Summary
Aims
Finasteride inhibits the conversion of testosterone to dihydrotestosterone. Because androgen regulates dopaminergic system in the brain, it could be hypothesized that finasteride may inhibit dopaminergic system. The present study therefore investigates the effects of finasteride in adolescent and early developmental rats on dopaminergic system, including contents of dopamine and its metabolites (dihydroxy phenyl acetic acid and homovanillic acid) and tyrosine hydroxylase expressions both at gene and protein levels. Meanwhile, open‐field behaviors of the rats are examined because of the regulatory effect of dopaminergic system on the behaviors.
Methods
Open‐field behaviors were evaluated by exploratory and motor behaviors. Dopamine and its metabolites were assayed by liquid chromatography‐mass spectrometry. Tyrosine hydroxylase mRNA and protein expressions were determined by real‐time qRT‐PCR and western blot, respectively.
Results
It was found that in adolescent male rats, administration of finasteride at doses of 25 and 50 mg/kg for 14 days dose dependently inhibited open‐field behaviors, reduced contents of dopamine and its metabolites in frontal cortex, hippocampus, caudate putamen, nucleus accumbens, and down‐regulated tyrosine hydroxylase mRNA and protein expressions in substantia nigra and ventral tegmental area. However, there was no significant change of these parameters in early developmental rats after finasteride treatment.
Conclusion
These results suggest that finasteride inhibits dopaminergic system and open‐field behaviors in adolescent male rats by inhibiting the conversion of testosterone to dihydrotestosterone, and imply finasteride as a potential therapeutic option for neuropsychiatric disorders associated with hyperactivities of dopaminergic system and androgen.
Keywords: adolescence, dopaminergic system, finasteride, open‐field behaviors, rat
1. INTRODUCTION
Androgen plays a pivotal role in the regulation of multiple neuropsychiatric behaviors in the central nervous system.1, 2, 3 Numerous studies have revealed that disturbance of central androgen contributes to the pathogenesis of neuropsychiatric disorders, such as Tourette syndrome, which usually occurs in the developmental children and characterized by multiple motor tics and vocal tics.4 For instance, application of androgen aggravated motor tics in Tourette syndrome patients,5, 6 while treatment with flutamide, an androgen receptor antagonist, significantly alleviated the Tourette syndrome symptoms.7, 8
Dopaminergic system in the brain also plays an important role in the regulation of behaviors besides its regulation on extra‐pyramidal system, and studies have shown that dopaminergic system can be regulated by androgen in the brain. For example, administration of nandrolone decanoate or testosterone significantly enhanced activity of dopaminergic system in adult rats, presented as increased dopamine transporter binding densities in caudate putamen (CPu).9, 10 Intranasal administration of testosterone facilitated the release of dopamine and increased contents of dopamine in the intercellular space.11 These studies suggest a possibility that the dopaminergic system might be involved in some androgen disturbance‐induced neuropsychiatric diseases. Indeed, aberrant metabolisms of dopamine have been shown to contribute to the development and maintenance of Tourette syndrome.12, 13, 14, 15
Finasteride is a type II 5α reductase inhibitor and inhibits the conversion of testosterone to dihydrotestosterone which is the potent androgen.16 Finasteride is mainly used for the treatment of high androgen‐induced illness, such as benign prostatic hyperplasia and alopecia.17 Preliminary study revealed that finasteride treatment to male adult patients with Tourette syndrome could significantly improve symptoms of Tourette syndrome, such as motor and vocal tics,17, 18 and elicit antipsychotic‐like effect in rats.19 Considering the regulatory effect of androgen on dopaminergic system in the brain, it could be hypothesized that finasteride might inhibit dopaminergic system by inhibiting androgen. Therefore, the present study was undertaken to investigate the effect of finasteride on brain dopaminergic system in adolescent and early developmental rats, including the contents of dopamine and its metabolites, tyrosine hydroxylase expressions at both gene and protein levels. Meanwhile, open‐field behaviors were tested because of the regulatory effect of dopaminergic system on the behaviors. This study would facilitate the understanding and clinical application of finasteride in the treatment of neuropsychiatric disorders.
2. MATERIALS AND METHODS
2.1. Animals
Healthy male Wistar rats during adolescence of postnatal day 35 and early developmental rats of postnatal day 7 were used. All rats were provided by The Experimental Animal Center of Hebei Medical University. Animals were housed in the room temperature of 22 ± 3°C, 12 hour light dark cycle, food and water ad libitum. All experimental procedures were in accordance with the Guide for the Care and Use of Laboratory Animals of Hebei Medical University. The protocol was approved by the Committee of Ethics on Animal Experiments of Hebei Medical University. All efforts were made to minimize animal suffering.
2.2. Groupings and protocols
The adolescent rats of postnatal day 35 (n = 28) were randomly divided into control and finasteride group. According to the dose of finasteride used, finasteride group was further divided into 3 subgroups of 3, 25 and 50 mg/kg (n = 7 for each dose). The doses of finasteride were determined based on the previous reports.20, 21, 22 Rats in finasteride group were repeatedly administrated with finasteride dissolved in sesame oil and ethanol (5% v/v) by subcutaneous injection on the back of rats once a day for 14 days from postnatal day 35‐48. Rats in control group were administrated with vehicle (sesame oil) as the same protocol to those in finasteride group. On the next day (postnatal day 49) after the completion of finasteride or vehicle administration, open‐field test was conducted to evaluate exploratory and motor behaviors and then rats were sacrificed by decapitation under anesthesia (Figure 1A). The frontal cortex, hippocampus, CPu and nucleus accumbens (Acb), which are the sites of dopaminergic projections located at, were dissected for the assay of contents of dopamine and its metabolites including dihydroxy phenyl acetic acid (DOPAC) and homovanillic acid (HVA). The substantia nigra (SN) and ventral tegmental area (VTA), which are the sites of dopaminergic neurons located at, were dissected for the assay of expressions of tyrosine hydroxylase (TH) at both gene and protein levels.
Figure 1.
