Summary
Aims
To investigate the efficacy of chitin biological absorbable catheters in a rat model of autologous nerve transplantation.
Methods
A segment of sciatic nerve was removed to produce a sciatic nerve defect, and the sural nerve was cut from the ipsilateral leg and used as a graft to bridge the defect, with or without use of a chitin biological absorbable catheter surrounding the graft. The number and morphology of regenerating myelinated fibers, nerve conduction velocity, nerve function index, triceps surae muscle morphology, and sensory function were evaluated at 9 and 12 months after surgery.
Results
All of the above parameters were improved in rats in which the nerve graft was bridged with chitin biological absorbable catheters compared with rats without catheters.
Conclusions
The results of this study indicate that use of chitin biological absorbable catheters to surround sural nerve grafts bridging sciatic nerve defects promotes recovery of structural, motor, and sensory function and improves muscle fiber morphology.
Keywords: chitin biological absorbable catheters, nerve defect, nerve transplantation, peripheral nerve regeneration, sensory function
1. INTRODUCTION
Nerve defects caused by peripheral nerve injury are common in the clinic, and autologous nerve transplantation is a common repair method.1 Various tube structures, such as veins, pseudo sheaths, and silicone catheters, are often used for bridging the donor nerve and the recipient nerve to avoid the formation of neuromas and prevent the proximal fibers from escaping to the periphery.2, 3, 4 Chitin biological absorbable catheters have shown some superiority in neural transplant models. Our previous study, which used a nerve magnification model in which the proximal common peroneal nerve was bridged to the distal tibial nerve by a chitin biological absorbable catheter, showed that the catheter could promote nerve regeneration by promoting the magnification effect of the regenerated fibers.5 In this study, we aimed to determine the effect of chitin biological absorbable catheters in a neural transplantation model. Smaller, less important nerves or cutaneous nerves are usually selected as donor nerves for neural transplantation to avoid a large loss of function in the area originally innervated by the donor nerve. In this experiment, sural nerves were selected as donors and bridged to injured sciatic nerves using chitin biological absorbable catheters, and the effect on nerve regeneration was examined.
2. MATERIALS AND METHODS
2.1. Animals and groups
Male SD rats weighing 200‐220 g were purchased from Peking University and maintained under specific pathogen‐free conditions and a 12‐hours light/dark cycle, with free access to pellet food and water. The rats were randomly classified as group A (normal group), group B (sciatic nerve graft orthotopic transplantation by adventitial suture), group C (sural nerve graft transplantation by adventitial suture), and group D (sural nerve graft transplantation with bridging catheter). There were 6 rats in each group in each survival period. All efforts were made to minimize animal suffering, and experimental procedures with animals were performed strictly in conformity with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication number 85‐23, revised 1985) and the related ethics regulations of Peking University.
2.2. Materials
The equipment used in the current research were as follows: a Synergy electrophysiological instrument (04oc003; Oxford Instrument Inc.); a Leica pathological tissue slicer (RM Z135; Leica); a Leica dissecting microscope (MZ75; Leica); a footprint measurement box (self‐made); a Leica tissue embedding machine (EG 1150H; Leica); and a Leica image collection and analysis system. Chitin biological absorbable catheters (ether‐free chitin biological catheters; length, 6 mm; wall thickness, 0.2 mm; inner diameter, 2 mm) were obtained from Peking University People ‘s Hospital (Patent number: 01136314.2).
2.3. Surgical procedures
2.3.1. Preparation of the sural nerve
Surgical procedures were performed in a specific pathogen‐free animal laboratory. Rats in groups C and D were anesthetized with sodium pentobarbital (30 mg/kg, intraperitoneally). The skin of the right limb was sterilized, and a longitudinal incision was made parallel to and slightly outside the right tibia, revealing the sural nerve below the shallow muscles. The shallow muscles were cut along the sural nerve, and the sural nerve was carefully separated, avoiding damage to its associated small vein. The sural nerve was cut out and then placed in saline for use. The proximal and distal ends were distinguished so that the nerve could be transplanted in an anterograde to retrograde direction. Finally, the wound was sutured with 4‐0 suture. All efforts were made to minimize the incision and damage to the animals.
2.3.2. Preparation of the neural transplantation model
In all groups except group A, the right sciatic nerve was exposed and cut 2 and 12 mm above the bifurcation, leaving a 10‐mm defect. In group A, the right sciatic and sural nerves were exposed as described above and the wound was closed without any further surgery (Figure 1A). In group B, the right sural nerve was exposed without any further surgery, and then, the wound was closed. A 10‐mm sciatic nerve segment was transplanted back into the sciatic nerve defect in an anterograde to retrograde orientation by adventitial suture. There were 2 anastomoses after the nerve segment was placed in the defect. In each anastomosis, the edge of the epineurium of each of the 2 nerve ends was penetrated by a microsurgical suture sequentially, and then, the 2 suture ends were ligated. Two stitches were made on opposite edges (Figure 1B). In group C, a 10‐mm segment of the sural nerve was cut and transplanted into the sciatic nerve defect in an anterograde to retrograde orientation by adventitial suture in the same way as in group B (Figure 1C). In group D, a 10‐mm sural nerve segment was transplanted into the sciatic nerve defect in an anterograde to retrograde orientation and bridged with 2 chitin biological absorbable catheters. The proximal end of the sural nerve and the proximal stump of the sciatic nerve were bridged with a catheter; the distal end of the sural nerve and the distal stump of sciatic nerve were bridged with another catheter. At each bridging location, the catheter wall was penetrated from the outside to the inside with a 8‐0 suture at the site 2 mm away from 1 end of the catheter. The suture then penetrated the epineurium of the proximal nerve stump, and finally, the catheter wall was penetrated from inside to outside. The 2 suture ends, now outside the wall, were pulled tight and ligated. At the same time, the proximal nerve stump was gently pulled 2 mm into the catheter. The distal nerve stump was similarly sutured so that the distal and proximal nerve stumps extended 2 mm into either end of the catheter, leaving a 2‐mm gap between the 2 nerve stumps (Figure 1D). Every effort was made to minimize the incision and reduce any damage to the animals. At 2 weeks, 9 and 12 months after surgery, the animals were used for morphological and functional analyses.
