Abstract
Background
Left ventricular ejection fraction (LVEF) is a major determinant of long‐term prognosis after ST‐segment elevation myocardial infarction (STEMI). STEMI patients with reduced LVEF have a poor prognosis, despite successful reperfusion and the use of renin‐angiotensin‐aldosterone inhibitors.
Hypothesis
Intracoronary infusion of bone marrow–derived mononuclear cells (BMMC) may improve LVEF in STEMI patients successfully reperfused.
Methods
The main inclusion criteria for this double‐blind, randomized, multicenter study were patient age 30 to 80 years, LVEF ≤50%, successful angioplasty of infarct‐related artery, and regional dysfunction in the infarct‐related area analyzed before cell injection. Cardiac magnetic resonance imaging was used to assess LVEF, left ventricular volumes, and infarct size at 7 to 9 days and 6 months post–myocardial infarction.
Results
One hundred and twenty‐one patients were included (66 patients in the BMMC group and 55 patients in the placebo group). The primary endpoint, mean LVEF, was similar between both groups at baseline (44.63% ± 10.74% vs 42.23% ± 10.33%; P = 0.21) and at 6 months (44.74% ± 12.95 % vs 43.50 ± 12.43%; P = 0.59). The groups were also similar regarding the difference between baseline and 6 months (0.11% ± 8.5% vs 1.27% ± 8.93%; P = 0.46). Other parameters of left ventricular remodeling, such as systolic and diastolic volumes, as well as infarct size, were also similar between groups.
Conclusions
In this randomized, multicenter, double‐blind trial, BMMC intracoronary infusion did not improve left ventricular remodeling or decrease infarct size.
Keywords: Acute Coronary Syndrome, Cardiomyopathy, Ischemic Heart Disease, Myocardial Infarction, Stem Cell Therapy
1. INTRODUCTION
Despite advances in cardiovascular disease care, ischemic heart disease is still the leading cause of death worldwide.1 In patients with ST‐segment elevation myocardial infarction (STEMI), left ventricle (LV) dysfunction is a major determinant of long‐term prognosis.2, 3, 4, 5 The most effective strategy to avoid LV dysfunction after STEMI is to promptly restore blood flow at the culprit artery.6 However, this is not achieved in many patients.7, 8 Further, even in those patients promptly reperfused, microvascular obstruction due to microthrombi can threaten infarcted muscle and compromise LV function.9, 10, 11
Once muscle dysfunction is established, pharmacological treatment is the best way to halt further progression of LV damage.12, 13, 14, 15 Additionally, implantable cardioverter‐defibrillators can further improve prognosis by decreasing sudden deaths.16, 17 However, they do not target ventricular failure itself; instead, they just treat one of its consequences: potentially fatal arrhythmias. Therefore, further improving prognosis of this population remains an unmet clinical need.
A new promising therapy is to implant stem cells into the myocardium after MI. Stem cells could have regenerative properties18 and potentiate neoangiogenesis.19 Their main mechanism seems to be paracrine actions, which decrease apoptosis and abate inflammatory response.20, 21
About a decade ago, 2 randomized clinical trials showed, in the short run (≤6 months), better LV remodeling in patients given intracoronary bone marrow–derived cells.22, 23, 24 By contrast, other trials failed to show LV function improvement, although they demonstrated reduction in infarct size,25, 26, 27, 28 whereas others failed to report any benefit.29, 30, 31, 32 Two recently published meta‐analyses33, 34 showed similar results: cell treatment led to significant improvement in LVEF (by 2%–5%) when measured by echocardiography, single‐photon emission computed tomography, and left ventriculography, but not with magnetic resonance imaging (MRI).
To help clarify these conflicting results, we evaluated whether autologous bone marrow–derived mononuclear cell (BMMC) therapy affects LV function in patients with STEMI successfully reperfused, via either primary percutaneous coronary intervention (PCI) or fibrinolytic therapy. Among other important characteristics (such as the double‐blind utilization of BMMC and depressed LVEF as an inclusion criterion), in the present study the baseline MRI was accomplished about 8 days after the culprit‐artery recanalization, taking into account that, at this time, most of the myocardial stunning has likely resolved.
