Abstract
The syndromes of myocardial infarction/myocardial ischemia with No Obstructive Coronary Artery Disease (MINOCA/INOCA) are increasingly evident. A majority of these patients have coronary microvascular dysfunction. These patients have elevated risk for a cardiovascular event (including acute coronary syndrome, myocardial infarction, stroke, and repeated cardiovascular procedures) and appear to be at higher risk for development of heart failure with preserved ejection fraction. Terminology such as coronary artery disease or coronary heart disease is often synonymous with obstructive atherosclerosis in the clinician's mind, leaving one at a loss to recognize or explain the phenomenon of MINOCA and INOCA with elevated risk. We review the available literature regarding stable and unstable ischemic heart disease that suggests that use of the ischemic heart disease (IHD) terminology matters for women, and should facilitate recognition of risk to provide potential treatment targets and optimized health.
Keywords: Coronary Microvascular Dysfunction, Myocardial Infarction/Myocardial Ischemia With No Obstructive Coronary Artery Disease
1. DEFINITION AND TERMINOLOGY
Patients presenting with myocardial infarction with no obstructive coronary artery disease (MINOCA) or symptoms and signs of ischemia but no obstructive coronary artery disease (INOCA) are increasingly recognized within acute coronary syndrome (ACS) and stable ischemic heart disease (SIHD) populations.1, 2 Evidence documents that this is associated with an adverse prognosis, yet no clinical practice management guidelines exist. There is likely overlap between MINOCA and INOCA.3
2. PREVALENCE, COSTS, AND PROGNOSTIC SIGNIFICANCE
2.1. INOCA
The American College of Cardiology–National Cardiovascular Data Registry and the National Heart, Lung and Blood Institute–sponsored Women's Ischemic Syndrome Evaluation (WISE) databases suggest that at least 3 to 4 million women and men with signs/symptoms suggestive of myocardial ischemia have no obstructive coronary artery disease (CAD).4 Such individuals incur healthcare costs and disabilities similar to obstructive CAD, in part due to angina and heart failure (HF) hospitalizations and repeated testing.4, 5 These hospitalizations and repeated angiography are confirmed by a European consecutive‐patient registry.6
Although approximately the same number of women than men die annually from cardiovascular disease (CVD),7 women presenting with INOCA/MINOCA are more likely to have no obstructive CAD on coronary angiography compared with men.8, 9 Such patients are often reassured but offered no specific management, yet have a heightened CVD event risk compared with age‐ and sex‐matched reference subjects.1, 10 An intermediate risk for a major adverse cardiac event (MACE) (death, nonfatal myocardial infarction [MI], nonfatal stroke, and HF hospitalization) rate exceeding 2.5% yearly by 5 years is observed, as well as elevated rates of readmission and repeat angiography triggered by symptom burden.10 At 10 years, CVD death or MI occurred in 6.7% of those with no evident angiographic CAD, and in 12.8% among those with nonobstructive CAD.11 Large, consecutive‐case registry reports have replicated this heightened risk for adverse prognosis and extended the findings to men.1, 2, 12 Table 1 demonstrates a summary of prognosis in INOCA subjects with CMD.13
Table 1.
Natural history studies of patients with coronary microvascular dysfunction13 (Reprinted with permission.)
| Author, Year | No. | Population | Method | Outcome Measure | Follow‐up | CMD Outcome Predictor | |
|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | ||||||
| Britten, 200481 | 120 | Post‐PCI/mild CAD | Intracoronary papaverine or adenosine CFR Doppler flow wire | Cardiac death, ACS, revascularization, stroke | 6.5 ± 3 years (14–125 months) | Yes | Yes |
| Schindler, 200682 | 72 | CAD risk factors without flow‐limiting stenosis |
CPT‐MBF increase with 13N‐NH3 PET | CV death, ACS, MI, PCI/CABG, stroke, PTA | 66 ± 8 months | Yes | No |
| Rigo, 200783 | 86 | CAD, LAD 51%–75% stenosis | Vasodilator LAD CFR, Doppler/TTE | Nonfatal MI | 30 months, 14 median | Yes | Yes |
| Nemes, 200884 | 397 | Hospitalized, angina, mostly severe CAD, TEE for AA | Vasodilator LAD CFR, Doppler/TEE | CV death, HF, thrombosis | 41 ± 12 months | Yes | Yes |
| Herzog, 200985 | 229 | Suspect CAD/66% had severe CAD | Vasodilator CFR with 13N‐NH3 PET | CV death, nonfatal MI, hospitalization, PCI/CABG | 5.5 ± 2.1 years | Yes | Yes |
| Tio, 200986 | 344 | Severe CAD, not revascularized, LV systolic dysfunction | Vasodilator CFR with 13N‐NH3 PET | Cardiac death | 85 months (1–138 months) | Yes | Yes |
| Cortigiani, 201087 | 1660 | Chest pain, normal DSE | Vasodilator LAD CFR, Doppler/TTE | Death, MI, revascularization | 19 months median | Yes | Yes |
| Pepine (WISE), 201088 | 189 | Women, angina/ischemia, most with obstructive CAD | Intracoronary Ado‐CFR, Doppler flow wire | Death, nonfatal MI, nonfatal stroke, HF hospitalization | 5.4 years (mean) | Yes | Yes |
| Ziadi, 201134 | 677 | Most had severe CAD | Vasodilator CFR with 82Rb PET | CV death, nonfatal MI | 387 days (375–416 days) | Yes | Yes |
| Balazs (SZEGED study), 201189 | 45 | Women, angina/ischemia, no obstructive CAD | Vasodilator CFR, Doppler/TEE, TTE | Death, CV hospitalization | 102 ± 26 months, 113 median | Yes | Yes |
| Murthy, 201490 | 1218; 813 women, 405 men | No obstructive CAD (excluded by CTA or PET) | Stress Perfusion, imaging (PET) | CV death, MI, late revascularization (>90 days) or HF hospitalization | 3 years | Yes | Yes |
Abbreviations: ACS, acute coronary syndrome; CABG, coronary artery bypass graft; CAD, coronary artery disease; CFR, coronary flow reserve; CMD, coronary microvascular dysfunction; CPT, cold pressor test; CTA, computed tomography angiography; CV, cardiovascular; DSE, dobutamine stress echo; HF, heart failure; LAD, left anterior descending; LV, left ventricular; MBF, myocardial blood flow; MI, myocardial infarction; PCI, percutaneous coronary intervention; PET, positron emission tomography; PTA, percutaneous transluminal angioplasty; SZEGED, SummariZation of long‐tErm prognostic siGnificance of coronary flow rEserve in special Disorders; TEE, transesophageal echocardiography; TTE, transthoracic echocardiography; WISE, Women's Ischemia Syndrome Evaluation.