Figure 1.
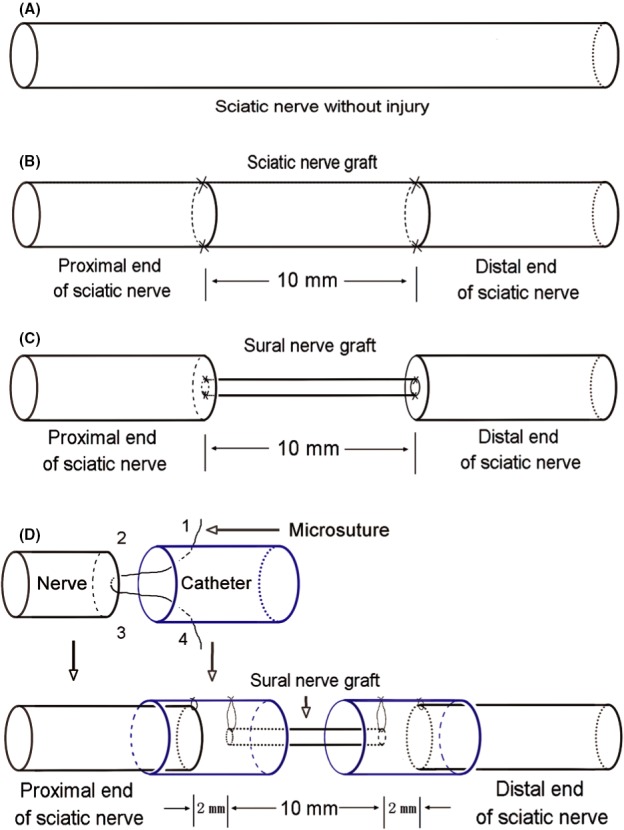
Neural transplantation model groups. A, sciatic nerve without surgical procedures, group A; B, sciatic nerve graft orthotopic transplantation by adventitial suture, group B; C, sural nerve graft transplantation by adventitial suture, group C; D, sural nerve graft transplantation by bridging with a chitin catheter, group D
2.4. Pain recovery test
Rats were placed in a cage with a grid floor, and a safety pin was used to press the central plantar surface of the right hind paw so that the surface of the paw was briefly stimulated at an intensity sufficient to dimple but not penetrate. Paw withdrawal duration (PWD) was recorded, with an arbitrary minimal time of 0.5 seconds and a maximal cutoff of 15 seconds.6
2.5. Walking track analysis
At 9 and 12 months after the operation, walking track analysis was performed on the animals in each group, as described previously,7 using a confined walking track (10 × 60 cm), darkened at 1 end. White paper (10 × 60 cm) was placed on the bottom of the track. The hind limbs were dipped into black ink before the animal was placed at the entrance of the walking track. Each rat then walked down the track 4 or 5 times, and the bilateral footprints were recorded. Clear and complete prints were chosen for measurement.
Paired footprint parameters for print length (PL; distance from heel to toe), toe spread (TS; distance from first to fifth toe) and intermediary toe spread (IT; distance from second to fourth toe) were recorded for the left normal control foot (NPL, NTS, NIT) and the corresponding right experimental foot (EPL, ETS, EIT) for each rat. Sciatic function index (SFI), tibial function index (TFI), and peroneal function index (PFI) were calculated according to the Bain‐Mackinnon‐Hunter formula: SFI = −38.3 ([EPL−NPL]/NPL) + 109.5 ([ETS−NTS]/NTS) + 13.3 ([EIT−NIT]/NIT) − 8.8; TFI = −37.2 ([EPL−NPL]/NPL) + 104.4 ([ETS−NTS]/NTS) + 45.6 ([EIT−NIT]/NIT) − 8.8; PFI = 174.9 ([EPL−NPL]/NPL) + 80.3 ([ETS−NTS]/NTS) − 13.4.
2.6. General observations of the catheters
When the right sciatic nerves were exposed 9 and 12 months postsurgery, the degradation and morphology of the catheters were noted.
2.7. Electrophysiological examination
Electrophysiological examination of the sciatic nerve was conducted 9 and 12 months postsurgery, as described previously.5 The right sciatic nerve was exposed, and stimulating bipolar electrodes were placed proximal and distal to the repaired site. The recording electrode was placed in the triceps surae muscle, while the ground electrode was placed in the subcutaneous tissue between the stimulating and recording electrodes. Rectangular pulses (0.1 mseconds, 10 Hz, 0.9 mA, and 6 continuous stimuli) were used. The nerve conduction velocity (NCV; m/s) was obtained semi‐automatically by dividing the distance between the 2 stimulating sites by the difference in the conduction time.
2.8. Regrowing axons evaluation in the early stage
Two weeks after surgery, the right sciatic nerves were exposed in groups B, C, and D. The proximal anastomotic nerve segments were harvested as shown in Figure 2, fixed in 4% paraformaldehyde for 24 hours, rinsed twice in water for 12 hours, and then embedded in paraffin. Sections (5 μm) were cut for immunohistochemical staining. The paraffin sections were reacted with primary GAP‐43 antibody (1:750) (ABCAM) and then with secondary biotinylated IgG (1:300) (ABCAM). The immunoreactivity was visualized using the diaminobenzidine method. The sections were observed under a microscope and photographed (400×) to measure the positive area ratio with Image J software. Positive area ratio = positive staining area/the total area of a field of view.
Figure 2.