2. METHODS
This trial reports the findings of the acute myocardial infarction (AMI) arm of the MiHeart study (known as the MiHeart/AMI study). The MiHeart study (http://www.clinicaltrials.gov NCT00350766) is a multicenter randomized trial of cell therapy in cardiomyopathies, which analyzed the role of stem cells in patients with dilated cardiomyopathy, Chagas disease, chronic ischemic cardiomyopathy, and AMI.35
Eighteen sites across Brazil participated in the MiHeart/AMI study and enrolled ≥1 patient (for names of facilities, along with the principal investigators' names, see Supporting Information, Appendix, in the online version of this article). Full eligibility criteria were previously published.36 The main inclusion criteria were patient age 30 to 80 years, successful (Thrombolysis In Myocardial Infarction flow grade 3) recanalization of the infarct‐related artery with abnormal contractility in the infarct‐related area assessed before BMMC injection, and LVEF of ≤50%. The LVEF for inclusion was initially evaluated by the Dodge method using contrasted ventriculography (17 patients), and after specific amendment to the protocol, by the Simpson method using echocardiography (104 patients).
Patients were excluded if they had an obstruction of >50% at the left main coronary artery or multivessel coronary disease requiring coronary artery bypass grafting; no need for coronary intervention with a stent; final diastolic LV pressure of >30 mmHg; creatinine of >2.0 mg/dL; cardiac arrest or Killip class IV at admission; significant valvular disease or mechanical complication; sustained ventricular tachycardia occurring >48 hours after MI; sepsis or myocarditis; severe extracardiac disease; chronic inflammatory or infectious disease; any comorbidity affecting survival in the following 2 years; or refusal to sign the informed consent form.
The study protocol conformed to the recommendations of the Helsinki Declaration and Good Clinical Practice norms on medical research in humans. The protocol was approved by each local ethics committee from the 18 participating centers. All patients signed the consent form before randomization.
Patients were randomly assigned (1:1) via computer‐generated block randomization using R software, version 1.9.0 (R Foundation for Statistical Computing, http://www.r-project.org), with variable block size (blocks of 2, 4, or 6 patients), to receive either BMMC or placebo. The complete sequence of randomization was recorded in 2 computer servers, one located at the National Institute of Cardiology and another at the Rio de Janeiro Federal University. Only the 2 statisticians responsible for the randomization program had access to all randomization sequences.
Randomization was done after bone‐marrow aspiration. Only the hematologist responsible for cell separation had access to the online randomization system and was not masked to treatment allocation. According to the assigned group, the hematologist prepared a darkened syringe containing BMMC or placebo and sent it to the catheterization laboratory for injection. Patients and study investigators were masked to treatment allocation.
All patients had coronary angiography. Those who were submitted to fibrinolytic therapy had angiography up to 72 hours afterward. All culprit coronary arteries received ≥1 VeriFLEX bare metal stent (Boston Scientific, Marlborough, MA).
Mononuclear cell and placebo infusions were done 6 to 9 days after AMI using the Maverick over‐the‐wire balloon catheter (Boston Scientific) to transiently interrupt anterograde blood flow during infusions. All patients received 10 000 IU of intravenous unfractionated heparin after sheath insertion. Cell solutions or placebo were infused through the central lumen of the balloon catheter, in about 40 seconds, during 3 coronary occlusions, each lasting 2 to 3 minutes, followed by 2 minutes of balloon deflation.
For stem‐cell treatment, 100 mL of bone‐marrow aspirate was obtained from the iliac crest under local anesthesia and sedation at an intensive care unit or surgical center. The BMMC were isolated by density gradient centrifugation on Ficoll‐Paque PLUS (GE Healthcare, Pittsburgh, PA) and manipulated under aseptic conditions at each participating center. 100 × 106 cells were re‐suspended in 10‐mL saline solution with 5% autologous serum and filtered through 10‐μm nylon mesh to remove cell aggregates for injection. Cell viability needed to be >90% to be injected (median, 97% in the treated group and 98% in the control group).
Two to three hours after processing (and 6 hours after bone‐marrow aspiration), a syringe containing the assigned treatment was sent to the catheterization laboratory for intracoronary infusion. Patients in the placebo group received 10 mL saline solution with 5% autologous serum.
The primary endpoint was mean improvement in LVEF at 6 months, analyzed by MRI. Secondary endpoints included other LV remodeling. Only patients with readable MRI results at baseline and 6 months were included in this analysis.
2.1. Statistical analysis
We originally calculated that we needed 300 patients to detect an absolute difference in LVEF of 5% between the groups34; but we had to stop recruitment at 160 patients because of difficulties in finding eligible patients, with consequent financial exhaustion. Considering that the mean LVEF from the initial echocardiogram of the population included in this analysis was 39.34% (± 6.5%), we recalculated a sample size of 84 patients (42 per group) to have 80% power to detect an absolute difference in LVEF of 5% (as in the original calculation) between the stem‐cell and control groups at an α level of 0.01.
