Summary
Aim
Different mechanisms may be involved in the antinociceptive effects of oxycodone (opioid) and venlafaxine (serotonin‐norepinephrine reuptake inhibitor), and the aim of this study was to investigate the effect of these drugs on brain functional connectivity.
Methods
Resting state functional magnetic resonance imaging was acquired in 20 healthy volunteers before and after a 5‐day treatment with oxycodone, venlafaxine, or placebo in a randomized, double‐blind, crossover study. Functional connectivity analyses were performed between four predefined seeds (dorsal anterior cingulate cortex, rostral anterior cingulate cortex, posterior insula, and prefrontal cortex), and the whole brain.
Results
The overall interpretation was that there were differences between the effects of oxycodone and venlafaxine on functional connectivity. Oxycodone mainly showed decreased functional connectivity between limbic structures and to supralimbic areas (all P < 0.05). Venlafaxine also showed decreased functional connectivity between limbic structures and to supralimbic areas, but increased functional connectivity to structures in the midbrain and brain stem was also found (all P < 0.05).
Conclusions
Oxycodone and venlafaxine showed differential effects on resting‐state functional connectivity as compared to placebo. This supports that the two drugs exert different mechanisms, and that the drugs in combination may exert additive effects and could potentially improve pain therapy.
Keywords: functional connectivity, magnetic resonance imaging, opioid, resting state, serotonin‐norepinephrine reuptake inhibitor
1. INTRODUCTION
Chronic pain is often treated with opioids, but other drug classes such as the serotonin‐norepinephrine reuptake inhibitors (SNRIs) have also shown analgesic effects. The effects of opioids are mediated by activation of opioid receptors, mainly present in supraspinal, spinal, and peripheral levels of the nervous system. SNRIs are thought to exert their effect primary on serotonergic and noradrenergic pathways.1 Thus, different mechanisms may be involved in the antinociceptive effect of the two drug classes. Oxycodone is a mu‐opioid receptor agonist, and venlafaxine is an SNRI (antidepressant) with an analgesic effect. The antinociceptive mechanisms of venlafaxine are not fully elucidated, but it has been suggested to be related to the opioidergic system.1, 2, 3, 4
Functional magnetic resonance imaging (fMRI) has been widely used to study brain activity, and the interest of using fMRI to study the modulation of neuronal activation by drug administration is growing. Resting‐state fMRI (RSfMRI) is one approach to investigate drug mechanisms in terms of functional connectivity between brain regions during resting state; however, limited studies on drug effects on functional connectivity exist5, 6, 7. Gorka et al investigated the effect of oxycodone on functional connectivity from two seeds of interest, the dorsal anterior cingulate cortex (dACC), and the rostral anterior cingulate cortex (rACC) and demonstrated that oxycodone decreased functional connectivity to insula.6
As oxycodone and potentially venlafaxine have impact on the opioidergic system, we hypothesized that oxycodone and venlafaxine reveal both similar, but to some extent also different effect on functional connectivity. Recently we used magnetic resonance spectroscopy to show that oxycodone and venlafaxine decreased the concentration of the neurotransmitter glutamate in the anterior cingulate cortex, insula, and prefrontal cortex.8 As these regions are also rich in opioid receptors, they were selected for the current study.9, 10, 11 Accordingly, the aim of this study was to investigate the effect of oxycodone and venlafaxine treatment on functional connectivity in these regions of interest in healthy volunteers as compared to placebo.
2. METHODS
Twenty healthy male subjects (mean age 24.6 ± 2.5 years) were MRI scanned in a randomized, double‐blind, three‐way crossover study. They were treated with oxycodone, venlafaxine, or placebo and scanned on day one (before each treatment) and after 5 days of treatment. The “wash‐out” periods between treatments were at least 1 week. The study was carried out at Department of Radiology, Aalborg University Hospital, Denmark and at Mech‐Sense, Department of Gastroenterology and Hepatology, Aalborg University Hospital, Denmark.
2.1. Experimental protocol
All subjects underwent a routine health screening conducted by a medical doctor to exclude subjects with any pain and nervous system related conditions, and subjects with a history of abuse or mental disorders were also excluded. Subjects gave written, informed consent before enrollment and could withdraw from the study at any time. Inclusion criteria were male, age between 20 and 35 years, normal medical examination, ability to read and understand Danish and of Scandinavian origin.