2.2. MINOCA
MINOCA represents up to 14% of all acute MIs,14, 15 and is diagnosed more frequently in younger patients and in women than MI in the setting of obstructive CAD.15 African American patients are more frequently diagnosed with MINOCA than those of other ethnicities.16 MINOCA patients may also exhibit a different cardiovascular risk profile than patients with obstructive CAD, as they are less likely to be diagnosed with hyperlipidemia15 and diabetes,17 but have a reported higher prevalence of hypertension.18 MINOCA patients report less angina prior to MI,18 and non–ST‐elevation MI accounts for two‐thirds of cases.15
Although MINOCA prognosis appears more favorable than obstructive CAD MI, it is not benign. The occurrence of recurrent MI and urgent revascularization, as reported by Planer et al., in a propensity‐matched cohort of 197 MINOCA patients was significantly lower than in those with obstructive CAD.19 In a systematic review, analysis of data from 8 studies found that, despite a lower mortality rate than those with obstructive CAD, MINOCA was nonetheless associated with an all‐cause mortality rate of 4.7% at the 12‐month follow‐up.15 Up to 25% of MINOCA patients report persistent angina following MI, and experience similar rates of hospitalization due to angina than their counterparts with obstructive CAD.18 Persistent angina represents an important socioeconomic personal and societal burden, with MINOCA patients reporting worse quality of life due to angina on the Seattle Angina Questionnaire and more dissatisfaction with medical management of their symptoms.18
3. PREDICTORS OF ADVERSE OUTCOMES
3.1. INOCA
Older age, hypertension, diabetes, and smoking have been associated with increased mortality, whereas sex, hyperlipidemia, family history of premature CAD, or pretest CAD likelihood have not.5 Risk‐adjusted analyses found that nonobstructive CAD conferred increased mortality risk vs that of patients with no evident CAD.5
Chest pain persisting at the 1‐year follow‐up predicted MACE among those with INOCA in the WISE study.20 Measures of nonobstructive CAD extent and severity (eg, WISE CAD severity score, number of vessels involved) also appear important in prognosis, but these measures are not well developed.1, 2, 11 A large cohort undergoing coronary computed tomographic angiography propensity matched for age, CAD risk factors, and angina typicality observed elevated death/MI rates in those with nonobstructive CAD vs normal angiograms.21
3.2. MINOCA
Female sex and a younger age (median age = 59 vs 64 years for MINOCA vs obstructive CAD, P < 0.0001) were the only independent clinical predictors of MINOCA in the CRUSADE (Can Rapid risk stratification of Unstable angina patients Suppress ADverse outcomes with Early implementation of the ACC/AHA Guidelines) registry of 38 301 non–ST‐elevation myocardial infarction patients.22 In an observational study of 131 patients diagnosed with MINOCA who underwent cardiac magnetic resonance imaging (cMRI), ST‐elevation myocardial infarction at presentation and late gadolinium enhancement (LGE) were associated with an increased risk of developing ventricular tachycardia or ventricular fibrillation during index hospitalization, but not with the occurrence of sudden death at 1 year.23 Repeat hospitalizations for chest pain are associated with increased risk of repeat investigations and procedures.10
4. PATHOPHYSIOLOGY
4.1. MINOCA/INOCA
Mechanisms contributing to INOCA appear multifactorial and may operate alone or in combination.24 Although these may include hypertension, severe aortic stenosis, severe anemia, type II MI, shunts, certain drugs, HF or cardiogenic shock, Prinzmetal variant angina (coronary spasm), myocardial diseases (eg, myocarditis), congenital heart disease, coronary anomalies, myocardial bridging, and other causes, underlying mechanisms and appropriate diagnostic and management strategies in these settings are often evident.
4.1.1. Atherosclerosis
The pathophysiology of atherosclerosis is now clearly related to chronic inflammation with periods of minor plaque rupture, erosion, and distal embolism resulting in MI.25 Evidence linking microvascular and inflammatory responses to risk factors indicates that oxidative stress, reduced nitric oxide (NO) bioavailability, and endothelial activation are common early features of coronary microvascular responses to atherosclerosis risk factors.26 Essentially, all INOCA patients with chronic angina studied by intravascular ultrasound (IVUS) to date have some coronary atherosclerosis.27, 28 A greater burden of risk factors is associated with more atherosclerosis, concealed by compensatory positive remodeling, yielding diffuse nonobstructive CAD.27 Two single‐center reports of nonobstructive CAD presenting with ACS suggest that plaque rupture is observed in the minority: 38% of 42 women29 and 37% of men and women.30 The former study found that plaque ulceration was also frequent, in addition to LGE.29 The latter study found plaque ruptures frequently appeared with larger plaque burden and positive remodeling.30
4.1.2. Coronary Microvascular Dysfunction
One proposed mechanism contributing to MINOCA/INOCA is coronary microvascular dysfunction (CMD),31 defined as epicardial and/or microvascular endothelial and/or nonendothelial dysfunction that limits myocardial perfusion, most often detected as reduced coronary flow reserve (CFR) detected by invasive doppler or noninvasive advanced imaging such as positron emission tomography (PET) or cMRI. CMD may occur in the absence of obstructive CAD and myocardial diseases, in myocardial diseases, in obstructive CAD, or may be iatrogenic.31 Coronary vasomotor dysfunction, even without flow‐limiting stenosis, identifies patients at risk for cardiac death.32, 33, 34 There is limited correlation between anatomic CAD severity and functional impairment, as reflected in the CFR.35 Diabetic patients without obstructive CAD but with impaired CFR experienced cardiac death rates similar to those for nondiabetic patients with CAD.36