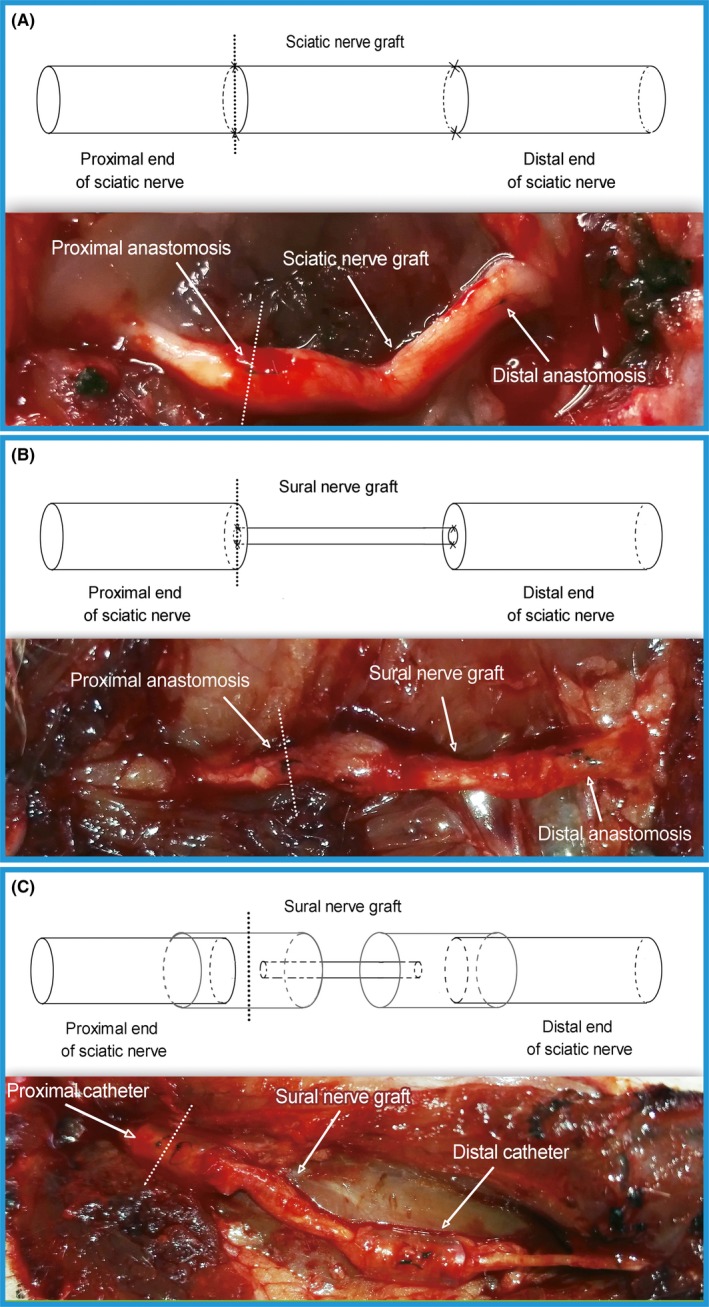
Nerve cutting position. The dotted line indicates the nerve cutting position; A, sciatic nerve graft orthotopic transplantation by adventitial suture, group B; B, sural nerve graft transplantation by adventitial suture, group C; C, sural nerve graft transplantation by bridging with a chitin catheter, group D
2.9. Histological analysis of regenerated myelinated fibers
The right sciatic nerve was exposed, and a nerve segment 5 mm distal to the distal catheter was cut out and divided into 2 sections; 1 was prepared for optical microscopy and the other for transmission electron microscope (TEM) specimen.
Optical microscope specimen: the sections were fixed in 4% paraformaldehyde in 0.1 mol/L phosphate buffer for 24 hours at 4°C, rinsed twice in water for 12 hours, and stained in 1% osmium tetroxide for 12 hours, dehydrated through a graded series of ethanol, immersed in xylene, embedded in paraffin, and sliced into 5‐μm cross‐sections.5 Micrographs were made and the myelinated axons were counted by a person who was totally blind to the experimental design and groups.
Transmission electron microscope specimen: the sections were fixed in 3% glutaraldehyde for 2 hours, then postfixed in 1% osmium tetroxide in 0.1 mol/L sodium cacodylate buffer (pH 7.3) for 1.5 hours, dehydrated in ethanol, immersed in a mixture of epoxy resin and acetone (1: 1) for 2 hours, and embedded in epoxy resin. Transverse ultrathin (thickness: 50.0 nm) sections were prepared. The ultrathin sections were stained with uranyl acetate and lead citrate and were finally examined under a transmission electron microscope (JEM‐100, Tokyo, Japan), from which the morphometrics of the myelinated axons were evaluated by a person who was blind to the experimental design.
2.10. Weight and histological analysis of the triceps surae muscle
Triceps surae muscles were freed and cut from the right and left limbs of the rats. To reduce statistical error and insure objectivity, the triceps surae muscles used for analysis included the tendons. Muscles were then immediately weighed with a microbalance. The recovery rate of muscle wet weight was calculated by dividing the wet weight of the affected muscle by that of the control contralateral muscle.
After weighing, the triceps surae muscles were immediately fixed in 4% paraformaldehyde in 0.1 mol/L phosphate buffer for 7 days, rinsed in water, dehydrated through a graded series of ethanol, immersed in xylene, and embedded in paraffin. Each muscle segment was sliced into 5‐μm cross‐sections and then stained in hematoxylin‐eosin solution. Images were acquired under a Leica dissecting microscope, from which the histomorphology of muscle fibers was observed.
2.11. Statistical analysis
Statistical analyses were performed with SPSS 19.0 software (IBM, Armonk, NY, USA). One‐way analysis of variance (ANOVA) was used for comparisons, and independent sample t test was used as posthoc test for intergroup comparisons. A value of P < 0.05 was considered to be statistically significant. All values are presented as the mean ± SD.
3. RESULTS
3.1. Pain recovery test
Nine months after surgery, PWDs in groups B, C, and D were significantly higher than in group A (P < 0.05). The PWDs in groups C and D were significantly higher than in group B (P < 0.05), while the value in group D was significantly lower than in group C (P < 0.05). At 12 months after surgery, PWDs in groups B, C, and D were significantly higher than in group A (P < 0.05), and the PWDs in groups C and D were significantly higher than in group B (P < 0.05), while the values between groups C and D were not significantly different (Figure 3).
Figure 3.