The study was approved by the local Ethics Committee (N‐20130011) and the Danish Medicines Agency (201300017030) and monitored by the Good Clinical Practice unit at Aalborg and Aarhus University Hospitals, Denmark. The study was conducted according to the Declaration of Helsinki and registered with the European Clinical Trials Database (EudraCT 2013‐000170‐30).
2.2. Drug administration
Oxycodone (10 mg extended release, “Accord,” Accord Healthcare, Salzburg, Austria), venlafaxine (37.5 mg extended release, “Stada,” Stada Nordic ApS, Herlev, Denmark), and placebo (8 mm tablets) were orally administered. Tablets were over‐encapsulated in DBcaps®, Swed.orange, size AA, “Capsugel®, Basel, Switzerland.” Capsules were administered once on day 1 and day 5, and twice a day on day 2‐4 (8 doses in total with twelve hours in between). Medication was handled, packed, and delivered by Hospital Pharmacy, Central Denmark Region, Denmark.
2.3. Brain imaging
Magnetic resonance imaging data were acquired on a 3T GE scanner (GE Signa HDxt, General Electric, Milwaukee, WI, USA) with a standard eight‐channel head coil. The head was fixed using foam pads. A high‐resolution T1‐weighted structural scan was acquired (TR/TE: 9.0 ms/3.6 ms, flip angle: 14°, field of view (FOV): 25 cm, matrix: 320 × 320, and voxel size: 0.8 × 0.8 × 1.0 mm). Resting‐state fMRI was acquired for 6:32 minutes as 192 volumes of gradient echo planar images (TR/TE: 2000 ms/30 ms, flip angle: 90°, FOV: 24 cm, matrix: 64 × 64, and voxel size: 2.5 × 2.5 × 3.8 mm). Four dummy scans were acquired for all functional scans prior to scanning of the 192 volumes. Volunteers were instructed to remain awake and to lie in the most relaxed position with closed eyes.
2.4. Resting‐state functional connectivity data analyses
The fMRI data were preprocessed and analyzed using SPM12 (Wellcome Trust Centre for Neuroimaging, London, UK). Images were slice timing corrected, realigned to correct for head movement, structural images and functional images were coregistered, segmented into CSF, white matter and gray matter, normalized to a standard brain in the MNI (Montreal Neurological Institute, Montreal, Canada), and smoothed (full width at half maximum = 8 mm). The functional connectivity (CONN) toolbox,12 www.nitrc.org/projects/conn, was used for connectivity analyses. Effects of nuisance covariates, cerebrospinal fluid, white matter, and motion parameters (including the first derivative), obtained during realignment were regressed from the data. The data were band‐pass filtered to 0.008‐0.09 Hz. Seed‐to‐voxel first‐level analyses for all subjects were performed by calculating the temporal correlation between blood‐oxygen‐level‐dependent signals from four predefined seeds to all voxels in the brain. The predefined seeds were: (i) the dorsal anterior cingulate cortex (dACC) (10 mm sphere around center MNI coordinates (0, 6, 40); (ii) rostral anterior cingulate cortex (rACC) (10 mm sphere around center MNI coordinates (0, 46, 2); (iii) right posterior insula (insula) (10 mm sphere around center MNI coordinates (37, −27, 12); and (iv) right prefrontal cortex (PFC) (10 mm sphere around center MNI coordinates (14, 48, 28)). The MarsBar toolbox was used to extract seeds.13 The second‐level analyses were performed in SPM12. Differences in functional connectivity across the three baselines (before treatments) were investigated for each seed using paired sample t tests. Treatment effects were investigated using paired sample t tests between treatment sessions (placebo vs oxycodone, placebo vs venlafaxine), and the directions of effects were investigated (placebo > oxycodone; placebo < oxycodone; placebo > venlafaxine; placebo < venlafaxine). We used a primary threshold (P < 0.005) and cluster‐extent based thresholding with k > 220 voxels. The MNI coordinates (X, Y, Z) for the maximum t‐value (obtained from the t tests) in each activated area were presented together with the number of voxels and the z‐value.
3. RESULTS
Due to logistic challenges, one subject missed an MRI scan before venlafaxine treatment, and another subject missed a scan after oxycodone treatment. Furthermore, one subject was excluded due to high level of movements (>2.5 mm and/or 2.5 degrees). Consequently, 18 subjects were included for analyses between baseline scans, 18 subjects were included for analyses of the oxycodone effects, and 19 subjects were included for analyses of the venlafaxine effects.