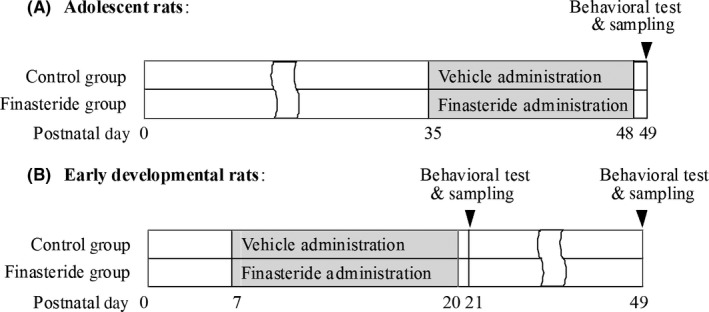
The schematic diagram shows animals’ age, time point of finasteride administration, behavioral tests and sampling
In addition, the effect of finasteride on early developmental rats of postnatal day 7 was also observed as the control of adolescent rats. The grouping and protocols were the same to those in adolescent rats, except that the initial administration time point of finasteride and vehicle in all groups were from postnatal day 7. In addition, besides the observation time point on postnatal day 21 (the next day after the completion of finasteride or vehicle administration), another observation time point on postnatal day 49 was conducted to evaluate whether the administration of finasteride in early developmental stage had delayed effect in adolescent stage (n = 7 for each dose and time point, total of 56 rats were used) (Figure 1B).
2.3. Open‐field test
The open field (100 × 100 × 40 cm) was made up of 4 black walls and a white bottom. The bottom consisted of 25 squares (20 × 20 cm), and each square was composed of 400 grills (1 × 1 cm). The open field was located at a sound‐attenuating chamber and illuminated with 20 lux light. A camera was installed above the open field. In order to eliminate odors, the field was cleaned with 70% alcohol before each animal was tested. The open‐field test was conducted according to the previous reports.23, 24 Briefly, on the test day, each rat was placed at the center of arena and was allowed to explore and videotaped for 15 minutes. Exploratory and motor behaviors (Table 1) were scored by observers blind to the experimental plan.
Table 1.
Behaviors observed in the open‐field test
| Open‐field behaviors | Descriptions of behaviors |
|---|---|
| Exploratory behavior | |
| Walking | The number of rats walk around while sniffing the environment50, 51, 52 |
| Sniffing | The number of rats sniff the environment while moving50, 51, 52, 53 |
| Climbing | The number of rats stand up with hind feet leaning against wall50, 51, 52 |
| Rearing | The number of rats stand up without rear feet leaning against wall50, 51, 52 |
| Motor behavior | |
| Vertical activity | The number of rats stand up with hind feet54, 55 |
| Horizontal activity | The number of rats cross the squares54, 55, 56, 57 |
| Total path length | The distance of rats cross the grills58 |
2.4. Liquid chromatography‐mass spectrometry (LC‐MS/MS)
Cerebral dopamine and its metabolites including DOPAC and HVA were determined by LC‐MS/MS. Tissues including frontal cortex, hippocampus, CPu and Acb were weighed and homogenized in 80% acetonitrile containing 0.1% formic acid (5 μL/mg). The homogenates were centrifuged at 21 100 g and 4°C for 10 minutes. The supernatant was collected and stored at −80°C. The LC separation was carried out on an Agileng 1200 LC system using a PhenomenexKinetex F5 column (100 × 2.1 mm, 2.6 μm). MS/MS detection was conducted on a 3200 QTRAP LC‐MS/MS system. The multiple‐reaction monitoring mode was used for the quantification.
2.5. Real‐Time Quantitative RT‐PCR
This method was used for the assay of TH gene expression in SN and VTA. Total RNA was extracted with E.Z.N.A. Total RNA Kit II (Omega Bio‐tek, Norcross, GA, USA), and RNA concentration was determined with the spectrophotometer (BioTek, Winooski, VT, USA). The first‐strand cDNA template was obtained by the reverse transcription from 2 μg total RNA. The sets of primers were 5′‐GCTTCTCTGACCAGGTGTATCG‐3′ and 5′‐GCAATCTCTTCCGCTGTGTAT‐3′ for TH, and 5′‐TGAACG GGAAGCTCACTG‐3′ and 5′‐GCTTCACCACCTTC‐ TTG ATG‐3′ for GAPDH. The real‐time quantitative PCR was performed with 0.8 μL cDNA, 2 μL specific primers and 2× SYBR green with final volume of 20 μL. The condition of PCR was initial cycle at 95°C for 10 minutes, 40 cycles at 95°C for 15 seconds, 58°C for 20 seconds, 72°C for 27 seconds. The PCR products were analyzed by melting curve to confirm the specificity of amplification. The relative quantification was calculated with 2−△△Ct. TH mRNA expression was normalized with GAPDH as the internal control. The ratio of TH to GAPDH was used to indicate the relative expression of TH mRNA.
2.6. Western blot
This method was used for the assay of TH protein expression. Tissues of SN and VTA were homogenized and sonicated in RIPA containing 10% protease inhibitors. The homogenates were centrifuged at 21 100 g and 4°C for 15 minutes. The supernatant was collected and store at −80°C. Protein sample concentration was determined by BCA method. Samples were loaded and electrophoresized in 5% stacking gel and 12% separating gel, and subsequently transferred to a polyvinylidene fluoride membrane. The membrane was incubated in 5% fetal bovine serum for 1 hour at 37°C and then was incubated overnight at 4°C with primary antibodies of rat anti‐TH monoclonal antibody (Sigma, 1:5000) and rat anti‐β‐actin monoclonal antibody (Sigma, 1:2000). The membrane was incubated for 1 hour in IRDye® 800‐conjugated goat antimouse secondary antibody (1:3000, Rockland) at room temperature. The immunoblot bands were scanned with Odyssey infrared scanner (LI‐COR, Lincoln, NE, USA). TH protein expression was normalized with β‐actin as the internal control. The ratio of TH to β‐actin was used to indicate the relative expression of TH protein.
2.7. Statistical analysis
Statistical analysis was carried out with SPSS 21.0. All data were presented as means ± SD. For data followed equal variance and normal distribution, 1‐way ANOVA was applied followed with post hoc Tukey's HSD. For data not in accordance with equal variance or normal distribution, Kruskal‐Wallis test was used followed by pairwise comparisons. P < 0.05 was considered to be significant.
3. RESULT
3.1. Open‐field behaviors
In adolescent rats, finasteride treatment both at doses of 25 and 50 mg/kg significantly decreased the numbers of walking, sniffing, climbing and rearing (only in 50 mg/kg group) in exploratory behaviors (Figure 2A) and decreased the numbers of vertical and horizontal activities and total path length in motor behaviors (Figure 3A) compared with control group. The decreasing percentage ranged from 31% to 68% in exploratory behaviors and from 33% to 66% in motor behaviors (Table 2). With greater doses of finasteride used in 25 and 50 mg/kg, exploratory and motor behaviors were inhibited more significantly (Table 2), which indicated the dose dependency of the decrease in open‐field behaviors to finasteride. The finasteride treatment at dose of 3 mg/kg had no effect on the open‐field behaviors.
Figure 2.
