Abstract
Background and objective
The scoring scales scoring system for targeting atrial fibrillation (STAF), left atrial diameter, age, diagnosis of stroke, and smoking status (LADS), and identified by past history of arrhythmia or antiarrhythmic agent use, atrial dilation, and elevation of Brain natriuretic peptide (iPAB) have been proposed for predicting atrial fibrillation in patients with acute cerebral infarction, but their relative accuracies are not clear. This prospective study compared STAF, LADS, and iPAB scores for predicting paroxysmal atrial fibrillation (PAF) in patients with acute cerebral infarction.
Methods
Patients with acute cerebral infarction (n = 744; 495 men, 249 women; aged 65 ± 12 years) were consecutively enrolled throughout the year 2016 at the Department of Neurology of Huizhou Municipal Central Hospital. Patients were followed for 3 months. The sensitivity, specificity, area under the receiver operating characteristic curve (AUC), and best cutoff points of STAF, LADS, and iPAB scores for predicting PAF were computed.
Results
Among the 744 patients, 37 patients had PAF. The AUCs of the STAF, LADS, and iPAB scores for predicting PAF were 0.87, 0.79, and 0.84, respectively, and with a cutoff at four points, the sensitivities were 73%, 70.3%, and 83.8%, and specificities were 92.1%, 82.2%, and 77%.
Conclusions
The STAF, LADS, and iPAB scores could satisfactorily predict PAF in patients with acute cerebral infarction. STAF was superior to the others in diagnostic performance.
Keywords: acute cerebral infarction, atrial fibrillation, iPAB score, LADS score, paroxysmal atrial fibrillation, STAF score
1. INTRODUCTION
Atrial fibrillation (AF) is an independent risk factor for cerebral infarction. Patients with AF are at four to five times greater risk of cerebral infarction.1 After ischemic stroke, the presence of AF is an important risk factor for recurrent stroke, whether the type is paroxysmal AF (PAF) or permanent AF. Although PAF has a short duration, the risk of cerebral infarction is the same as that of chronic AF,2, 3 and PAF is the main cause of cryptogenic stroke.4, 5
The risk of cerebral infarction caused by AF can be reduced by anticoagulation therapy.6 Current guidelines from Europe, the United States, China, and Japan each recommend the use of oral anticoagulation for the prevention of AF‐induced cerebral infarction.7, 8, 9, 10 It is also important to start anticoagulant therapy in patients with AF who have already experienced cerebral infarction. However, 81% of patients with PAF are asymptomatic.11 The 12‐lead electrocardiogram (ECG) can reveal permanent AF, but PAF is not as easily diagnosed. The wearable ECG is limited by an allergic reaction in some patients and is uncomfortable in the long term.
Three scoring systems have been proposed to predict PAF. In 2011, Suissa et al.12 developed scoring system for targeting AF (STAF) to predict PAF in patients with cerebral infarction. When the STAF score is >5 points, the sensitivity and specificity for predicting PAF is 90% and 77%, respectively. In 2010, Malik et al.13 proposed the based on left atrial diameter, age, diagnosis of stroke, and smoking status (LADS) scale to predict both PAF and chronic AF after acute cerebral infarction or transient ischemic attack. When the LADS score is >4 points, the sensitivity and specificity for predicting PAF is 85.5% and 53.1%. More recently, Yoshioka et al.14 developed the identified by past history of arrhythmia or antiarrhythmic agent use, atrial dilation, and elevation of brain natriuretic peptide (iPAB) scale to predict PAF after acute cerebral infarction. When the iPAB score is >2 points, the sensitivity and specificity for predicting PAF is 93% and 71%, and when the score is >4, the sensitivity and specificity is 60% and 95%.
However, the sensitivity and specificity of the three scoring systems have been questioned,12, 15 and they are not widely used in clinical practice. In mainland China, STAF scores might theoretically be obtained for all patients with acute stroke and used as a reference to evaluate the accuracy of LADS and iPAB.16 However, the annual incidence of stroke in mainland China is 1596.0/100 000 population, and epidemiological studies have shown that 69.6 to 77.8% are due to cerebral infarction.17 Since long‐term electrocardiogram examination for all stroke patients will cost huge medical resources, it is of vital importance to screen patients with potential AF before performing ECG examination. Thus, it is mandatory to compare the utility of the STAF, LADS, and iPAB scoring for their sensitivity and specificity. To the best of our knowledge, there has been no comparative study to determine which is best for predicting PAF in patients with acute cerebral infarction. Thus, the primary objective of the present study was to compare the diagnostic accuracies of these scores for predicting PAF in patients with acute cerebral infarction.
