:1171‐1176. [DOI] [PubMed] [Google Scholar]
- 23. Saito Y. Hypoglycemic attack: a rare triggering factor for takotsubo cardiomyopathy. Intern Med. 2005;44:171‐172. [DOI] [PubMed] [Google Scholar]
- 24. Lavie CJ, Cahalin LP, Chase P, et al. Impact of cardiorespiratory fitness on the obesity paradox in patients with heart failure. Mayo Clin Proc. 2013;88:251‐258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lamb HJ, Beyerbacht HP, van der Laarse A, et al. Diastolic dysfunction in hypertensive heart disease is associated with altered myocardial metabolism. Circulation. 1999;99:2261‐2267. [DOI] [PubMed] [Google Scholar]
- 26. Lauer MS, Anderson KM, Kannel WB, et al. The impact of obesity on left ventricular mass and geometry: the Framingham Heart Study. JAMA. 1991;266:231‐236. [PubMed] [Google Scholar]
- 27. Engeli S, Sharma AM. The renin‐angiotensin system and natriuretic peptides in obesity‐associated hypertension. J Mol Med (Berl). 2001;79:21‐29. [DOI] [PubMed] [Google Scholar]
- 28. Vincent HK, Powers SK, Stewart DJ, et al. Obesity is associated with increased myocardial oxidative stress. Int J Obes Relat Metab Disord. 1999;23:67‐74. [DOI] [PubMed] [Google Scholar]
- 29. Komamura K, Fukui M, Iwasaku T, et al. Takotsubo cardiomyopathy: pathophysiology, diagnosis and treatment. World J Cardiol. 2014;6:602‐609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Watanabe H, Kodama M, Okura Y, et al. Impact of earthquakes on Takotsubo cardiomyopathy. JAMA. 2005;294:305‐307. [DOI] [PubMed] [Google Scholar]
- 31. Wittstein IS, Thiemann DR, Lima JA, et al. Neurohumoral features of myocardial stunning due to sudden emotional stress. N Engl J Med. 2005;352:539‐548. [DOI] [PubMed] [Google Scholar]
- 32. Santoro F, Tarantino N, Ferraretti A, et al. Serum interleukin 6 and 10 levels in Takotsubo cardiomyopathy: increased admission levels may predict adverse events at follow‐up. Atherosclerosis. 2016;254:28‐34. [DOI] [PubMed] [Google Scholar]
- 33. Eder K, Baffy N, Falus A, Fulop AK. The major inflammatory mediator interleukin‐6 and obesity. Inflamm Res. 2009;58:727‐736. [DOI] [PubMed] [Google Scholar]
- 34. Yudkin JS, Kumari M, Humphries SE, et al. Inflammation, obesity, stress and coronary heart disease: is interleukin‐6 the link? Atherosclerosis. 2000;148:209‐214. [DOI] [PubMed] [Google Scholar]
- 35. Agarwal MA, Shah M, Garg L, et al. Relationship between obesity and survival in patients hospitalized for hypertensive emergency. Mayo Clin Proc. 2018;93:263‐265. [DOI] [PubMed] [Google Scholar]
- 36. Hales CM, Fryar CD, Carroll MD, et al. Trends in obesity and severe obesity prevalence in us youth and adults by sex and age, 2007–2008 to 2015–2016. JAMA. 2018;319:1723‐1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1 International Classification of Disease ninth Revision ‐Clinical Modification Codes (ICD‐9 CM) and Clinical Classifications Software (CCS) and procedure (PR) codes used for Comorbidities and Complications not provided by NIS database.