Categorical variables are described as absolute numbers and percentages and were compared using the χ2 test or Fisher exact test when indicated. Continuous variables are described as mean ± SD; we used the Student t test (normal distribution) or Mann–Whitney U test (non‐Gaussian distribution) to compare the stem‐cell and placebo groups, and the Kolmogorov–Smirnov test to assess normality of the distribution.
Logistic regression was used to explore the eventual interaction between ΔLVEF (calculated from LVEF at 6 months minus LVEF at baseline) and different subgroups. For interaction analyses, ΔLVEF was categorized as either above the median of 2% (which included 44.6% of patients) or below or equal to 2% (55.4% of patients).
All tests were 2‐tailed, and a P value of <0.05 was considered statistically significant. We used SPSS version 21.0 (IBM Corp., Armonk, NY) for statistical calculations.
3. RESULTS
We screened 538 patients for eligibility, of whom 160 were randomly assigned to the stem‐cell or placebo groups between September 2006 and July 2013 (Figure 1).
Figure 1.

Trial profile. Abbreviations: BMT, bone marrow transplantation; EF, ejection fraction; LTFU, lost to follow‐up; MRI, magnetic resonance imaging; PCI, percutaneous coronary intervention
At 6 months, 121 patients were eligible to be included in the final analysis. The mean age of this population was 59.02 years (SD, 9.17 years), and 93 patients (81.0%) were male. Table 1 shows the baseline characteristics of the population. The stem‐cell and placebo groups were well matched in terms of their characteristics and medical history. Moreover, they were well treated by the time of randomization, with >90% using statin and dual antiplatelet therapy, and >80% taking β‐blockers and angiotensin inhibitors (angiotensin‐converting enzyme inhibitor or angiotensin receptor blocker). In terms of initial recanalization therapy, 57.0% had fibrinolytic therapy and the remainder had primary PCI.
Table 1.
Baseline patient characteristics
| Stem‐Cell Group, n = 66 | Placebo Group, n = 55 | |
|---|---|---|
| Age at randomization, y | 59.23 (9.44) | 58.72 (9.30) |
| Time to hospital arrival, ha | 7.34 (11.72) | 8.14 (14.57) |
| Time to recanalization, hb | 9.82 (13.30) | 11.25 (19.56) |
| DAP, mm Hg | 82.40 (20.38) | 80.91 (12.24) |
| SAP, mm Hg | 130.24 (29.72) | 129.89 (27.54) |
| LVEF (Simpson method), % | 41.74 (9.97) | 41.93 (8.21) |
| TIMI 3 flow after PCI | 61 (93.84) | 50 (90.90) |
| Male sex | 53 (80.30) | 45 (81.81) |
| Anterior‐wall AMI location | 57 (89.06) | 51 (92.73) |
| Killip class I at admission | 54 (81.82) | 44 (80.00) |
| Microvascular obstruction at baseline MRIc | 34 (69.39) | 26 (72.22) |
| Medical history | ||
| Previous PCId | 7 (10.60) | 1 (1.82) |
| Previous ischemic stroke | 1 (1.52) | 3 (5.45) |
| Previous DM | 15 (22.73) | 6 (10.91) |
| Previous HTN | 38 (57.57) | 28 (50.91) |
| Previous dyslipidemia | 24 (36.36) | 13 (23.64) |
| Previous AMI | 9 (13.64) | 2 (3.64) |
| Family history of CAD | 25 (37.88) | 17 (30.91) |
| Previous HF | 2 (3.03) | 0 (0) |
| Medical therapies at randomization | ||
| DAPT | 60 (90.91) | 54 (98.18) |
| β‐Βlocker | 55 (85.94) | 44 (80.00) |
| ACEI/ARB | 60 (90.91) | 47 (85.45) |
| Statin | 62 (95.38) | 54 (98.18) |
| Furosemide | 20 (31.75) | 20 (37.04) |
| Fibrinolytic therapy | 36 (54.45) | 33 (60.00) |
Abbreviations: ACEI, angiotensin‐converting enzyme inhibitor; AMI, acute myocardial infarction; ARB, angiotensin II receptor blocker; CAD, coronary artery disease; DAP, diastolic arterial pressure; DAPT, dual antiplatelet therapy; DM, diabetes mellitus; HF, heart failure; HTN, hypertension; LVEF, left ventricular ejection fraction; MRI, magnetic resonance imaging; PCI, percutaneous coronary intervention; SAP, systolic arterial pressure; SD, standard deviation; TIMI, Thrombolysis In Myocardial Infarction.
