Abstract
Purpose: Nr5a2 (nuclear receptor subfamily 5 group A member 2, also known as LRH-1), which belongs to the NR5A (Ftz-F1) subfamily of nuclear receptors, is a key regulator in stem cell pluripotency and the development of several types of cancer. However, the data are controversial. Since Nr5a2 plays different roles in multiple types of cancer and the function of Nr5a2 in gastric cancer (GC) has not been revealed, we studied the role and molecular mechanism of Nr5a2 in GC.
Methods: In this study, we have investigated the effect of Nr5a2 on tumor growth and metastasis by in vivo and in vitro models.
Results: The results showed that knockdown of Nr5a2 could inhibit cell proliferation via arresting the cell cycle in the G2/M phase and suppress cell mobility through preventing the epithelial-mesenchymal transition (EMT) process in AGS cells. In addition, knockdown of Nr5a2 could suppress tumorigenesis and metastasis of AGS cells in vivo. We also demonstrated that knockdown of Nr5a2 inhibited cellular proliferation and mobility by suppressing the Wnt/beta-catenin signaling pathway.
Conclusion: Nr5a2 may act as an oncogene in GC development. The EMT process and the Wnt/beta-catenin signaling pathway play an important role in the Nr5a2 induced GC development.
Keywords: Nr5a2, gastric cancer, proliferation, metastasis, Wnt/beta-catenin
Introduction
Gastric cancer (GC) is the fourth most common cancer and the second leading cause of cancer death worldwide. The outcomes of metastatic GC are very poor, with median survival being around 1 year.1 It is important to explore the molecular mechanisms underlying GC development to identify novel therapeutic targets or prognostic markers.
Nr5a2 (nuclear receptor subfamily 5 group A member 2, also known as LRH-1), which belongs to the NR5A (Ftz-F1) subfamily of nuclear receptors, is an orphan nuclear receptor predominantly expressed in the enterohepatic axis and in the ovary.2 Previous studies have reported that Nr5a2 plays a role in reverse cholesterol transport and bile acid metabolism in the liver.3–7 More recent studies have identified Nr5a2 as a key regulator in stem cell pluripotency and the development of several types of cancer,8–16 especially breast cancer and pancreatic cancer.17–30 Nr5a2 promotes cancer cell proliferation via regulating cell cycle. In colon cancer cell lines, Nr5a2 knockdown leads to an impairment of cell proliferation and Nr5a2 silencing can prolong the G0/G1 phase by cell-cycle analysis.15 In pancreatic cancer cell lines, Nr5a2 overexpression enhanced the expression of Cyclin D1/E1 and stimulated cell proliferation.23 In addition, Nr5a2 promotes cell proliferation through mitochondrial glutaminase 2 (GLS2) - mTORC1 pathway in hepatoma carcinoma cells.13 Nr5a2 inhibition affects cell proliferation of different types of breast cancer cells by regulating CDKN1A transcription.20 Nr5a2 also play an important role in cell mobility and invasion. Nr5a2 promotes pancreatic cancer metastasis through enhanced transcriptional activity of beta-catenin and the expression of downstream target genes (c-Myc, MMP2/9).29 Remodelling of the actin cytoskeleton and E-cadherin cleavage was observed in Nr5a2 over-expressed breast cancer cells, contributing to increased cell mobility and invasion.17 Moreover. Nr5a2 mediates cancer cells chemoresistance via multiple mechanisms. Nr5a2 enhances breast cancer cell chemoresistance by upregulating MDC1 and attenuating DNA damage.27 Nr5a2 exhibits an increased expression pattern in castration-resistant prostate cancer (CRPC) xenograft models, and it promotes the intratumoral androgen biosynthesis in CRPC via its direct transcriptional control of steroidogenesis.14
Although most studies have shown that Nr5a2 may play an oncogenic role and may correlate with poor prognosis, there are also some reports indicating the opposite role of Nr5a2.28 These studies indicate that Nr5a2 plays different roles in multiple types of cancer. Moreover, Nr5a2 regulates the established tumor marker AFP in the liver, whose expression is reinduced not only in hepatic but also in gastric cancers.31,32 Does Nr5a2 also play a role in the process of gastric cancer? In addition, Nr5a2 play a central role in intestinal tumorigenesis, and intestinal metaplasia of the stomach, a mucosal change characterized by the conversion of gastric epithelium into an intestinal phenotype, is a precancerous lesion from which intestinal-type gastric cancer.33 In view of this, we wonder the effects and molecular mechanisms of Nr5a2 on gastric cancer.
Material and methods
Cell culture
The human GC cell line AGS was obtained from the American Type Culture Collection (ATCC, Manassas, VA) and cultured in F-12K medium. Human gastric cancer cell lines BGC-823, KATO III, MGC-803 and SGC-7901 were purchased from the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China) and cultured in RPMI 1640 medium. All media were supplemented with 10% FBS, 100 U/ml penicillin and 100 μg/ml streptomycin. HiPerFect Transfection Reagent (QIAGEN, Hilden, Germany) was used to transfect cells with siRNA and Attractene Transfection Reagent (QIAGEN, Hilden, Germany) was used to transfect cells with plasmid DNA.
Establishment of stable shRNA-Nr5a2 AGS cell lines by lentivirus
Lentiviral vectors with shRNA-Nr5a2 (shNr5a2) and negative control (shNC) were purchased from the GenePharma Company (Shanghai, China). The oligonucleotide encoding sequences are as follows: shRNA-Nr5a2 5ʹ-GCAGCAGACAGAGAAATTT-3ʹ, and negative control 5ʹ-TTCTCCGAACGTGTCACGT-3ʹ. Cells in the logarithmic growth phase were collected and inoculated into a 24-well plate (5×104 cells/well). After adherence, the cells were transfected with lentivirus and screened using 2 μg/ml puromycin 2 days later. Cells from each clone were analyzed for Nr5a2 expression by RT-qPCR and western blotting, and the most efficiently knocked-down cells were used in subsequent tests.
RNA extraction and RT-qPCR
Total RNA was extracted using TRIZOL Reagent (Invitrogen, CA, USA) and reverse transcription was performed using PrimeScript RT Master Mix Kit (Takara, Dalian, China). For RT-qPCR, the cDNA was amplified using SYBR Premix Ex Taq (Takara, Dalian, China), and detected by a LightCycler 480 II (Roche, Mannheim, Germany). Relative gene expression was determined using the comparative delta-delta Cq method (2-ΔΔCq).34 The housekeeping gene GAPDH was used as an internal control and all of the RT-qPCR reactions were performed in triplicates. The primer sequences are as follows, Nr5a2 (5ʹ to 3ʹ, Forward: GCCACCCTCAACAACCTCAT, Reverse: CTGCTGCGGGTAGTTACACA), GAPDH (5ʹ to 3ʹ, Forward: GAAGGTGAAGGTCGGAGTC, Reverse: GAAGATGGTGATGGGATTTC).