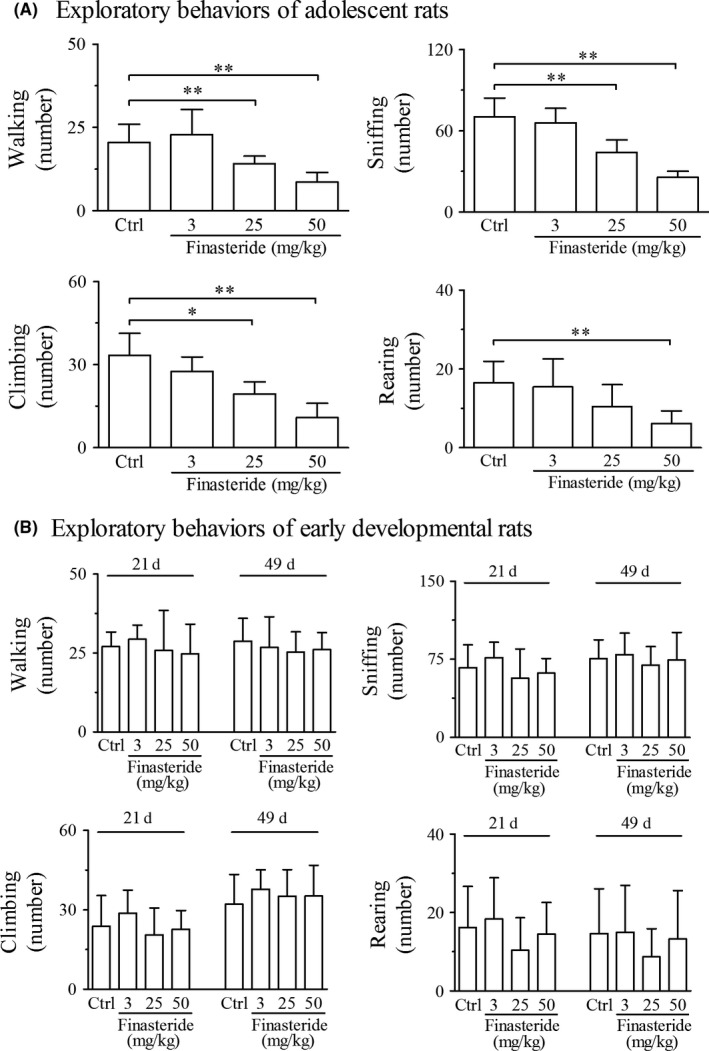
Open‐field test shows the effect of finasteride on exploratory behaviors in adolescent (A) and early developmental rats (B). **P < 0.01, *P < 0.05. It is shown that finasteride treatment at doses of 25 and 50 mg/kg significantly inhibits the exploratory behaviors in adolescent rats (A) in comparison with control group, while has no effect in early developmental rats (B). (n = 7 for each group)
Figure 3.
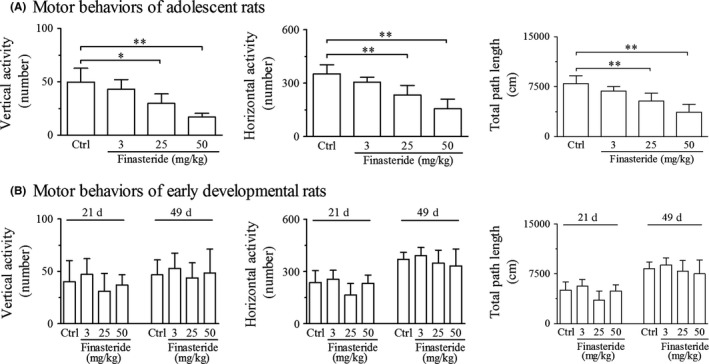
Open‐field test shows the effect of finasteride on motor behaviors in adolescent (A) and early developmental rats (B). **P < 0.01, *P < 0.05. It is shown that finasteride treatment at doses of 25 and 50 mg/kg significantly inhibits the motor behaviors in adolescent rats (A) in comparison with control group, while has no effect in early developmental rats (B). (n = 7 for each group)
Table 2.
Alterations of open‐field behaviors, dopamine and its metabolites and TH expressions after finasteride treatment to postnatal day 35 rats
| Indices | Decreasing percentage in comparison with control group | |
|---|---|---|
| 25 mg/kg finasteride | 50 mg/kg finasteride | |
| Exploratory behaviors | ||
| Walking | 31 (P < 0.01) | 58 (P < 0.01) |
| Sniffing | 38 (P < 0.01) | 64 (P < 0.01) |
| Climbing | 42 (P < 0.05) | 68 (P < 0.01) |
| Rearing | N/A | 63 (P < 0.01) |
| Motor behaviors | ||
| Vertical activity | 40 (P < 0.05) | 66 (P < 0.01) |
| Horizontal activity | 34 (P < 0.01) | 56 (P < 0.01) |
| Total length path | 33 (P < 0.01) | 54 (P < 0.01) |
| Content levels of dopamine (upper panel), DOPAC (middle panel), HVA (lower panel) | ||
| Frontal cortex | 35 (P < 0.01) | 59 (P < 0.01) |
| N/A | N/A | |
| N/A | N/A | |
| Hippocampus | 34 (P < 0.05) | 63 (P < 0.01) |
| N/A | N/A | |
| N/A | N/A | |
| CPu | 35 (P < 0.05) | 55 (P < 0.01) |
| 23 (P < 0.05) | 45 (P < 0.01) | |
| 23 (P < 0.05) | 51 (P < 0.01) | |
| Acb | 34 (P < 0.05) | 57 (P < 0.01) |
| 23 (P < 0.05) | 38 (P < 0.01) | |
| N/A | 50 (P < 0.01) | |
| Expression levels of TH mRNA (upper panel) TH protein (lower panel) | ||
| SN | 33 (P < 0.01) | 37 (P < 0.01) |
| 40 (P < 0.01) | 57 (P < 0.01) | |
| VTA | 35 (P < 0.01) | 42 (P < 0.01) |
| 45 (P < 0.01) | 64 (P < 0.01) | |
DA, dopamine; DOPAC, dihydroxy phenyl acetic acid; HVA, homovanillic acid; Hip. Hippocampus; CPu, caudate putamen; Acb, nucleus accumbens; TH, tyrosine hydroxylase; SN, substantia nigra; VTA, ventral tegmental area; N/A, not applicable.
In early developmental rats, there was no significant change in exploratory and motor behaviors at any dose of finasteride or at any observation time point of postnatal day 21 or 49 compared with control group (Figures 2B and 3B).