3.1. Treatment effects on functional connectivity
To verify the changes in functional connectivity to be treatment related, functional connectivity of measurements before treatments were compared. No significant differences in functional connectivity of areas relevant for the treatment response (as reported below for oxycodone and venlafaxine treatment) were seen between the baseline scans. Differences in baseline connectivity were confined to the occipital cortex, lingual gyrus, intracalcarine cortex, middle temporal gyrus, parahippocampal gyrus, and temporal pole (all P < 0.05), see Table S1.
The effects of oxycodone and venlafaxine treatment on functional connectivity from the predefined seeds (dACC, rACC, insula, and PFC) to other brain regions are presented in Tables 1, 2, 3, 4, respectively and in Figure 1. To provide an overview, the overall trends of the treatment effects are summarized in Table 5.
Table 1.
Functional connectivity between dACC and other voxels in the brain
| Region name | X | Y | Z | Voxels | Z‐score | P uncorr |
|---|---|---|---|---|---|---|
| Oxycodone<placebo | ||||||
| Superior parietal lobule, left | −28 | −48 | 76 | 908 | 4.21 | <0.001 |
| Supramarginal gyrus, right | 64 | −34 | 38 | 813 | 4.08 | <0.001 |
| Insula | 42 | 2 | 2 | 325 | 3.32 | 0.010 |
| Oxycodone > placebo | ||||||
| Precentral gyrus | −4 | −22 | 58 | 598 | 3.91 | 0.001 |
| Anterior cingulate gyrus | 2 | 40 | 18 | 245 | 3.69 | 0.021 |
| Venlafaxine < placebo | ||||||
| Superior frontal gyrus, left | −8 | 44 | 34 | 637 | 3.95 | 0.001 |
| Supramarginal gyrus, left | −68 | −48 | 26 | 512 | 3.84 | 0.002 |
| Middle frontal gyrus, left | −52 | 26 | 40 | 311 | 3.72 | 0.010 |
| Posterior cingulate gyrus | 2 | −24 | 38 | 278 | 3.66 | 0.014 |
| Venlafaxine > placebo | ||||||
| Superior frontal gyrus, right | 22 | 5 | 54 | 223 | 3.61 | 0.024 |
Oxycodone < placebo, decreased functional connectivity; Oxycodone > placebo, increased functional connectivity, same nomenclature for venlafaxine.
Table 2.
Functional connectivity between rACC and other voxels in the brain
| Region name | X | Y | Z | Voxels | Z‐score | P uncorr |
|---|---|---|---|---|---|---|
| Oxycodone < placebo | ||||||
| Middle temporal gyrus, left | −64 | −8 | −22 | 381 | 4.15 | 0.006 |
| Frontal medial cortex, left | 14 | 44 | −6 | 544 | 4.02 | 0.002 |
| Oxycodone > placebo | ||||||
| Middle frontal gyrus, left | −18 | 12 | 36 | 767 | 4.28 | <0.001 |
| Middle frontal gyrus, right | 22 | 4 | 40 | 791 | 3.80 | <0.001 |
| Venlafaxine < placebo | ||||||
| Insula, left | −44 | −22 | 2 | 1384 | 4.94 | <0.001 |
| Parietal operculum, right | 58 | −32 | 22 | 374 | 4.64 | 0.007 |
| Insula, right | 54 | 2 | −8 | 1356 | 4.08 | <0.001 |
| Superior frontal gyrus, right | 16 | 2 | 70 | 292 | 3.76 | 0.014 |
| Frontal orbital gyrus/inferior frontal gyrus | 44 | 30 | −6 | 231 | 3.23 | 0.026 |
| Venlafaxine > placebo | ||||||
| Cerebellum | −26 | −94 | −38 | 480 | 4.47 | 0.003 |
| Superior frontal gyrus | 2 | 56 | 46 | 361 | 3.49 | 0.007 |
| Lingual gyrus | 0 | −76 | −2 | 432 | 3.34 | 0.004 |
Table 3.