Western blotting
Western blotting analyses were performed following standard protocols. Briefly, cells were lysed in RIPA Lysis Buffer (Beyotime, Jiangsu, China), which contained Protease Inhibitor Cocktail (Roche, Mannheim, Germany). Protein concentrations were measured using a BCA Protein Assay Reagent (Thermo, MA, USA). Equal amounts of cell lysate were loaded onto SDS-PAGE gels and then transferred to PVDF membranes. Membranes were blocked with 5% fat-free milk and incubated with primary antibodies at 4 °C overnight. The membranes were incubated with horseradish peroxidase-conjugated species-specific secondary antibodies. Bands were visualized with enhanced chemiluminescence reagent (Millipore, MA, USA). The following commercial antibodies were used in this study: Nr5a2 (1:1000, Sigma, MO, USA), E-cadherin, N-cadherin, Twist1, Vimentin, MMP-2, beta-Catenin, Wnt3a, c-Myc and Cyclin D1 (1:1000, Cell Signaling Technology, MA, USA), and Snail2 and GAPDH (1:1000, Proteintech, Wuhan, China).
Cell proliferation assay
Cell proliferation rates were measured using a Cell Counting Kit-8 (CCK-8) (Dojindo Laboratories, Japan) according to the manufacturer’s instructions. Cells were plated into 96-well plates (5×103 cells/well) and the cell proliferation assay was performed at 0, 2, 48, 72, 96, and 120 h. The absorbance was measured by the EnSpire Multimode Plate Reader (PerkinElmer, CA, USA). Each sample was assayed in six repeated wells and the experiment was performed three times independently.
Colony-formation assay
Cells were plated into 6-well plates (500 cells/well) and incubated for 10–14 days. The medium was changed every 3 days. At the endpoint of incubation, the cells were fixed with paraformaldehyde and stained with crystal violet. Colonies (≥50 cells/colony) were counted.
Cell cycle analysis
Cells were collected at 72 h after siRNA-Nr5a2 or siRNA-control transfection for flow cytometry analysis. Cells were incubated with 50 µg/ml RNase A for 30 min at room temperature, and then stained with 50 µg/ml propidium iodide for 15 min at room temperature in the dark before flow cytometry analysis. A total of 1×104 cells were subjected to cell cycle analysis by the flow cytometer (Becton Dickinson, NJ, USA). Each set was repeated three times.
Cell migration and invasion assay
Cell migration and invasion assay were performed using a 24-well migration chamber (Corning, NY, USA) with or without Matrigel. Then, 5×104 cells were seeded in the top chamber with 200 μl medium containing 5% FBS. The bottom chamber was filled with 600 μl medium containing 20% FBS. After incubation for 24 h, the cells remaining at the upper surface of the membrane were removed with a cotton swab, and those that adhered to the lower surface were fixed with paraformaldehyde and stained with crystal violet. The number of cells that had invaded through the membrane per field were counted and imaged under a microscope (Leica Microsystems, Wetzlar, Germany). Each experiment was performed three times independently.
Wound healing assay
Cells were plated into 6-well plates. After cells were grown to 90% confluence, a scratch was made by a sterile pipette tip. After washing, cells were incubated in medium containing 5% FBS. After incubation for 24 h plates were photographed. Images were analyzed by Image J software, and wound healing was calculated as the proportion of remaining cell-free area compared with the initial wound area. Each experiment was performed three times independently.
TOP flash/FOP-flash reporter assay
Cells were plated into 24-well plates (5×104 cells/well) and co-transfected with 0.4 μg beta-catenin reporter plasmid (TOP-flash; Sino Biological Inc., Beijing, China) or its mutant control (FOP-flash; Sino Biological Inc., Beijing, China) and 0.04 μg pRLTK (Renilla TK-luciferase vector; Promega, WI, USA) using Attractene Transfection Reagent (QIAGEN, Hilden, Germany). Cells were collected at 48 h after transfection, and the activities of both firefly and Renilla luciferase reporters were determined using a dual-luciferase reporter assay kit (Promega, WI, USA) according to the manufacturer’s instructions. Reporter activity was normalized to the control Renilla, and the TOP/FOP ratio was used as a measure of beta-catenin-driven transcriptional activity.
In vivo tumor xenograft assays and metastasis assays
For the in vivo xenograft assay, 2×106 AGS-shNr5a2 or AGS-NC cells were subcutaneously injected into the left or right dorsalflank, respectively, in nude mice. Tumor sizes were measured with calipers every 3 days, and the volumes were calculated with the formula (length × width2)/2. The mice were sacrificed at 26 days after injection, and the tumors were excised for photography. For lung metastasis formation, 1×106 AGS-shNr5a2 and AGS-NC cells were injected into the tail veins of nude mice. Mice were euthanized 8 weeks after injection, and the lungs of the mice were fixed with formaldehyde, followed by H&E staining. The metastatic foci in the lungs were counted under a microscope. All experimental procedures were approved by the Animal Ethics Committee of the West China Hospital and were performed in accordance with the Guide for the Care and Use of Laboratory Animals.
GC patient samples and immunohistochemistry (IHC) staining
A total of 72 GC tissues and paired adjacent noncancerous tissues were purchased from Shanghai Outdo Biotech Company and were approved by the Ethics Committee of Shanghai Outdo Biotech Company (Shanghai, China). All tissue samples were double examined with a hematoxylin & eosin staining method by two individual pathologists. Immunohistochemistry staining of Nr5a2 was performed using a standard biotin-streptavidin method with a monoclonal antibody against human Nr5a2 (1:200 dilution, Sigma, MO, USA). For evaluating the expression levels of Nr5a2, the Nr5a2 staining intensities were independently scored by two experienced pathologists. Then, the immunohistochemical staining of cells was evaluated according to a score that added a scale of intensity of staining to the proportion of positively-stained cells. The intensity of staining was scored as follows: 0, no staining; 1, weak staining; 2, moderate staining; and 3, strong staining. The proportion of stained cells was scored as follows: 0, no cells stained; 1, <10% of cells stained positive; 2, 10–50% of cells stained positive; and 3, >50% of cells stained positive. The final score was determined by combining the two scores. A score of ≤3 was considered low expression, and a score of 4–9 was considered high expression.35
Statistical analysis
Statistical analyses were performed using unpaired Student’s t-tests and paired Student’s t-tests on GraphPad Prism Software (GraphPad Software Inc., CA, USA). All of the experiments were repeated at least three times, and the data were expressed as means ± SD. The P-values were denoted as * P<0.05, ** P<0.01, and *** P<0.001 in all figures.