3.2. Dopamine and its metabolites
In adolescent rats, finasteride treatment both at doses of 25 and 50 mg/kg significantly reduced the contents of dopamine in frontal cortex, hippocampus, CPu and Acb compared with control group (Figure 4A‐D). The metabolites of dopamine including DOPAC and HVA were also reduced in CPu and Acb (HVA only in 50 mg/kg group) after finasteride treatment at both doses of 25 and 50 mg/kg compared with control group (Figure 4E‐H). With greater doses of finasteride used in 25 and 50 mg/kg, the contents of dopamine and its metabolites were decreased more significantly (Table 2), which indicated the dose dependency of the decrease in contents to finasteride. The levels of DOPAC and HVA in the frontal cortex and hippocampus were too low to be detected. The finasteride treatment at dose of 3 mg/kg had no effect on the contents of dopamine and its metabolites.
Figure 4.
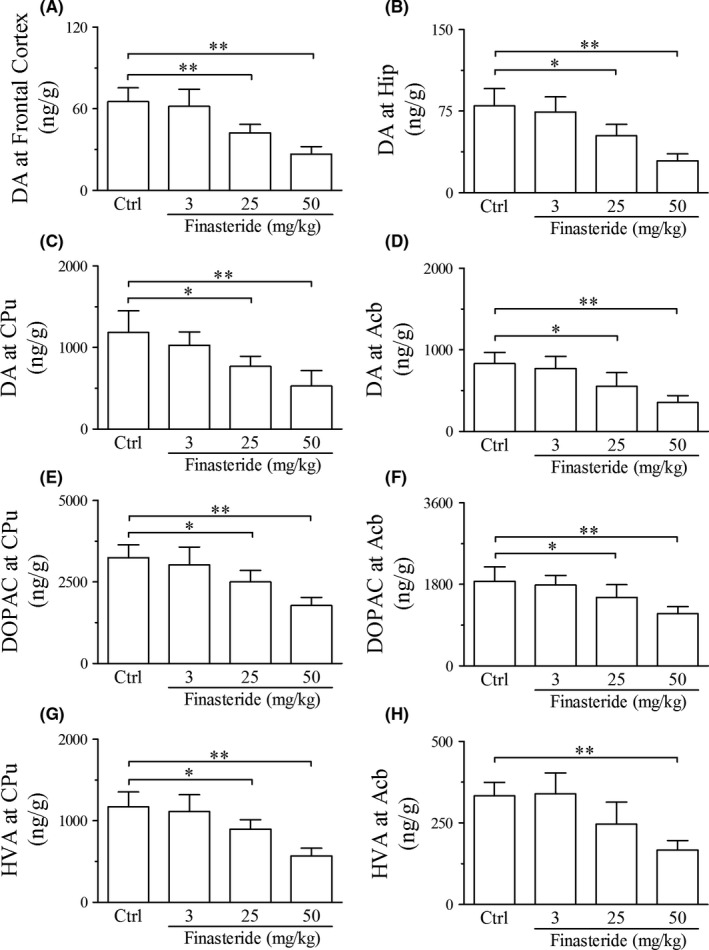
Liquid chromatography‐mass spectrometry analysis shows the effect of finasteride on the contents of dopamine and its metabolites in adolescent rats. **P < 0.01, *P < 0.05. It is shown that finasteride treatment at doses of 25 and 50 mg/kg significantly reduces the contents of dopamine (A‐D) in frontal cortex, hippocampus, CPu and Acb and its metabolites including DOPAC (E‐F) and HVA (G‐H) in CPu and Acb compared to control group. Abbreviations: DA, dopamine; DOPAC, dihydroxy phenyl acetic acid; HVA, homovanillic acid; Hip. Hippocampus; CPu, caudate putamen; Acb, nucleus accumbens. (n = 7 for each group)
In early developmental rats, there was no significant change in the contents of dopamine, DOPAC and HVA at any dose of finasteride group or any observation time point of postnatal day 21 or 49 compared with control group (Figure 5).
Figure 5.
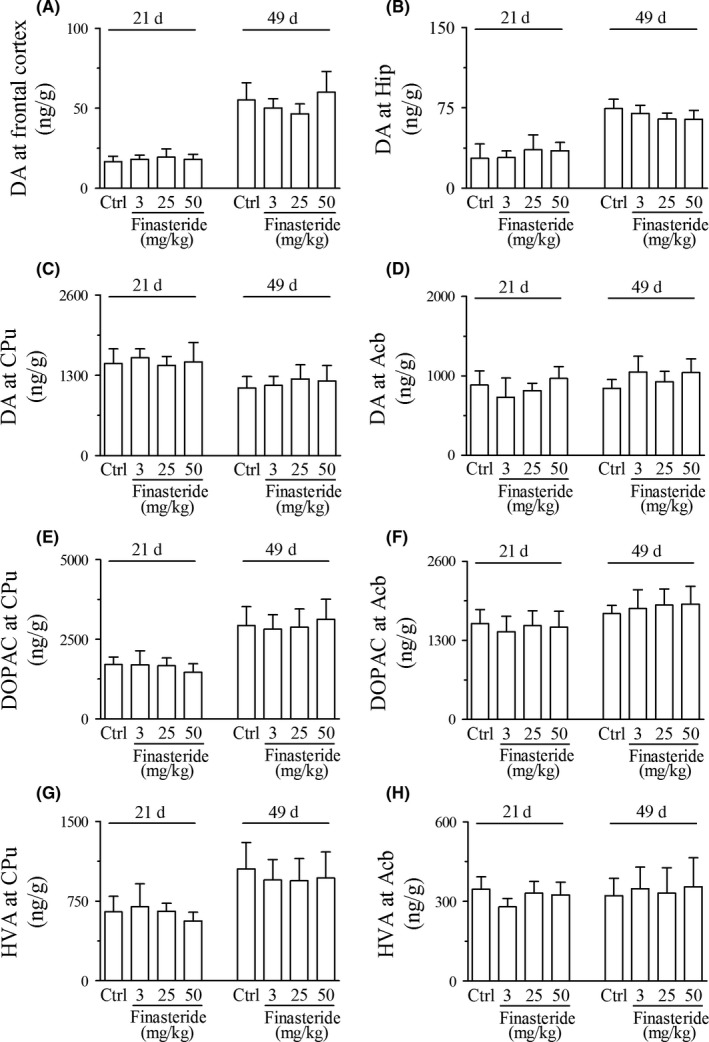
Liquid chromatography‐mass spectrometry analysis shows the effect of finasteride on the contents of dopamine and its metabolites in early developmental rats. It is shown that finasteride treatment at any dose or any observing time point has no effect on the contents of dopamine (A‐D) in frontal cortex, hippocampus, CPu and Acb and its metabolites including DOPAC (E‐F) and HVA (G‐H) in CPu and Acb compared to control group. Abbreviations used are the same to Figure 4. (n = 7 for each group)
3.3. TH mRNA and protein expressions
In adolescent rats, finasteride treatment both at doses of 25 and 50 mg/kg significantly down‐regulated TH expression both at mRNA and protein levels in SN and VTA (Figure 6). With greater doses of finasteride used in 25 and 50 mg/kg, the expression of TH both at mRNA and protein levels were down‐regulated more significantly (Table 2), which indicated the dose dependency of the down‐regulation of TH expressions to finasteride. The finasteride treatment at dose of 3 mg/kg had no effect on the TH expressions both at gene and protein levels.
