Abstract
Background
This study sought to analyze in‐hospital outcomes associated with preexisting and newly implanted permanent pacemaker (PPM) in patients who underwent transcatheter aortic valve replacement (TAVR). PPM implantation following the development of conduction abnormalities is a common adverse event following TAVR. Furthermore, PPM implantation rates are higher in TAVR hospitalizations compared with the surgical alternative, thus we have analyzed the predictors of pacing post‐TAVR.
Hypothesis
We hypothesize that incidence of arrhythmias are high post‐TAVR and have worse adverse outcomes after receiving PPM.
Methods
The study population was identified from the National Inpatient Sample database between 2012 and 2014. TAVR population was identified using ICD‐9‐CM procedure codes 35.05 and 35.06. Hospitalizations were divided into 3 group: (1) with preexisting PPM, (2) with newly implanted PPM, and (3) without any PPM.
Results
Overall, 0.8% of hospitalizations presented with preexisting PPM and 23.7% of hospitalizations received new PPM. The overall incidence of atrial fibrillation was 44.5%, left bundle branch block 8.9%, complete atrioventricular block 9.5%, and right bundle branch block 2.7%. In‐hospital mortality was higher in hospitalizations receiving PPM compared with those without (4.9% vs 4.0%; P = 0.05). Length of stay and cost were higher in the group receiving new PPM. Female sex, atrial fibrillation, left bundle branch block, and second‐degree and complete atrioventricular block were significant predictors for receiving PPM after TAVR.
Conclusions
A risk stratification for hospitalizations with conduction disorders is necessary to avoid longer hospital stays, added costs, and mortality. Further research is warranted to investigate additional predictors for PPM after TAVR.
Keywords: TAVR, Permanent Pacemaker, Conduction Abnormalities, In‐hospital Outcomes
1. INTRODUCTION
Transcatheter aortic valve replacement (TAVR) is a therapeutic option for patients with severe aortic stenosis and high surgical risk. Many TAVR patients are octogenarians and nonagenarians with significant comorbidities, including some with preexisting pacemakers.1 Despite the growing popularity of TAVR, the need for a permanent pacemaker (PPM) following the procedure represents a frequently encountered complication and often occurs following the development of atrioventricular (AV) conduction abnormalities.2 Rates of PPM implantation post‐TAVR range from 2% to 51%3, 4; however, the bulk of patients receive a PPM either during or within the acute period following the valve replacement.5
PPM implantation is significantly higher in patients undergoing TAVR compared with the surgical replacement method.6 Previous studies have reported that one of the most commonly encountered complication types is a conduction abnormality, such as left bundle branch block (LBBB), right bundle branch block (RBBB), and atrioventricular block (AVB).7, 8 These abnormalities often require subsequent PPM implantation to prevent further adverse events. Most conduction abnormalities generally arise within the first week following TAVR and require a PPM; however, it remains unclear what variables can predict PPM implantation in real‐world patients.2, 9 Therefore, it is necessary to assess the predictors of PPM insertion to stratify the post‐TAVR risk for unfavorable outcomes.10 Identification of these high‐risk patients would be of great clinical importance.
Much of the clinical evidence regarding various predictors of PPM implantation is based on studies with small patient populations or from clinical trials; therefore, this large, real‐world study cohort may expose predictors that would help identify certain high‐risk patients post‐TAVR. Operative mortality can be significant; therefore, the main goal of this study was to evaluate the incidence, predictors, and clinical effects of PPM implantation following TAVR.
2. METHODS
Data were obtained from the National Inpatient Sample (NIS) files from January 2012 to December 2014.11 The NIS database is sponsored by Healthcare Cost and Utilization Project (HCUP). The description of this database has been published in previous studies.12, 13 Briefly, NIS includes >1000 hospitals across the United States, representing >95% of the US population. NIS is a 20% stratified sample, which excludes rehabilitation admissions and long‐term acute‐care hospitals. The institutional review board deemed this study exempt from its approval requirements because of the use of de‐identified observational data from the NIS database. The principles underlying the Declaration of Helsinki were adhered to in this study.