Results
Knockdown of Nr5a2 reduced cellular proliferation in AGS cells
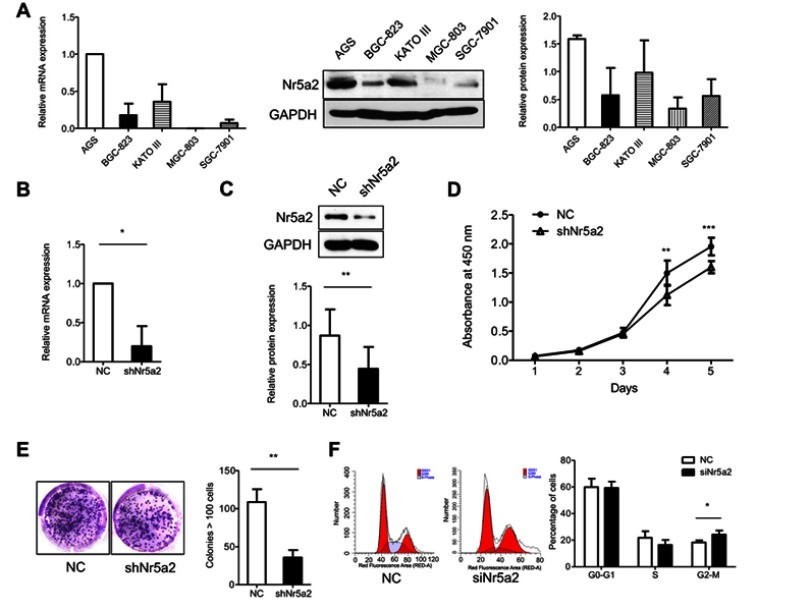
In order to explore the role of Nr5a2 in the proliferation of GC cells, we detected the mRNA and protein expression level of Nr5a2 in five GC cell lines (AGS, BGC-823, KATO III, MGC-803, and SGC-7901) (Figure 1A). The Nr5a2 high-expression GC cell line AGS was infected with the shNr5a2 lentivirus. The transfection efficiency was validated by RT-qPCR and western blotting. The results showed that infection with shNr5a2 lentivirus efficiently decreased the mRNA (Figure 1B) and protein expression level (Figure 1C) of Nr5a2 in AGS cells. The effect of Nr5a2 knockdown on the proliferation of AGS cell lines was examined by a CCK-8 proliferation assay and a clone formation assay. The results showed that knockdown of Nr5a2 in AGS cells caused a significant inhibition of cell growth at 4 days after seeding (P<0.01) (Figure 1D). This suppressive effect was further confirmed by a colony formation assay, in which Nr5a2 knockdown significantly decreased colony formation of AGS cells when compared with control (P<0.01) (Figure 1E). Further, we examined the effect of Nr5a2 knockdown on cell cycle progression in AGS cells using PI staining in combination with flow cytometric analyses. Compared to the control group, Nr5a2 knockdown cells showed a significant increase in the fraction of cells in the G2/M phase (P<0.05) (Figure 1F). These results suggested that Nr5a2 knockdown arrested cells in the G2/M phase. These results revealed that Nr5a2 knockdown suppressed the proliferation of AGS cells and suggested that Nr5a2 functions as an oncogenic molecule in GC.
Figure 1.
Effect of Nr5a2 on proliferation of gastric cancer (GC) cells. (A) Nr5a2 mRNA abundance and protein expression levels in 5 GC cell lines were detected by RT-qPCR and western blotting (B and C). Nr5a2 mRNA abundance and protein expression levels in the control and knockdown groups were confirmed by RT-qPCR and western blotting. (D) The effect of Nr5a2 knockdown on cell viability was analyzed by a CCK-8 assay in AGS cells. (E) The effect of Nr5a2 knockdown on cell proliferation was analyzed by a colony formation assay in AGS cells. Representative images of cell colonies in control and shNr5a2 cells are shown. (F) The effect of Nr5a2 knockdown on cell cycle was performed using flow cytometry. Representative images in control and siNr5a2 cells are shown. The results are expressed as mean ± SD of a triplicate assay, *P<0.05, **P<0.01, ***P<0.001. Abbreviation: NC, negative control.
Knockdown of Nr5a2 suppressed cellular mobility and inhibited EMT in AGS cells
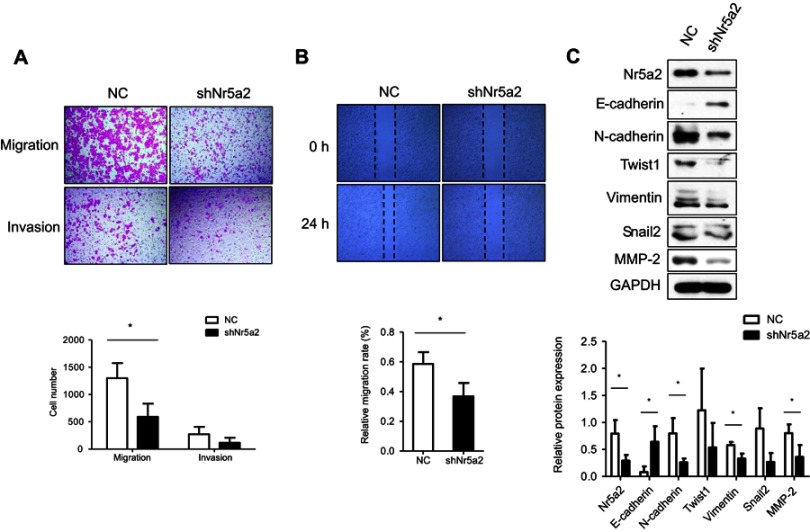
To further examine the effect of Nr5a2 on AGS cell mobility, the Nr5a2 knockdown and shRNA control cells were used for Transwell assays. The Transwell migration assays showed that the migration ability of Nr5a2 knockdown cells was obviously suppressed compared with control cells (P<0.05). The Matrigel invasion assays indicated that the tendency of the number of Nr5a2 knockdown cells that invaded through Matrigel appeared to be less than that of control cells, but there was no statistical difference (Figure 2A). The wound healing assays showed a consistent result, which was that the Nr5a2 knockdown cells demonstrated lower migration rates compared to the control cells (P<0.05) (Figure 2B).