2. METHODS
Patients with acute cerebral infarction admitted to the Department of Neurology in Huizhou Municipal Central Hospital from 1 January to December 31, 2016 were potentially eligible. STAF, LADS, and iPAB scores were immediately obtained after hospital admission. Remote ECG monitoring or 24‐hour Holter were performed for patients who fulfilled any of the following criteria: STAF score > 5; LADS score > 4; or iPAB score > 4. All the patients were followed for 3 months to increase the probability of PAF detection. Not all patients could complete Holter or remote ECG monitoring because of the shortage of funds and equipment. The Ethics Committee of Huizhou Municipal Central Hospital approved this study.
All the patients were followed in the community, by contacting patients or their family members through telephone call, on 15 days before, and 3 months after the onset of cerebral infarction. Clinical data generated during the 3‐month follow‐up period were collected for both outpatients and inpatients.
During follow‐up, ECG was performed at home by primary care physicians in the community. Older patients and stroke patients are the focus of community healthcare service. There is evidence that for patients older than 65 years who demonstrate an irregular pulse, community doctors can increase the identification of AF through ECG by 50%.18 Influenced by traditional Chinese medicine (TCM), the pulse of every patient is monitored (eg, the pulse profile is vital sign in the TCM framework). Stroke patients are visited by community doctors ≥3 times per week for 14 days after discharge, and ≥1 time per week thereafter. If an irregular pulse is identified, a 12‐lead ECG examination is immediately performed. The results are recorded and given to doctors to implement stroke treatment. In this way, AF is identified.
Inclusion criteria of the study were: acute cerebral infarction; <2 weeks between disease onset and hospital visit; and older than 18 years.
The diagnosis of acute cerebral infarction was made in accordance with the Baltimore‐Washington Cooperative Young Stroke Study standard criteria. That is, nerve function defect lasted >24 hours, and clinical manifestations and brain computed tomography (CT) and/or magnetic renascence imaging (MRI) findings were consistent with acute cerebral infarction.19 The diagnosis of acute lacunar infarction was made based on the findings of isolated infarction with diameters <15 mm in the subcortical or brainstem on MRI. Otherwise, the lesion was defined as non‐lacunar cerebral infarction.
Patients with history of chronic AF, PAF before admission, or missing baseline values were excluded from this study. AF was categorized as either PAF or chronic AF.20 Chronic AF included persistent AF, long‐standing persistent AF, and permanent AF.21 Remote ECG monitoring was performed using a Beijing Maibang telemetry monitoring system MB800 (Beijing M&B Electronic Instruments Co., Ltd, Beijing, China). Bedside ECG monitoring was performed with a Shenzhen MINDRAY iPM10 patient monitor (Mindray Automation Equipment Co., Ltd, Shenzhen, China). The 24‐hour Holter was performed using a Tim dynamic ECG recorder DMS300‐4 from the United States (DM Software Inc., Davis, California).
2.1. Baseline characteristics
The following baseline characteristics of the patients were collected: age, gender, blood uric acid, and total cholesterol, history of stroke, arrhythmia, antiarrhythmic drug use, smoking, hypertension, and diabetes. In addition, the following were included: brain natriuretic peptide, left atrial diameter, 12‐lead ECG and ECG monitoring data and 24‐hour Holter; and results of neck vascular ultrasound, MRI, cranial CT or cranial magnetic resonance angiography, CT angiography of the head and neck, or digital subtraction angiography. The National Institutes of Health Stroke Scale (NIHSS) score on the first admission due to cerebral infarction, Trial of ORG 10172 in Acute Stroke Treatment (TOAST ) classification, and the scores for LADS, STAF, and iPAB were also noted.