To understand CMD mechanistic pathways, WISE investigation has explored genotypic pathways of arterial vasomotion, including bradykinin and related peptides (terminal kinins), which promote vasodilation, endothelial NO production, and vascular permeability. We hypothesized that the kinin system's protective effect on the coronary circulation may be reduced in patients with polymorphic (deficient) alleles of the Bradykinin B1R gene. Evaluation of 141 WISE women who underwent quantitative coronary angiography and genetic analysis for the guanine (G)–cytosine (C) polymorphism at position −699 of the gene demonstrated a lesser CFR to adenosine associated with the B1R gene polymorphism (CG genotype). The mean CFR was 2.05 ± 0.30 (abnormal is <2.32), while wildtype women (GG genotype) had a normal mean CFR of 2.78 ± 0.15 (P = 0.047). This change in flow ratio was due to a decrease in velocity (P = 0.022), rather than to a change in coronary cross‐sectional area (P = 0.78). A significant difference in flow ratio by genotype was also seen in response to nitroglycerin, where women with the polymorphism had a flow ratio of 2.47 ± 0.24 versus 3.36 ± 0.14 for the wildtype genotype (P = 0.029). When acetylcholine (an endothelial mediator) was tested, no difference was seen by genotype (P = 0.55). No difference by genotype was seen for age, severity of angiographic CAD, hypertension, diabetes, dyslipidemia, or smoking. We concluded that in addition to early atherosclerosis, these data suggest that intrinsic genetic factors contribute to coronary smooth muscle reactivity as well.37 Furthermore, in 667 WISE women, diabetics were compared to nondiabetics in terms of survival and NOS3 genetic polymorphism. The median follow‐up was 5.9 years. In nondiabetics the Asp298 variant was associated with poor survival (n = 504, percent survival 1, 3, and 5 years: Glu298Glu = 98%/97%/96%, Glu298Asp = 99%/96%/94%, Asp298Asp = 94%/94%/86%, P = 0.048). This interaction was not seen in diabetics (n = 160, P = 0.92). This survival impact was not evident in subjects with no obstructive CAD at entry (n = 239), as the obstructive CAD subset Asp298 was exclusively associated with poor outcomes (% survival: Glu298Glu = 97%/90%/88%; Glu298Asp = 97%/86%/82%; Asp298Asp = 92%/83%/65%; P = 0.02). As in the larger cohort, the impact of Asp298 in women with obstructive CAD was exclusively in nondiabetics (P = 0.002) and was not evident in subjects with diabetes (P = 0.57). The impact of this allele was eliminated by the presence of diabetes, and this genetic interaction suggests a role of NO imbalance in diabetic vasculopathy.38
5. DIAGNOSIS
5.1. Invasive testing
Assessment of coronary vascular function includes measurements of coronary blood flow (CBF) and epicardial coronary artery diameter with endothelium‐dependent probes: acetylcholine (Ach), bradykinin, substance‐P, L‐NMMA, shear‐stress, and CFR with predominantly endothelium‐independent probes: adenosine or nitroglycerin. Exercise, pacing‐induced tachycardia, cold pressor test (CPT), and mental stress have also been used to elicit abnormalities in CBF. WISE‐CVD project data suggest a strong correlation between Ach and CPT coronary artery diameter changes in women.28 Reports from testing over 1500 ACS and SIHD patients indicate an excellent safety record, with no deaths and <1% procedure‐related adverse experiences like those observed with coronary angiography.39, 40, 41
IVUS can be useful in the proximal portions of epicardial coronary artery vessels in search of potential etiologies of MINOCA, including plaque rupture or ulceration, presence of thrombus, or spontaneous coronary artery dissection.29 IVUS identified plaque disruption in 38% of patients and plaque ulceration in 10% of a cohort of MINOCA patients, and in the hands of experienced operators, is a tool of interest in identifying precise etiologies in this patient population. Optical coherence tomography (OCT) has a 10‐fold higher resolution than IVUS42 and has a 92% sensitivity and 75% specificity for identification of plaques with a large lipid pool and thin fibrous cap.43 These lesions are associated with a similar risk of cardiovascular events at follow‐up as patients with ACS and obstructive CAD. IVUS and OCT have become key imaging modalities during invasive angiography to better define MINOCA.
5.2. Noninvasive testing
PET is a highly accurate, reproducible, and modifiable procedure providing comprehensive evaluation of CBF, including myocardial perfusion, left ventricular (LV) function, and CFR. There is a strong association between impaired CFR and impaired LV myocardial relaxation or elevated filling pressures, strongest among those with cardiac troponin elevations.44 Transthoracic echo Doppler can measure coronary flow velocity (CFV), by pulsed‐wave Doppler of the left anterior descending coronary artery at rest and after dipyridamole, and a prior publication in INOCA patients demonstrated that 26% had and abnormal CFV reserve <2.0.45 Those with low CFV reserve had significantly greater physical limitation and disease perception scores using the Seattle Angina Questionnaire. cMRI can detect failure of subendocardial perfusion to increase appropriately in response to stress in INOCA subjects.46, 47 A semiquantitative approach with measurement of myocardial perfusion reserve index (MPRI) detects CMD in women with INOCA.48 cMRI can also be used to detect and further characterize myocardial edema and scarring in patients diagnosed with MINOCA, providing insight into potential pathophysiological mechanisms. In a study conducted by Reynolds et al., women with MINOCA were prospectively enrolled and underwent cMRI within 1 week of diagnosis. Twenty‐six of the 44 (59%) patients who underwent cMRI had abnormal findings. T2 signal hyperintensity (T2+) indicating edema was found in 9 patients and LGE compatible with myocardial scarring in 17 patients.29 These findings indicate that MINOCA is associated to true myocardial anomalies detectable on cMRI, suggestive of ischemic but also nonischemic etiologies that need to be considered in patient management.
Mauricio et al. examined whether there was evidence of abnormal perfusion compatible with CMD on cMRI in INOCA patients.49 Forty patients underwent adenosine stress cMRI, with 63% of patients exhibiting abnormal stress perfusion. There was a trend toward higher probability of T2+ among those with stress perfusion abnormalities (P = 0.06), which matched the location of T2+ in all patients who were found to have both. Semiquantitative perfusion analysis was also performed, and a quarter of patients had abnormal MPRI, although this was not associated with LGE or T2+. CMD may represent an underlying pathological substratum predisposing the myocardium to edema and potential necrosis as detected by cMRI, and subsequent MINOCA, in the setting of vasospasm, endothelial dysfunction, or coronary microemboli.29
6. MANAGEMENT
Potential therapies for MINOCA/INOCA with evidence of CMD include therapeutic lifestyle change, management of risk factors, and lifestyle modifications such as weight loss,50 smoking cessation, high‐fiber diet, fruits and vegetables consumption, and regular physical activity.50, 51
Figure 1.