Figure 2.
Effects of Nr5a2 on migration of GC cells. (A) The effect of Nr5a2 knockdown on cell migration and invasion ability in AGS cells was evaluated by Transwell assay. (B) Wound healing assay was performed to assess the migratory potential of control and Nr5a2-downregulated AGS cells. (C) The effect of Nr5a2 knockdown on expression of epithelial-to-mesenchymal transition (EMT)-related proteins was assessed by western blotting. The results are expressed as mean ± SD of three independent experiments, *P<0.05. Abbreviation: NC, negative control.
The role of Nr5a2 in cell migration led us to examine if Nr5a2 had any effect on the EMT process in AGS cells. We measured the expression levels of multiple EMT-related factors in Nr5a2 knockdown and control cells by western blotting. The results showed that Nr5a2 knockdown induced the expression of epithelial cell marker (E-cadherin) and reduced the expression of mesenchymal cell markers (N-cadherin and Vimentin) in AGS cells. The tendency of the expression levels of two other typical mesenchymal cell markers (Snail2 and Twist1) also appeared to be reduced in Nr5a2 knockdown cells, but there were no statistical differences. Furthermore, we detected the expression of the MMP family member MMP2, which has previously been reported to induce EMT in variety of cancer. The results revealed that the expression of MMP2 was remarkably suppressed in Nr5a2 knockdown AGS cells (Figure 2C). Taken together, these data suggest that Nr5a2 regulates the EMT process, and downregulation of Nr5a2 inhibits AGS cells migration in vitro.
Knockdown of Nr5a2 suppressed tumorigenesis and metastasis of AGS cells in vivo
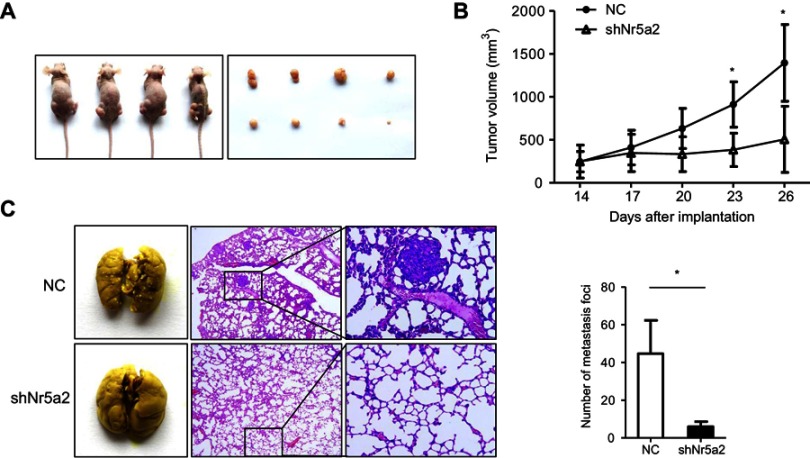
To investigate whether Nr5a2 knockdown could also suppress AGS cells growth and metastasis in vivo, we used the Nr5a2-knockdown AGS cells to establish xenograft tumor models in nude mice. We found that the mean volume of tumors infected with shNr5a2 was 2.8-fold smaller than the volume of tumors in the control group at 26 days after inoculation (Figure 3A). Tumor growth curves show that the AGS-shNr5a2 group exhibited a markedly decreased growth rate after inoculation for 23 days (P<0.05) in contrast to the control group (Figure 3B). Taken together, these findings demonstrate that Nr5a2 knockdown can obviously suppress tumorigenic ability in AGS cells.
Figure 3.
Effects of Nr5a2 on growth and metastasis of gastric cancer (GC) cells in vivo. (A) Subcutaneous xenograft assay was performed to assess the proliferation potential of control (left flank) and Nr5a2 downregulated AGS cells (right flank) in vivo. An image of subcutaneous tumors from the control and shNr5a2 groups is shown. (B) Tumor volumes were measured on the indicated days, and tumor growth curves were drawn. The data points represent the mean tumor volumes ± SD. (C) Tumor cell tail vein injection was performed to assess the effect of Nr5a2 knockdown on GC cell metastasis in vivo. This representative image shows the metastatic foci on the lung surfaces of mice after tail vein injection. H&E staining of the lung slices shows that Nr5a2 knockdown in AGS cells decreased the metastatic capacity in vivo. The number of metastatic tumor foci was counted in the control and shNr5a2 groups. The data express the mean ± SD from triplicate measurements. *P<0.05. NC, negative control.
Further, to test whether Nr5a2 knockdown could influence the metastatic potential of AGS cells in vivo, AGS-shNr5a2 and control cells were injected into nude mice’s tail veins. The mice were sacrificed 2 months after injection, and we examined the metastatic nodules in the lung. H&E staining showed that downregulated expression of Nr5a2 led to a significantly decreased number of metastatic nodules in the lung compared to the vector group (P<0.05) (Figure 3C). All these findings suggest that Nr5a2 knockdown could suppress the metastatic properties of AGS cells in vivo.