2.2. Details of the 3 scores
2.2.1. STAF
The STAF scale is scored as follows: age > 62 years, 2 points; NIHSS > 8, 1 point; left atrial enlargement, 2 points; and absence of extracranial artery stenosis, 3 points. Intracranial and extracranial vascular lesions were defined as: culprit vascular stenosis ≥50% and symptomatic artery dissection or lacunar infarction. The optimal cutoff was ≥5 points.22
2.2.2. LADS
Specific items that determined the LADS score are as follows: 0, 1, or 2 points assigned for left atrial diameter <35, 35 to 45, and >45 mm, respectively; 0, 1, or 2 points for age <60, 60 to 79, and > 80 years; 0 or 1 point for diagnosis of transient ischemic attack or stroke; and 1 point for positive smoking status. The optimal cutoff point was ≥4 points.13
2.2.3. iPAB
Specific items for the iPAB score are: history of arrhythmia or antiarrhythmic drug use, 3 points; left atrial enlargement ≥40 mm, 1 point; and 1, 2, or 3 points for brain natriuretic peptide ≥50, ≥90, and ≥150 pg/mL, respectively.14
2.3. Statistical analysis
All data were analyzed with SPSS 22 software. The continuous variables with normal distribution were tested by t test, and the continuous variables of non‐normal distribution were tested by Mann‐Whitney U test.23 The categorical variables were tested using the χ 2 test. Receiver operating characteristic (ROC) curves were constructed for each of the three scoring systems, and the sensitivity, specificity, and area under the ROC curve (AUC) of each were computed. The optimal cutoff points of the three scores were determined using the Youden index. The respective sensitivities and specificities were estimated at these cutoff points. Two‐tailed P < 0.05 was considered statistically significant.
3. RESULTS
Initially, 883 patients with cerebral infarction were identified. Forty‐two patients with onset time > 2 weeks, 57 with incomplete baseline data, and 40 with a history of definite chronic AF or PAF were excluded. The remaining 744 cases met the diagnostic criteria of acute cerebral infarction and thus were included in this study: 495 (66.5%) men and 239 (33.5%) women, aged 65 ± 12 years (Table 1). The frequency distributions of the STAF, LADS, and iPAB scores are shown in Table 2.
Table 1.
Baseline characteristics of patients with acute cerebral infarction
| Total | PAF | Non‐AF | P‐value | |
|---|---|---|---|---|
| Subjects (n) | 744 | 37 | 707 | — |
| Male | 495 (66.5%) | 23 (62.2%) | 472 (66.8%) | 0.563 |
| Age (years) | 65 ± 12 | 72 ± 10 | 64 ± 12 | 0.000 |
| Hypertension | 523 (70.3%) | 20 (54.1%) | 503 (71.1%) | 0.000 |
| Diabetes | 180 (24.2%) | 6 (16.2%) | 174 (24.6%) | 0.010 |
| Blood uric acid | 315.1 ± 105.4 | 323.6 ± 118.2 | 314.5 ± 105.1 | 0.120 |
| Total cholesterol | 4.56 ± 1.18 | 4.35 ± 1.17 | 4.58 ± 1.22 | 0.005 |
| Diameter of left atrium (cm) | 3.2 ± 5 | 3.6 ± 0.6 | 3.2 ± 0.4 | 0.000 |
| Smoking history | 271 (36.4%) | 4 (10.8%) | 267 (37.8%) | 0.001 |
| NIHSS | 5 ± 4 | 8 ± 6 | 5 ± 4 | 0.004 |
| Absence of vascular lesion | 277 (37.2%) | 23 (62.2%) | 254 (60.6%) | 0.000 |
| Past history of arrhythmia | 45 (6.0%) | 12 (32.4%) | 33 (4.7%) | 0.000 |
| Brain natriuretic peptide (pg/mL) | 110 ± 244 | 405 ± 688 | 94 ± 184 | 0.000 |
| TOAST classification | ||||
| 1 | 196 (26.3%) | 0 | 196 (27.7%) | 0.000 |
| 2 | 50 (6.7%) | 36 (97.3%) | 14 (2%) | — |
| 3 | 225 (30.2%) | 0 | 225 (31.8%) | — |
| 4 | 19 (2.6%) | 0 | 19 (2.7%) | — |
| 5 | 254 (34.1%) | 1 (2.7%) | 253(35.8%) | — |
| Holter (days) | 57 (7.6%) | 2 (5.4%) | 55 (7.7%) | — |
| ECG (person) | 258 (34.7%) | 35 (94.6%) | 223 (31.5%) | — |
| PAF | 37 (4.9%) | 37 (100%) | 0 | — |
Abbreviations: ECG, electrocardiogram; NIHSS, National Institutes of Health Stroke Scale; PAF, paroxysmal atrial fibrillation; TOAST: Trial of ORG 10172 in acute stroke treatment.
Table 2.