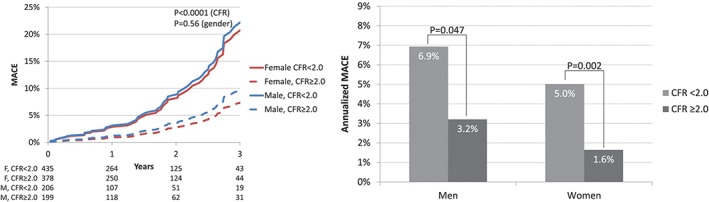
Annualized major adverse cardiac event (MACE) rates by sex and coronary flow reserve (CFR).90 Abbreviations: CAD, coronary artery disease; CMDPET, coronary microvascular dysfunction positron emission tomography; ds, days; HF, heart failure; MI, myocardial infarction (Reprinted with permission
Prior statin trials using IVUS have documented prevention of progression, or even regression, of atherosclerosis in coronary arteries, as well as coronary endothelial and/or vascular smooth muscle function in subjects with nonobstructive CAD.52 Fluvastatin and the combination of fluvastatin and diltiazem improved CFR.53 Two small pilot studies have shown administration of atorvastatin improved CFR after 2 months54 and 6 months.55 Angiotensin converting enzyme (ACE) inhibitors have been shown to improve exercise tolerance and angina symptoms.56 In a WISE ancillary trial, women who received quinapril had improved CFR and angina symptoms.57 Patients with essential hypertension had marked improved CBF after 12 months of treatment with perindopril, with regression of periarteriolar fibrosis seen on biopsy.58 In patients already on an ACE inhibitor, the addition of an aldosterone blocker did not improve endothelial function,59 although in subjects with diabetes, the addition of spironolactone has been shown to improve coronary microvascular function.60 A statin plus ACE inhibitor (atorvastatin and ramipril) at 6 months improved Seattle Angina Questionnaire scores and exercise duration vs placebo. Mechanistically, the combination produced greater increases in brachial artery flow‐mediated dilation vs placebo and reduced extracellular superoxide dismutase.56
Additional approaches to CMD treatment are listed in Table 2. β‐blockers reduce myocardial oxygen consumption and increase diastolic filling time, reducing the number of angina episodes in patients46 and improving ischemic threshold. Carvedilol has been shown to improve endothelial function.61 One study of intracoronary diltiazem did not improve CFR in CMD patients, but rather had a predominant vasodilatory effect on the epicardial arteries.62 Despite these findings, patients with abnormal vasodilator reserve have improved symptoms, less nitrate usage, and improved exercise tolerance after being treated with verapamil or nifedipine.63 The use of nitrates may improve patient's symptoms, but there are limited data on their effect on endothelial function. Ranolazine is an antianginal that inhibits the late sodium current; however, results on CMD have been conflicting.64, 65, 66 Another drug, ivabradine, which reduces heart rate through its effect on If of the sinoatrial node, was found to improve symptoms but had no effect on coronary microvascular function, as reported by Villano et al.66 Aminophylline, a nonselective adenosine‐receptor antagonist, blocks the mediation of nociception, and some improvement in symptoms and exercise capacity were seen with short‐term intravenous67 and oral aminophylline68 in these patients. Fasudil, a ρ kinase inhibitor, has been shown to be effective for vasospastic angina. Two studies have found improvement of CFR with infusion of L‐arginine.69 However Lerman et al. found that after oral supplementation for 6 months, there was no improvement in CFR, only a significant improvement in CBF.70 Imipramine has been shown to reduce frequency of pain.71, 72
Table 2.
Treatment of subjects with angina, evidence of myocardial ischemia, and no obstructive coronary artery disease91 (Reprinted with permission.)
| Microvascular Coronary Dysfunction |
| Abnormal endothelial function |
| Angiotensin converting enzyme inhibitors |
| HMG CoA reductase inhibitors (statins) |
| L‐arginine supplementation |
| Aerobic exercise |
| Enhanced external counterpulsation |
| Abnormal nonendothelial function |
| β‐blockers/α‐ and β‐blockers |
| Nitrates |
| Antianginal |
| Ranolazine |
| Ivabradine |
| Xanthine derivatives |
| Abnormal smooth muscle function (Prinzmetal's angina) |
| Calcium channel blockers |
| Nitrates |
| Abnormal cardiac nociception |
| Low dose tricyclic medication |
| Spinal cord stimulation |
| Cognitive behavioral therapy |
Abbreviations: HMG CoA, 3‐hydroxy‐3‐methyl‐glutaryl‐coenzyme A.
Spinal cord stimulation has been shown to normalize abnormal pain perception73 and improve angina symptoms and increase exercise tolerance.74 Enhanced external counterpulsation uses pneumatic cuffs applied to the patient's legs. Sequential inflation and deflation synchronized to the cardiac cycle improves hemodynamics.75 It has been shown to improve angina in a small case series.76 Cognitive behavioral therapy can reduce symptom severity and frequency.77 Cardiac rehabilitation can be helpful as it improves blood pressure, BMI, and exercise capacity.78
Patients with MINOCA are less likely to be treated and discharged on guideline‐recommended therapies than those with obstructive CAD, including β‐blockers, statins, ACE inhibitors/angiotensin receptor blockers (ARB), and dual antiplatelet therapy.17 Although no randomized controlled trials regarding the use of these therapies in the context of MINOCA exist, Lindahl et al. recently conducted an observational study of medical therapy for secondary prevention in 9466 MINOCA patients.79 MACEs, including all‐cause mortality, hospitalization for MI, ischemic stroke, and heart failure were lower in all patients who were discharged on statins (hazard ratio [HR]: 0.77, 95% confidence interval [CI]: 0.68‐0.87) and ACE inhibitor/ARB (HR: 0.82, 95% CI: 0.73‐0.93), and this was also true for both women and men. β‐blockers were not associated with lower MACE overall, but with lower incidence of MI (HR: 0.74, 95% CI: 0.56‐0.97), and patients discharged with dual antiplatelet therapy seemed to have a similar rate of MACEs than those without such therapy.79 The association of statins and ACE inhibitors/ARB to lower rates of adverse outcomes may be in part explained by the efficient treatment of underlying atherosclerosis and endothelial dysfunction with these agents in a proportion of these patients, which prevents disease progression, plaque rupture, and further cardiovascular events, and has been shown to improve outcomes in those with CMD as described above. Prospective studies are needed to confirm these associations and improve management of MINOCA patients.