Figure 6.
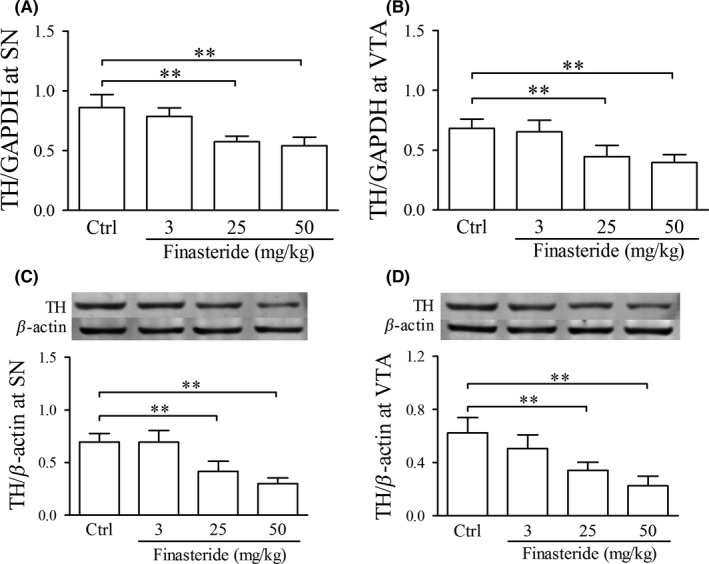
Real‐time quantitative RT‐PCR and western blot show the effect of finasteride on TH mRNA and protein expression, respectively, in adolescent rats. A and B are the relative expression of TH mRNA with the ratio of TH to GAPDH. In C and D, the upper panel is the immunoblot bands of TH and β‐actin, and the lower panels are the relative expression of TH with the density ratio of immunoblot bands of TH to β‐actin. **P < 0.01. It is shown that finasteride treatment at doses of 25 and 50 mg/kg significantly down‐regulated TH expression both at mRNA and protein levels in SN and VTA compared to control group. Abbreviations: TH, tyrosine hydroxylase; SN, substantia nigra; VTA, ventral tegmental area. (n = 7 for each group)
In early developmental rats, there was no significant change in TH expressions at either mRNA or protein level at any dose of finasteride group or any observation time point of postnatal day 21 or 49 compared with control group (Figure 7).
Figure 7.
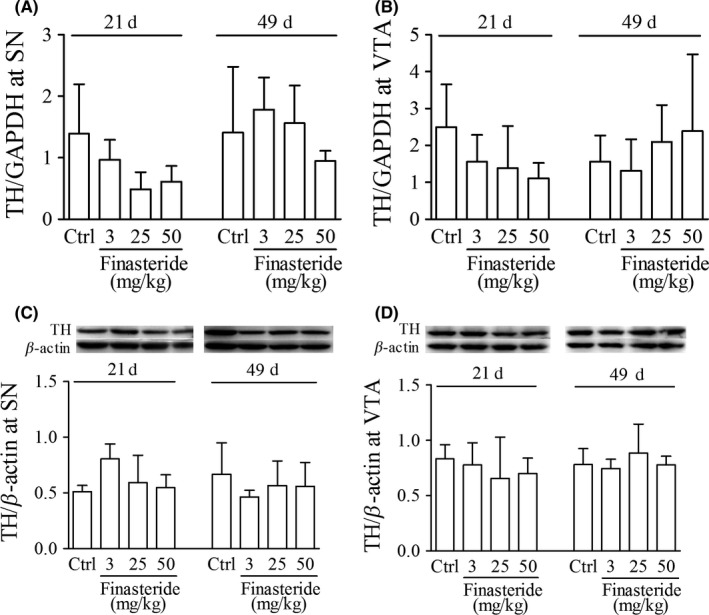
Real‐time quantitative RT‐PCR and western blot show the effect of finasteride on TH mRNA and protein expression, respectively, in early developmental rats. A and B are the relative expression of TH mRNA with the ratio of TH to GAPDH. In C and D, the upper panel is the immunoblot bands of TH and β‐actin, and the lower panels are the relative expression of TH with the density ratio of immunoblot bands of TH to β‐actin. It is shown that finasteride treatment has no effect on the TH expression at any dose or any observing time point at gene or protein level compared to control group. Abbreviations used are the same to Figure 6. (n = 7 for each group)
4. DISCUSSION
The present study indicated that finasteride inhibited brain dopaminergic system and exploratory and motor behaviors in adolescent male rats. Dopaminergic system exerts important modulating effect on neuropsychiatric behaviors besides its regulation on motor control and learning new motor skills.25 Many studies indicate that nigrostriatal dopamine pathway takes part in the drive of exploratory and locomotion behaviors.26, 27, 28, 29, 30 For example, dopamine transporter inhibitor GBR‐12909 and amphetamine increased open‐field ambulation in rats31 and preference for novel options in monkeys.26 Furthermore, dopamine D4 receptor knockout mice showed reduced exploration in open‐field test.28 Therefore, it was suggested that the inhibition of exploratory and motor behaviors after finasteride treatment in the present study may be the result of, at least in part, the inhibition of dopaminergic system.
A series of studies suggest the involvement of dysfunction of dopaminergic system in the pathogenesis of neuropsychiatric disorders, such as Tourette syndrome and schizophrenia.32, 33 For instance, hyperactivity and aberrant metabolisms of dopaminergic system, including high level of dopamine transporters, high densities of dopamine receptor D1 and D2 in frontal cortex, were found in the development and maintenance of Tourette syndrome.12, 13, 14, 15 On the other hand, loss of dopamine in ventral striatum, amygdala, thalamus and cingulated cortex was shown to be associated with depression and anxiety in Parkinson disease.34 Experimental studies revealed that decreased dopaminergic activity was associated with the reduction of exploratory and locomotor behaviors.35 Ablation of dopamine D2 receptor or age‐related reduction of the brain dopaminergic activity was involved in motor dysfunction.36, 37 Thus, the present finding that finasteride treatment inhibited brain dopaminergic system, implies finasteride as a potential therapeutic option for neuropsychiatric disorders with hyperactivities of dopaminergic system and androgen.