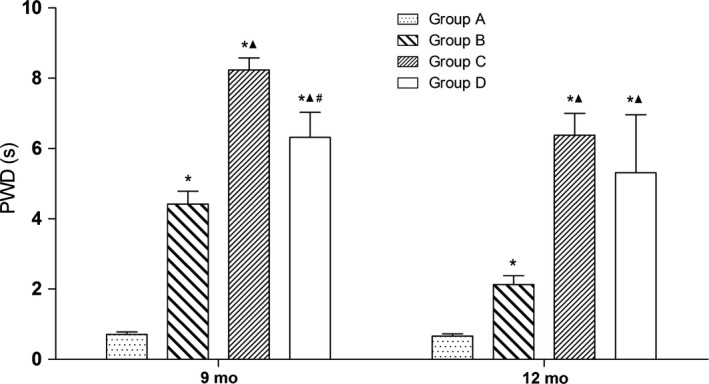
Pain recovery test; *P < 0.05 vs group A; ▲ P < 0.05 vs group B; # P < 0.05 vs group C. Group A, sciatic nerve without surgical procedures; group B, sciatic nerve graft orthotopic transplantation by adventitial suture; group C, sural nerve graft transplantation by adventitial suture; group D, sural nerve graft transplantation by bridging with a chitin catheter
3.2. Walking track analysis
3.2.1. SFI
Nine months after surgery, the SFIs were significantly lower in groups B, C, and D than in group A (P < 0.05), and the SFIs were significantly lower in groups C and D than in group B (P < 0.05), while the value in group D was significantly higher than in group C (P < 0.05). At 12 months after surgery, the SFIs in groups B, C, and D were significantly lower than in group A (P < 0.05), and the SFIs in groups C and D were significantly lower than in group B (P < 0.05), while the values between groups C and D were not significantly different (Figure 4).
Figure 4.
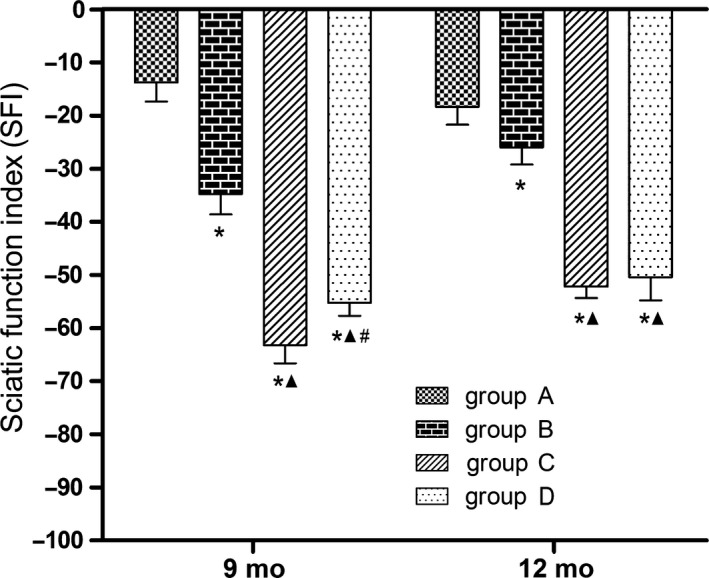
Motor function‐SFI; *P < 0.05 vs group A; ▲ P < 0.05 vs group B; # P < 0.05 vs group C. Group A, sciatic nerve without surgical procedures; group B, sciatic nerve graft orthotopic transplantation by adventitial suture; group C, sural nerve graft transplantation by adventitial suture; group D, sural nerve graft transplantation by bridging with a chitin catheter
3.2.2. TFI
Nine and 12 months after surgery, the TFIs were significantly lower in groups B, C, and D than in group A (P < 0.05), and the TFIs were significantly lower in groups C and D than in group B (P < 0.05), while the values in groups C and D did not significantly differ (Figure 5).
Figure 5.
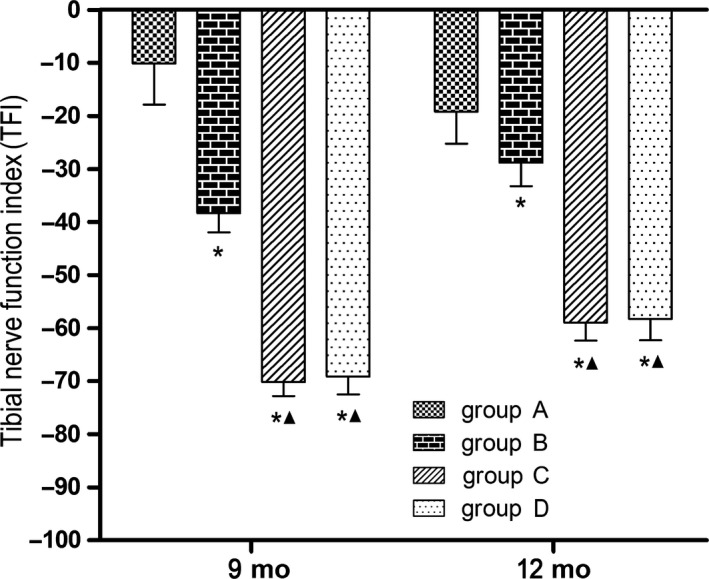
Motor function‐TFI; *P < 0.05 vs group A; ▲ P < 0.05 vs group B. Group A, sciatic nerve without surgical procedures; group B, sciatic nerve graft orthotopic transplantation by adventitial suture; group C, sural nerve graft transplantation by adventitial suture; group D, sural nerve graft transplantation by bridging with a chitin catheter
3.2.3. PFI
Nine months after surgery, the PFIs in groups C and D were significantly lower than in group A (P < 0.05), the value in group C was significantly lower than in group B (P < 0.05), and the value in group D was significantly higher than in group C (P < 0.05), while the values between groups A and B and the values between groups B and D were not significantly different. At 12 months after surgery, the PFI in group D was significantly higher than in group C (P < 0.05). The PFIs did not differ significantly between any pairwise comparison among groups A, B, and C. The values did not differ significantly between any pairwise comparison among groups A, B, and D (Figure 6).
Figure 6.