The International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM) procedure codes 35.06 (nontransfemoral implantation) and 35.05 (transfemoral implantation) were used to identify hospitalizations undergoing TAVR. The study population was divided into 3 groups: (1) preexisting PPM, (2) new implantation of PPM, and (3) no need for PPM (Figure 1). The Elixhauser method was used to define comorbidities in this study.14 Baseline characteristics not provided by NIS were identified using appropriate ICD‐9‐CM codes (see Supporting Information, Table 1, in the online version of this article. Additionally, ICD‐9‐CM codes for conduction disorders are included in this table). In‐hospital mortality, length of stay, and median cost of hospitalization for each group were measured. Cost was calculated using cost‐to‐charge ratio files provided by the sponsors and was merged with the original NIS files. The final cost was calculated by multiplying the total cost by the cost‐to‐charge ratio.
Figure 1.
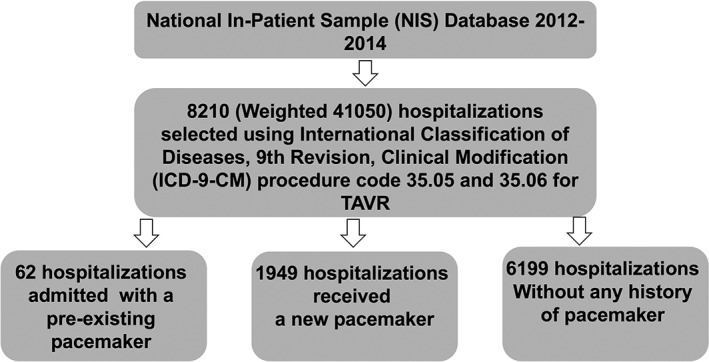
Flowchart for the selection of study cohorts. Abbreviations: TAVR, transcatheter aortic valve replacement
2.1. Statistical analysis
The entire statistical analysis was performed using SAS software, version 9.4 (SAS Institute Inc., Cary, NC). Continuous variables were analyzed using the Student t test or Wilcoxon rank‐sum test and are represented as mean ±SD. Categorical variables were analyzed using the χ2 test or Fisher exact test and were represented as frequencies and percentages. All P values were 2‐sided, and a value of <0.05 was considered statistically significant. A multivariate logistic regression model was generated to identify the predictors for receiving a new PPM implantation following TAVR. Those hospitalizations with preexisting PPM were excluded from the model. This model included age, sex, race, Elixhauser, other comorbidities, and procedural details.
3. RESULTS
This study included 0.8% hospitalizations with a preexisting PPM, 23.7% of hospitalizations with newly implanted PPM, and 75.5% of hospitalizations with no need for PPM post‐TAVR. Baseline differences existed and are shown in Table 1. Hospitalizations who received newly implanted PPM after TAVR presented with higher age as compared with those without a pacemaker (81.7 vs 80.8 years, respectively; P < 0.001). Also, hospitalizations who presented with preexisting pacemakers had a higher age than hospitalizations who did not (85.1 vs 80.8 years, respectively; P < 0.001). Distribution of sex and race was equal in all 3 groups. There was no difference in the Elixhauser comorbidities between the 3 groups, except for chronic lung disease, which was highest in the group that received a new PPM. A greater number of hospitalizations receiving PPM were treated at urban teaching hospitals compared with rural and nonteaching urban hospitals.
Table 1.