Distribution of STAF, LADS, and iPAB scores, n (%)
| STAF | LADS | iPAB | |
|---|---|---|---|
| 0 | 198 (26.6%) | 8 (1.1%) | 302 (40.6%) |
| 1 | 41 (5.5%) | 86 (11.6%) | 249 (33.5%) |
| 2 | 291 (39.1%) | 247 (33.2%) | 55 (7.4%) |
| 3 | 135 (18.2%) | 252 (33.9%) | 92 (12.4%) |
| 4 | 25 (3.4%) | 120 (16.1%) | 14 (1.9%) |
| 5 | 44 (5.9%) | 24 (3.2%) | 11 (1.5%) |
| 6 | 3 (0.4%) | 7 (0.9%) | 17 (2.3%) |
| 7 | 6 (0.8%) | — | 4 (0.5%) |
| 8 | 1 (0.1%) | — | — |
Abbreviations: AUC, area under curve; iPAB, identified by past history of arrhythmia or antiarrhythmic agent use, atrial dilation, and elevation of Brain natriuretic peptide; LADS, based on left atrial diameter, age, diagnosis of stroke, and smoking status; STAF, scoring system for targeting atrial fibrillation
Each of the 744 patients (100%) underwent a 12‐lead ECG examination at bedside. Fifty‐seven patients (7.6%) underwent 24‐hour Holter, and 258 (34.7%) underwent either ECG or continuous bedside ECG monitoring. The median time of ECG or continuous bedside ECG monitoring was 4 days, and the mean was 8.25 days per person. A total number of 37 patients were found to have PAF, of which there were 29 (78.4%) identified during hospitalization, and 8 (21.6%) identified during follow‐up.
Imaging studies of the patients included neck vascular color Doppler ultrasound in 740 patients (99.5%), cranial magnetic resonance angiography in 555 (74.6%), head neck CT angiography in 342 (46%), and digital subtraction angiography in 55 (7.4%).
3.1. ROCs of the three scores
The AUCs of the STAF, LADS, and iPAB scores were 0.872, 0.794, and 0.844, respectively (Table 3,Figure 1). The optimal cutoff points of the STAF, LADS, and iPAB scores at the maximum Youden index (0.525, 0.651, and 0.605) were 4, 4, and 2 points, respectively.
Table 3.
Area under the receiver operating characteristic
| AUC | Se | P | 95% CI | |
|---|---|---|---|---|
| LADS | 0.794 | 0.039 | 0.000 | 0.718‐0.871 |
| STAF | 0.872 | 0.033 | 0.000 | 0.807‐0.937 |
| iPAB | 0.844 | 0.034 | 0.000 | 0.778‐0.910 |
Abbreviations: AUC, iPAB, identified by past history of arrhythmia or antiarrhythmic agent use, atrial dilation, and elevation of Brain natriuretic peptide; LADS, based on left atrial diameter, age, diagnosis of stroke, and smoking status; STAF, scoring system for targeting atrial fibrillation.
Figure 1.
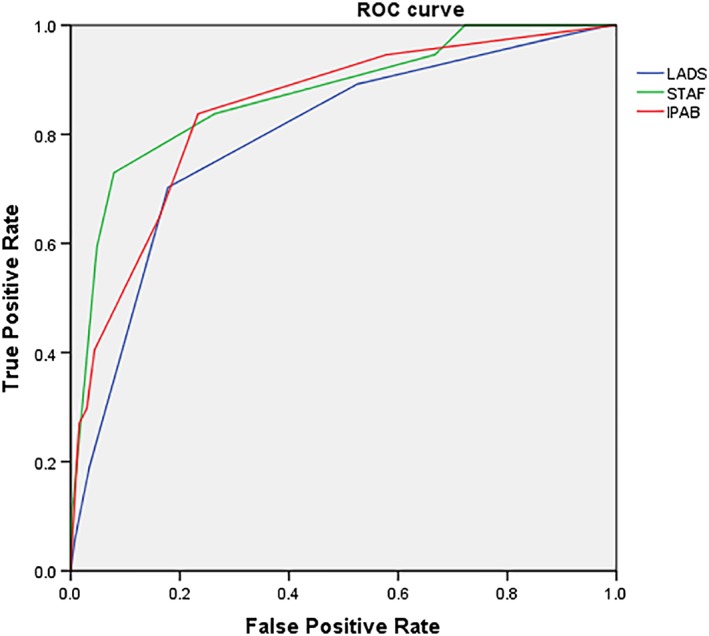
ROC: Receiver operating characteristic; STAF: Scoring system for targeting atrial fibrillation; LADS: Based on left atrial diameter, age, diagnosis of stroke, and smoking status; iPAB: Identified by past history of arrhythmia or antiarrhythmic agent use, atrial dilation, and elevation of brain natriuretic peptide
4. DISCUSSION
PAF is an occult cause of ischemic stroke that is clinically challenging to identify.24 In recent years, the scoring systems STAF, LADS, and iPAB scoring systems were developed for the prediction of AF (especially PAF) after stroke. It is important to identify AF so that anticoagulant therapy can be initiated to prevent stroke recurrence. However, the predictive values of the three scores had not been validated and compared in a Chinese population. This prospective study compared the efficacies of the three scores for predicting PAF after acute cerebral infarction in a Chinese population.