P values for the difference between the stem cell and placebo groups are >0.05 for all characteristics.
Data was missing for TIMI flow (1 patient), infarct location (2 patients), β‐blocker (2 patients), statin (1 patient), furosemide (4 patients), and fibrinolytic (1 patient).
Data are presented as n (%) or mean (SD).
Time to hospital arrival is the time between initiation of symptoms and the patient arriving at hospital.
Time to recanalization is the time between initiation of symptoms and the patient having the recanalization procedure (fibrinolytic or primary angioplasty).
This information was available for 85 patients (49 in stem cell group and 36 in placebo group).
No patient had been submitted to surgical revascularization.
The primary endpoint of mean LVEF was similar between the 2 groups at baseline and 6‐month follow‐up, as was ΔLVEF. We found no statistical differences in other indicators of LV remodeling, such as systolic and diastolic volumes and infarct size (Table 2, Figure 2).
Table 2.
MRI results for indicators of LV remodeling (whole population)
| Stem‐Cell Group, n = 66 | Placebo Group, n = 55 | P Value | 95% CI of the Difference | |
|---|---|---|---|---|
| LVEF, % | ||||
| Baseline | 44.63 (10.74) | 42.23 (10.33) | 0.21 | –6.21 to 1.40 |
| 6 mo | 44.74 (12.95) | 43.50 (12.43) | 0.59 | –5.82 to 3.34 |
| Δa | 0.11 (8.5) | 1.27 (8.93) | 0.46 | –1.98 to 4.31 |
| EDVi, mL/m2 | ||||
| Baseline | 86.21 (24.53) | 84.27 (26.00) | 0.67 | –11.05 to 7.17 |
| 6 mo | 97.13 (28.70) | 98.53 (24.59) | 0.78 | –8.34 to 11.12 |
| Δ | 10.92 (31.83) | 14.25 (27.88) | 0.46 | –7.55 to 14.21 |
| ESVi, mL/m2 | ||||
| Baseline | 48.15 (19.36) | 49.35 (20.87) | 0.41 | –6.05 to 8.45 |
| 6 mo | 56.22 (26.65) | 58.93 (25.35) | 0.57 | –6.71 to 12.14 |
| Δ | 8.06 (23.59) | 9.58 (20.02) | 0.71 | –6.45 to 9.49 |
| Infarct size, g | ||||
| Baseline | 44.58 (22.89) | 47.53 (26.65) | 0.53 | –6.25 to 12.24 |
| 6 mo | 33.98 (17.88) | 37.67 (19.84) | 0.30 | –3.28 to 10.66 |
| Δ | –9.44 (26.91) | –9.31 (23.27) | 0.94 | –9.03 to 9.28 |
Abbreviations: CI, confidence interval; EDVi, end‐diastolic volume indexed; ESVi, end‐systolic volume indexed; LV, left ventricular; LVEF, left ventricular ejection fraction; MRI, magnetic resonance imaging; SD, standard deviation.
Indexed refers to absolute value divided by each individual body area.
Data are presented as mean (SD).
Δ refers to the value at 6 months minus value at baseline.
Figure 2.
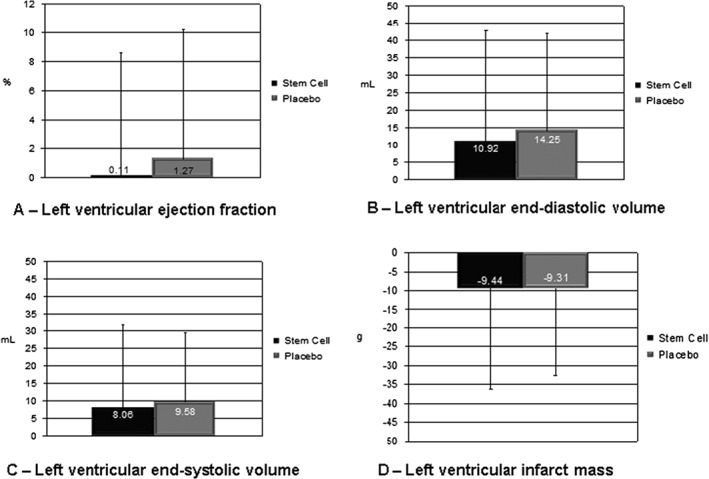
Difference in MRI results between baseline and 6 months. P values for the difference between the stem‐cell and placebo groups are >0.05 for all indicators. Abbreviations: MRI, magnetic resonance imaging
Seventeen out of the 121 patients had LVEF >50% on initial echocardiography and were included based on the contrasted ventriculography analysis that showed LVEF of ≤50%. We analyzed the MRI results excluding these 17 patients and, as for the whole population, we found no significant differences between the stem‐cell and placebo groups for any indicators of LV remodeling (see Supporting Information, Table S1, in the online version of this article).