7. CONCLUSIONS
The prevalence of no obstructive CAD among clinically ordered coronary angiograms conducted for myocardial infarction or evidence of suspected myocardial ischemia (MINOCA/INOCA) is increasing.1, 2, 8, 80 A majority of these patients have CMD, an elevated risk for a cardiovascular event (including ACS, MI, HF, and anginal hospitalization and repeated cardiovascular procedures). At present, there is no uniform, comprehensive diagnostic strategy or algorithm for risk stratification for these patients; however, invasive and noninvasive coronary flow reserve testing can be useful. Although small trials have suggested benefit from ACE inhibitors and statins, there is a lack of appropriately designed clinical outcome trials to inform evidence‐based therapeutic strategies. The next steps needed to address knowledge gaps include evidence‐based approaches to the definition, diagnostic evaluation, risk stratification, and management of MINOCA/INOCA patients, including large outcome clinical trials.
Conflicts of interest
The authors declare no potential conflicts of interest.
Pacheco Claudio C, Quesada O, Pepine CJ, Noel Bairey Merz C. Why names matter for women: MINOCA/INOCA (myocardial infarction/ischemia and no obstructive coronary artery disease). Clin Cardiol. 2018;41:185–193. 10.1002/clc.22894
Funding information This work was supported by contracts from the National Heart, Lung and Blood Institutes, (N01‐HV‐68161, N01‐HV‐68162, N01‐HV‐68163, N01‐HV‐68164, RO1‐HL‐073412‐01), grants U01‐64829, U01‐HL649141, U01‐HL649241, UL1‐TR001427, and grants from the Gustavus and Louis Pfeiffer Research Foundation, Danville, New Jersey; The Women's Guild of Cedars‐Sinai Medical Center, Los Angeles, California; The Ladies Hospital Aid Society of Western Pennsylvania, Pittsburgh, Pennsylvania; QMED, Inc., Laurence Harbor, New Jersey; the Edythe L. Broad Endowment, the Barbra Streisand Women's Cardiovascular Research and Education Program, the Linda Joy Pollin Women's Heart Health Program, and the Constance Austin Fellowship Endowment, Cedars‐Sinai Medical Center, Los Angeles; and the Erika Glazer Women's Heart Health Project, Cedars‐Sinai Medical Center, Los Angeles, California.
REFERENCES
- 1. Jespersen L, Hvelplund A, Abildstrom SZ, et al. Stable angina pectoris with no obstructive coronary artery disease is associated with increased risks of major adverse cardiovascular events. Eur Heart J. 2012;33:734–744. [DOI] [PubMed] [Google Scholar]
- 2. Maddox TM, Stanislawski MA, Grunwald GK, et al. Nonobstructive coronary artery disease and risk of myocardial infarction. JAMA. 2014;312:1754–1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Agewall S, Beltrame JF, Reynolds HR, et al. ESC working group position paper on myocardial infarction with non‐obstructive coronary arteries. Eur Heart J. 2017;38:143–153. [DOI] [PubMed] [Google Scholar]
- 4. Shaw LJ, Shaw RE, Merz CN, et al. Impact of ethnicity and gender differences on angiographic coronary artery disease prevalence and in‐hospital mortality in the American College of Cardiology‐National Cardiovascular Data Registry. Circulation. 2008;117:1787–1801. [DOI] [PubMed] [Google Scholar]
- 5. Min JK, Dunning A, Lin FY, et al. Age‐ and sex‐related differences in all‐cause mortality risk based on coronary computed tomography angiography findings results from the International Multicenter CONFIRM (Coronary CT Angiography Evaluation for Clinical Outcomes: an International Multicenter Registry) of 23,854 patients without known coronary artery disease. J Am Coll Cardiol. 2011;58:849–860. [DOI] [PubMed] [Google Scholar]
- 6. Jespersen L, Abildstrom SZ, Hvelplund A, et al. Burden of hospital admission and repeat angiography in angina pectoris patients with and without coronary artery disease: a registry‐based cohort study. PLoS One. 2014;9:e93170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart disease and stroke statistics‐2017 update: a report from the American Heart Association. Circulation. 2017;135:e146–e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pepine CJ, Ferdinand KC, Shaw LJ, et al. Emergence of nonobstructive coronary artery disease: a woman's problem and need for change in definition on angiography. J Am Coll Cardiol. 2015;66:1918–1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sullivan AK, Holdright DR, Wright CA, Sparrow JL, Cunningham D, Fox KM. Chest pain in women: clinical, investigative, and prognostic features. BMJ. 1994;308:883–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gulati M, Cooper‐DeHoff RM, McClure C, et al. Adverse cardiovascular outcomes in women with nonobstructive coronary artery disease: a report from the Women's Ischemia Syndrome Evaluation Study and the St James Women Take Heart Project. Arch Intern Med. 2009;169:843–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sharaf B, Wood T, Shaw L, et al. Adverse outcomes among women presenting with signs and symptoms of ischemia and no obstructive coronary artery disease: findings from the National Heart, Lung, and Blood Institute‐sponsored Women's Ischemia Syndrome Evaluation (WISE) angiographic core laboratory. Am Heart J. 2013;166:134–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sedlak TL, Lee M, Izadnegahdar M, Merz CN, Gao M, Humphries KH. Sex differences in clinical outcomes in patients with stable angina and no obstructive coronary artery disease. Am Heart J. 2013;166:38–44. [DOI] [PubMed] [Google Scholar]
- 13. Bairey Merz CN, Pepine CJ, Walsh MN, Fleg JL. Ischemia and no obstructive coronary artery disease (INOCA): developing evidence‐based therapies and research agenda for the next decade. Circulation. 2017;135:1075–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gehrie ER, Reynolds HR, Chen AY, et al. Characterization and outcomes of women and men with non‐ST‐segment elevation myocardial infarction and nonobstructive coronary artery disease: results from the Can Rapid Risk Stratification of Unstable Angina Patients Suppress Adverse Outcomes with Early Implementation of the ACC/AHA Guidelines (CRUSADE) quality improvement initiative. Am Heart J. 2009;158:688–694. [DOI] [PubMed] [Google Scholar]
- 15. Pasupathy S, Air T, Dreyer RP, Tavella R, Beltrame JF. Systematic review of patients presenting with suspected myocardial infarction and nonobstructive coronary arteries. Circulation. 2015;131:861–870. [DOI] [PubMed] [Google Scholar]