Baseline and procedural characteristics of preprocedural pacing, new pacing, and nonpacing groups
| Variable | Pacing on Admission, n = 62 | New Pacing, n = 1949 | No Pacing, n = 6199 | P Value |
|---|---|---|---|---|
| Weighted frequency | 310 | 9745 | 30 995 | N/A |
| Age, y | 85.1 ± 5.9 | 81.7 ± 7.9 | 80.8 ± 8.9 | <0.001 |
| >85 y | 66.1 | 45 | 42.1 | <0.001 |
| Female sex | 43.5 | 49.6 | 47.1 | 0.14 |
| Race | 0.24 | |||
| White | 86.7 | 87.1 | 87.5 | |
| Black | 1.7 | 3.3 | 4.1 | |
| Elixhauser comorbidities | ||||
| DM | 35.5 | 28.8 | 28.4 | 0.45 |
| HTN | 80.6 | 79.4 | 79.4 | 0.97 |
| Liver disease | 1.6 | 2.3 | 2.5 | 0.76 |
| Chronic lung disease | 17.7 | 34.1 | 33 | 0.024 |
| CKD | 38.7 | 38.9 | 37 | 0.31 |
| Obesity | 14.5 | 15.1 | 13.8 | 0.37 |
| Dialysis | 6.4 | 5.3 | 4.4 | 0.18 |
| Other comorbidities | <0.001 | |||
| Ischemic cardiomyopathy | 1.6 | 6.8 | 7.2 | 0.21 |
| Mitral stenosis | 0 | 1.6 | 1.2 | 0.31 |
| Mitral insufficiency | 11.3 | 16.7 | 16.9 | 0.49 |
| Hospital characteristics | <0.001 | |||
| Rural | 0 | 0.4 | 0.9 | |
| Urban (nonteaching) | 11.3 | 8.1 | 11.1 | |
| Urban (teaching) | 88.7 | 91.6 | 88 | |
| Conduction abnormality | ||||
| AF | 54.8 | 45.7 | 44 | 0.11 |
| LBBB | 11.3 | 13 | 7.6 | <0.001 |
| RBBB | 1.6 | 4.9 | 2 | <0.001 |
| Complete AVB | 38.7 | 30.7 | 2.5 | <0.001 |
| First‐degree AVB | 4.8 | 6.5 | 3.5 | <0.001 |
| Second‐degree AVB (Mobitz type I) | 1.6 | 0.7 | 0.1 | <0.001 |
| Second‐degree AVB (Mobitz type II) | 4.8 | 2.3 | 0.6 | <0.001 |
| Other conduction disorders | 22.6 | 23.1 | 11.6 | <0.001 |
| Procedural detail | ||||
| Transfemoral approach | 77.4 | 82.9 | 79.4 | 0.003 |
| Use of MCS | 0 | 0.6 | 0.6 | 0.83 |
| Concomitant PCI | 3.2 | 3.8 | 3.3 | 0.54 |
| Concomitant CABG | 0 | 0.3 | 0.7 | 0.08 |
| Concomitant TMVR | 0 | 0 | 0.1 | 0.52 |
| Conversion to SAVR | 0 | 0.3 | 0.4 | 0.78 |
| In‐hospital parameters | ||||
| In‐hospital mortality | 3.2 | 4.9 | 4 | 0.21 |
| Length of stay, d | 6 (4–9) | 7 (4–10) | 6 (4–9) | <0.001 |
| Cost, $ | 62 353 (22524) | 56 493 (31474) | 49 873 (30989) | <0.001 |
Abbreviations: AF, atrial fibrillation; AVB, atrioventricular block; CABG, coronary artery bypass grafting; CKD, chronic kidney disease; DM, diabetes mellitus; HTN, hypertension; IQR, interquartile range; LBBB, left bundle branch block; MCS, mechanical circulatory support; N/A, not applicable; PCI, percutaneous coronary intervention; RBBB, right bundle branch block; SAVR, surgical aortic valve replacement; SD, standard deviation; TMVR, transcatheter mitral valve repair.
Data are presented as %, mean ± SD, or median (IQR).
The overall incidence of atrial fibrillation (AF) was 44.5%, with no difference between the groups. The overall incidence of LBBB was 8.9%, with the highest being in the hospitalizations that received a new PPM. Additionally, 11.3% of hospitalizations with a previously implanted PPM and new‐onset LBBB underwent TAVR. The overall incidence of RBBB was 2.7%, with the highest being in the hospitalizations group that received a new PPM (4.9%). The overall incidence of complete AVB was 9.5%; however, 38.7% of these hospitalizations presented with a preexisting PPM, and 30.7% of these hospitalizations without a preexisting pacemaker received a new PPM following TAVR. No differences existed in any of the procedural details except for the transfemoral approach, which was highest in those hospitalizations receiving new PPM. A total of 3.5% of hospitalizations were treated with percutaneous coronary intervention in the same admission as TAVR with no significant differences between the 3 groups. No differences in the in‐hospital mortality rates existed between the 3 groups; however, the in‐hospital mortality was significantly higher in those hospitalizations receiving a new PPM compared with those who did not (4.9% vs 4.0%, respectively; P = 0.05]. The length of hospitalization stay was longer in the group receiving new PPM compared with those who did not (7 vs 6 days, respectively; P < 0.001). This translated into an added $6620 for hospitalizations receiving new PPM and an added $12 480 for hospitalizations presenting with preexisting PPM (Table 1).