Functional connectivity between insula and other voxels in the brain
| Region name | X | Y | Z | Voxels | Z‐score | P uncorr |
|---|---|---|---|---|---|---|
| Oxycodone < placebo | ||||||
| Opercular cortex, right | 58 | −10 | 12 | 1944 | 4.04 | <0.001 |
| Postcentral gyrus, left | −40 | −24 | 40 | 676 | 3.65 | 0.001 |
| Superior temporal gyrus, right | −70 | −30 | 2 | 587 | 3.53 | 0.001 |
| Precentral gyrus, left | −40 | −18 | 58 | 227 | 3.15 | 0.030 |
| Oxycodone > placebo | NS | |||||
| Venlafaxine < placebo | ||||||
| Postcentral gyrus, left | −52 | −28 | 44 | 877 | 5.03 | <0.001 |
| Venlafaxine > placebo | ||||||
| Thalamus | 6 | −16 | 10 | 475 | 4.29 | 0.003 |
| Cerebellum | 24 | −54 | −48 | 549 | 3.68 | 0.001 |
| Brain stem | 8 | −14 | −18 | 225 | 3.60 | 0.026 |
| Cerebellum | 18 | −86 | −52 | 424 | 3.54 | 0.004 |
NS, nonsignificant.
Table 4.
Functional connectivity between PFC and other voxels in the brain
| Region name | X | Y | Z | Voxels | Z‐score | P uncorr |
|---|---|---|---|---|---|---|
| Oxycodone < placebo | ||||||
| Precuneus/posterior cingulate gyrus, left | −18 | −50 | 8 | 379 | 4.10 | 0.004 |
| Temporal occipital cortex, left | −20 | −52 | −16 | 445 | 3.92 | 0.002 |
| Postcentral gyrus, right | 12 | −44 | 78 | 260 | 3.84 | 0.014 |
| Paracingulate gyrus | 6 | 42 | −10 | 256 | 3.54 | 0.015 |
| Cerebellum | 10 | −40 | −28 | 402 | 3.48 | 0.003 |
| Oxycodone > placebo | ||||||
| Precentral gyrus, left | −40 | −6 | 46 | 1954 | 5.05 | <0.001 |
| Superior parietal gyrus, right | 38 | −48 | 48 | 361 | 4.39 | 0.005 |
| Precentral gyrus, right | 46 | 4 | 34 | 351 | 3.97 | 0.006 |
| Middle temporal gyrus, left | −64 | −60 | 4 | 293 | 3.64 | 0.010 |
| Superior parietal lobule, left | −38 | −54 | 50 | 270 | 3.60 | 0.013 |
| Anterior cingulate/paracingulate | −16 | 12 | 38 | 251 | 3.34 | 0.016 |
| Venlafaxine < placebo | ||||||
| Opercular cortex, left | −42 | −10 | 18 | 276 | 3.55 | 0.016 |
| Venlafaxine > placebo | ||||||
| Supramarginal gyrus, left | −64 | −44 | 44 | 248 | 3.36 | 0.021 |
Figure 1.
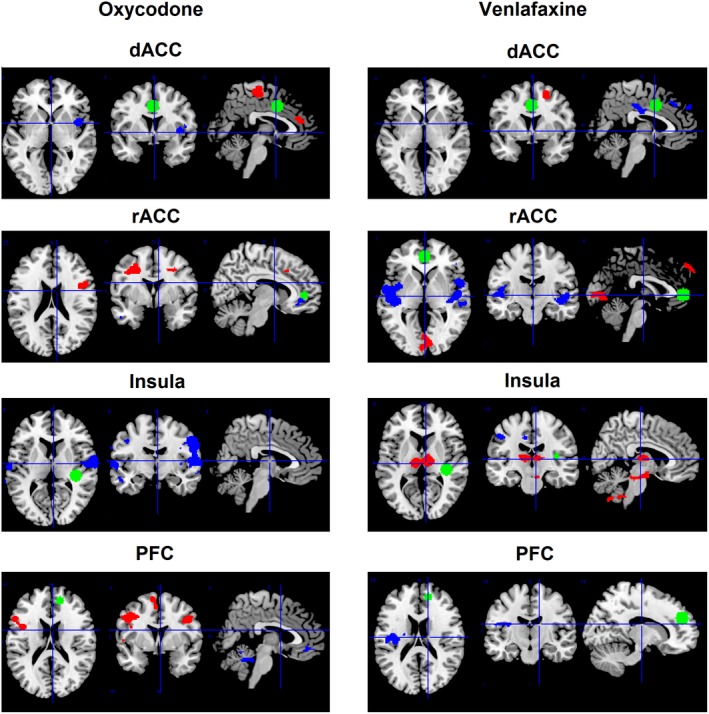
Treatment effects on functional connectivity between preselected seeds (the dorsal anterior cingulate cortex (dACC), the rostral anterior cingulate cortex (rACC), the right posterior insula (insula) and the right prefrontal cortex (PFC)) and other voxels in the brain for oxycodone (left column) and venlafaxine (right column) as compared to placebo. Green: preselected seed; Blue: decreased functional connectivity as compared to placebo; Red: increased functional connectivity as compared to placebo. The presented results are cluster‐extent based thresholded (k > 220) with primary threshold of P < 0.005. Note that the seeds of interest (green) are the same size (10 mm sphere) for all preselected seeds, and not visible in all slices as the selected slices focus on the brain regions with changes in connectivity
Table 5.