The present study demonstrated that administration of finasteride during adolescence inhibited dopaminergic system in late adolescent male rats. It was already revealed that testosterone levels in the plasma and testes elevate rapidly between postnatal day 20 and 30 and reach maxima between postnatal day 30 and 40.38 In the present study, for adolescent rats, finasteride was initially delivered at postnatal day 35 and repeatedly for 14 days while the testosterone level was maximal. During this period of high testosterone level, the inhibition of 5α‐reductase by the administration of finasteride inhibited the upregulation of potent dihydrotestosterone which is normally converted from testosterone and then elicited inhibition of dopaminergic system. However, for early developmental rats, finasteride was administrated from postnatal day 7‐21, neither early adolescent (postnatal day 21) nor late adolescent male rats (postnatal day 49) showed significant change in the dopaminergic system and exploratory and motor behaviors. It was reported that testosterone levels in the plasma and testes drop from postnatal day 1 and remain low level until day 20 and only tiny amount of testosterone is secreted by the testes during this period.38 Even the 5α‐reductase is active, there would not be much dihydrotestosterone generated in the plasma because of the low level of testosterone during postnatal day 7‐21. Therefore, the inhibition of 5α‐reductase by administration of finasteride during this developmental stage had no effect on androgen activity and dopaminergic system in early adolescence (postnatal day 21). In addition, rats received finasteride from postnatal day 7‐21 did not show significant change in dopaminergic system or exploratory and motor behaviors in late adolescence (postnatal day 49) as well. This result indicated that the administration of finasteride in early developmental stage had no delayed effect on dopaminergic system and exploratory and motor behaviors in late adolescence, because the half‐life period of finasteride is around 6 hours only according to the instruction, and finasteride administrated at early developmental stage had been metabolized before rats developed to adolescent stage. A recent study showed that administration of 3 mg/kg finasteride to young adult rats for 20 days failed to induce changes of dihydrotestosterone level in the cerebral spinal fluid and brain tissue.39 In the present study, the same dose of finasteride treatment also did not elicit significant change in dopaminergic system or open‐field behaviors in adolescent rats. Taken together, the present findings that finasteride treatment inhibited dopaminergic system only in adolescent rats rather than early developmental rats suggested that finasteride inhibited dopaminergic system by inhibiting the activity of androgen.
Androgen signaling is critical for central nervous system function,40 and converging studies indicate the androgen stimulation on the activity of dopaminergic system. For example, administration of testosterone inhibited the reduction of dopamine and DOPAC in castrated male rats.10 Castration decreased the activities of TH in caudate putamen,41 and supplement with testosterone completely prevented castration‐induced reduction in striatal TH activity.41 Testosterone propionate treatment induced alterations in dopaminergic signaling pathway in the brain,42, 43, 44 which increased the risk of mental illness.42 These reports support the present conclusion that finasteride inhibited dopaminergic system by inhibiting the activity of androgen.
Although the present study suggested the effect of finasteride on dopaminergic system via the inhibition of androgen activity by comparing the effects of finasteride on adolescent and early developmental rats, one concern remains whether finasteride directly affects the dopaminergic system. Indeed, there is a report that rats at postnatal day 5‐9 received 50 mg/kg finasteride decreased novelty‐exploratory at adolescent age.45 Thus, it cannot be denied that finasteride directly impacted brain dopaminergic system. More detailed studies are favorable to elucidate this concern.
Colocalization of androgen receptor with dopaminergic neurons might be important for the mechanisms underlying the stimulation of androgen on dopaminergic system. Immunocytochemistry and situ hybridization demonstrated numerous androgen receptors in VTA and SN,46, 47 and one‐third androgen receptor bearing cells in retrorubral fields were TH immune‐positive.46, 48 These findings suggest that androgen receptors are colocalized with dopamine neurons, and androgen can directly bind with androgen receptor located on the surface of dopaminergic neurons in VTA and SN and modulate dopamine production and metabolism. In addition, immunohistochemical analysis showed that 5α reductase II localized in the prefrontal cortex, basal ganglia, basolateral amygdala and hippocampus,49 where the dopaminergic neurons or their projections are abundantly located, so finasteride is able to inhibit dihydrotestosterone production in these areas. The above evidence provides neuroanatomical basis for the stimulation of androgen on dopaminergic system. Thus, in the present study, when finasteride was delivered to postnatal day 35 rats, dihydrotestosterone production was reduced. The stimulation of androgen binding with its receptor located on dopaminergic neurons in VTA and SN was decreased, which reduced dopamine production, and inhibited open‐field behaviors.
In conclusion, the present study demonstrated that administration of finasteride to adolescent male rats remarkably decreased activities of dopaminergic system and open‐field behaviors. The result of this study provided neurobiological evidence for finasteride as a potential therapeutic option for neuropsychiatric disorders associated with hyperactivities of dopaminergic system and androgen.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ACKNOWLEDGMENTS
This study was supported by funds from National Natural Science Foundation of China (No. 31240033) and Natural Science Foundation of Hebei Province of China (No. C2012206033).