Multivariate predictors for receiving new PPM in hospitalizations undergoing TAVR were analyzed. Hospitalizations with a preexisting PPM on admission were excluded. Female sex (odds ratio [OR]: 1.15, 95% confidence interval [CI]: 1.02–1.30, P = 0.016) was a significant predictor for receiving new PPM. Additionally, AF (OR: 1.20, 95% CI: 1.07–1.35, P = 0.001), LBBB (OR: 1.67, 95% CI: 1.38–2.01, P < 0.001), and complete AVB (OR: 17.6, 95% CI: 14.5–21.3, P < 0.001) significantly predicted new PPM implantation post‐TAVR. Type I second‐degree AVB (Mobitz type I; OR: 6.12, 95% CI: 1.60–23.37, P = 0.008) and type II second‐degree AVB (Mobitz type II; OR: 1.80, 95% CI: 1.07–3.05, P = 0.026) were significant predictors for receiving new PPM as well. RBBB was not found to predict new PPM implantation in this study. Finally, treatment at an urban teaching hospital (OR: 2.60, 95% CI: 1.07–6.29, P = 0.033), compared with rural hospitals, was found to be a significant predictor (Table 2).
Table 2.
Multivariate logistic regression analysis of factors influencing new pacing in patients undergoing TAVRa
| Variable | OR (95% CI) | P Value |
|---|---|---|
| Age > 85 y | 1.12 (0.99–1.26) | 0.06 |
| Female vs male sex | 1.15 (1.02–1.30) | 0.016b |
| Black vs white race | 0.92 (0.66–1.27) | 0.61 |
| Comorbidities | ||
| DM | 1.00 (0.87–1.13) | 0.99 |
| HTN | 0.99 (0.86–1.14) | 0.93 |
| Liver disease | 1.04 (0.72–1.51) | 0.81 |
| Chronic lung disease | 1.09 (0.96–1.23) | 0.14 |
| CKD | 1.08 (0.96–1.22) | 0.19 |
| Obesity | 1.11 (0.93–1.31) | 0.22 |
| Ischemic cardiomyopathy | 1.03 (0.82–1.30) | 0.74 |
| Mitral stenosis | 1.14 (0.70–1.84) | 0.58 |
| Mitral insufficiency | 0.90 (0.77–1.05) | 0.21 |
| Conduction abnormalities | ||
| AF | 1.20 (1.07–1.35) | 0.001b |
| LBBB | 1.67 (1.38–2.01) | <0.001b |
| RBBB | 1.37 (0.98–1.93) | 0.06 |
| Complete AVB | 17.58 (14.52–21.28) | <0.001b |
| First‐degree AVB | 0.57 (0.39–0.85) | 0.005 |
| Second‐degree AVB (Mobitz type I) | 6.12 (1.60–23.37) | 0.008b |
| Second‐degree AVB (Mobitz type II) | 1.80 (1.07–3.05) | 0.026b |
| Other conduction disorders | 3.05 (2.22–4.20) | <0.001b |
| Procedural details | ||
| Transfemoral vs other approach | 1.12 (0.96–1.30) | 0.13 |
| Use of MCS | 0.97 (0.69–1.36) | 0.85 |
| Concomitant PCI | 1.29 (0.96–1.74) | 0.08 |
| Concomitant CABG | 0.50 (0.18–1.35) | 0.17 |
| Concomitant TMVR | 0.91 (0.80–1.87) | 0.95 |
| Conversion to SAVR | 0.73 (0.26–2.03) | 0.55 |
| Hospital characteristics | ||
| Rural | Ref | |
| Urban (nonteaching) | 1.79 (0.72–4.42) | 0.20 |
| Urban (teaching) | 2.60 (1.07–6.29) | 0.033b |
Abbreviations: AF, atrial fibrillation; AVB, atrioventricular block; CABG, coronary artery bypass grafting; CI, confidence interval; CKD, chronic kidney disease; DM, diabetes mellitus; HTN, hypertension; LBBB, left bundle branch block; MCS, mechanical circulatory support; OR, odds ratio; PCI, percutaneous coronary intervention; PPM, permanent pacemaker; RBBB, right bundle branch block; Ref, referent; SAVR, surgical aortic valve replacement; TAVR, transcatheter aortic valve replacement; TMVR, transcatheter mitral valve repair.
C‐statistic = 0.7.