Summary of the overall treatment effects (as compared to placebo) on functional connectivity between dACC, rACC, insula, and PFC cortex and relevant brain regions
| dACC | rACC | Insula | PFC | |
|---|---|---|---|---|
| Insula/operculum regions | Oxycodone ↓ | Venlafaxine ↓ | Oxycodone ↓ | Venlafaxine↓ |
| Cingulate regions |
Oxycodone ↑ (a) Venlafaxine ↓ (p) |
Oxycodone ↓ (p) ↑ (a) | ||
| Parietal regions |
Oxycodone ↓ Venlafaxine ↓ |
Oxycodone ↓ Venlafaxine ↓ |
Oxycodone ↓↑ Venlafaxine ↓ |
|
| Frontal regions | Venlafaxine ↓↑ |
Oxycodone ↓↑ Venlafaxine ↓↑ |
||
| Thalamus/brain stem | Venlafaxine ↑ |
dACC, dorsal anterior cingulate cortex; rACC, rostral anterior cingulate cortex; PFC, right prefrontal cortex; ↓, decreased functional connectivity; ↑, increased functional connectivity; ↓↑, decreased and increased functional connectivity has been found within the region; (a), anterior region; (p), posterior region.
3.2. Oxycodone
Significant decreased functional connectivity (compared to placebo) was found for oxycodone treatment (i) between dACC and insula and parietal regions, (ii) between rACC and frontal regions, (iii) between insula and operculum and parietal regions, and (iv) between PFC and posterior cingulate and parietal regions (all P < 0.05). Significant increased functional connectivity was found (i) between dACC and anterior cingulate regions, (ii) between rACC and frontal regions, and (iii) between PFC and anterior cingulate and parietal regions (all P < 0.05). Overall, oxycodone mostly affected functional connectivity in limbic and supralimbic regions.
3.3. Venlafaxine
Significant decreased functional connectivity (compared to placebo) was found for venlafaxine treatment (i) between dACC and posterior cingulate, parietal regions, and frontal regions, (ii) between rACC and insula/operculum and frontal regions, (iii) between insula and parietal regions, and (iv) between PFC and insula/operculum and parietal regions (all P < 0.05). Increased functional connectivity was found (i) between dACC and frontal regions, rACC, and frontal regions and (ii) between insula and thalamus and brain stem (all P < 0.05). Overall, venlafaxine mostly affected functional connectivity in the limbic system and in deeper structures (thalamus and brain stem).
4. DISCUSSION
We investigated the effect of oxycodone and venlafaxine on cingulate, insula, and prefrontal functional connectivity to other brain regions. In comparison with placebo, functional connectivity was affected by both active treatments and involved all the predefined brain areas. The effect of oxycodone on functional connectivity was more pronounced in the limbic and supralimbic system, whereas the effect of venlafaxine on functional connectivity was more pronounced in the limbic system and in deeper structures (thalamus and brain stem).