Li L, Kang Y‐X, Ji X‐M, et al. Finasteride inhibited brain dopaminergic system and open‐field behaviors in adolescent male rats. CNS Neurosci Ther. 2018;24:115–125. 10.1111/cns.12781
REFERENCES
- 1. Frye CA, Seliga AM. Testosterone increases analgesia, anxiolysis, and cognitive performance of male rats. Cogn Affect Behav Neurosci. 2001;1:371‐381. [DOI] [PubMed] [Google Scholar]
- 2. Perry PJ, Kutscher EC, Lund BC, Yates WR, Holman TL, Demers L. Measures of aggression and mood changes in male weightlifters with and without androgenic anabolic steroid use. J Forensic Sci. 2003;48:646‐651. [PubMed] [Google Scholar]
- 3. Edinger KL, Frye CA. Testosterone's anti‐anxiety and analgesic effects may be due in part to actions of its 5α‐reduced metabolites in the hippocampus. Psychoneuroendocrinology. 2005;30:418‐430. [DOI] [PubMed] [Google Scholar]
- 4. Castiglia PT. Tourette syndrome. J Pediatr Health Care. 1997;11:189‐191. [DOI] [PubMed] [Google Scholar]
- 5. Martino D, Macerollo A, Leckman JF. Neuroendocrine aspects of Tourette syndrome. Int Rev Neurobiol. 2013;112:239‐279. [DOI] [PubMed] [Google Scholar]
- 6. Zou LP, Wang Y, Zhang LP, et al. Tourette syndrome and excitatory substances: is there a connection? Childs Nerv Syst. 2011;27:793‐802. [DOI] [PubMed] [Google Scholar]
- 7. Peterson BS, Leckman JF, Scahill L, et al. Steroid hormones and Tourette's syndrome: early experience with antiandrogen therapy. J Clin Psychopharmacol. 1994;14:131‐135. [PubMed] [Google Scholar]
- 8. Peterson BS, Zhang H, Anderson GM, Leckman JF. A double‐blind, placebo‐controlled, crossover trial of an antiandrogen in the treatment of Tourette's syndrome. J Clin Psychopharmacol. 1998;18:324‐331. [DOI] [PubMed] [Google Scholar]
- 9. Kindlundh AM, Rahman S, Lindblom J, Nyberg F. Increased dopamine transporter density in the male rat brain following chronic nandrolone decanoate administration. Neurosci Lett. 2004;356:131‐134. [DOI] [PubMed] [Google Scholar]
- 10. Mitchell JB, Stewart J. Effects of castration, steroid replacement, and sexual experience on mesolimbic dopamine and sexual behaviors in the male rat. Brain Res. 1989;491:116‐127. [DOI] [PubMed] [Google Scholar]
- 11. de Souza Silva MA, Mattern C, Topic B, Buddenberg TE, Huston JP. Dopaminergic and serotonergic activity in neostriatum and nucleus accumbens enhanced by intranasal administration of testosterone. Eur Neuropsychopharmacol. 2009;19:53‐63. [DOI] [PubMed] [Google Scholar]
- 12. Yoon DY, Gause CD, Leckman JF, Singer HS. Frontal dopaminergic abnormality in Tourette syndrome: a postmortem analysis. J Neurol Sci. 2007;255:50‐56. [DOI] [PubMed] [Google Scholar]
- 13. Wong DF, Brai JR, Singer HS, et al. Mechanisms of dopaminergic and serotonergic neurotransmission in Tourette syndrome: clues from an in vivo neurochemistry study with PET. Neuropsychopharmacology. 2008;33:1239‐1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Singer HS, Walkup JT. Tourette syndrome and other tic disorders. Diagnosis, pathophysiology, and treatment. Medicine (Baltimore). 1991;70:15‐32. [DOI] [PubMed] [Google Scholar]
- 15. Minzer K, Lee O, Hong JJ, Singer HS. Increased prefrontal D2 protein in Tourette syndrome: a postmortem analysis of frontal cortex and striatum. J Neurol Sci. 2004;219:55‐61. [DOI] [PubMed] [Google Scholar]
- 16. Jin Y, Penning TM. Steroid 5α‐reductases and 3α‐hydroxysteroid dehydrogenases: key enzymes in androgen metabolism. Best Pract Res Clin Endocrinol Metab. 2001;15:79‐94. [DOI] [PubMed] [Google Scholar]
- 17. Bortolato M, Muroni A, Marrosu F. Treatment of Tourette's syndrome with finasteride. Am J Psychiatry. 2007;164:1914‐1915. [DOI] [PubMed] [Google Scholar]
- 18. Muroni A, Paba S, Puligheddu M, Marrosu F, Bortolato M. A preliminary study of finasteride in Tourette syndrome. Mov Disord. 2011;26:2146‐2147. [DOI] [PubMed] [Google Scholar]
- 19. Bortolato M, Frau R, Orrù M, et al. Antipsychotic‐like properties of 5‐alpha‐reductase inhibitors. Neuropsychopharmacology. 2008;33:3146‐3156. [DOI] [PubMed] [Google Scholar]
- 20. Giatti S, Foglio B, Romano S, et al. Effects of subchronic finasteride treatment and withdrawal on neuroactive steroid levels and their receptors in the male rat brain. Neuroendocrinology. 2016;103:746‐757. [DOI] [PubMed] [Google Scholar]
- 21. Frau R, Abbiati F, Bini V, et al. Targeting neurosteroid synthesis as a therapy for schizophrenia‐related alterations induced by early psychosocial stress. Schizophr Res. 2015;168:640‐648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Darbra S, Pallarès M. Alterations in neonatal neurosteroids affect exploration during adolescence and prepulse inhibition in adulthood. Psychoneuroendocrinology. 2010;35:525‐535. [DOI] [PubMed] [Google Scholar]
- 23. Glenn MJ, Kirby ED, Gibson EM, et al. Age‐related declines in exploratory behavior and markers of hippocampal plasticity are attenuated by prenatal choline supplementation in rats. Brain Res. 2008;1237:110‐123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sanchez HL, Silva LB, Portiansky EL, Herenu CB, Goya RG, Zuccolilli GO. Dopaminergic mesencephalic systems and behavioral performance in very old rats. Neuroscience. 2008;154:1598‐1606. [DOI] [PubMed] [Google Scholar]
- 25. Molina‐Luna K, Pekanovic A, Röhrich S, et al. Dopamine in motor cortex is necessary for skill learning and synaptic plasticity. PLoS One. 2009;4:e7082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Costa VD, Tran VL, Turchi J, Averbeck BB. Dopamine modulates novelty seeking behavior during decision making. Behav Neurosci. 2014;128:556‐566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Patel JC, Rossignol E, Rice ME, Machold RP. Opposing regulation of dopaminergic activity and exploratory motor behavior by forebrain and brainstem cholinergic circuits. Nat Commun. 2012;3:1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dulawa SC, Grandy DK, Low MJ, Paulus MP, Geyer MA. Dopamine D4 receptor‐knock‐out mice exhibit reduced exploration of novel stimuli. J Neurosci. 1999;19:9550‐9556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhuang X, Oosting RS, Jones SR, et al. Hyperactivity and impaired response habituation in hyperdopaminergic mice. Proc Natl Acad Sci U S A. 2001;98:1982‐1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Beninger RJ. The role of dopamine in locomotor activity and learning. Brain Res. 1983;287:173‐196. [DOI] [PubMed] [Google Scholar]