This multivariate model included age > 85 y, sex, race, Elixhauser comorbidities, other comorbidities, conduction abnormality, and procedural details.
Significant predictor for receiving new PPM in patients undergoing TAVR.
4. DISCUSSION
This study highlighted numerous key points in patients receiving a new PPM following TAVR. A small percentage (0.80%) of patients presented with preexisting pacemakers and 23.7% received a new PPM after their TAVR procedure. Patients receiving a new PPM had a higher incidence of conduction defects such as LBBB, RBBB, AVB, and other conduction disorders; however, only AVB (complete and second degree), LBBB, and other conduction disorders were significant predictors for pacing. Furthermore, compared with patients without any history of a pacemaker, patients who received a new PPM or had a preexisting PPM had higher in‐hospital mortality rates, longer hospitalization stays, and higher associated cost. Other predictors of PPM implantation included AF, female sex, and treatment at an urban teaching hospital. This discussion aims to analyze the significant predictors and possible indicators for pacing after TAVR in this patient population.
The incidence of new‐onset LBBB post‐TAVR ranges from 5% to 65% and has resulted in the insertion of a PPM in 15% to 20% of patients in the acute period; however, the incidence of late appearance, from discharge to 1 year, ranges between a mere 0% and 2.9%.15, 16, 17, 18 In this study, 8.9% of the entire patient cohort developed new‐onset LBBB, 13% of whom received a new PPM. Pacemakers are implanted relatively quickly to reduce the possibility of the LBBB evolving into a complete AVB; however, a short waiting period may eliminate the need for a PPM because of possible regression of the LBBB due to acute edema.16 It is suggested that a proximal lesion of the left bundle branch at the immediate exit of the bundle of His is an indicator for LBBB.16 The close anatomical location of the aortic annulus to the AV nodal‐Hisian system may explain why the valve may disrupt the intra‐ and infra‐Hisian conduction system, leading to the development of a new LBBB. The progression of the LBBB into a complete AVB may help explain the association with adverse events.5
Complete AVB is a significant independent predictor of pacing following TAVR, which is consistent with previous studies.2, 8 The development of AVB occurred in 9.5% of this patient population; however, it has been reported as high as 30%.7 The mechanical pressure generated on the conduction system by native valve calcium and the prosthesis may induce injury to the tissue and yield AVB.19 It has been suggested that a higher burden of comorbidities may play a role in causing higher postprocedural complications and subsequent PPM implantation. Treatment for a complete block usually involves implantation of a pacemaker, and Steinberg et al showed that 39% of patients with a newly developed or worsening AVB required PPM implantation post‐TAVR.7 Of the patients in this study with an AVB, 30.7% received a new pacemaker and 38.7% had preexisting pacemakers. In a study by Guetta et al, 96% of patients who developed high‐degree AVB required a PPM within 5 days of TAVR.2 It should be noted, however, that the block is often transient and does not always require a long‐term pacemaker.20 Studies have shown complete recovery of the block within 1 month following implantation in up to 65% of patients.15, 20 Therefore, caution should be taken when considering PPM implantation for patients with a complete AVB because of the possibility that recovery will reduce the need for long‐term pacing. Future studies should investigate the timeline of PPM implantation following the development of AVB.
Many TAVR patients are diagnosed with AF before their valve replacement procedure or during the postoperative period. Studies have indicated the incidence of AF in 22% to 39% of patients.4 In this study, a slightly higher rate of 44.5% was observed. AF is a major predictor of death, stroke, PPM implantation, and congestive heart failure; therefore, it represents an important comorbidity to consider.21 The development of AF can extend the patient's hospital visit by 6 to 9 days, thus increasing the cost of the visit.21 Motloch et al. found that all episodes of AF occurred within 24 hours post‐TAVR, and patients with preexisting AF revealed a stabilized sinus rhythm 72 hours post‐TAVR.22 It was suggested that this stabilization may be due to the subsequent reduction of intracardiac pressure. This study indicates that preexisting AF is a significant predictor of pacing post‐TAVR; however, adequate recovery time may reduce the need for PPM implantation.22
The prevalence of RBBB ranges from 0.5% to 1.5% and is known to increase to 2.2% in patients age > 55 years23, 24; however, Siontis et al. reported a prevalence as high as 10% in patients with a mean age of 81 years undergoing TAVR.8 This study indicated a 2.7% prevalence with a mean age of 83 years. The development of a RBBB may be associated with procedural injury and fibrosis of the right intraventricular conduction system25; however, the exact mechanism is still in question. Previous studies have indicated preexisting RBBB to be a significant predictor of pacing post‐TAVR, but the database used for this analysis lacks information on preexisting conduction abnormalities.8 The absence of data indicating patients with preexisting RBBB, more commonly observed than new‐onset RBBB, may help explain the low prevalence and why it was not found to be an independent predictor of pacing following TAVR.