Among the 744 consecutively enrolled patients in our study population, 66.5% were men and 33.5% with women (a male‐to‐female ratio of 2.01:1), with a mean age of 65 ± 12 years. These demographic variables are consistent with other reports. In 2009, Feigin et al.25 conducted a systematic review of 56 countries and regions, and found that the male‐to‐female ratio was 1.41:1, and the prevalence of stroke was higher in men than women. In 2011, a study of data in the Chinese National Stroke Registry database (n = 21 902) reported that Chinese male stroke patients (61.2%) significantly outnumbered the women (38.8%).26
In this study, the AUC of the STAF score was 0.87, with an optimal cutoff of 4 points. At ≥4, the sensitivity and specificity were 73% and 92.1%, respectively. The predictive accuracy of the present study was lower than that reported by Suissa et al.,12 in which the data of 500 patients with acute cerebral infarction showed that the AUC of STAF scores for predicting PAF was 0.907, and the optimal cutoff was 5 points. When the STAF scores were ≥5, the sensitivity and specificity were 90% and 77%, which was different from our results. These differences may be because of, first, in the present study 10.3% of the patients had AF, including 37 with PAF (4.9%), while in Suissaet al.12 the corresponding percentages were 29% and 13%. Thus, the percentages in the latter study were much higher.
Another study reported that in a Chinese population, the prevalence of AF was only 0.77%, while the rate increased to 7.5% for patients older than 85 years27; these results are significantly lower than reported in other countries. A large epidemiological study from Israel (n = 2 414 282) reported a prevalence of AF of 3%.28 A study from Rotterdam Holland showed that the prevalence of AF among people older than 55 years was 5.5% and 17.8% in those older than 85 years.29 Furthermore, a cross‐sectional study conducted in China involving medical centers devoted to stroke patients from 19 cities showed that the prevalence of AF and transient ischemic attack in patients with cerebral infarction was only 4.7% (108/2283).30 In contrast, a German quality control study reported prevalences from 23.2% to 28%.31
The TOAST classification of our study also differed from that of Suissa et al.12 In our study, 26.4%, 6.7%, and 30.2% of our patients suffered from large artery atherosclerotic stroke, cardiogenic cerebral embolism, and arterial occlusion, respectively, and 2.6% and 34.1% from ischemic stroke or other causes of unexplained ischemic stroke. In contrast, the corresponding percentages in Suissa et al.'s study12 were 23.8%, 35.4%, 8.8%, 8%, and 23.6%. Thus, the proportion of small artery occlusion in our study was significantly higher than that of Suissa et al. This may be attributable to the different ethnicities of the populations. The proportion of arterial occlusive stroke in a Chinese population has been reported at ~33.1%, which is significantly higher than that reported in western countries.32
Horstmann et al.15 evaluated the STAF scoring system in a prospective cohort and found that it was of limited value for predicting PAF after acute cerebral infarction. The study included 584 patients with acute cerebral infarction, and 31.3% were identified to have AF. The AUC of the STAF score to predict AF was 0.84, with a sensitivity and specificity of 79% and 75%, respectively, at a cutoff value of ≥5. When subjects with a past history of AF were excluded, the AUC was 0.71. A STAF score >5 points showed a sensitivity and specificity for predicting PAF of 58% and 74%.
In addition, Yoshioka et al.14 showed that the AUC of STAF for predicting PAF after cerebral infarction was 0.77, which is of moderate predictive value. Liu et al.33 also showed that the STAF score was of limited value for predicting PAF, or new onset AF, after cerebral infarction; the AUCs for predicting AF and PAF were 0.842 and 0.763, respectively. When the score was >5 points, the sensitivity and specificity were 76.92% and 78.68%. The study by Lin et al.34 found that the STAF score could differentiate cardioembolic stroke from other types of stroke in the hospital, with an AUC of 0.98. When the score was ≥5 points, the sensitivity and specificity for diagnosing cardiogenic stroke were 90% and 95%.