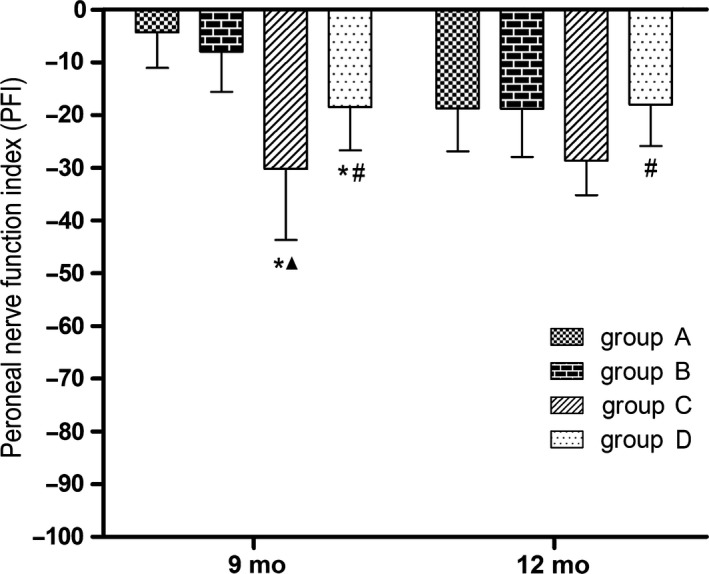
Motor function‐PFI; *P < 0.05 vs group A; ▲ P < 0.05 vs group B; # P < 0.05 vs group C. Group A, sciatic nerve without surgical procedures; group B, sciatic nerve graft orthotopic transplantation by adventitial suture; group C, sural nerve graft transplantation by adventitial suture; group D, sural nerve graft transplantation by bridging with a chitin catheter
3.3. General observations of the catheters
Nine months after operation, the catheters in group D had degraded to a certain extent, but the sural and sciatic nerves had integrated well. The local blood supply appeared to be good at the catheter sites.
The catheters had degraded further 12 months after surgery but still retained their outlines. Nerve regeneration was good and orderly.
3.4. Electrophysiology of the sciatic nerve
As shown in Figure 7, 9 months after surgery, the NCVs were significantly lower in groups B, C, and D than in group A (P < 0.05) and were significantly lower in groups C and D than in group B (P < 0.05), while the values in groups C and D did not significantly differ. At 12 months after surgery, the values were significantly lower in groups B, C, and D than in group A (P < 0.05) and were significantly lower in groups C and D than in group B (P < 0.05), while the value in group D was significantly higher than in group C (P < 0.05).
Figure 7.

Electrophysiology of sciatic nerve; *P < 0.05 vs group A; ▲ P < 0.05 vs group B; # P < 0.05 vs group C. Group A, sciatic nerve without surgical procedures; group B, sciatic nerve graft orthotopic transplantation by adventitial suture; group C, sural nerve graft transplantation by adventitial suture; group D, sural nerve graft transplantation by bridging with a chitin catheter
3.5. Regrowing axons evaluation in the early stage
The protein expression of GAP‐43 was visualized in the proximal anastomosis in all groups with immunohistochemical staining method 2 weeks after surgery (Figure 8A‐C). The expression of GAP‐43 in group C appeared less than in groups B and D. The GAP‐43 immunoreactive substances in groups B and D were evenly distributed, whereas the immunoreactive substances in group C were distributed sparsely.
Figure 8.
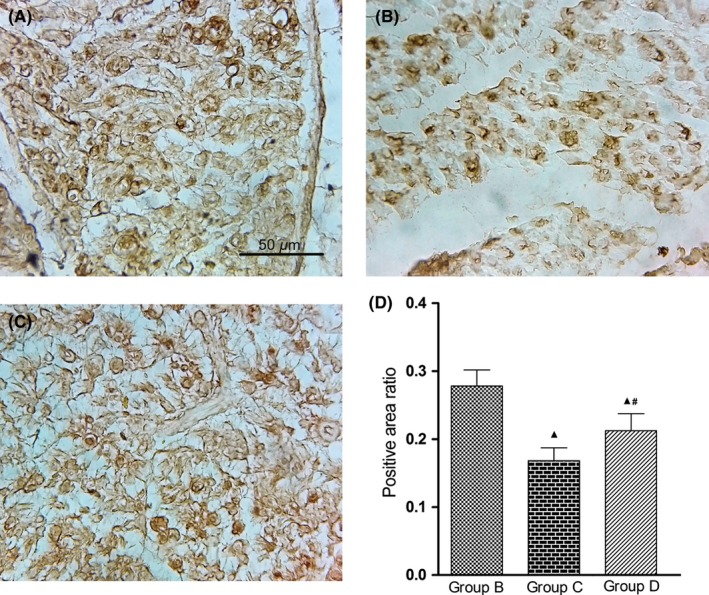
Regrowth of axons at an early stage. A‐C, microscopic evaluation; immunohistochemical staining; original magnification ×400; scale bar = 50 μm. A, sciatic nerve graft orthotopic transplantation by adventitial suture, group B; B, sural nerve graft transplantation by adventitial suture, group C; C, sural nerve graft transplantation by bridging with a chitin catheter, group D; D, positive area ratios of GAP‐43 immunoreactive substance; ▲ P < 0.05 vs group B; # P < 0.05 vs group C
Measurements of the GAP‐43 immunoreactive substance in the proximal anastomosis were used to calculate the positive area ratios. The ratios in groups C and D were both lower than in group B (P < 0.05), but the ratio in group D was higher than in group C (P < 0.05) (Figure 8D).
3.6. Morphology and morphometrics of regenerated myelin sheaths
3.6.1. Morphology of myelin sheaths
The images acquired from optical microscopy and TEM (Figure 9) show that, 9 months after surgery, the myelin sheaths were evenly distributed and appeared uniform and normal in group A. The myelin sheaths in group B were almost evenly distributed and appeared almost uniform. In groups C and D, myelin regeneration looked poor and myelin distribution looked uneven. The diameter of the myelin sheaths appeared larger in group D than in group C. At 12 months after surgery, the myelin sheaths in groups A and B were evenly distributed and appeared uniform, while myelin regeneration appeared poorer in groups C and D. The diameter of the myelin sheaths appeared larger in group D than in group C.