Patients receiving a new PPM and those with in‐situ PPM on admission experience a greater healthcare cost and longer length of hospitalization, as demonstrated in this study.4 It should be noted that a PPM implantation can raise the hospitalization cost by 36%.26 However, PPM implantation has shown to improve mortality rates as well. A meta‐analysis concluded that there was a reduced mortality rate from sudden death 30 days post‐TAVR in patients receiving PPM.27 A complete AVB or new‐onset LBBB may often increase mortality rates; therefore, a reduction due to pacing would be expected. Urena et al. also reported a reduced 30‐day unexpected mortality rate in the PPM patients.28 However, several other studies have indicated no statistical difference between pacing and no‐pacing groups in terms of all‐cause mortality, cardiovascular mortality, stroke, or myocardial infarction at 30 days or 1 year follow‐ups.27, 28 Recognizing the common predictors of PPM implantation following TAVR and understanding their mechanisms of development may alter the time period for pacing and could potentially reduce the financial burden the implantation has on a patient's visit. As indications for TAVR continue to grow in low‐ to intermediate‐risk subset, we must keep in mind risk important predictors for PPM to avoid excess mortality associated with it.29
4.1. Study limitations
This study has several limitations inherent to any retrospective observational analysis, such as coding errors and under‐ or over‐reporting of comorbidities. Additionally, this study lacked information on the valve type, defect, size used, and echocardiographic parameters. It has been noted that the valve type significantly affects the risk of PPM implantation and thus would affect the incidence of PPM post‐TAVR between the groups.30, 31 Moreover, echocardiographic parameters such as intraventricular septum >17 mm and noncoronary aortic cusp thickness > 8 mm predicted PPM in earlier studies.32 The NIS database does not provide information on preexisting conduction disorders; therefore, this study did not account for them. Finally, this study does not have relevant follow‐up data, which prevents the analysis of long‐term clinical outcomes. Most commonly, patients are likely to receive PPM 7 days following the TAVR procedure, which suggests that this data may under‐represent the PPM implantation rates. However, this study had the opportunity to analyze the biggest “all‐comer” database of patients undergoing TAVR and receiving pacemakers, representing a real‐world patient population.
5. CONCLUSION
This study indicated that incidence of LBBB, AVB and new‐onset AF is very high post‐TAVR. This study also indicated that the major predictors of new PPM implantation include female sex, AF, AVB (complete and second degree), LBBB, and treatment at an urban teaching hospital. A risk stratification for such patients is necessary to avoid longer hospital stays, added costs, and, ultimately, mortality.
Conflicts of interest
The authors declare no potential conflicts of interest.
Supporting information
Supplementary Table 1 International Classification of Disease, Version 9 (ICD‐9) Codes Used in this analysis
Doshi R, Decter DH, Meraj P. Incidence of arrhythmias and impact of permanent pacemaker implantation in hospitalizations with transcatheter aortic valve replacement. Clin Cardiol. 2018;41:640–645. 10.1002/clc.22943
Rajkumar Doshi, MD, MPH, and Dean H. Decter contributed equally to this manuscript. Both authors should be considered primary authors.