In the present study, the AUC of the LADS system for predicting AF was 0.79, with an optimal cutoff of 4 points; the sensitivity and specificity were 70.3% and 82.2%, respectively, at the cutoff point. Malik et al.13 evaluated the diagnostic performance of the LADS in 970 patients with acute cerebral infarction and transient ischemic attack. In this cohort, there were 145 patients (15%) with AF (including chronic AF and PAF), and the best cutoff was 4 points. When the scores were ≥4 points, the sensitivity and specificity for predicting AF were 85.5% and 53.1%, respectively.
In the present study, the prevalence of AF (10.3%) was slightly lower than that reported by Malik et al.13 (15%). However, the specificity of our result was significantly higher, which is attributable to the large proportion of non‐AF cases in our study. Furthermore, our study excluded all patients with chronic AF, whereas Malik et al.13 included both PAF and chronic AF cases. Another reason for the difference may be that Malik et al.13 included patients with both acute cerebral infarction and transient ischemic attack, with the latter accounting for 21% of the overall study population. In contrast, our study included only patients with acute cerebral infarction.
The AUC of iPAB in the study was 0.84, with an optimal cutoff of 2 points. At this cutoff, the sensitivity and specificity were 83.8% and 76.7%, respectively, whereas at a cutoff of 4 points, the sensitivity and specificity were 40.5% and 95.6%, respectively. Furthermore, our study population had a large proportion of patients that scored zero (40.6%) or 1 point (33.5%) according to the iPAB system. Yoshioka et al.14 evaluated the iPAB score in 494 patients with cerebral infarction and found that the AUC of iPAB for predicting PAF after cerebral infarction was 0.93. At a cutoff of 2 points, the sensitivity and specificity were 93% and 71%, respectively, and at a cutoff of 4 points, the sensitivity and specificity were 60% and 95%, respectively. The lower diagnostic accuracy of our study may be because of arrhythmia was not clearly defined, and many patients did not know they were taking anti‐arrhythmia drugs. There was no electronic healthcare record in the region and the family members could not provide these medical histories. The history of arrhythmia and use of relevant drugs is an important component of the total iPAB score, and missing data may significantly compromise the sensitivity of the iPAB score. The specificity of the score was comparable to that reported in Yoshioka et al.'s14 study.
In the present study, the AUCs of the STAF, LADS, and iPAB scores were 0.872, 0.794, and 0.844, respectively. The maximum Youden’s indices of the STAF, LADS, and iPAB scores were 0.525, 0.651, and 0.605, respectively. Thus, the diagnostic accuracies of the three scores were moderate. Further studies are warranted to identify subgroups of patients for whom predictive values are better.35
Several limitations of the present study must be acknowledged. First, only 42.3% of the patients completed the bedside or remote ECG or 24‐hour Holter, and not all of the patients completed the follow‐up. The ECG examinations of the high‐risk patients were not complete, as indicated by the three scores, which explains the low incidence of AF. On the other hand, the identification of PAF was challenging because the patients could not be followed closely enough. Indeed, it was impossible to identify all the patients with PAF accurately. Second, the duration of bedside or remote monitoring ranged between 1 and 180 days per person, and thus was not standardized; the median and mean numbers of days were 4 and 8.25, respectively. This may also contribute to the heterogeneity of the study population. Third, patients with incomplete data were excluded from the analysis, which may be considered selection bias.36 Finally, although the sensitivity and specificity for predicting AF was good for those above the cutoff point, for those below the cutoff point evidence is lacking for predicting performance. This may be the reason why, for those below the cutoff, AF maybe overlooked. The cutoff point explored in the present study was the preliminary result of risk stratification, and needs to be validated in future studies with larger sample sizes. However, the proportion of patients who were excluded was not large and the results can still be considered robust.
5. CONCLUSION
STAF, LADS, and iPAB scores were able to predict PAF in patients with acute cerebral infarction. The STAF score had the highest predictive value, and then iPAB and LADS.