4.1. Drug effects on functional connectivity
Previous studies have shown MRI resting‐state functional connectivity to be useful to evaluate drug action on the central nervous system.5, 14, 15 Oxycodone is a mu‐opioid receptor agonist, and a number of previous studies have reported that cingulate, insula, and prefrontal cortex are rich in opioid receptors.9, 10, 11 ACC, especially, has high opioidergic binding potential, and it has previously been demonstrated that oxycodone treatment reduced functional connectivity between dACC/rACC and insula.6 In this study, we demonstrated that oxycodone decreased functional connectivity in the limbic system. In line with Gorka et al we found reduced functional connectivity between dACC and insula for oxycodone treatment and furthermore functional connectivity was decreased between insula and opercular cortex. ACC and insula work together in the salience network and integrate interoceptive information with emotional salience and awareness.16, 17 Moreover, ACC and insula are regions well known to be important in pain processing.18 Increased functional connectivity between ACC and insula during rest has been demonstrated in chronic pain conditions,19, 20 and in an acute pain model, we showed increased activity in ACC, insula, and thalamus in healthy volunteers, whereas morphine (mu‐opioid receptor agonist) decreased activity in insula, ACC, and inferior parietal cortex.21 Even in absence of painful stimulation, we demonstrated in the present study that oxycodone decreased functional connectivity in these brain regions. Hence, we may infer that modulation of functional connectivity in these brain regions could be related to activation of opioid receptors and hence changed neuronal activity in these regions. Furthermore, in our recent magnetic resonance spectroscopy study, decreased concentration of the neurotransmitter glutamate was observed in response to oxycodone treatment (and a trend for venlafaxine treatment) in the insula, ACC, and prefrontal cortex.8 In addition to this, in two EEG source localization studies, which used the same cohort of healthy volunteers as this study, we showed frontal shift of cingulate activity in response to oxycodone treatment in the cingulate‐operculum network underlying nociceptive withdrawal reflex evoked potentials and an increase in cingulate activity coupled with a decrease in operculum activity,22 and we observed a decrease in insula and frontal gyrus activity underlying tonic pain following oxycodone treatment.23 It can also be proposed then, that the shifts and changes of activity seen in the previous surface EEG studies could be related to the changes in functional connectivity as observed in our present MRI study. We found both increased and decreased functional connectivity from cingulate to frontal regions. Increased functional connectivity between dACC and frontal regions has previously been shown in response to oxycodone treatment, and this has been suggested to reflect acutely increased cognitive control over subjective pain unpleasantness.6 As overall reduced functional connectivity was seen for all seeds of interest to parietal regions, this may indicate an inhibition of neuronal activity, which may be central in inhibition of pain processing, when pain is present.
Venlafaxine is an antidepressant drug, which modulates the serotonergic and noradrenergic pathways, and is believed to involve descending pain inhibitory systems.3 It is still not elucidated how this mechanism differentiates from opioidergic pathways, and a complex interaction between several neurotransmitter systems might be involved in the underlying mechanisms for the antinociceptive effects of venlafaxine. Similar to oxycodone, venlafaxine demonstrated decreased functional connectivity in the limbic system. In particular, decreased functional connectivity was observed between rACC and insula, which may indicate the involvement of opioidergic pathways at least to some extent. Previous studies have proposed that venlafaxine improves attention, motor activity, and response time.24 To observe the effects of venlafaxine on the human motor cortex, a study using fMRI in combination with different motor tasks showed that one‐week treatment improved the finger‐tapping rate and increased the activations of contralateral primary sensorimotor cortex, contralateral premotor cortex, and contralateral supplementary motor area.25 On the other hand, the authors found the activation of the parietal cortices was decreased. Another study in duloxetine (SNRI) in healthy volunteers showed reduced functional connectivity between the medial prefrontal cortex and the lateral parietal cortex.26 We also found venlafaxine to reduce functional connectivity to parietal regions. Additionally, using the same cohort of healthy volunteers as in the present study, in our previous EEG study involving spinal and cortical evoked potentials, we observed a decrease in latencies induced by venlafaxine treatment,27 and in an EEG study involving tonic pain, we observed a decrease in alpha activity induced by venlafaxine treatment, which was correlated to decrease in pain scores.23 The observed changes in the surface EEG could be related to changes in functional connectivity observed in our present MRI study. Consequently, it could be speculated that these changes in functional connectivity to venlafaxine treatment are centrally involved in the mechanisms of pain relief. Finally, we observed that venlafaxine increased functional connectivity from insula to thalamus and the brain stem. This increased functional connectivity may present a part of the thalamic feedback loop of descending pain inhibition resulting in reduction of pain signaling. However, experiments involving pain are needed to verify this.
4.2. Methodological considerations
In this study, we investigated the effect of oxycodone and venlafaxine on functional connectivity during resting state, and we demonstrated drug induced changed functional connectivity. An important advantage of estimation of functional connectivity during rest, and not during task (eg, painful stimulation), is that the estimated effects are not confounded by, for example, anticipation, performance, or other task‐induced confounders. On the other hand, as this study did not include pain models or subjects with chronic pain, the analgesic effects of the drugs cannot be distinguished and further research is needed to address that aspect.