Knockdown of Nr5a2 inhibited cellular proliferation and mobility by suppressing the Wnt/beta-catenin signaling pathway
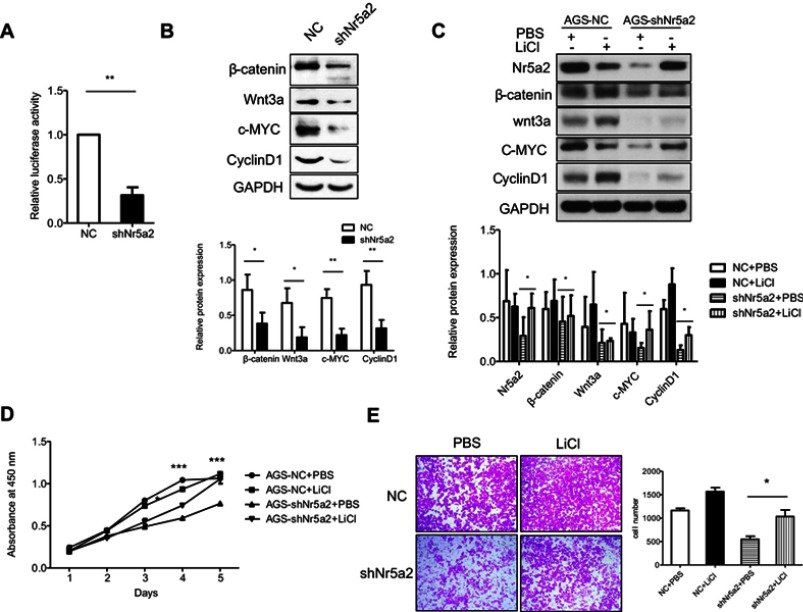
The Wnt/beta-catenin signaling pathway plays an important role in maintaining an epithelial cell phenotype,36 and previous studies have reported that Nr5a2 could drive cell cycle progression and tumorigenesis by synergizing with the beta-catenin signaling pathway in gut,37 so we detected the activity of the Wnt/beta-catenin signaling pathway in AGS cells. Our data showed that the activities of Wnt/beta-catenin signaling were significantly decreased in shNr5a2 groups compared with the vector group by a TOP/FOP luciferase reporter assay (P<0.01) (Figure 4A). Western blotting analysis showed that Nr5a2 knockdown decreased the expression of beta-catenin and Wnt3a in AGS cells. In accordance with this, Cyclin D1 and c-Myc expression levels were also decreased (Figure 4B). These are Wnt/beta-catenin signaling pathway target genes. Therefore, these results indicated that Nr5a2 may promote cellular proliferation and mobility by regulating Wnt/beta-catenin signaling. To further confirm this hypothesis, we treated AGS-NC and AGS-shNr5a2 cells with or without LiCl (an activator of the Wnt/beta-catenin signaling pathway), and then detected cellular proliferation and mobility by a CCK-8 proliferation assay and a Transwell assay, respectively. The results showed that Nr5a2 knockdown-induced downregulation of proteins related to the Wnt/beta-catenin signaling pathway could be rescued by LiCl (Figure 4C). Moreover, the results of the CCK-8 proliferation assay showed that LiCl restored the cells’ proliferation property in the shNr5a2 group (Figure 4D). Further, the results of the Transwell assay indicated that LiCl significantly increased the cell migration ability of Nr5a2 knockdown AGS cells (P<0.05) (Figure 4E). All these findings suggest that Nr5a2 promotes cellular proliferation and mobility by regulating the Wnt/beta-catenin signaling pathway in AGS cells.
Figure 4.
The oncogenic effect of Nr5a2 is mediated by activating the Wnt/β-catenin signaling pathway. (A) The effect of Nr5a2 knockdown on the activity of Wnt/β-catenin signaling pathway was assessed by dual-luciferase reporter assays in AGS cells. The results are expressed as mean ± SD of three independent experiments. (B) The expression levels of β-catenin, Wnt3a, and the Wnt/β-catenin signaling pathway target proteins c-Myc and CyclinD1 in control and Nr5a2 knockdown cells were detected by western blotting. (C) AGS-shNr5a2 cells were treated with or without 10 mM LiCl (an activator of the Wnt/β-catenin signaling pathway), and the expression levels of Wnt/β-catenin signaling pathway-related proteins were examined by western blotting. (D) The effect of LiCl on cell proliferation in Nr5a2-downregulated AGS cells was evaluated by a CCK-8 assay. The differences between the shNr5a2+ PBS and shNr5a2+ LiCl groups were analyzed byunpaired t-tests. (E) The effect of LiCl on cell migration in Nr5a2-downregulated AGS cells was evaluated by Transwell assay. The results are expressed as mean ± SD of three independent experiments. *P<0.05, **P<0.01, ***P<0.001. NC, negative control.
There was no significant difference in Nr5a2 expression in GC tissues compared with adjacent noncancerous tissues
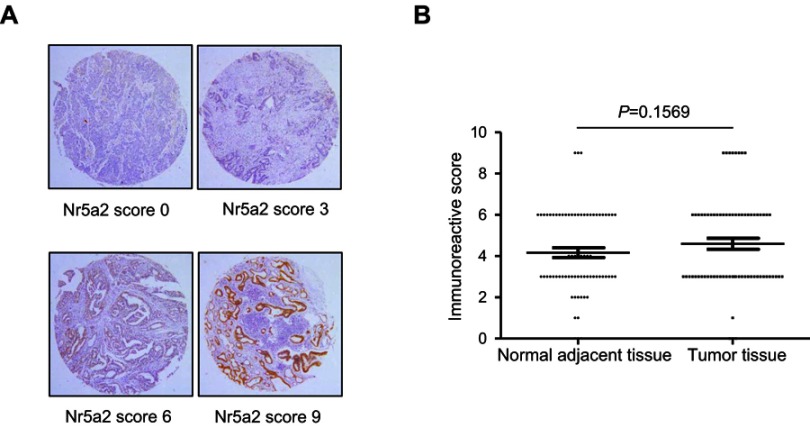
To determine the expression levels of Nr5a2 in GC tissues, tissue microarray (TMA) - immunohistochemistry (IHC) was performed in 72 paired primary GC tissues and adjacent noncancerous tissues. The expression of Nr5a2 is mainly exhibited in the nucleus and/or the cytoplasm. Strong or moderate staining of Nr5a2 was observed in 34/72 (47.2%) GC tissues, whereas weak or no staining was observed in 38/72 (52.8%) of them (Figure 5A). The mean expression level of Nr5a2 in the GC tissues (4.60±0.27) was higher than in the paired adjacent noncancerous tissues (4.16±0.24), but there was no significant difference between them (P>0.05) (Figure 5B).
Figure 5.
Nr5a2 expression in gastric cancer (GC) tissues and paired adjacent noncancerous tissues. (A) Representative immunohistochemistry images of Nr5a2 expression in GC (upper left: negative staining, upper right: weakly positive staining, lower left: moderately positive staining, and lower right: strongly positive staining). (B) The scores of the Nr5a2 expression levels in 72 paired GC tissues versus noncancerous tissues were analyzed by paired t-tests. The results are expressed as mean ± SD.
Discussion
It is well known that Nr5a2 plays an important role in the regulation of genes involved in reverse cholesterol transport and bile acid metabolism in the liver.5,7 In addition, more recent studies have identified Nr5a2 as a key regulator in the development of several types of cancer.11–16 Most of these studies have shown that Nr5a2 plays an oncogenic role and correlates with poor prognosis, although some reports have indicated the opposite role of Nr5a2.28 In our study, we demonstrated that Nr5a2 plays an important role in regulating proliferation and metastasis of AGS cells in vitro and in vivo. We showed that knockdown of Nr5a2 could inhibit the EMT process and suppress the activity of the Wnt/beta-catenin signaling pathway. These results suggest that Nr5a2 plays an important role in promoting GC progression.