- 16. Chokshi NP, Iqbal SN, Berger RL, et al. Sex and race are associated with the absence of epicardial coronary artery obstructive disease at angiography in patients with acute coronary syndromes. Clin Cardiol. 2010;33:495–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. De Ferrari GM, Fox KA, White JA, et al. Outcomes among non‐ST‐segment elevation acute coronary syndromes patients with no angiographically obstructive coronary artery disease: observations from 37,101 patients. Eur Heart J Acute Cardiovasc Care. 2014;3:37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Grodzinsky A, Arnold SV, Gosch K, et al. Angina frequency after acute myocardial infarction in patients without obstructive coronary artery disease. Eur Heart J Qual Care Clin Outcomes. 2015;1:92–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Planer D, Mehran R, Ohman EM, et al. Prognosis of patients with non‐ST‐segment‐elevation myocardial infarction and nonobstructive coronary artery disease: propensity‐matched analysis from the Acute Catheterization and Urgent Intervention Triage Strategy trial. Circ Cardiovasc Interv. 2014;7:285–293. [DOI] [PubMed] [Google Scholar]
- 20. Johnson BD, Shaw LJ, Pepine CJ, et al. Persistent chest pain predicts cardiovascular events in women without obstructive coronary artery disease: results from the NIH‐NHLBI‐sponsored Women's Ischaemia Syndrome Evaluation (WISE) study. Eur Heart J. 2006;27:1408–1415. [DOI] [PubMed] [Google Scholar]
- 21. Leipsic J, Taylor CM, Gransar H, et al. Sex‐based prognostic implications of nonobstructive coronary artery disease: results from the international multicenter CONFIRM study. Radiology. 2014;273:393–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Patel MR, Chen AY, Peterson ED, et al. Prevalence, predictors, and outcomes of patients with non‐ST‐segment elevation myocardial infarction and insignificant coronary artery disease: results from the Can Rapid risk stratification of Unstable angina patients Suppress ADverse outcomes with Early implementation of the ACC/AHA Guidelines (CRUSADE) initiative. Am Heart J. 2006;152:641–647. [DOI] [PubMed] [Google Scholar]
- 23. Biere L, Niro M, Pouliquen H, et al. Risk of ventricular arrhythmia in patients with myocardial infarction and non‐obstructive coronary arteries and normal ejection fraction. World J Cardiol. 2017;9:268–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pepine CJ. Multiple causes for ischemia without obstructive coronary artery disease: not a short list. Circulation. 2015;131:1044–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Libby P, Pasterkamp G. Requiem for the 'vulnerable plaque'. Eur Heart J. 2015;36:2984–2987. [DOI] [PubMed] [Google Scholar]
- 26. Vitiello L, Spoletini I, Gorini S, et al. Microvascular inflammation in atherosclerosis. IJC Metab Endocr. 2014;3:1–7. [Google Scholar]
- 27. Khuddus MA, Pepine CJ, Handberg EM, et al. An intravascular ultrasound analysis in women experiencing chest pain in the absence of obstructive coronary artery disease: a substudy from the National Heart, Lung and Blood Institute‐Sponsored Women's Ischemia Syndrome Evaluation (WISE). J Interv Cardiol. 2010;23:511–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lee BK, Lim HS, Fearon WF, et al. Invasive evaluation of patients with angina in the absence of obstructive coronary artery disease. Circulation. 2015;131:1054–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Reynolds HR, Srichai MB, Iqbal SN, et al. Mechanisms of myocardial infarction in women without angiographically obstructive coronary artery disease. Circulation. 2011;124:1414–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ouldzein H, Elbaz M, Roncalli J, et al. Plaque rupture and morphological characteristics of the culprit lesion in acute coronary syndromes without significant angiographic lesion: analysis by intravascular ultrasound. Ann Cardiol Angeiol (Paris). 2012;61:20–26. [DOI] [PubMed] [Google Scholar]
- 31. Camici PG, Crea F. Coronary microvascular dysfunction. N Engl J Med. 2007;356:830–840. [DOI] [PubMed] [Google Scholar]
- 32. Fukushima K, Javadi MS, Higuchi T, et al. Prediction of short‐term cardiovascular events using quantification of global myocardial flow reserve in patients referred for clinical 82Rb PET perfusion imaging. J Nucl Med. 2011;52:726–732. [DOI] [PubMed] [Google Scholar]
- 33. Murthy VL, Naya M, Foster CR, et al. Improved cardiac risk assessment with noninvasive measures of coronary flow reserve. Circulation. 2011;124:2215–2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ziadi MC, Dekemp RA, Williams KA, et al. Impaired myocardial flow reserve on rubidium‐82 positron emission tomography imaging predicts adverse outcomes in patients assessed for myocardial ischemia. J Am Coll Cardiol. 2011;58:740–748. [DOI] [PubMed] [Google Scholar]
- 35. Taqueti VR, Hachamovitch R, Murthy VL, et al. Global coronary flow reserve is associated with adverse cardiovascular events independently of luminal angiographic severity and modifies the effect of early revascularization. Circulation. 2015;131:19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Murthy VL, Naya M, Foster CR, et al. Association between coronary vascular dysfunction and cardiac mortality in patients with and without diabetes mellitus. Circulation. 2012;126:1858–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Brown Z, Kerensky RP, McGorray SP, et al. Bradykinin B1 receptor gene polymorphism is associated with impaired coronary reactivity in women: a report from the NHLBI WISE study. J Am Coll Cardiol. 2002;39:A217. [Google Scholar]
- 38. McNamara DM, Holubkov R, Wang JJ, Palmer A, Merz NB, Sharaf BL. The eNOS Asp298 variant is not associated with atherosclerosis or endothelial dysfunction: results from the NHLBI WISE study. Circulation. 1999;S718. [Google Scholar]
- 39. Ong P, Athanasiadis A, Borgulya G, et al. Clinical usefulness, angiographic characteristics, and safety evaluation of intracoronary acetylcholine provocation testing among 921 consecutive white patients with unobstructed coronary arteries. Circulation. 2014;129:1723–1730. [DOI] [PubMed] [Google Scholar]