As we used a hypothesis‐driven seed‐based method and a relatively small sample size, we used a liberal primary threshold (P < 0.005)12 followed by a cluster‐extent based thresholding method. An advantage of using cluster‐extent based thresholding is higher sensitivity in identifying significant regions, but low spatial specificity is a disadvantage, and accurate inferences about the true activation are difficult.28
The treatment period of 5 days for venlafaxine may not be sufficient to reach the maximal effect in the brain and should optimally have been longer (ie, at least 2 weeks).29 However, this study was conducted in healthy subjects, and it was not feasible to treat for longer than 5 days for ethical reasons. Moreover, effects of venlafaxine on the pain system have been observed already after few days of treatment.30 Another study found that a single dose of a serotonin reuptake inhibitor dramatically altered functional connectivity in the human brain.31 Hence, although the longer treatment is desired for the maximal clinical effect, we believe that the five‐day treatment in this study was long enough to observe relevant changes in the central nervous system.
5. CONCLUSIONS
In this placebo‐controlled study in healthy subjects, the effects of oxycodone and venlafaxine treatment on functional connectivity were investigated. Functional connectivity was affected by both treatments involving all the investigated brain regions. Differential effects of oxycodone and venlafaxine on resting‐state functional connectivity were found supporting that venlafaxine exerts different mechanisms compared to oxycodone. Thus, future drug development for pain treatment could be improved as both drugs can be used as monotherapy, but also in combination with different mechanisms can be targeted. However, future studies involving pain are needed to support this idea.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supporting information
ACKNOWLEDGMENTS
The study was supported by Innovation Fund Denmark—Individuals, Disease, and Society [#0603‐00411B]; The Obel Family Foundation; Karen Elise Jensen's Foundation; and a free grant from Grünenthal.
Hansen TM, Lelic D, Olesen AE, Drewes AM, Frøkjær JB. Differential effects of oxycodone and venlafaxine on resting state functional connectivity—A randomized placebo‐controlled magnetic resonance imaging study. CNS Neurosci Ther. 2018;24:820–827. 10.1111/cns.12827
REFERENCES
- 1. Mika J, Zychowska M, Makuch W, Rojewska E, Przewlocka B. Neuronal and immunological basis of action of antidepressants in chronic pain – clinical and experimental studies. Pharmacol Rep. 2013;65:1611‐1621. [DOI] [PubMed] [Google Scholar]
- 2. Gültekin H, Ahmedhov V. Roles of the opioidergic system and nitric oxide in the analgesic effect of venlafaxine. Yakugaku Zasshi. 2006;126:117‐121. [DOI] [PubMed] [Google Scholar]
- 3. Schreiber S, Bleich A, Pick CG. Venlafaxine and mirtazapine. J Mol Neurosci. 2002;18:143‐149. [DOI] [PubMed] [Google Scholar]
- 4. Sikka P, Kaushik S, Kumar G, Kapoor S, Bindra VK, Saxena KK. Study of antinociceptive activity of SSRI (fluoxetine and escitalopram) and atypical antidepressants (venlafaxine and mirtazapine) and their interaction with morphine and naloxone in mice. J Pharm Bioallied Sci. 2011;3:412‐416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Khalili‐Mahani N, Zoethout RM, Beckmann CF, et al. Effects of morphine and alcohol on functional brain connectivity during “resting state”: a placebo‐controlled crossover study in healthy young men. Hum Brain Mapp. 2012;33:1003‐1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gorka SM, Fitzgerald DA, de Wit H, Angstadt M, Phan KL. Opioid modulation of resting‐state anterior cingulate cortex functional connectivity. J Psychopharmacol. 2014;28:1115‐1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Scheidegger M, Walter M, Lehmann M, et al. Ketamine decreases resting state functional network connectivity in healthy subjects: implications for antidepressant drug action. PLoS ONE. 2012;7:e44799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hansen TM, Olesen AE, Simonsen CW, et al. Acute metabolic changes associated with analgesic drugs: an MR spectroscopy study. J Neuroimaging. 2016;26:545‐551. [DOI] [PubMed] [Google Scholar]
- 9. Jones AK, Luthra SK, Maziere B, et al. Regional cerebral opioid receptor studies with [11C]diprenorphine in normal volunteers. J Neurosci Methods. 1988;23:121‐129. [DOI] [PubMed] [Google Scholar]
- 10. Baumgärtner U, Buchholz HG, Bellosevich A, et al. High opiate receptor binding potential in the human lateral pain system. NeuroImage. 2006;30:692‐699. [DOI] [PubMed] [Google Scholar]
- 11. Zubieta JK, Bueller JA, Jackson LR, et al. Placebo effects mediated by endogenous opioid activity on mu‐opioid receptors. J Neurosci. 2005;25:7754‐7762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Whitfield‐Gabrieli S, Nieto‐Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012;2:125‐141. [DOI] [PubMed] [Google Scholar]
- 13. Brett M, Anton J, Valabregue R, Poline J. Presented at the 8th International Conference on Functional Mapping of the Human Brain, June 2–6, 2002, Sendai, Japan. Available on CD‐ROM in NeuroImage, Vol 16, No 2.