REFERENCES
- 1. Barbanti M, Buccheri S, Rodés‐Cabau J, et al. Transcatheter aortic valve replacement with new‐generation devices: a systematic review and meta‐analysis. Int J Cardiol. 2017;245:83–89. [DOI] [PubMed] [Google Scholar]
- 2. Guetta V, Goldenberg G, Segev A, et al. Predictors and course of high‐degree atrioventricular block after transcatheter aortic valve implantation using the CoreValve Revalving System. Am J Cardiol. 2011;108:1600–1605. [DOI] [PubMed] [Google Scholar]
- 3. Savino DC, McCarthy FH, Spragan DD, et al. Permanent pacemaker implantation following transcatheter aortic valve replacement. JACC Cardiovasc Interv. 2017;10:1276–1278. [DOI] [PubMed] [Google Scholar]
- 4. Fadahunsi OO, Olowoyeye A, Ukaigwe A, et al. Incidence, predictors, and outcomes of permanent pacemaker implantation following transcatheter aortic valve replacement: analysis from the U.S. Society of Thoracic Surgeons/American College of Cardiology TVT Registry. JACC Cardiovasc Interv. 2016;9:2189–2199. [DOI] [PubMed] [Google Scholar]
- 5. Bagur R, Rodés‐Cabau J, Gurvitch R, et al. Need for permanent pacemaker as a complication of transcatheter aortic valve implantation and surgical aortic valve replacement in elderly patients with severe aortic stenosis and similar baseline electrocardiographic findings. JACC Cardiovasc Interv. 2012;5:540–551. [DOI] [PubMed] [Google Scholar]
- 6. Ando T, Briasoulis A, Holmes AA, et al. Transcatheter aortic valve replacement versus surgical aortic valve replacement in patients with previous coronary artery bypass surgery: a systematic review and meta‐analysis. Int J Cardiol. 2016;215:14–19. [DOI] [PubMed] [Google Scholar]
- 7. Steinberg BA, Harrison JK, Frazier‐Mills C, et al. Cardiac conduction system disease after transcatheter aortic valve replacement. Am Heart J. 2012;164:664–671. [DOI] [PubMed] [Google Scholar]
- 8. Siontis GC, Jüni P, Pilgrim T, et al. Predictors of permanent pacemaker implantation in patients with severe aortic stenosis undergoing TAVR: a meta‐analysis. J Am Coll Cardiol. 2014;64:129–140. [DOI] [PubMed] [Google Scholar]
- 9. Tamburino C, Barbanti M, D'Errigo P, et al. 1‐Year outcomes after transfemoral transcatheter or surgical aortic valve replacement: results from the Italian OBSERVANT Study. J Am Coll Cardiol. 2015;66:804–812. [DOI] [PubMed] [Google Scholar]
- 10. Nazif TM, Dizon JM, Hahn RT, et al; PARTNER Publications office. Predictors and clinical outcomes of permanent pacemaker implantation after transcatheter aortic valve replacement: the PARTNER (Placement of Aortic Transcatheter Valves) trial and registry. JACC Cardiovasc Interv. 2015;8(1 part A):60–69. [DOI] [PubMed] [Google Scholar]
- 11. Cost Healthcare and Project Utilization, US Agency for Healthcare Research and Quality. NIS Database Documentation Archive; Rockville, MD; June 2016. http://www.hcup-us.ahrq.gov/db/nation/nis/nisarchive.jsp. Accessed December 6, 2017. [Google Scholar]
- 12. Doshi R, Shah J, Patel V, et al. Transcatheter or surgical aortic valve replacement in patients with advanced kidney disease: a propensity score‐matched analysis. Clin Cardiol. 2017;40:1156–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Doshi R, Shlofmitz E, Meraj P. Comparison of outcomes and complications of transcatheter aortic valve implantation in women versus men (from the National Inpatient Sample). Am J Cardiol. 2018;121:73–77. [DOI] [PubMed] [Google Scholar]
- 14. Elixhauser A, Steiner C, Harris DR, et al. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. [DOI] [PubMed] [Google Scholar]
- 15. Roten L, Wenaweser P, Delacrétaz E, et al. Incidence and predictors of atrioventricular conduction impairment after transcatheter aortic valve implantation. Am J Cardiol. 2010;106:1473–1480. [DOI] [PubMed] [Google Scholar]
- 16. Massoullié G, Bordachar P, Ellenbogen KA, et al. New‐onset left bundle branch block induced by transcutaneous aortic valve implantation. Am J Cardiol. 2016;117:867–873. [DOI] [PubMed] [Google Scholar]