CONFLICTS OF INTEREST
The authors declare no potential conflict of interests
Chen X, Luo W, Li J, et al. Diagnostic accuracy of STAF, LADS, and iPAB scores for predicting paroxysmal atrial fibrillation in patients with acute cerebral infarction. Clin Cardiol. 2018;41:1507–1512. 10.1002/clc.23080
REFERENCES
- 1. Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham study. Stroke. 1991;22(8):983‐988. [DOI] [PubMed] [Google Scholar]
- 2. Hohnloser SH, Pajitnev D, Pogue J, et al. ACTIVE W Investigators Incidence of stroke in paroxysmal versus sustained atrial fibrillation in patients taking oral anticoagulation or combined antiplatelet therapy: an ACTIVE W substudy. J Am Coll Cardiol. 2007;50(22):2156‐2161. [DOI] [PubMed] [Google Scholar]
- 3. Achanta SS, Chicago I. USA. Risk of stroke with paroxysmal and permanent atrial fibrillation is the same. BMJ. 2015;335(7616):383‐386. [Google Scholar]
- 4. Christensen LM, Krieger DW, Højberg S, et al. Paroxysmal atrial fibrillation occurs often in cryptogenic ischaemic stroke. Final results from the SURPRISE study. Eur J Neurol. 2014;21:884‐889. [DOI] [PubMed] [Google Scholar]
- 5. Wohlfahrt J, Stahrenberg R, Weber‐Krüger M, et al. Clinical predictors to identify paroxysmal atrial fibrillation after ischaemic stroke. Eur J Neurol. 2014;21:21‐27. [DOI] [PubMed] [Google Scholar]
- 6. Camm AJ, Lip GY, De Caterina R, et al. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J. 2012;33:2719‐2747. [DOI] [PubMed] [Google Scholar]
- 7. January CT, Wann LS, Alpert JS, et al. American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association task force on practice guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64(21):e1‐e76. [DOI] [PubMed] [Google Scholar]
- 8. Neurology CSO. Guidelines for the secondary prevention of ischemic stroke and transient ischemic attack in China 2014. Chinese J Neurol. 2015;48:258‐273. [Google Scholar]
- 9. National Clinical Guideline Centre (UK) . Atrial Fibrillation: The Management of Atrial Fibrillation. London, UK: National Institute for Health and Care Excellence (UK); 2014:1‐420. [PubMed] [Google Scholar]
- 10. JCS Joint Working Group . Guidelines for pharmacotherapy of atrial fibrillation (JCS 2013). Circ J. 2014;78:1997‐2021. [DOI] [PubMed] [Google Scholar]
- 11. Quirino G, Giammaria M, Corbucci G, et al. Diagnosis of paroxysmal atrial fibrillation in patients with implanted pacemakers: relationship to symptoms and other variables. Pacing Clin Electrophysiol. 2009;32(1):91‐98. [DOI] [PubMed] [Google Scholar]
- 12. Suissa L, Mahagne MH, Lachaud S. Score for the targeting of atrial fibrillation: a new approach to diagnosing paroxysmal atrial fibrillation. Cerebrovasc Dis. 2011;31(5):442‐447. [DOI] [PubMed] [Google Scholar]
- 13. Malik S, Hicks WJ, Schultz L, et al. Development of a scoring system for atrial fibrillation in acute stroke and transient ischemic attack patients: the LADS scoring system. J Neurol Sci. 2011;301(1‐2):27‐30. 10.1016/j.jns.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 14. Yoshioka K, Watanabe K, Zeniya S, et al. A score for predicting paroxysmal atrial fibrillation in acute stroke patients: iPAB score. J Stroke Cerebrovasc Dis. 2015;24(10):2263‐2269. [DOI] [PubMed] [Google Scholar]
- 15. Horstmann S, Rizos T, Güntner J, et al. Does the STAF score help detect paroxysmal atrial fibrillation in acute stroke patients. Eur J Neurol. 2013;20(1):147‐152. [DOI] [PubMed] [Google Scholar]
- 16. The Center for Quality Control of Stroke Management , State Health and Family Planning Commission , Specialized Committee of Stroke Prevention and Control of the Chinese Society of Preventive Medicine . Chinese Expert Consensus on the Screening of Atrial Fibrillation and antithrombotic therapy in patients with ischemic stroke/transient ischemic attack. J Intern Med. 2014;8:665‐671. [Google Scholar]
- 17. Wang W, Jiang B, Sun H, et al. NESS‐China Investigators. Prevalence, incidence, and mortality of stroke in China: results from a Nationwide population‐based survey of 480 687 adults. Circulation. 2017;135(8):759‐771. [DOI] [PubMed] [Google Scholar]