Figure 3 shows the results for different subgroups that could have influenced the main results. We found no significant interaction between any of the analyzed subgroups and ΔLVEF. Of note, there was no interaction with sex, STEMI location (anterior vs nonanterior), or method of reperfusion (primary PCI vs fibrinolytic).
Figure 3.

Forest plot showing the ORs for the proportion of patients with ΔLVEF above the median of 2% between treated and placebo group across prespecified subgroups. Interaction with statin and DAPT was not analyzed because of the very small number of patients not taking these drugs. Δ = LVEF at 6 months minus LVEF at baseline. Abbreviations: AMI, acute myocardial infarction; CI, confidence interval; DAPT, dual antiplatelet therapy; LVEF, left ventricular ejection fraction; OR, odds ratio
4. DISCUSSION
The use of stem‐cell therapies for STEMI has been extensively investigated since the beginning of the millennium. At first, small trials examined the safety of intracoronary injection of BMMC; then the safety profile, combined with putative positive effects on LVEF, led to larger trials designed to evaluate the efficacy of the procedure. Wollert and colleagues22 were among the first to report beneficial effects of intracoronary injection of BMMC from a randomized controlled trial of 60 patients with STEMI who had been successfully treated with PCI.
In 2006, 2 papers published back‐to‐back in the New England Journal of Medicine reported conflicting results. In a multicenter, double‐blind, randomized trial of 204 patients, Schächinger and colleagues found that patients who received intracoronary injection of BMMC had significant improvement in LVEF, measured by ventricular angiography.23 By contrast, in a 2‐center, single‐blind randomized study of 100 patients, Lunde and colleagues could not detect any improvement in LVEF after 6 months by single‐photon emission computed tomography, echocardiography, or MRI (with baseline MRI obtained 2 to 3 weeks after cell infusion) in patients who received the same treatment.29 Since then, several positive studies have been published either reporting increases in LVEF or decreases in scar tissue, by various methods. Meta‐analyses of these trials, although mostly favoring cell therapy,33, 34 also have been controversial.37
These conflicting reports have led to many different theories to justify the discrepancy in results. For example, the results could be affected by baseline LVEF, the presence of heparin in the stem‐cell solution, the stem‐cell separation method, and the time between initiation of symptoms and stem‐cell injection.23, 30, 31, 38 To investigate these factors, the Cardiovascular Cell Therapy Research Network (CCTRN) examined the effect of intracoronary BMMC injection 2 to 3 weeks after PCI in 87 patients, who were randomly assigned in a 2:1 ratio to receive stem cells or placebo. In this multicenter, double‐blind, placebo‐controlled trial, Traverse and colleagues could not find differences in global and regional LV function by MRI.30 In another CCTRN trial, the timing of intracoronary BMMC injection at 3 or 7 days after PCI for anterior STEMI was compared with placebo in 120 patients. Again, MRI results of global and regional LV function showed no difference between the 2 groups.31
Our results are similar to those found by the CCTRN. Cell injection times were roughly equivalent (3–6 days after PCI in our study), but injected cell numbers were 50% higher in CCTRN (108 cells in our study). As with the Autologous Stem‐Cell Transplantation in Acute Myocardial Infarction (ASTAMI),29 Timing In Myocardial Infarction Evaluation (TIME),31 and Swiss Multicenter Intracoronary Stem Cells Study in Acute Myocardial Infarction (SWISS‐AMI)39 studies, we could not find differences between the stem‐cell and placebo groups regarding global LVEF, LV volumes, or infarct size using MRI at 6 to 9 days after stem‐cell or placebo injection and at 6‐month follow‐up. Furthermore, we could not find interactions between ΔLVEF and clinically relevant variables (such as age, sex; presence of diabetes mellitus; use of β‐blockers, angiotensin‐converting enzyme inhibitors, and furosemide; anterior‐wall infarct; and baseline LVEF) that were higher or lower than 40%.