Many studies have shown that Nr5a2 can promote cell proliferation in multiple types of tumors. In ER-positive breast cancer cells, Nr5a2 promotes cell proliferation by enhancing ERalpha mediated transcription of target genes such as GREB-1.19 Nr5a2 inhibition affects cell proliferation of different types of breast cancer cells by regulating CDKN1A transcription.20 In pancreatic cancer cell lines, Nr5a2 overexpression enhanced the expression of downstream target genes (Cyclin D1/E1) and stimulated cell proliferation.23 Selective blocking of Nr5a2 significantly inhibits pancreatic cancer cell proliferation in vitro. The inhibition effect is partly due to the attenuation of the receptor’s target genes (including Cyclin D1, E1, and c-Myc), which control cell growth, proliferation, and differentiation.30 Nr5a2 knockdown leads to significant impairment of proliferation in a colon cancer cell line with high Nr5a2 expression, and results in more modest impairment in a cell line with moderate Nr5a2 expression. Nr5a2 silencing can prolong the G0/G1 phase by cell-cycle analysis.15 Nr5a2, in complex with co-repressors, suppresses p53 action at the cell cycle inhibitor p21 gene, allowing colorectal cancer cells to evade cell cycle arrest mediated by p21.16 In our study, we found that knockdown Nr5a2 inhibited cellular proliferation in AGS cells. Cell cycle assays indicated that downregulation of Nr5a2 arrested AGS cells in the G2/M phase.
The epithelial to mesenchymal transition (EMT) plays an important role in carcinogenesis and metastatic progression. Previous literature has reported that NR5A2 could play a role in cancer stem cell (CSC) stemness and EMT in pancreatic cancer. NR5A2 knockdown resulted in increased expression of the epithelial marker E-cadherin and reduced expression of the mesenchymal marker Vimentin.25 Recent studies have confirmed that aberrant EMT activation is closely associated with gastric carcinogenesis and progression.38,39 Our results showed that Nr5a2 knockdown increased the expression of the epithelial marker E-cadherin and decreased the expression of the typical mesenchymal markers N-cadherin, Snail2, Vimentin and Twist1. Moreover, Nr5a2 knockdown resulted in a remarkably reduced expression of MMP2, which has previously been shown to induce EMT in a variety of cancers.40 These results suggest that Nr5a2 may promote cellular mobility and metastasis through regulating the process of EMT in GC.
The Wnt/beta-catenin signaling pathway is activated in multiple types of tumors. Particularly, activation of this pathway is found in about 30–50% of GC tissues and in many kinds of GC cell lines.41–43 Furthermore, the Wnt/beta-catenin pathway closely relates to EMT and plays a critical role in metastasis.44 Nr5a2 promotes cell cycle progression by two distinct mechanisms. Nr5a2 acts as co-activator of beta-catenin/Tcf4 and induces Cyclin D1 and c-Myc expression in a DNA binding-independent manner. Nr5a2 also binds the promoter of cyclin E1 and SHP. A combination of these two activities contributes to induce G1 cyclin and leads to accelerated cell cycle progression.45 Recent study has revealed that miR-219-5p may regulate cell proliferation, migration, and invasion by directly targets the 3ʹ-UTR of Nr5a2 and suppresses the Wnt/beta-catenin signaling pathway in human GC cells. Further, overexpression of Nr5a2 rescues miR-219-5p-mediated tumor suppression.46 In accordance with this, our data showed that Nr5a2 knockdown significantly decreased the activity of Wnt/beta-catenin signaling. The expression levels of Cyclin D1 and c-Myc, downstream targets of the Nr5a2 and beta-catenin signaling pathways, were decreased as well. However, as an established regulators for G1 progression, depressed cyclin D1 induced by Nr5a2 knockdown leads cells be arrested in the G2/M phase instead of the G1 phase, we speculated that cyclin D1 affects cell cycle together with other cell cycle regulators. Moreover, treating AGS-shNr5a2 cells with LiCl, an activator of the Wnt/beta-catenin signaling pathway, restored the cells’ proliferation, migration, and invasion properties. All these findings suggest that Nr5a2 promotes cellular proliferation and mobility by regulating the Wnt/beta-catenin signaling pathway in AGS cells. Nr5a2 is a constitutively active transcription factor, and previous studies have shown that b-catenin functions as its coactivator.47 LRH-1 coordinates intestinal cell renewal by controlling cell proliferation through crosstalk with the Wnt/b-catenin pathway.45 In the present study, as shown in Figure 4C, enhance the activaty of the Wnt/beta-catenin pathway by LiCl could also enhanced the level of Nr5a2. These results indicate that there may be a feedback loop between Nr5a2 and Wnt/beta-catenin pathway, and they may play a synergistic effect in GC development.Although the results showed that Nr5a2 could promote tumorigenesis and metastasis of AGS cells in vitro and in vivo, there was no significant difference of Nr5a2 expression in GC tissues compared with adjacent noncancerous tissues. It is probably because that the sample in this research is a little less (72 cases). The tendency of the expression level of Nr5a2 in the GC tissues is higher than in the paired adjacent noncancerous tissues, but there was no statistical difference. Moreover, many studies have reported that Nr5a2 plays an important role in cell stemness and embryonic development,8–10 so the difference of the expression level of Nr5a2 between GC tissues and adjacent noncancerous tissues might not be very great.However, the activation of the Wnt/beta-catenin pathway is found in about 30–50% of GC tissues.41–43 It is possible because that as a transcription factor, a minimal change in the expression level of Nr5a2 may lead to cascade reaction of downstream target genes. Therefore, the expression level of Nr5a2 in the GC tissues, and the relationship between Nr5a2 and clinicopathological characteristics of GC patients are worth further investigation.
Conclusion
Our study demonstrates that knockdown of Nr5a2 inhibits cell proliferation via arresting the cell cycle in the G2/M phase and suppressing cell mobility through blocking the Wnt/beta-catenin pathway and preventing the EMT process in AGS cells. These findings strongly indicate that Nr5a2 may act as an oncogene in GC development.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81402013), the Application and Basic Research Foundation of Science and Technology Department of Sichuan Province (2017JY0094), the Popularization and Application Foundation of Health and Family Planning Commission of Sichuan Province (17PJ484), the Youth Innovation Foundation of Medical Association of Sichuan Province (Q16006), and the Foundation of Chengdu Municipal Science and Technology Bureau (2018-YF05-00227-SN; 2015-HM01-00372-SF).