- 18. Lowres N, Neubeck L, Redfern J, Freedman SB. Screening to identify unknown atrial fibrillation. A systematic review. Thromb Haemost. 2013;110(2):213‐222. [DOI] [PubMed] [Google Scholar]
- 19. Johnson CJ, Kittner SJ, McCarter RJ, et al. Interrater reliability of an etiologic classification of ischemic stroke. Stroke. 1995;26:46‐51. [DOI] [PubMed] [Google Scholar]
- 20. Kallenberger SM, Schmid C, Wiedmann F, et al. A simple, non‐invasive score to predict paroxysmal atrial fibrillation. PLoS One. 2016;11(9):e0163621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Camm AJ, Kirchhof P, Lip GY, et al. Guidelines for the management of atrial fibrillation: the task force for the management of atrial fibrillation of the European Society of Cardiology (ESC). Eur Heart J. 2010;31:2369‐2429. [DOI] [PubMed] [Google Scholar]
- 22. Suissa L, Bertora D, Lachaud S, Mahagne MH. Score for the targeting of atrial fibrillation (STAF): a new approach to the detection of atrial fibrillation in the secondary prevention of ischemic stroke. Stroke. 2009;40(8):2866‐2868. [DOI] [PubMed] [Google Scholar]
- 23. Zhang Z. Univariate description and bivariate statistical inference: the first step delving into data. Ann Transl Med. 2016;4:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Flint AC, Banki NM, Ren X, Rao VA, Go AS. Detection of paroxysmal atrial fibrillation by 30‐day event monitoring in cryptogenic ischemic stroke: the stroke and monitoring for PAF in real time (SMART) registry. Stroke. 2012;43:2788‐2790. [DOI] [PubMed] [Google Scholar]
- 25. Feigin VL, Lawes CM, Bennett DA, Barker‐Collo SL, Parag V. Worldwide stroke incidence and early case fatality reported in 56 population‐based studies: a systematic review. Lancet Neurol. 2009;8(4):355‐369. [DOI] [PubMed] [Google Scholar]
- 26. Wang Y, Cui L, Ji X, et al. on behalf of the investigators for the China National Stroke Registry (CNSR) investigators. The China National Stroke Registry for patients with acute cerebrovascular events: design, rationale, and baseline patient characteristics. Int J Stroke. 2011;6:355‐361. [DOI] [PubMed] [Google Scholar]
- 27. Zhou Z, Hu D, Chen J, Zhang R, Li K, Zhao X. An epidemiological survey of atrial fibrillaiton in China. Chi J Int Med. 2004;43(7):491‐494. [PubMed] [Google Scholar]
- 28. Haim M, Hoshen M, Reges O, Rabi Y, Balicer R, Leibowitz M. Prospective national study of the prevalence, incidence, management and outcome of a large contemporary cohort of patients with incident non‐valvular atrial fibrillation. J Am Heart Assoc. 2015;4(1):e001486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Heeringa J, van der Kuip DA, Hofman A, et al. Prevalence, incidence and lifetime risk of atrial fibrillation: the Rotterdam study. Eur Heart J. 2006;27(8):949‐953. [DOI] [PubMed] [Google Scholar]
- 30. Wang YL, Wu D, Nguyen‐Huynh MN, et al. Prevention of Recurrences of Stroke Study in China Investigators. Antithrombotic management of ischaemic stroke and transient ischaemic attack in China: a consecutive cross‐sectional survey. Clin Exp Pharmacol Physiol. 2010;37(8):775‐781. [DOI] [PubMed] [Google Scholar]
- 31. Grau AJ, Eicke M, Biegler MK, et al. Quality monitoring of acute stroke care in Rhineland‐Palatinate, Germany, 2001‐2006. Stroke. 2010;41(7):1495‐1500. [DOI] [PubMed] [Google Scholar]
- 32. Tsai CF, Thomas B, Sudlow CL. Epidemiology of stroke and its subtypes in Chinese vs white populations: a systematic review. Neurology. 2013;81:264‐272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Liu XY, Li YX, Fu YG, et al. The value of the score for the targeting of atrial fibrillation (STAF) screening in acute stroke patients. J Stroke Cerebrovasc Dis. 2017;26:1280‐1286. [DOI] [PubMed] [Google Scholar]
- 34. Lin SP, Long Y, Chen XH, et al. STAF score is a new simple approach for diagnosing cardioembolic stroke. Int J Neurosci. 2016;127(3):261‐266. [DOI] [PubMed] [Google Scholar]
- 35. Zhang Z. Big data and clinical research: perspective from a clinician. J Thorac Dis. 2014;6:1659‐1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhang Z. Missing values in big data research: some basic skills. Ann Transl Med. 2015;3:323. [DOI] [PMC free article] [PubMed] [Google Scholar]