4.1. Study limitations
The strengths of our study rely on the use of MRI to assess ventricular function and infarct size; the large number of patients enrolled (almost 50% more than the recalculated number needed to detect a 5% increase in LVEF with an α level of 0.01); the equal randomization using variable block sizes; the low LVEF at baseline (near 40%); and the multicenter, double‐blind, placebo‐controlled design. The main limitations were the lack of a core cell‐processing laboratory, the unbalanced enrollment by the centers, and the use of the surrogate endpoint of LVEF. This last issue is common to all published stem‐cell trials in STEMI; that is, LVEF improvement may not be the ideal endpoint to investigate cell‐infusion efficacy due to its dynamic changes in the acute phase.
The Effect of Intracoronary Reinfusion of Bone Marrow–Derived Mononuclear Cells on All Cause Mortality in Acute Myocardial Infarction (BAMI; http://www.clinicaltrials.gov NCT01569178) probably will be the definitive test for BMMC therapy in AMI, because it will rely on the hard endpoint of mortality. Nonetheless, the use of other cell types—such as mesenchymal stem cells, endogenous cardiac progenitors, and those derived from pluripotent cells—is ongoing in preclinical and initial clinical studies, bringing hope that stem‐cell therapies will in fact be proven as an effective treatment for patients with MI and other cardiac diseases.
5. CONCLUSION
In the present study, intracoronary delivery of autologous BMMC to patients with STEMI did not improve LV function or decrease scar size.
CONFLICTS OF INTEREST
The authors declare no potential conflicts of interest.
Supporting information
Appendix S1 Appendix: Participant sites
Table S1 MRI results for indicators of left ventricular remodeling in patients with LVEF of 50% or less on initial echocardiogram
ACKNOWLEDGMENTS
The authors are indebted to Katrina Phillips for her editorial support of the manuscript, to Clerio Azevedo for the MRI analyses, and to Bernardo Tura and Eduardo Hill for the coordination of randomization and data‐management processes.
Nicolau JC, Furtado RHM, Silva SA, et al. Stem‐cell therapy in ST‐segment elevation myocardial infarction with reduced ejection fraction: A multicenter, double‐blind randomized trial. Clin Cardiol. 2018;41:392–399. 10.1002/clc.22882
Funding information This work was supported by a grant from the Ministry of Health–Brazil and the Finance Agency for Studies and Projects (FINEP).
REFERENCES
- 1. Global Burden of Disease 2013 Mortality and Causes of Death Collaborators . Global, regional, and national age‐sex specific all‐cause and cause‐specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385:117–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Volpi A, De Vita C, Franzosi MG, et al; The Ad Hoc Working Group of the Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto Miocardico (GISSI)‐2 Database . Determinants of 6‐month mortality in survivors of myocardial infarction after thrombolysis: results of the GISSI‐2 database. Circulation. 1993;88:416–429. [DOI] [PubMed] [Google Scholar]
- 3. Richards AM, Nicholls MG, Espiner EA, et al. B‐type natriuretic peptides and ejection fraction for prognosis after myocardial infarction. Circulation. 2003;107:2786–2792. [DOI] [PubMed] [Google Scholar]
- 4. Dagres N, Hindricks G. Risk stratification after myocardial infarction: is left ventricular ejection fraction enough to prevent sudden cardiac death? Eur Heart J. 2013;34:1964–1971. [DOI] [PubMed] [Google Scholar]
- 5. Brooks GC, Lee BK, Rao R, et al; PREDICTS Investigators . Predicting persistent left ventricular dysfunction following myocardial infarction: the PREDICTS Study. J Am Coll Cardiol. 2016;67:1186–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schömig A, Ndrepepa G, Mehilli J, et al. Therapy‐dependent influence of time‐to‐treatment interval on myocardial salvage in patients with acute myocardial infarction treated with coronary artery stenting or thrombolysis. Circulation. 2003;108:1084–1088. [DOI] [PubMed] [Google Scholar]
- 7. Alter DA, Ko DT, Newman A, et al. Factors explaining the under‐use of reperfusion therapy among ideal patients with ST‐segment elevation myocardial infarction. Eur Heart J. 2006;27:1539–1549. [DOI] [PubMed] [Google Scholar]