Figure 9.
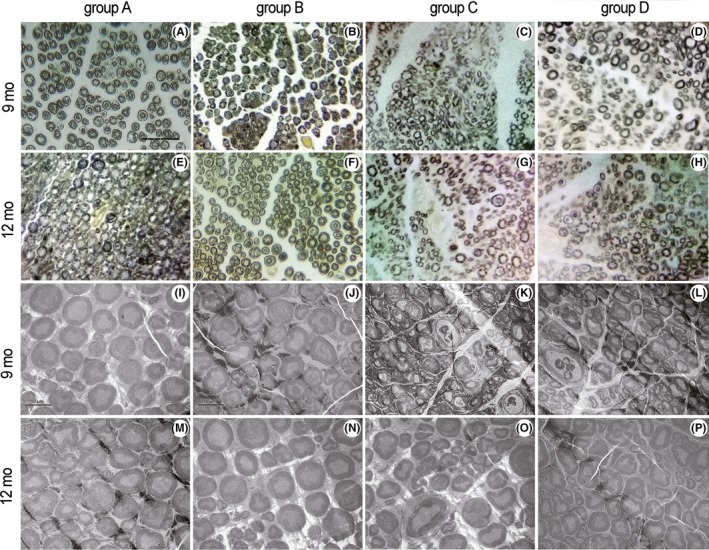
Morphology of myelinated fibers. Osmium tetroxide staining, original magnification ×400, and Scale bar = 50 μm (A‐H); TEM specimens, original magnification ×1200, and Scale bar = 10 μm (I‐P). Group A, sciatic nerve without surgical procedures (A, E, I, M); group B, sciatic nerve graft orthotopic transplantation by adventitial suture (B, F, J, N); group C, sural nerve graft transplantation by adventitial suture (C, G, K, O); group D, sural nerve graft transplantation by bridging with a chitin catheter (D, H, L, P)
3.6.2. Quantification of myelinated nerve fibers
As shown in Figure 10, 9 months after surgery, the numbers of regenerated myelinated fibers were significantly lower in groups B, C, and D than in group A (P < 0.05) and significantly lower in groups C and D than in group B (P < 0.05), while the value in group D was significantly higher than in group C (P < 0.05). At 12 months after surgery, the numbers of regenerated myelinated fibers were significantly lower in groups C and D than in groups A and B (P < 0.05), the value was significantly higher in group D than in group C (P < 0.05). The values between groups A and B were not significantly different.
Figure 10.

Quantification of myelinated nerve fibers; *P < 0.05 vs group A; ▲ P < 0.05 vs group B; # P < 0.05 vs group C. Group A, sciatic nerve without surgical procedures; group B, sciatic nerve graft orthotopic transplantation by adventitial suture; group C, sural nerve graft transplantation by adventitial suture; group D, sural nerve graft transplantation by bridging with a chitin catheter
3.6.3. Morphometrics of myelin sheaths
Nine months after surgery, mean axon diameters were significantly smaller in groups B, C, and D than in group A (P < 0.05) and significantly smaller in groups C and D than in group B (P < 0.05), while significantly greater in group D than in group C (P < 0.05) (Figure 11A). At 12 months after surgery, axon diameters did not differ significantly between any pairwise comparison among groups A, B, C, and D.
Figure 11.
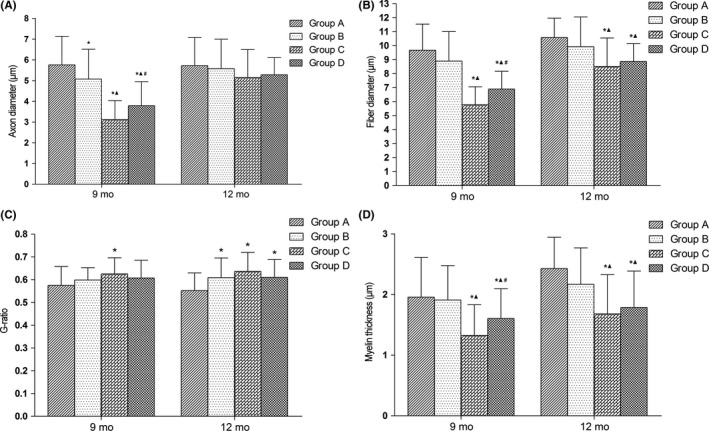
Morphometrics of myelin sheaths; *P < 0.05 vs group A; ▲ P < 0.05 vs group B; # P < 0.05 vs group C. A, axon diameter; B, fiber diameter; C, g‐ratio; D, myelin thickness. Group A, sciatic nerve without surgical procedures; group B, sciatic nerve graft orthotopic transplantation by adventitial suture; group C, sural nerve graft transplantation by adventitial suture; group D, sural nerve graft transplantation by bridging with a chitin catheter
Fiber diameters of myelinated nerves were significantly smaller, 9 months after surgery, in groups C and D than in groups A and B (P < 0.05) and significantly higher in group D than in group C (P < 0.05) (Figure 11B). Fiber diameters were not significantly different between groups A and B. At 12 months after surgery, fiber diameters were significantly smaller in groups C and D compared with groups A and B (P < 0.05); however, the values did not differ significantly between groups A and B or between groups C and D.
The g‐ratios (ratio between the inner and outer diameters of the myelin sheath) were significantly higher in group C than in group A (P < 0.05), 9 months after surgery, but the values did not differ significantly between any pairwise comparison among groups A, B, and D (Figure 11C). The values between groups B and C and the values between groups C and D did not differ significantly. At 12 months after surgery, the g‐ratios were significantly higher in groups B, C, and D than in group A (P < 0.05), but the values did not differ significantly between any pairwise comparisons among groups B, C, and D.