- 17. Nazif TM, Williams MR, Hahn RT, et al. Clinical implications of new‐onset left bundle branch block after transcatheter aortic valve replacement: analysis of the PARTNER experience. Eur Heart J. 2014;35:1599–1607. [DOI] [PubMed] [Google Scholar]
- 18. Houthuizen P, van der Boon RM, Urena M, et al. Occurrence, fate and consequences of ventricular conduction abnormalities after transcatheter aortic valve implantation. EuroIntervention. 2014;9:1142–1150. [DOI] [PubMed] [Google Scholar]
- 19. Bleiziffer S, Ruge H, Hörer J, et al. Predictors for new‐onset complete heart block after transcatheter aortic valve implantation. JACC Cardiovasc Interv. 2010;3:524–530. [DOI] [PubMed] [Google Scholar]
- 20. Raelson CA, Gabriels J, Ruan J, et al. Recovery of atrioventricular conduction in patients with heart block after transcatheter aortic valve replacement. J Cardiovasc Electrophysiol. 2017;28:1196–1202. [DOI] [PubMed] [Google Scholar]
- 21. Maan A, Heist EK, Passeri J, et al. Impact of atrial fibrillation on outcomes in patients who underwent transcatheter aortic valve replacement. Am J Cardiol. 2015;115:220–226. [DOI] [PubMed] [Google Scholar]
- 22. Motloch LJ, Reda S, Rottlaender D, et al. Postprocedural atrial fibrillation after transcatheter aortic valve implantation versus surgical aortic valve replacement. Ann Thorac Surg. 2012;93:124–131. [DOI] [PubMed] [Google Scholar]
- 23. Haataja P, Nikus K, Kähönen M, et al. Prevalence of ventricular conduction blocks in the resting electrocardiogram in a general population: the Health 2000 Survey. Int J Cardiol. 2013;167:1953–1960. [DOI] [PubMed] [Google Scholar]
- 24. Bussink BE, Holst AG, Jespersen L, et al. Right bundle branch block: prevalence, risk factors, and outcome in the general population. Results from the Copenhagen City Heart Study. Eur Heart J. 2013;34:138–146. [DOI] [PubMed] [Google Scholar]
- 25. Krongrad E, Hefler SE, Bowman FO Jr, et al. Further observations on the etiology of the right bundle branch block pattern following right ventriculotomy. Circulation. 1974;50:1105–1113. [DOI] [PubMed] [Google Scholar]
- 26. Chevreul K, Brunn M, Cadier B, et al; FRANCE Registry Investigators . Cost of transcatheter aortic valve implantation and factors associated with higher hospital stay cost in patients of the FRANCE (French Aortic National CoreValve and Edwards) registry. Arch Cardiovasc Dis. 2013;106:209–219. [DOI] [PubMed] [Google Scholar]
- 27. Mohananey D, Jobanputra Y, Kumar A, et al. Clinical and echocardiographic outcomes following permanent pacemaker implantation after transcatheter aortic valve replacement: meta‐analysis and meta‐regression. Circ Cardiovasc Interv. 2017;10:pii:e005046. 10.1161/CIRCINTERVENTIONS.117.005046 [DOI] [PubMed] [Google Scholar]
- 28. Urena M, Webb JG, Tamburino C, et al. Permanent pacemaker implantation after transcatheter aortic valve implantation: impact on late clinical outcomes and left ventricular function. Circulation. 2014;129:1233–1243. [DOI] [PubMed] [Google Scholar]
- 29. Khan SU, Lone AN, Saleem MA, et al. Transcatheter vs surgical aortic‐valve replacement in low‐ to intermediate‐surgical‐risk candidates: a meta‐analysis and systematic review. Clin Cardiol. 2017;40:974–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tarantini G, Mojoli M, Purita P, et al. Unravelling the (arte)fact of increased pacemaker rate with the Edwards SAPIEN 3 valve. EuroIntervention. 2015;11:343–350. [DOI] [PubMed] [Google Scholar]
- 31. Muñoz‐García AJ, Hernández‐García JM, Jiménez‐Navarro MF, et al. Factors predicting and having an impact on the need for a permanent pacemaker after CoreValve prosthesis implantation using the new Accutrak delivery catheter system. JACC Cardiovasc Interv. 2012;5:533–539. [DOI] [PubMed] [Google Scholar]
- 32. Jilaihawi H, Chin D, Vasa‐Nicotera M, et al. Predictors for permanent pacemaker requirement after transcatheter aortic valve implantation with the CoreValve bioprosthesis. Am Heart J. 2009;157:860–866. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1 International Classification of Disease, Version 9 (ICD‐9) Codes Used in this analysis