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Van Cutsem E, Sagaert X, Topal B, Haustermans K, Prenen H. Gastric cancer. Lancet. 2016;388(10060):2654–2664. doi: 10.1016/S0140-6736(16)30354-3 [DOI] [PubMed] [Google Scholar]
- 2.Fayard E, Auwerx J, Schoonjans K. LRH-1: an orphan nuclear receptor involved in development, metabolism and steroidogenesis. Trends Cell Biol. 2004;14(5):250–260. doi: 10.1016/j.tcb.2004.03.008 [DOI] [PubMed] [Google Scholar]
- 3.Goodwin B, Jones SA, Price RR, et al. A regulatory cascade of the nuclear receptors FXR, SHP-1, and LRH-1 represses bile acid biosynthesis. Mol Cell. 2000;6(3):517–526. [DOI] [PubMed] [Google Scholar]
- 4.Luo Y, Liang CP, Tall AR. The orphan nuclear receptor LRH-1 potentiates the sterol-mediated induction of the human CETP gene by liver X receptor. J Biol Chem. 2001;276(27):24767–24773. doi: 10.1074/jbc.M100912200 [DOI] [PubMed] [Google Scholar]
- 5.Chen F, Ma L, Dawson PA, et al. Liver receptor homologue-1 mediates species- and cell line-specific bile acid-dependent negative feedback regulation of the apical sodium-dependent bile acid transporter. J Biol Chem. 2003;278(22):19909–19916. doi: 10.1074/jbc.M207903200 [DOI] [PubMed] [Google Scholar]
- 6.Mataki C, Magnier BC, Houten SM, et al. Compromised intestinal lipid absorption in mice with a liver-specific deficiency of liver receptor homolog 1. Mol Cell Biol. 2007;27(23):8330–8339. doi: 10.1128/MCB.00852-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee YK, Schmidt DR, Cummins CL, et al. Liver receptor homolog-1 regulates bile acid homeostasis but is not essential for feedback regulation of bile acid synthesis. Mol endocrinol. 2008;22(6):1345–1356. doi: 10.1210/me.2007-0565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gu P, Goodwin B, Chung AC, et al. Orphan nuclear receptor LRH-1 is required to maintain Oct4 expression at the epiblast stage of embryonic development. Mol Cell Biol. 2005;25(9):3492–3505. doi: 10.1128/MCB.25.9.3492-3505.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heng JC, Feng B, Han J, et al. The nuclear receptor Nr5a2 can replace Oct4 in the reprogramming of murine somatic cells to pluripotent cells. Cell Stem Cell. 2010;6(2):167–174. doi: 10.1016/j.stem.2009.12.009 [DOI] [PubMed] [Google Scholar]
- 10.Wagner RT, Xu X, Yi F, Merrill BJ, Cooney AJ. Canonical Wnt/beta-catenin regulation of liver receptor homolog-1 mediates pluripotency gene expression. Stem Cells. 2010;28(10):1794–1804. doi: 10.1002/stem.502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dube C, Bergeron F, Vaillant MJ, Robert NM, Brousseau C, Tremblay JJ. The nuclear receptors SF1 and LRH1 are expressed in endometrial cancer cells and regulate steroidogenic gene transcription by cooperating with AP-1 factors. Cancer Lett. 2009;275(1):127–138. doi: 10.1016/j.canlet.2008.10.008 [DOI] [PubMed] [Google Scholar]
- 12.Franca MM, Ferraz-de-Souza B, Lerario AM, Fragoso MC, Lotfi CF. POD-1/TCF21 reduces SHP expression, affecting LRH-1 regulation and cell cycle balance in adrenocortical and hepatocarcinoma tumor cells. Biomed Res Int. 2015;2015:841784. doi: 10.1155/2015/841784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu P, Oosterveer MH, Stein S, et al. LRH-1-dependent programming of mitochondrial glutamine processing drives liver cancer. Genes Dev. 2016;30(11):1255–1260. doi: 10.1101/gad.277483.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xiao L, Wang Y, Xu K, et al. Nuclear receptor LRH-1 functions to promote castration-resistant growth of prostate cancer via its promotion of intratumoral androgen biosynthesis. Cancer Res. 2018;78(9):2205–2218. doi:10.1158/0008-5472.CAN-17-2341 [DOI] [PubMed] [Google Scholar]
- 15.Bayrer JR, Mukkamala S, Sablin EP, Webb P, Fletterick RJ. Silencing LRH-1 in colon cancer cell lines impairs proliferation and alters gene expression programs. Proc Natl Acad Sci U S A. 2015;112(8):2467–2472. doi: 10.1073/pnas.1500978112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kramer HB, Lai CF, Patel H, et al. LRH-1 drives colon cancer cell growth by repressing the expression of the CDKN1A gene in a p53-dependent manner. Nucleic Acids Res. 2016;44(2):582–594. doi: 10.1093/nar/gkv948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chand AL, Herridge KA, Thompson EW, Clyne CD. The orphan nuclear receptor LRH-1 promotes breast cancer motility and invasion. Endocr Relat Cancer. 2010;17(4):965–975. doi: 10.1677/ERC-10-0179 [DOI] [PubMed] [Google Scholar]
- 18.Chand AL, Herridge KA, Howard TL, Simpson ER, Clyne CD. Tissue-specific regulation of aromatase promoter II by the orphan nuclear receptor LRH-1 in breast adipose stromal fibroblasts. Steroids. 2011;76(8):741–744. doi: 10.1016/j.steroids.2011.02.024 [DOI] [PubMed] [Google Scholar]
- 19.Chand AL, Wijayakumara DD, Knower KC, et al. The orphan nuclear receptor LRH-1 and ERalpha activate GREB1 expression to induce breast cancer cell proliferation. PLoS One. 2012;7(2):e31593. doi: 10.1371/journal.pone.0031593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bianco S, Jangal M, Garneau D, Gevry N. LRH-1 controls proliferation in breast tumor cells by regulating CDKN1A gene expression. Oncogene. 2015;34(34):4509–4518. doi: 10.1038/onc.2014.382 [DOI] [PubMed] [Google Scholar]