The mean thickness of myelin was significantly smaller in groups C and D than in group A and B (P < 0.05) and significantly higher in group D than in group C (P < 0.05) 9 months after surgery (Figure 11D). The values were not significantly different between groups A and B. At 12 months after surgery, myelin thicknesses were significantly smaller in groups C and D compared with group A and B (P < 0.05); however, the values did not differ significantly between groups A and B or between groups C and D.
3.7. Recovery of triceps surae muscle mass
As shown in Figure 12, at 9 and 12 months after surgery, recovery rates of muscle wet weight were significantly lower in groups B, C, and D than in group A (P < 0.05) and significantly lower in groups C and D than in group B (P < 0.05). The values were not significantly different between groups C and D.
Figure 12.
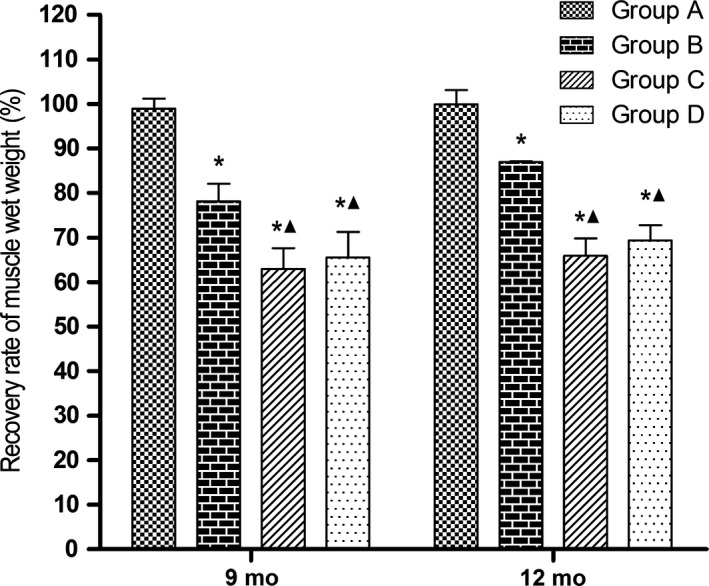
Recovery of triceps surae muscle mass; *P < 0.05 vs group A; ▲ P < 0.05 vs group B. Group A, sciatic nerve without surgical procedures; group B, sciatic nerve graft orthotopic transplantation by adventitial suture; group C, sural nerve graft transplantation by adventitial suture; group D, sural nerve graft transplantation by bridging with a chitin catheter
3.8. Histomorphology of the triceps surae muscle
At 9 and 12 months after surgery, the muscle fibers in all groups were evenly distributed, with a polygonal morphology. The muscle fiber cross‐sectional area in groups C and D looked smaller than in groups A and B, while the cross‐sectional area in group D appeared larger than in group C at the same time points (Figure 13).
Figure 13.
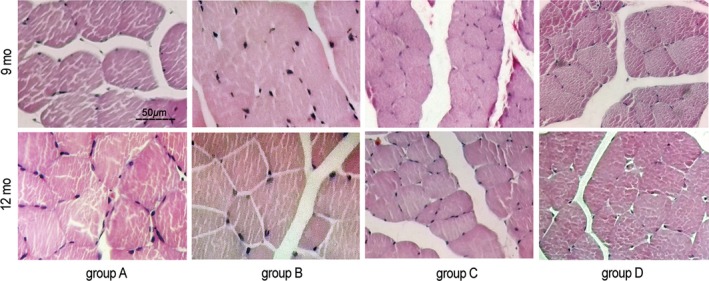
Histomorphology of the triceps surae muscle. Hematoxylin‐eosin staining, original magnification ×400. Scale bar = 50 μm. Group A, sciatic nerve without surgical procedures; group B, sciatic nerve graft orthotopic transplantation by adventitial suture; group C, sural nerve graft transplantation by adventitial suture; group D, sural nerve graft transplantation by bridging with a chitin catheter
4. DISCUSSION
When there is a large defect in a nerve, it needs to be repaired by transplantation of another nerve segment. In the traditional nerve adventitial suture model, donor nerve is implanted into the defect, the nerve ends are apposed with each other, and the epineurium is sutured, when sutured in position, at the proximal end, the regenerated fibers are inevitably scattered and grow into adjacent tissue. This may form a neuroma, or the surrounding tissue may grow into the anastomosis to form a scar. The above pathological processes hinder effective regeneration of the nerves. The application of nerve catheters solves the above problems. The catheter bridges the ends of the 2 nerves, forming a closed space around the anastomosis which prevents the regenerating fibers from growing into the adjacent tissue and prevents the adjacent tissue from growing into the anastomosis, thus, insuring more effective regeneration of the nerve.
Most nerve catheters in clinical use are made of silicone. This can trigger immune responses because they are not biocompatible, and they become fragile and vulnerable in the wet environment. The ideal nerve catheter material should have good biocompatibility and be able to be completely degraded and absorbed in the body when nerve regeneration is completed. This would also avoid secondary surgery for removal of the catheter. Chitin bioabsorbable catheters conform to the above characteristics. The catheter used in this study was developed by Peking University People's Hospital and the China Academy of Textile Sciences (Patent number: 01136314.2) and has been used in a variety of neural repair models with a positive effect.5,7, 8, 9 In this study, the chitin catheter was used in another model of nerve repair, autologous nerve transplantation, to explore its efficacy.
In the autologous nerve transplantation model, less important autonomic nerves are often used as donors, such as the femoral nerve sensory branch and the sural nerve,10 to avoid secondary damage to the body. In this study, the sural nerve was used to repair a sciatic nerve defect by bridging with a chitin absorbable catheter, to investigate the effect of the catheter on nerve regeneration in a neural transplantation model. The results of this experiment showed that nerve regeneration was enhanced, and morphological and functional recovery were promoted in group D, in which catheters were used, compared with group C without catheters.