- 31. van den Buuse M, de Jong W. Differential effects of dopaminergic drugs on open‐field behavior of spontaneously hypertensive rats and normotensive Wistar‐Kyoto rats. J Pharmacol Exp Ther. 1989;248:1189‐1196. [PubMed] [Google Scholar]
- 32. Buse J, Schoenefeld K, Münchau A, Roessner V. Neuromodulation in Tourette syndrome: dopamine and beyond. Neurosci Biobehav Rev. 2013;37:1069‐1084. [DOI] [PubMed] [Google Scholar]
- 33. Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III–the final common pathway. Schizophr Bull. 2009;35:549‐562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Remy P, Doder M, Lees A, Turjanski N, Brooks D. Depression in Parkinson's disease: loss of dopamine and noradrenaline innervation in the limbic system. Brain. 2005;128(Pt 6):1314‐1322. [DOI] [PubMed] [Google Scholar]
- 35. Rodríguez VM, Limón‐Pacheco JH, Mendoza‐Trejo MS, González‐Gallardo A, Hernández‐Plata I, Giordano M. Repeated exposure to the herbicide atrazine alters locomotor activity and the nigrostriatal dopaminergic system of the albino rat. Neurotoxicology. 2013;34:82‐94. [DOI] [PubMed] [Google Scholar]
- 36. Volkow ND, Gur RC, Wang GJ, et al. Association between decline in brain dopamine activity with age and cognitive and motor impairment in healthy individuals. Am J Psychiatry. 1998;155:344‐349. [DOI] [PubMed] [Google Scholar]
- 37. Bello EP, Casas‐Cordero R, Galiñanes GL, et al. Inducible ablation of dopamine D2 receptors in adult mice impairs locomotion, motor skill learning and leads to severe parkinsonism. Mol Psychiatry. 2017;22:595‐604. [DOI] [PubMed] [Google Scholar]
- 38. Jean‐Faucher C, Berger M, de Turckheim M, Veyssiere G, Jean C. Developmental patterns of plasma and testicular testosterone in mice from birth to adulthood. Acta Endocrinol. 1978;89:780‐788. [DOI] [PubMed] [Google Scholar]
- 39. Giatti S, Foglio B, Romano S, et al. Effects of subchronic finasteride treatment and withdrawal on neuroactive steroid levels and their receptors in the male rat brain. Neuroendocrinology. 2016;103:746‐757. [DOI] [PubMed] [Google Scholar]
- 40. Patchev VK, Schroeder J, Goetz F, Rohde W, Patchev AV. Neurotropic action of androgens: principles, mechanisms and novel targets. Exp Gerontol. 2004;39:1651‐1660. [DOI] [PubMed] [Google Scholar]
- 41. Abreu P, Hernandez G, Calzadilla CH, Alonso R. Reproductive hormones control striatal tyrosine hydroxylase activity in the male rat. Neurosci Lett. 1988;95:213‐217. [DOI] [PubMed] [Google Scholar]
- 42. Purves‐Tyson TD, Handelsman DJ, Double KL, Owens SJ, Bustamante S, Weickert CS. Testosterone regulation of sex steroid‐related mRNAs and dopamine‐related mRNAs in adolescent male rat substantia nigra. BMC Neurosci. 2012;13:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Purvestyson TD, Owens SJ, Double KL, Desai R, Handelsman DJ, Weickert CS. Testosterone induces molecular changes in dopamine signaling pathway molecules in the adolescent male rat nigrostriatal pathway. PLoS One. 2014;9:e91151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Vermes I, Várszegi M, Tóth EK, Telegdy G. Action of androgenic steroids on brain neurotransmitters in rats. Neuroendocrinology. 1979;28:386‐393. [DOI] [PubMed] [Google Scholar]
- 45. Darbra S, Pallarãs M. Alterations in neonatal neurosteroids affect exploration during adolescence and prepulse inhibition in adulthood. Psychoneuroendocrinology. 2010;35:525‐535. [DOI] [PubMed] [Google Scholar]
- 46. Kritzer MF. Selective colocalization of immunoreactivity for intracellular gonadal hormone receptors and tyrosine hydroxylase in the ventral tegmental area, substantia nigra, and retrorubral fields in the rat. J Comp Neurol. 1997;379:247‐260. [DOI] [PubMed] [Google Scholar]
- 47. Simerly RB, Chang C, Muramatsu M, Swanson LW. Distribution of androgen and estrogen receptor mRNA‐containing cells in the rat brain: an in situ hybridization study. J Comp Neurol. 1990;294:76‐95. [DOI] [PubMed] [Google Scholar]
- 48. Creutz LM, Kritzer MF. Mesostriatal and mesolimbic projections of midbrain neurons immunoreactive for estrogen receptor beta or androgen receptors in rats. J Comp Neurol. 2004;476:348‐362. [DOI] [PubMed] [Google Scholar]
- 49. Castelli MP, Casti A, Casu A, et al. Regional distribution of 5α‐reductase type 2 in the adult rat brain: an immunohistochemical analysis. Psychoneuroendocrinology. 2013;38:281‐293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Casarrubea M, Sorbera F, Crescimanno G. Multivariate analysis of the modifications induced by an environmental acoustic cue on rat exploratory behavior. Physiol Behav. 2008;93:687‐696. [DOI] [PubMed] [Google Scholar]
- 51. Casarrubea M, Sorbera F, Crescimanno G. Structure of rat behavior in hole‐board: I) multivariate analysis of response to anxiety. Physiol Behav. 2009;96:174‐179. [DOI] [PubMed] [Google Scholar]
- 52. Casarrubea M, Sorbera F, Crescimanno G. Structure of rat behavior in hole‐board: II) multivariate analysis of modifications induced bydiazepam. Physiol Behav. 2009;96:683‐692. [DOI] [PubMed] [Google Scholar]
- 53. Meyerson BJ, Hoglund A. Exploratory and socio‐sexual behaviour in the male laboratory rat: a methodological approach for the investigation of drug action. Acta Pharmacol Toxicol (Copenh). 1981;48:168‐180. [DOI] [PubMed] [Google Scholar]
- 54. Ericson E, Samuelsson J, Ahlenius S. Photocell measurements of rat motor activity: a contribution to sensitivity and variation in behavioral observations. J Pharmacol Methods. 1991;25:111‐122. [DOI] [PubMed] [Google Scholar]
- 55. Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety‐like behaviors: a review. Eur J Pharmacol. 2003;463:3‐33. [DOI] [PubMed] [Google Scholar]
- 56. Hillegaart V, Wadenberg M, Ahlenius S. Effects of 8‐OH‐DPAT on motor activity in the rat. Pharmacol Biochem Behav. 1989;32:797‐800. [DOI] [PubMed] [Google Scholar]
- 57. Wultz B, Sagvolden T, Moser EI, Moser MB. The spontaneously hypertensive rat as an animal model of attention‐deficit hyperactivity disorder: effects of methylphenidate on exploratory behavior. Behav Neural Biol. 1990;53:88‐102. [DOI] [PubMed] [Google Scholar]
- 58. Li JS, Huang YC. Early androgen treatment influences the pattern and amount of locomotion activity differently and sexually differentially in an animal model of ADHD. Behav Brain Res. 2006;175:176‐182. [DOI] [PubMed] [Google Scholar]