- 21.Chang LY, Liu LY, Roth DA, et al. The major prognostic features of nuclear receptor NR5A2 in infiltrating ductal breast carcinomas. Int J Genomics. 2015;2015:403576. doi: 10.1155/2015/403576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pang JB, Molania R, Chand A, et al. LRH-1 expression patterns in breast cancer tissues are associated with tumour aggressiveness. Oncotarget. 2017;8(48):83626–83636. doi: 10.18632/oncotarget.18886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin Q, Aihara A, Chung W, et al. LRH1 as a driving factor in pancreatic cancer growth. Cancer Lett. 2014;345(1):85–90. doi: 10.1016/j.canlet.2013.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.von Figura G, Morris J, Wright CV, Hebrok M. Nr5a2 maintains acinar cell differentiation and constrains oncogenic Kras-mediated pancreatic neoplastic initiation. Gut. 2014;63(4):656–664. doi: 10.1136/gutjnl-2012-304287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luo Z, Li Y, Zuo M, et al. Effect of NR5A2 inhibition on pancreatic cancer stem cell (CSC) properties and epithelial-mesenchymal transition (EMT) markers. Mol Carcinog. 2017;56(5):1438–1448. doi: 10.1002/mc.22604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cobo I, Martinelli P, Flandez M, et al. Transcriptional regulation by NR5A2 links differentiation and inflammation in the pancreas. Nature. 2018;554(7693):533–537. doi: 10.1038/nature25751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang S, Zou Z, Luo X, Mi Y, Chang H, Xing D. LRH1 enhances cell resistance to chemotherapy by transcriptionally activating MDC1 expression and attenuating DNA damage in human breast cancer. Oncogene. 2018;37:3243–3259. doi: 10.1038/s41388-018-0193-4 [DOI] [PubMed] [Google Scholar]
- 28.Flandez M, Cendrowski J, Canamero M, et al. Nr5a2 heterozygosity sensitises to, and cooperates with, inflammation in KRas(G12V)-driven pancreatic tumourigenesis. Gut. 2014;63(4):647–655. doi: 10.1136/gutjnl-2012-304381 [DOI] [PubMed] [Google Scholar]
- 29.Lin Q, Aihara A, Chung W, et al. LRH1 promotes pancreatic cancer metastasis. Cancer Lett. 2014;350(1–2):15–24. doi: 10.1016/j.canlet.2014.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Benod C, Vinogradova MV, Jouravel N, Kim GE, Fletterick RJ, Sablin EP. Nuclear receptor liver receptor homologue 1 (LRH-1) regulates pancreatic cancer cell growth and proliferation. Proc Natl Acad Sci U S A. 2011;108(41):16927–16931. doi: 10.1073/pnas.1112047108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matsunou H, Konishi F, Jalal RE, Yamamichi N, Mukawa A. Alpha-fetoprotein-producing gastric carcinoma with enteroblastic differentiation. Cancer. 1994;73(3):534–540. [DOI] [PubMed] [Google Scholar]
- 32.Debruyne EN, Delanghe JR. Diagnosing and monitoring hepatocellular carcinoma with alpha-fetoprotein: new aspects and applications. Clin Chim Acta. 2008;395(1–2):19–26. doi: 10.1016/j.cca.2008.05.010 [DOI] [PubMed] [Google Scholar]
- 33.Noto JM, Peek RM Jr. Gastric-to-intestinal transdifferentiation and cancer. Proc Natl Acad Sci U S A. 2012;109(50):20173–20174. doi: 10.1073/pnas.1218345110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang L, Zhao Z, Feng W, et al. Long non-coding RNA TUG1 promotes colorectal cancer metastasis via EMT pathway. Oncotarget. 2016;7(32):51713–51719. doi: 10.18632/oncotarget.10563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wood LD, Calhoun ES, Silliman N, et al. Somatic mutations of GUCY2F, EPHA3, and NTRK3 in human cancers. Hum Mutat. 2006;27(10):1060–1061. doi: 10.1002/humu.9452 [DOI] [PubMed] [Google Scholar]
- 36.Ghahhari NM, Babashah S. Interplay between microRNAs and WNT/beta-catenin signalling pathway regulates epithelial-mesenchymal transition in cancer. Eur J Cancer. 2015;51(12):1638–1649. doi: 10.1016/j.ejca.2015.04.021 [DOI] [PubMed] [Google Scholar]
- 37.Fernandez-Marcos PJ, Auwerx J, Schoonjans K. Emerging actions of the nuclear receptor LRH-1 in the gut. Biochim Biophys Acta. 2011;1812(8):947–955. doi: 10.1016/j.bbadis.2010.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsuji T, Ibaragi S, Hu GF. Epithelial-mesenchymal transition and cell cooperativity in metastasis. Cancer Res. 2009;69(18):7135–7139. doi: 10.1158/0008-5472.CAN-09-1618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roussos ET, Keckesova Z, Haley JD, Epstein DM, Weinberg RA, Condeelis JS. AACR special conference on epithelial-mesenchymal transition and cancer progression and treatment. Cancer Res. 2010;70(19):7360–7364. doi: 10.1158/0008-5472.CAN-10-1208 [DOI] [PubMed] [Google Scholar]
- 40.Qiao B, Johnson NW, Gao J. Epithelial-mesenchymal transition in oral squamous cell carcinoma triggered by transforming growth factor-beta1 is Snail family-dependent and correlates with matrix metalloproteinase-2 and −9 expressions. Int J Oncol. 2010;37(3):663–668. [DOI] [PubMed] [Google Scholar]
- 41.Ooi CH, Ivanova T, Wu J, et al. Oncogenic pathway combinations predict clinical prognosis in gastric cancer. PLoS Genet. 2009;5(10):e1000676. doi: 10.1371/journal.pgen.1000676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Clements WM, Wang J, Sarnaik A, et al. beta-catenin mutation is a frequent cause of Wnt pathway activation in gastric cancer. Cancer Res. 2002;62(12):3503–3506. [PubMed] [Google Scholar]
- 43.Ikenoue T, Ijichi H, Kato N, et al. Analysis of the beta-catenin/T cell factor signaling pathway in 36 gastrointestinal and liver cancer cells. Jpn J Cancer Res. 2002;93(11):1213–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Talbot LJ, Bhattacharya SD, Kuo PC. Epithelial-mesenchymal transition, the tumor microenvironment, and metastatic behavior of epithelial malignancies. Int J Biochem Mol Biol. 2012;3(2):117–136. [PMC free article] [PubMed] [Google Scholar]
- 45.Botrugno OA, Fayard E, Annicotte JS, et al. Synergy between LRH-1 and beta-catenin induces G1 cyclin-mediated cell proliferation. Mol Cell. 2004;15(4):499–509. doi: 10.1016/j.molcel.2004.07.009 [DOI] [PubMed] [Google Scholar]
- 46.Li C, Dong J, Han Z, Zhang K. MicroRNA-219-5p represses the proliferation, migration, and invasion of gastric cancer cells by targeting the LRH-1/Wnt/beta-catenin signaling pathway. Oncol Res. 2017;25(4):617–627. doi: 10.3727/096504016X14768374457986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lapointe E, Boerboom D. WNT signaling and the regulation of ovarian steroidogenesis. Front Biosci (Schol Ed). 2011;3:276–285. [DOI] [PubMed] [Google Scholar]