- 40. Reriani M, Sara JD, Flammer AJ, et al. Coronary endothelial function testing provides superior discrimination compared with standard clinical risk scoring in prediction of cardiovascular events. Coron Artery Dis. 2016;27:213–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wei J, Mehta PK, Johnson BD, et al. Safety of coronary reactivity testing in women with no obstructive coronary artery disease: results from the NHLBI‐sponsored WISE (Women's Ischemia Syndrome Evaluation) study. JACC Cardiovasc interv. 2012;5:646–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kubo T, Imanishi T, Takarada S, et al. Assessment of culprit lesion morphology in acute myocardial infarction: ability of optical coherence tomography compared with intravascular ultrasound and coronary angioscopy. J Am Coll Cardiol. 2007;50:933–939. [DOI] [PubMed] [Google Scholar]
- 43. Rathore S, Terashima M, Matsuo H, et al. Association of coronary plaque composition and arterial remodelling: a optical coherence tomography study. Atherosclerosis. 2012;221:405–415. [DOI] [PubMed] [Google Scholar]
- 44. Taqueti VR, Everett BM, Murthy VL, et al. Interaction of impaired coronary flow reserve and cardiomyocyte injury on adverse cardiovascular outcomes in patients without overt coronary artery disease. Circulation. 2015;131:528–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mygind ND, Michelsen MM, Pena A, et al. Coronary microvascular function and cardiovascular risk factors in women with angina pectoris and no obstructive coronary artery disease: the iPOWER study. J Am Heart Assoc. 2016;5:e003064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lanza GA, Buffon A, Sestito A, et al. Relation between stress‐induced myocardial perfusion defects on cardiovascular magnetic resonance and coronary microvascular dysfunction in patients with cardiac syndrome X. J Am Coll Cardiol. 2008;51:466–472. [DOI] [PubMed] [Google Scholar]
- 47. Panting JR, Gatehouse PD, Yang GZ, et al. Abnormal subendocardial perfusion in cardiac syndrome X detected by cardiovascular magnetic resonance imaging. N Engl J Med. 2002;346:1948–1953. [DOI] [PubMed] [Google Scholar]
- 48. Thomson LE, Wei J, Agarwal M, et al. Cardiac magnetic resonance myocardial perfusion reserve index is reduced in women with coronary microvascular dysfunction. A National Heart, Lung, and Blood Institute‐sponsored study from the Women's Ischemia Syndrome Evaluation. Circ Cardiovasc Imaging. 2015;8(4):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mauricio R, Srichai MB, Axel L, Hochman JS, Reynolds HR. Stress cardiac MRI in women with myocardial infarction and nonobstructive coronary artery disease. Clin Cardiol. 2016;39:596–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lim TK, Choy AJ, Khan F, Belch JJ, Struthers AD, Lang CC. Therapeutic development in cardiac syndrome X: a need to target the underlying pathophysiology. Cardiovasc Ther. 2009;27:49–58. [DOI] [PubMed] [Google Scholar]
- 51. Parikh P, McDaniel MC, Ashen MD, et al. Diets and cardiovascular disease: an evidence‐based assessment. J Am Coll Cardiol. 2005;45:1379–1387. [DOI] [PubMed] [Google Scholar]
- 52. Ballantyne CM, Raichlen JS, Nicholls SJ, et al. Effect of rosuvastatin therapy on coronary artery stenoses assessed by quantitative coronary angiography: a study to evaluate the effect of rosuvastatin on intravascular ultrasound‐derived coronary atheroma burden. Circulation. 2008;117:2458–2466. [DOI] [PubMed] [Google Scholar]
- 53. Zhang X, Li Q, Zhao J, et al. Effects of combination of statin and calcium channel blocker in patients with cardiac syndrome X. Coron Artery Dis. 2014;25:40–44. [DOI] [PubMed] [Google Scholar]
- 54. Caliskan M, Erdogan D, Gullu H, et al. Effects of atorvastatin on coronary flow reserve in patients with slow coronary flow. Clin Cardiol. 2007;30:475–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Eshtehardi P, McDaniel MC, Dhawan SS, et al. Effect of intensive atorvastatin therapy on coronary atherosclerosis progression, composition, arterial remodeling, and microvascular function. J Invasive Cardiol. 2012;24:522–529. [PubMed] [Google Scholar]
- 56. Pizzi C, Manfrini O, Fontana F, Bugiardini R. Angiotensin‐converting enzyme inhibitors and 3‐hydroxy‐3‐methylglutaryl coenzyme A reductase in cardiac Syndrome X: role of superoxide dismutase activity. Circulation. 2004;109:53–58. [DOI] [PubMed] [Google Scholar]
- 57. Pauly DF, Johnson BD, Anderson RD, et al. In women with symptoms of cardiac ischemia, nonobstructive coronary arteries, and microvascular dysfunction, angiotensin‐converting enzyme inhibition is associated with improved microvascular function: a double‐blind randomized study from the National Heart, Lung and Blood Institute Women's Ischemia Syndrome Evaluation (WISE). Am Heart J. 2011;162:678–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Schwartzkopff B, Brehm M, Mundhenke M, Strauer BE. Repair of coronary arterioles after treatment with perindopril in hypertensive heart disease. Hypertension. 2000;36:220–225. [DOI] [PubMed] [Google Scholar]
- 59. Bavry AA, Handberg EM, Huo T, et al. Aldosterone inhibition and coronary endothelial function in women without obstructive coronary artery disease: an ancillary study of the national heart, lung, and blood institute‐sponsored women's ischemia syndrome evaluation. Am Heart J. 2014;167:826–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Garg R, Rao AD, Baimas‐George M, et al. Mineralocorticoid receptor blockade improves coronary microvascular function in individuals with type 2 diabetes. Diabetes. 2015;64:236–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Nishioka K, Nakagawa K, Umemura T,et al. Carvedilol improves endothelium‐dependent vasodilation in patients with dilated cardiomyopathy. Heart. 2007;93:247–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Sutsch G, Oechslin E, Mayer I, Hess OM. Effect of diltiazem on coronary flow reserve in patients with microvascular angina. Int J Cardiol. 1995;52:135–143. [DOI] [PubMed] [Google Scholar]
- 63. Cannon RO III, Watson RM, Rosing DR, Epstein SE. Efficacy of calcium channel blocker therapy for angina pectoris resulting from small‐vessel coronary artery disease and abnormal vasodilator reserve. Am J Cardiol. 1985;56:242–246. [DOI] [PubMed] [Google Scholar]