- 14. Gear R, Becerra L, Upadhyay J, et al. Pain facilitation brain regions activated by nalbuphine are revealed by pharmacological fMRI. PLoS ONE. 2013;8:e50169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Upadhyay J, Maleki N, Potter J, et al. Alterations in brain structure and functional connectivity in prescription opioid‐dependent patients. Brain. 2010;133:2098‐2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Taylor KS, Seminowicz DA, Davis KD. Two systems of resting state connectivity between the insula and cingulate cortex. Hum Brain Mapp. 2009;30:2731‐2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Seeley WW, Menon V, Schatzberg AF, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349‐2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Apkarian AV, Bushnell MC, Treede RD, Zubieta JK. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain. 2005;9:463‐484. [DOI] [PubMed] [Google Scholar]
- 19. Ichesco E, Quintero A, Clauw D, et al. Altered functional connectivity between the insula and the cingulate cortex in patients with TMD – a pilot study. Headache. 2012;52:441‐454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cifre I, Sitges C, Fraiman D, et al. Disrupted functional connectivity of the pain network in fibromyalgia. Psychosom Med. 2012;74:55‐62. [DOI] [PubMed] [Google Scholar]
- 21. Hansen TM, Olesen AE, Graversen C, Drewes AM, Frøkjaer JB. The effect of oral morphine on pain‐related brain activation ‐ an experimental functional magnetic resonance imaging study. Basic Clin Pharmacol Toxicol. 2015;117:316‐322. [DOI] [PubMed] [Google Scholar]
- 22. Lelic D, Fischer IW, Olesen AE, et al. Venlafaxine and oxycodone effects on human spinal and supraspinal pain processing: a randomized cross‐over trial. Eur J Neurosci. 2016;44:2966‐2974. [DOI] [PubMed] [Google Scholar]
- 23. Lelic D, Hansen TM, Mark EB, Olesen AE, Drewes AM. The effects of analgesics on central processing of tonic pain: a cross‐over placebo controlled study. Neuropharmacology. 2017;123:455‐464. [DOI] [PubMed] [Google Scholar]
- 24. Saletu B, Grunberger J, Anderer P, Linzmayer L, Semlitsch HV, Magni G. Pharmacodynamics of venlafaxine evaluated by EEG brain mapping, psychometry and psychophysiology. Br J Clin Pharmacol. 1992;33:589‐601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Li C, Song X, Han L, et al. The effects of venlafaxine on cortical motor area activity in healthy subjects: a pilot study. J Clin Psychopharmacol. 2014;34:93‐98. [DOI] [PubMed] [Google Scholar]
- 26. Van Wingen GA, Tendolkar I, Urner M, et al. Short‐term antidepressant administration reduces default mode and task‐positive network connectivity in healthy individuals during rest. NeuroImage. 2014;88:47‐53. [DOI] [PubMed] [Google Scholar]
- 27. Lelic D, Valeriani M, Fischer IW, Dahan A, Drewes AM. Venlafaxine and oxycodone have different effects on spinal and supraspinal activity in man: a somatosensory evoked potential study. Br J Clin Pharmacol. 2016;83:764‐776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Woo CW, Krishnan A, Wager TD. Cluster‐extent based thresholding in fMRI analyses: pitfalls and recommendations. NeuroImage. 2014;91:412‐419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Haanpää ML, Gourlay GK, Kent JL, et al. Treatment considerations for patients with neuropathic pain and other medical comorbidities. Mayo Clin Proc. 2010;85:S15‐S25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Enggaard TP, Klitgaard NA, Gram LF, Arendt‐Nielsen L, Sindrup SH. Specific effect of venlafaxine on single and repetitive experimental painful stimuli in humans. Clin Pharmacol Ther. 2001;69:245‐251. [DOI] [PubMed] [Google Scholar]
- 31. Schaefer A, Burmann I, Regenthal R, et al. Serotonergic modulation of intrinsic functional connectivity. Curr Biol. 2014;24:2314‐2318. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