Two weeks after surgery, the positive area ratio was significantly higher in rats given a sural graft using the chitin catheters (group D) compared with the rats given a sural graft without catheters (group C). Our results demonstrated that the catheter promoted axonal regrowth along the correct path. Nine months after surgery, PWD was significantly lower in rats given a sural graft using catheters, demonstrating that this method promotes recovery of sensory function. SFI in group D at 9 months after surgery and PFI in group D at 9 and 12 months after surgery were significantly greater than in group C, demonstrating that the catheter method promotes recovery of motor function. NCVs were significantly higher 12 months after surgery in group D rats given sural nerve transplants with a catheter than those in group C given sural nerve transplants joined by adventitial suture. The numbers of myelinated nerve fibers were significantly greater in group D than in group C at 9 and 12 months after surgery, as were the axon diameters, fiber diameters, and myelin thicknesses of myelinated nerve fibers at 9 months after surgery. These anatomical, behavioral, physiological, and histological investigations suggest that the chitin catheter method promotes the recovery of nerve structure.
The above experimental results show the feasibility of reconstructing nerve defects with autologous cutaneous nerves, avoiding rejection responses caused by allogeneic nerve transplantation and related treatments.11, 12 Autologous grafts allow the body to recover important functions at a lower cost, and sometimes, the function at the site of the donor nerve can be compensated for by an adjacent nerve.
Our experimental results also show that neural structure and functional recovery in the group given sural nerve transplant with catheters (Group D) were inferior to the group given sciatic nerve grafts (group B). The PWDs in group B were significantly smaller than in group D at 9 and 12 months after surgery. The SFI, TFI, and NCV in the group B were significantly larger than in the group D at 9 and 12 months after surgery. The number of myelinated nerve fibers, fiber diameter, myelin thickness and the recovery rate of muscle wet weights were also significantly larger in group B compared with group D. The positive area ratio in group B was significantly higher than in the group D at 2 weeks after surgery, as were the axon diameters at 9 months after surgery. All these results indicated that a larger graft enhances nerve regeneration. Our future studies will aim for a larger graft, by combining several segments of the sural nerve in parallel as a graft within a chitin absorbable catheter, to repair the defect and improve regeneration.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ACKNOWLEDGMENTS
This research was continuously funded by Chinese National Key Basic Research 973 Program (2014CB542201); Beijing Science and Technology New Star Cross Subject (2018); National Natural Science Foundation of China (81401007, 31571235, 31771322, 51373023, 21171019, 31671248, 31640045, 31571236, 81171146, 31471144, 30971526, 31100860, 31040043, 31371210, 81372044); Natural Science Foundation of Beijing Municipality (7162098); Fostering Young Scholars of Peking University Health Science Center (BMU2017PY013), Chinese National General Program of National Natural Science Fund (31571235, 31771322) and Beijing City Science & Technology New Star Cross Project (201819).
Wang Z‐Y, Wang J‐W, Qin L‐H, Zhang W‐G, Zhang P‐X, Jiang B‐G. Chitin biological absorbable catheters bridging sural nerve grafts transplanted into sciatic nerve defects promote nerve regeneration. CNS Neurosci Ther. 2018;24:483–494. 10.1111/cns.12820
REFERENCES
- 1. Pilanci O, Ozel A, Basaran K, et al. Is there a profit to use the lateral antebrachial cutaneous nerve as a graft source in digital nerve reconstruction? Microsurgery. 2014;34:367‐371. [DOI] [PubMed] [Google Scholar]
- 2. Karacaoglu E, Yüksel F, Peker F, Güler MM. Nerve regeneration through an epineurial sheath: its functional aspect compared with nerve and vein grafts. Microsurgery. 2001;21:196‐201. [DOI] [PubMed] [Google Scholar]
- 3. Mohammadi R, Mahmoodi H. Improvement of peripheral nerve regeneration following nerve repair by silicone catheter filled with curcumin: a preliminary study in the rat model. Int J Surg. 2013;11:819‐825. [DOI] [PubMed] [Google Scholar]
- 4. Heijke GC, Klopper PJ, Baljet B, van Doorn IB. Silicone rubber tubulization in peripheral sensory nerve reconstruction: an experimental study in rabbits. Microsurgery. 2001;21:306‐316. [DOI] [PubMed] [Google Scholar]
- 5. Wang ZY, Zhang PX, Han N, Kou YH, Yin XF, Jiang BG. Effect of modified formula radix hedysari on the amplification effect during peripheral nerve regeneration. Evid Based Complement Alternat Med. 2013;2013:647982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Décosterd I, Buchser E, Gilliard N, Saydoff J, Zurn AD, Aebischer P. Intrathecal implants of bovine chromaffin cells alleviate mechanical allodynia in a rat model of neuropathic pain. Pain. 1998;76:159‐166. [DOI] [PubMed] [Google Scholar]
- 7. Zhang P, Wang Z, Kou Y, et al. Role of lumbricus extract in the nerve amplification effect during peripheral nerve regeneration. Am J Transl Res. 2014;6:876‐885. [PMC free article] [PubMed] [Google Scholar]
- 8. Zhang P, Xue F, Kou Y, et al. The experimental study of absorbable chitin conduit for bridging peripheral nerve defect with nerve fasciculu in rats. Artif Cells Blood Substit Immobil Biotechnol. 2008;36:360‐371. [DOI] [PubMed] [Google Scholar]
- 9. Zhang Z, Li X, Zuo S, Xin J, Zhang P. Bridging peripheral nerves using a deacetyl chitin conduit combined with short‐term electrical stimulation. Neural Regen Res. 2014;9:1075‐1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tzou CH, Aszmann OC, Frey M. Bridging peripheral nerve defects using a single‐fascicle nerve graft. Plast Reconstr Surg. 2011;128:861‐869. [DOI] [PubMed] [Google Scholar]
- 11. Strasberg SR, Mackinnon SE, Genden EM, et al. Long‐segment nerve allograft regeneration in the sheep model: experimental study and review of the literature. J Reconstr Microsurg. 1996;12:529‐537. [DOI] [PubMed] [Google Scholar]
- 12. Arai T, Lundborg G, Dahlin LB. Bioartificial nerve graft for bridging extended nerve defects in rat sciatic nerve based on resorbable guiding filaments. Scand J Plast Reconstr Surg Hand Surg. 2000;34:101‐108. [DOI] [PubMed] [Google Scholar]