- 8. Nicolau JC, Franken M, Lotufo PA, et al. Use of demonstrably effective therapies in the treatment of acute coronary syndromes: comparison between different Brazilian regions. Analysis of the Brazilian Registry on Acute Coronary Syndromes (BRACE) [article in English, Portuguese, and Spanish]. Arq Bras Cardiol. 2012;98:282–289. [DOI] [PubMed] [Google Scholar]
- 9. Wu KC, Zerhouni EA, Judd RM, et al. Prognostic significance of microvascular obstruction by magnetic resonance imaging in patients with acute myocardial infarction. Circulation. 1998;97:765–772. [DOI] [PubMed] [Google Scholar]
- 10. Nicolau JC, Maia LN, Vitola J, et al. ST‐segment resolution and late (6‐month) left ventricular remodeling after acute myocardial infarction. Am J Cardiol. 2003;91:451–453. [DOI] [PubMed] [Google Scholar]
- 11. de Lemos JA, Antman EM, Giugliano RP, et al; Thrombolysis in Myocardial Infarction (TIMI) 14 investigators . ST‐segment resolution and infarct‐related artery patency and flow after thrombolytic therapy. Am J Cardiol. 2000;85:299–304. [DOI] [PubMed] [Google Scholar]
- 12. Pfeffer MA, Braunwald E, Moyé LA, et al; SAVE Investigators . Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction: results of the Survival and Ventricular Enlargement trial. N Engl J Med. 1992;327:669–677. [DOI] [PubMed] [Google Scholar]
- 13. Dargie HJ. Effect of carvedilol on outcome after myocardial infarction in patients with left‐ventricular dysfunction: the CAPRICORN randomised trial. Lancet. 2001;357:1385–1390. [DOI] [PubMed] [Google Scholar]
- 14. Pitt B, Remme W, Zannad F, et al; Eplerenone Post‐Acute Myocardial Infarction Heart Failure Efficacy and Survival Study Investigators . Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction [published correction appears in N Engl J Med. 2003;348:2271]. N Engl J Med. 2003;348:1309–1321. [DOI] [PubMed] [Google Scholar]
- 15. Pfeffer MA, McMurray JJ, Velazquez EJ, et al. Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both. N Engl J Med. 2003;349:1893–1906. [DOI] [PubMed] [Google Scholar]
- 16. Moss AJ, Zareba W, Hall WJ, et al; Multicenter Automatic Defibrillator Implantation Trial II Investigators . Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877–883. [DOI] [PubMed] [Google Scholar]
- 17. Hohnloser SH, Israel CW. Current evidence base for use of the implantable cardioverter‐defibrillator. Circulation. 2013;128:172–183. [DOI] [PubMed] [Google Scholar]
- 18. Makkar RR, Smith RR, Cheng K, et al. Intracoronary cardiosphere‐derived cells for heart regeneration after myocardial infarction (CADUCEUS): a prospective, randomised phase 1 trial. Lancet. 2012;379:895–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kamihata H, Matsubara H, Nishiue T, et al. Implantation of bone marrow mononuclear cells into ischemic myocardium enhances collateral perfusion and regional function via side supply of angioblasts, angiogenic ligands, and cytokines. Circulation. 2001;104:1046–1052. [DOI] [PubMed] [Google Scholar]
- 20. Kelkar AA, Butler J, Schelbert EB, et al. Mechanisms contributing to the progression of ischemic and nonischemic dilated cardiomyopathy: possible modulating effects of paracrine activities of stem cells. J Am Coll Cardiol. 2015;66:2038–2047. [DOI] [PubMed] [Google Scholar]
- 21. Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815–1822. [DOI] [PubMed] [Google Scholar]
- 22. Wollert KC, Meyer GP, Lotz J, et al. Intracoronary autologous bone‐marrow cell transfer after myocardial infarction: the BOOST randomised controlled clinical trial. Lancet. 2004;364:141–148. [DOI] [PubMed] [Google Scholar]
- 23. Schächinger V, Erbs S, Elsässer A, et al. REPAIR‐AMI Investigators. Intracoronary bone marrow–derived progenitor cells in acute myocardial infarction. N Engl J Med. 2006;355:1210–1221. [DOI] [PubMed] [Google Scholar]
- 24. Hare JM, Traverse JH, Henry TD, et al. A randomized, double‐blind, placebo‐controlled, dose‐escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J Am Coll Cardiol. 2009;54:2277–2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Janssens S, Dubois C, Bogaert J, et al. Autologous bone marrow–derived stem‐cell transfer in patients with ST‐segment elevation myocardial infarction: double‐blind, randomised controlled trial. Lancet. 2006;367:113–121. [DOI] [PubMed] [Google Scholar]