- 64. Bairey Merz CN, Handberg EM, Shufelt CL, et al. A randomized, placebo‐controlled trial of late Na current inhibition (ranolazine) in coronary microvascular dysfunction (CMD): impact on angina and myocardial perfusion reserve. Eur Heart J. 2016;37:1504–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Mehta PK, Goykhman P, Thomson LE, et al. Ranolazine improves angina in women with evidence of myocardial ischemia but no obstructive coronary artery disease. JACC Cardiovasc Imaging. 2011;4:514–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Villano A, Di Franco A, Nerla R, et al. Effects of ivabradine and ranolazine in patients with microvascular angina pectoris. Am J Cardiol. 2013;112:8–13. [DOI] [PubMed] [Google Scholar]
- 67. Yesildag O, Yazici M, Yilmaz O, Ucar R, Sagkan O. The effect of aminophylline infusion on the exercise capacity in patients with syndrome X. Acta Cardiol. 1999;54:335–337. [PubMed] [Google Scholar]
- 68. Elliott PM, Krzyzowska‐Dickinson K, Calvino R, Hann C, Kaski JC. Effect of oral aminophylline in patients with angina and normal coronary arteriograms (cardiac syndrome X). Heart. 1997;77:523–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Gellman J, Hare JM, Lowenstein CJ, et al. L‐arginine ameliorates the abnormal sympathetic response of the dysfunctional human coronary microvasculature. Angiology. 2004;55:1–8. [DOI] [PubMed] [Google Scholar]
- 70. Lerman A, Burnett JC Jr, Higano ST, McKinley LJ, Holmes DR Jr. Long‐term L‐arginine supplementation improves small‐vessel coronary endothelial function in humans. Circulation. 1998;97:2123–2128. [DOI] [PubMed] [Google Scholar]
- 71. Cannon RO III, Quyyumi AA, Mincemoyer R, et al. Imipramine in patients with chest pain despite normal coronary angiograms. N Engl J Med. 1994;330:1411–1417. [DOI] [PubMed] [Google Scholar]
- 72. Cox ID, Hann CM, Kaski JC. Low dose imipramine improves chest pain but not quality of life in patients with angina and normal coronary angiograms. Eur Heart J. 1998;19:250–254. [DOI] [PubMed] [Google Scholar]
- 73. Sestito A, Lanza GA, Le Pera D, et al. Spinal cord stimulation normalizes abnormal cortical pain processing in patients with cardiac syndrome X. Pain. 2008;139:82–89. [DOI] [PubMed] [Google Scholar]
- 74. Lanza GA, Sestito A, Sandric S, et al. Spinal cord stimulation in patients with refractory anginal pain and normal coronary arteries. Ital Heart J. 2001;2:25–30. [PubMed] [Google Scholar]
- 75. Kitsou V, Xanthos T, Roberts R, Karlis GM, Padadimitriou L. Enhanced external counterpulsation: mechanisms of action and clinical applications. Acta Cardiol. 2010;65:239–247. [DOI] [PubMed] [Google Scholar]
- 76. Kronhaus KD, Lawson WE. Enhanced external counterpulsation is an effective treatment for Syndrome X. Int J Cardiol. 2009;135:256–257. [DOI] [PubMed] [Google Scholar]
- 77. Asbury EA, Kanji N, Ernst E, Barbir M, Collins P. Autogenic training to manage symptomology in women with chest pain and normal coronary arteries. Menopause. 2009;16:60–65. [DOI] [PubMed] [Google Scholar]
- 78. Samim A, Nugent L, Mehta PK, Shufelt C, Bairey Merz CN. Treatment of angina and microvascular coronary dysfunction. Curr Treat Options Cardiovasc Med. 2010;12:355–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Lindahl B, Baron T, Erlinge D, et al. Medical therapy for secondary prevention and long‐term outcome in patients with myocardial infarction with nonobstructive coronary artery disease. Circulation. 2017;135:1481–1489. [DOI] [PubMed] [Google Scholar]
- 80. Douglas PS, Hoffmann U, Patel MR, et al. Outcomes of anatomical versus functional testing for coronary artery disease. N Engl J Med. 2015;372:1291–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Britten MB, Zeiher AM, Schachinger V. Microvascular dysfunction in angiographically normal or mildly diseased coronary arteries predicts adverse cardiovascular long‐term outcome. Coron Artery Dis. 2004;15:259–264. [DOI] [PubMed] [Google Scholar]
- 82. Schindler TH. Relationship between increasing body weight, insulin resistance, inflammation, adipocytokine leptin, and coronary circulatory function. J Am Coll Cardiol. 2006;47:1188–1195. [DOI] [PubMed] [Google Scholar]
- 83. Rigo F, Sicari R, Gherardi S, Djordjevic‐Dikic A, Cortigiani L, Picano E. Prognostic value of coronary flow reserve in medically treated patients with left anterior descending coronary disease with stenosis 51% to 75% in diameter. Am J Cardiol. 2007;100:1527–1531. [DOI] [PubMed] [Google Scholar]
- 84. Nemes A, Forster T, Geleijnse ML, Soliman OI, Cate FJ, Csanady M. Prognostic role of aortic atherosclerosis and coronary flow reserve in patients with suspected coronary artery disease. Int J Cardiol. 2008;131:45–50. [DOI] [PubMed] [Google Scholar]
- 85. Herzog BA. Long‐term prognostic value of 13N‐ammonia myocardial perfusion positron emission tomography added value of coronary flow reserve. J Am Coll Cardiol. 2009;54:150–156. [DOI] [PubMed] [Google Scholar]
- 86. Tio RA, Dabeshlim A, H‐MJ Siebelink, et al. Comparison between the prognostic value of left ventricular function and myocardial perfusion reserve in patients with ischemic heart disease. J Nucl Med. 2009;50:214–219. [DOI] [PubMed] [Google Scholar]
- 87. Cortigiani L, Rigo F, Gherardi S, et al. Prognostic effect of coronary flow reserve in women versus men with chest pain syndrome and normal dipyridamole stress echocardiography. Am J Cardiol. 2010;106:1703–1708. [DOI] [PubMed] [Google Scholar]
- 88. Pepine CJ, Anderson RD, Sharaf BL, et al. Coronary microvascular reactivity to adenosine predicts adverse outcome in women evaluated for suspected ischemia results from the National Heart, Lung and Blood Institute WISE (Women's Ischemia Syndrome Evaluation) study. J Am Coll Cardiol. 2010;55:2825–2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Balazs E, Pinter KS, Egyed A, Csanady M, Forster T, Nemes A. The independent long‐term prognostic value of coronary flow velocity reserve in female patients with chest pain and negative coronary angiograms (results from the Szeged study). Int J Cardiol. 2011;146:259–261. [DOI] [PubMed] [Google Scholar]
- 90. Murthy VL, Naya M, Taqueti VR, et al. Effects of sex on coronary microvascular dysfunction and cardiac outcomes. Circulation. 2014;129:2518–2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Mehta PK, Bairey Merz CN. Treatment of angina in subjects with evidence of myocardial ischemia and no obstructive coronary artery disease. In: Braunwald's Heart Disease. Philadelphia, PA: Elsevier; 2011. [Google Scholar]