- 26. Tendera M, Wojakowski W, Ruzyłło W, et al; REGENT Investigators . Intracoronary infusion of bone marrow–derived selected CD34+CXCR4+ cells and nonselected mononuclear cells in patients with acute STEMI and reduced left ventricular ejection fraction: results of randomized, multicentre Myocardial Regeneration by Intracoronary Infusion of Selected Population of Stem Cells in Acute Myocardial Infarction (REGENT) Trial. Eur Heart J. 2009;30:1313–1321. [DOI] [PubMed] [Google Scholar]
- 27. San Roman JA, Sánchez PL, Villa A, et al. Comparison of different bone marrow–derived stem cell approaches in reperfused STEMI: a multicenter, prospective, randomized, open‐labeled TECAM trial. J Am Coll Cardiol. 2015;65:2372–2382. [DOI] [PubMed] [Google Scholar]
- 28. Choudry F, Hamshere S, Saunders N, et al. A randomized double‐blind control study of early intracoronary autologous bone marrow cell infusion in acute myocardial infarction: the REGENERATE‐AMI clinical trial. Eur Heart J. 2016;37:256–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lunde K, Solheim S, Aakhus S, et al. Intracoronary injection of mononuclear bone marrow cells in acute myocardial infarction. N Engl J Med. 2006;355:1199–1209. [DOI] [PubMed] [Google Scholar]
- 30. Traverse JH, Henry TD, Ellis SG, et al; Cardiovascular Cell Therapy Research Network . Effect of intracoronary delivery of autologous bone marrow mononuclear cells 2 to 3 weeks following acute myocardial infarction on left ventricular function: the LateTIME randomized trial. JAMA. 2011;306:2110–2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Traverse JH, Henry TD, Pepine CJ, et al. Cardiovascular Cell Therapy Research Network (CCTRN) . Effect of the use and timing of bone marrow mononuclear cell delivery on left ventricular function after acute myocardial infarction: the TIME randomized trial [published correction appears in JAMA. 2013;309:343]. JAMA. 2012;308:2380–2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Meyer GP, Wollert KC, Lotz J, et al. Intracoronary bone marrow cell transfer after myocardial infarction: eighteen months' follow‐up data from the randomized, controlled BOOST (Bone Marrow Transfer to Enhance ST‐Elevation Infarct Regeneration) trial. Circulation. 2006;113:1287–1294. [DOI] [PubMed] [Google Scholar]
- 33. Fisher SA, Zhang H, Doree C, et al. Stem cell treatment for acute myocardial infarction. Cochrane Database Syst Rev. 2015;CD006536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. de Jong R, Houtgraaf JH, Samiei S, et al. Intracoronary stem cell infusion after acute myocardial infarction: a meta‐analysis and update on clinical trials [published correction appears in Circ Cardiovasc Interv. 2014;7:424]. Circ Cardiovasc Interv. 2014;7:156–167. [DOI] [PubMed] [Google Scholar]
- 35. Tura BR, Martino HF, Gowdak LH, et al. Multicenter randomized trial of cell therapy in cardiopathies—MiHeart Study. Trials. 2007;8:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dohmann HF, Silva SA, Sousa AL, et al. Multicenter double blind trial of autologous bone marrow mononuclear cell transplantation through intracoronary injection post acute myocardium infarction—MiHeart/AMI study. Trials. 2008;9:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nowbar AN, Mielewczik M, Karavassilis M, et al; DAMASCENE Writing Group . Discrepancies in autologous bone marrow stem cell trials and enhancement of ejection fraction (DAMASCENE): weighted regression and meta‐analysis. BMJ. 2014;348:g2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Seeger FH, Tonn T, Krzossok N, et al. Cell isolation procedures matter: a comparison of different isolation protocols of bone marrow mononuclear cells used for cell therapy in patients with acute myocardial infarction. Eur Heart J. 2007;28:766–772. [DOI] [PubMed] [Google Scholar]
- 39. Sürder D, Manka R, Lo Cicero V, et al. Intracoronary injection of bone marrow–derived mononuclear cells early or late after acute myocardial infarction: effects on global left ventricular function. Circulation. 2013;127:1968–1979. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Appendix: Participant sites
Table S1 MRI results for indicators of left ventricular remodeling in patients with LVEF of 50% or less on initial echocardiogram


